The Impact of the Reduction in Environmental Pollution during COVID-19 Lockdown on Healthy Individuals
Abstract
:1. Introduction
2. Material and Methods
2.1. Study Design and Subjects
2.2. Oxidative Stress Biomarker Measurement
2.3. Cytokine Measurement
2.4. Data Analysis
3. Results
3.1. Demographic Characteristics of the Study Population
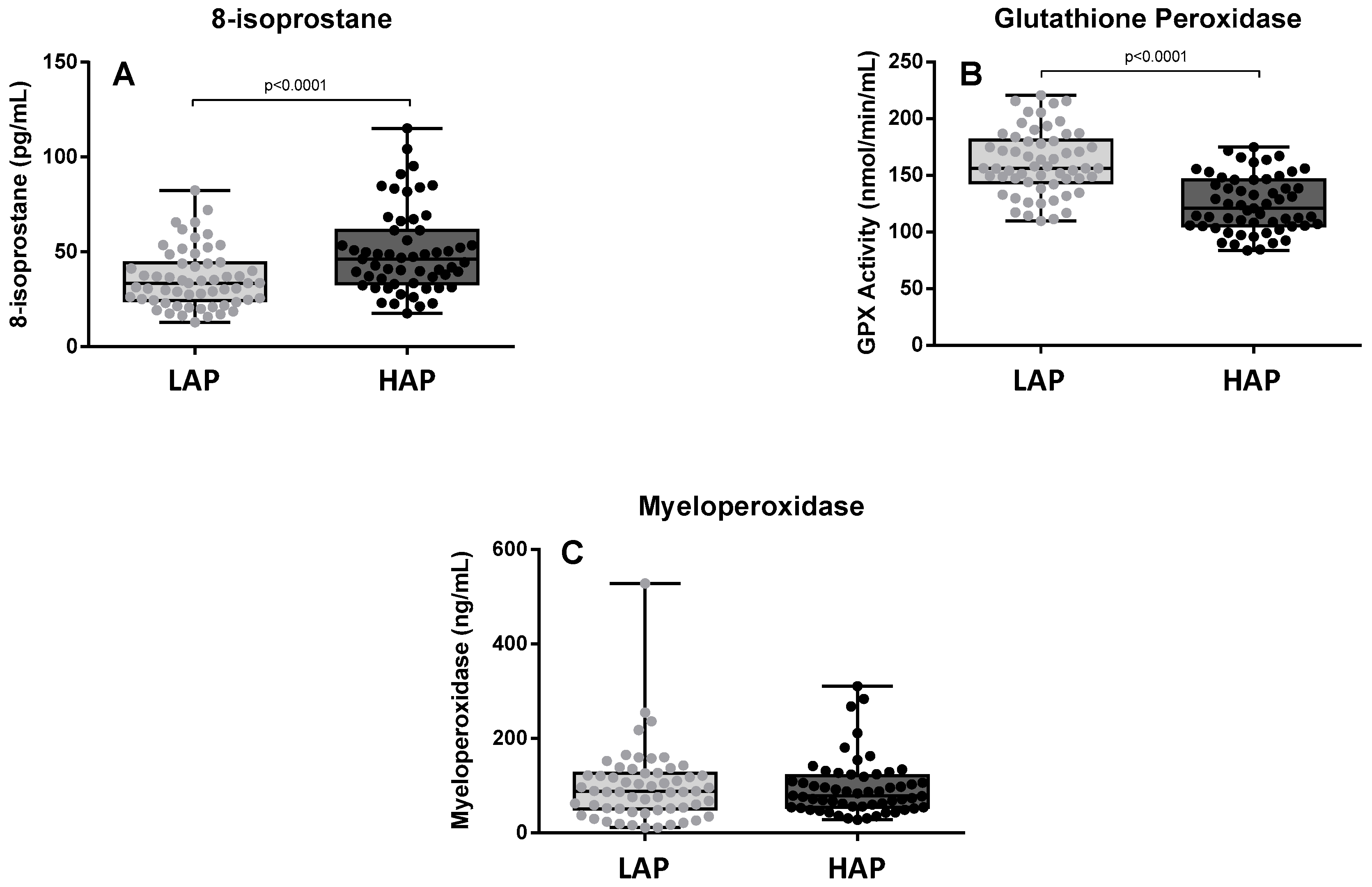
3.2. Oxidative Stress Levels
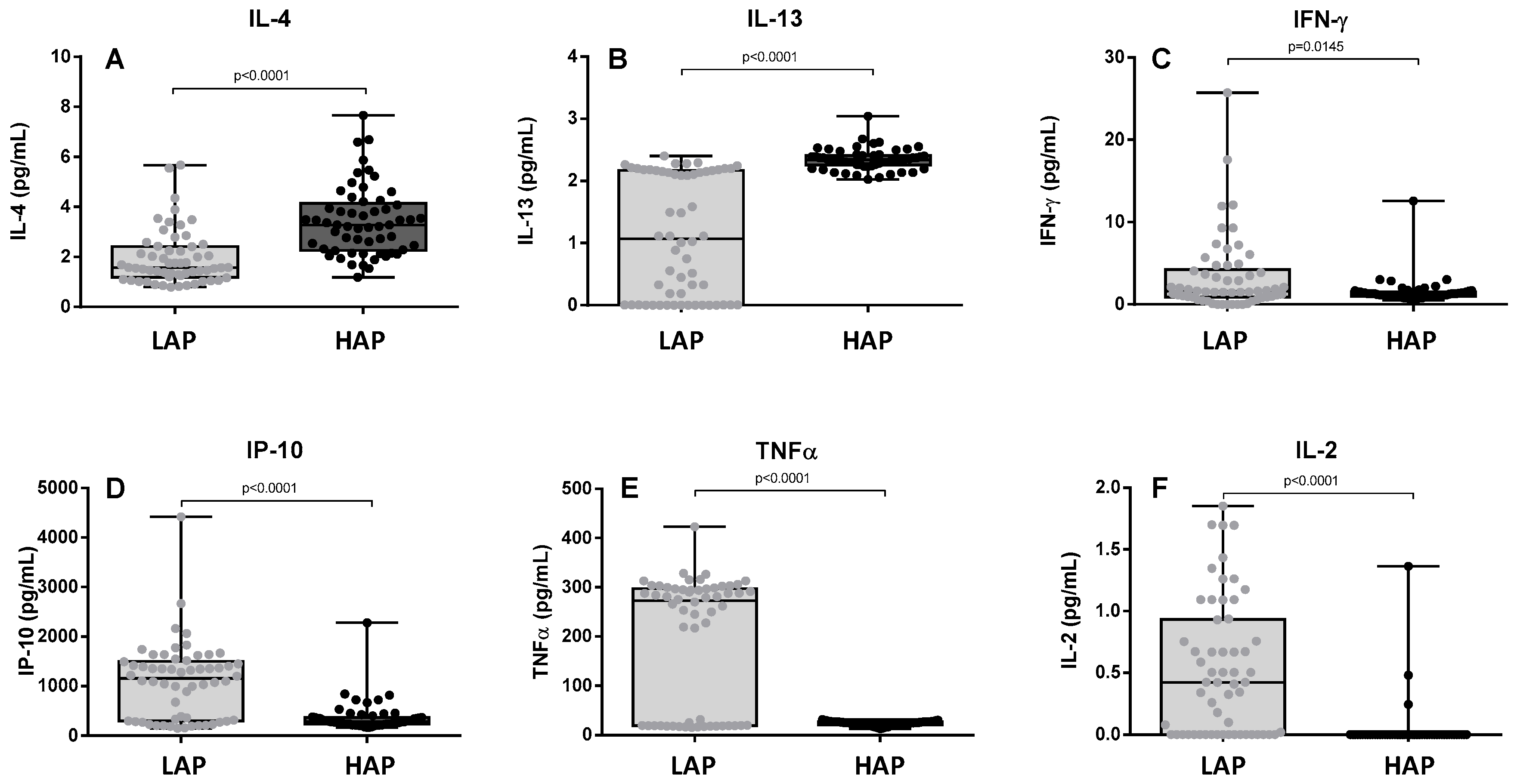
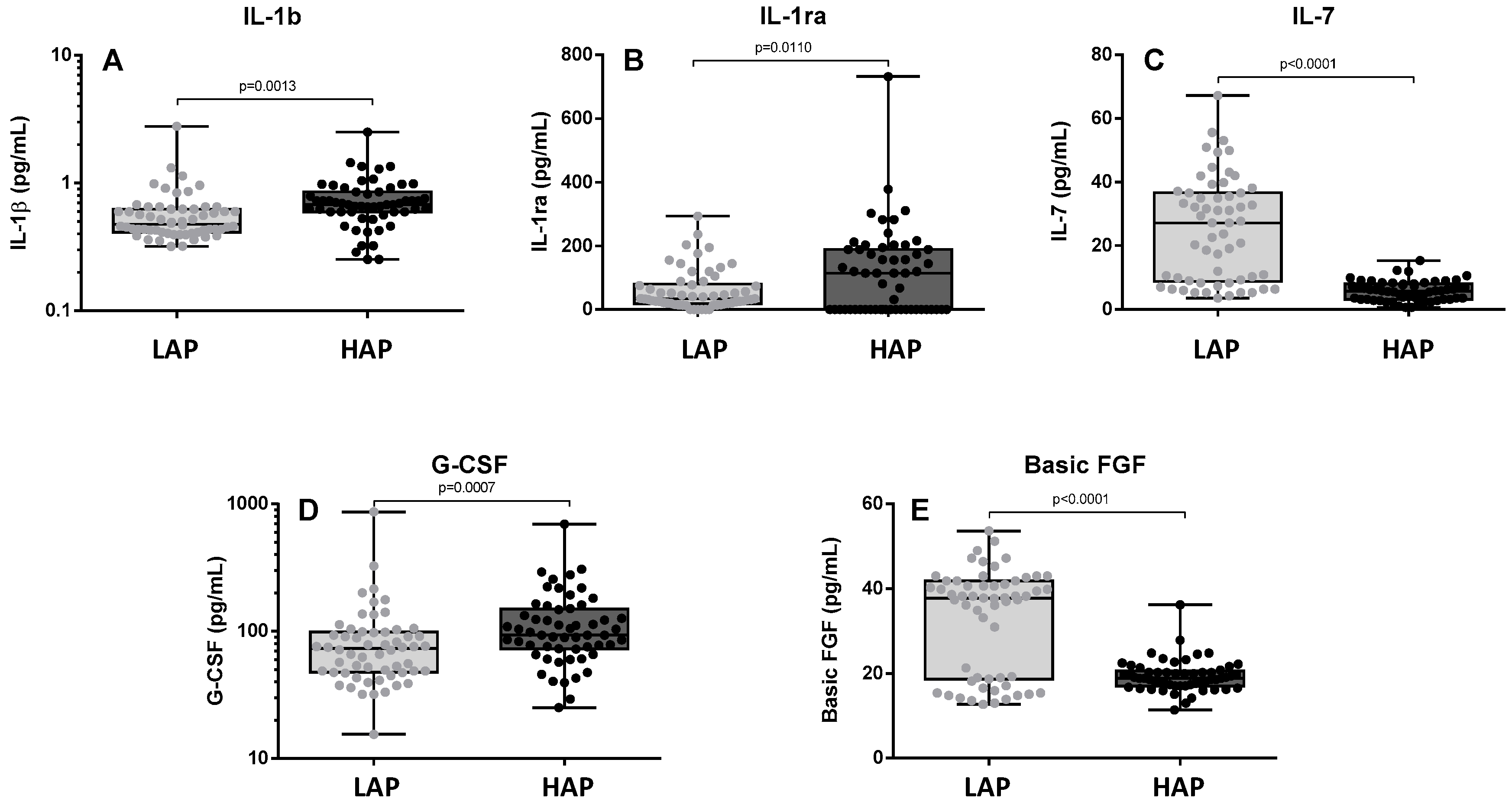
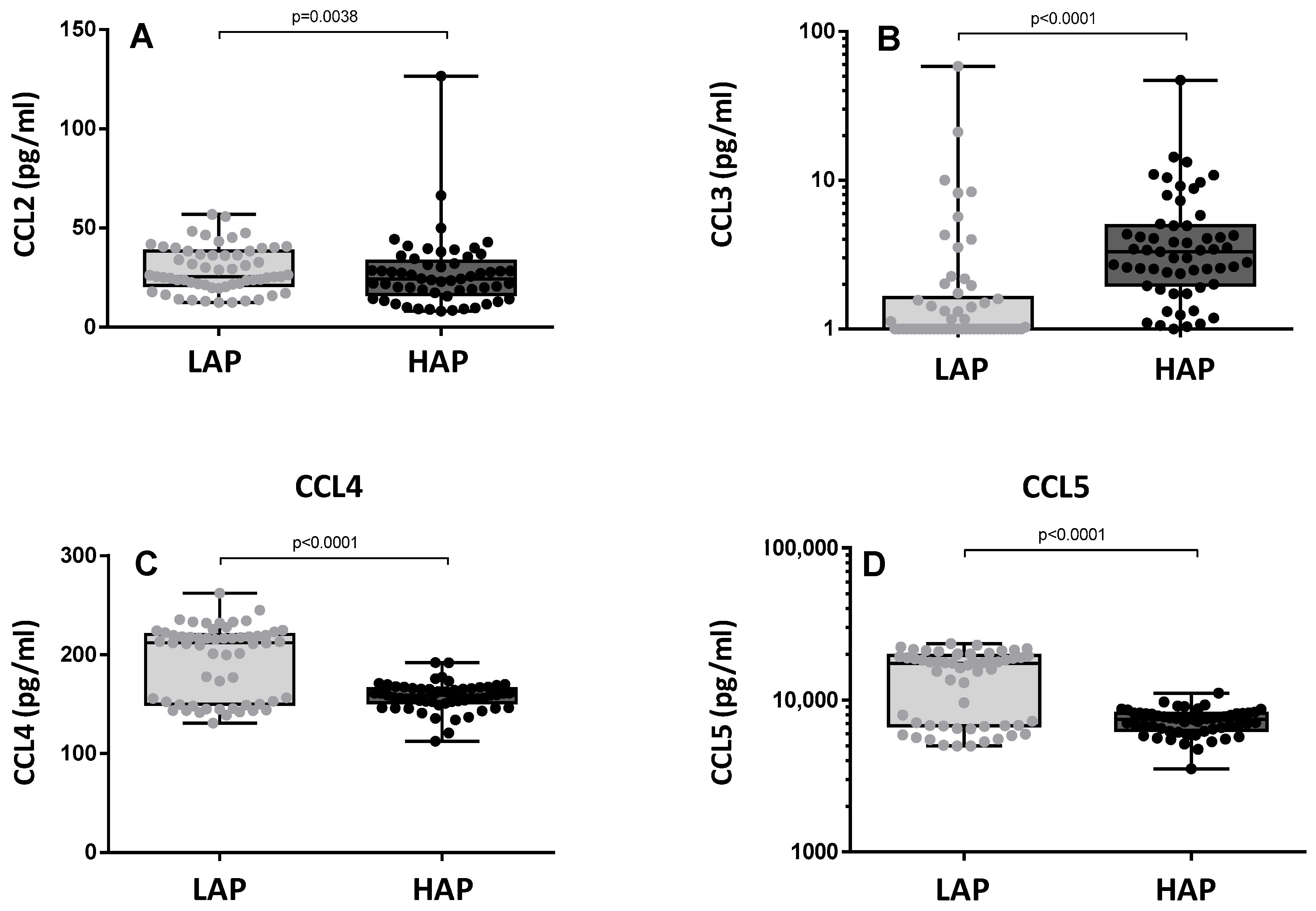
3.3. Cytokine Levels
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Thurston, G.D.; Kipen, H.; Annesi-Maesano, I.; Balmes, J.; Brook, R.D.; Cromar, K.; De Matteis, S.; Forastiere, F.; Forsberg, B.; Frampton, M.W.; et al. A joint ERS/ATS policy statement: What constitutes an adverse health effect of air pollution? An analytical framework. Eur. Respir. J. 2017, 49, 1600419. [Google Scholar] [CrossRef] [PubMed]
- GBD 2019 Risk Factors Collaborators. Global burden of 87 risk factors in 204 countries and territories, 1990–2019: A systematic analysis for the Global Burden of Disease Study 2019. Lancet 2020, 396, 1223–1249. [Google Scholar] [CrossRef] [PubMed]
- Samoli, E.; Rodopoulou, S.; Schneider, A.; Morawska, L.; Stafoggia, M.; Renzi, M.; Breitner, S.; Lanki, T.; Pickford, R.; Schikowski, T.; et al. Meta-analysis on short-term exposure to ambient ultrafine particles and respiratory morbidity. Eur. Respir. Rev. 2020, 29, 200116. [Google Scholar] [CrossRef] [PubMed]
- Olesiejuk, K.; Chałubiński, M. How does particulate air pollution affect barrier functions and inflammatory activity of lung vascular endothelium? Allergy 2023, 78, 629–638. [Google Scholar] [CrossRef] [PubMed]
- Moufarrej, L.; Verdin, A.; Cazier, F.; Ledoux, F.; Courcot, D. Oxidative stress response in pulmonary cells exposed to different fractions of PM2.5-0.3 from urban, traffic and industrial sites. Environ. Res. 2023, 216 Pt 2, 114572. [Google Scholar] [CrossRef] [PubMed]
- Xu, R.; Li, Z.; Zhu, X.; Guo, C.; Su, Q.; Peng, J.; Wang, Z.; Qian, Y.; Li, X.; Xu, Q.; et al. Acute effects of exposure to fine particulate matter and ozone on lung function, inflammation and oxidative stress in healthy adults. Ecotoxicol. Environ. Saf. 2022, 243, 114013. [Google Scholar] [CrossRef]
- Zhang, W.; Ma, R.; Wang, Y.; Jiang, N.; Zhang, Y.; Li, T. The relationship between particulate matter and lung function of children: A systematic review and meta-analysis. Environ. Pollut. 2022, 309, 119735. [Google Scholar] [CrossRef]
- Zong, Z.; Zhao, M.; Zhang, M.; Xu, K.; Zhang, Y.; Zhang, X.; Hu, C. Association between PM1 Exposure and Lung Function in Children and Adolescents: A Systematic Review and Meta-Analysis. Int. J. Environ. Res. Public Health 2022, 19, 15888. [Google Scholar] [CrossRef]
- Andrade, A.; D‘Oliveira, A.; De Souza, L.C.; Bastos, A.C.R.F.; Dominski, F.H.; Stabile, L.; Buonanno, G. Effects of Air Pollution on the Health of Older Adults during Physical Activities: Mapping Review. Int. J. Environ. Res. Public Health 2023, 20, 3506. [Google Scholar] [CrossRef]
- García-Dalmau, M.; Udina, M.; Bech, J.; Sola, Y.; Montolio, J.; Jaén, C. Pollutant Concentration Changes During the COVID-19 Lockdown in Barcelona and Surrounding Regions: Modification of Diurnal Cycles and Limited Role of Meteorological Conditions. Bound. Layer Meteorol. 2022, 183, 273–294. [Google Scholar] [CrossRef]
- Gorrochategui, E.; Hernandez, I.; Pérez-Gabucio, E.; Lacorte, S.; Tauler, R. Temporal air quality (NO2, O3, and PM10) changes in urban and rural stations in Catalonia during COVID-19 lockdown: An association with human mobility and satellite data. Environ. Sci. Pollut. Res. Int. 2022, 29, 18905–18922. [Google Scholar] [CrossRef] [PubMed]
- Prüss-Ustün, A.; Wolf, J.; Corvalán, C.; Neville, T.; Bos, R.; Neira, M. Diseases due to unhealthy environments: An updated estimate of the global burden of disease attributable to environmental determinants of health. J. Public Health 2017, 39, 464–475. [Google Scholar] [CrossRef] [PubMed]
- Muñoz, X.; Barreiro, E.; Bustamante, V.; Lopez-Campos, J.L.; González-Barcala, F.J.; Cruz, M.J. Diesel exhausts particles: Their role in increasing the incidence of asthma. Reviewing the evidence of a causal link. Sci. Total Environ. 2019, 652, 1129–1138. [Google Scholar] [CrossRef] [PubMed]
- Roberts, L.J., II; Morrow, J.D. The generation and actions of isoprostanes. Biochim. Biophys. Acta 1997, 1345, 121–135. [Google Scholar] [CrossRef]
- Delanty, N.; Reilly, M.; Practicò, D.; Fitzgerald, D.J.; Lawson, J.A.; FitzGerald, G.A. 8-Epi-PGF2a: Specific analysis of an isoeicosanoid as an index of oxidant stress in vivo. Br. J. Clin. Pharmacol. 1996, 42, 15–19. [Google Scholar] [CrossRef]
- Xiao, G.G.; Wang, M.; Li, N.; Loo, J.A.; Nel, A.E. Use of Proteomics to Demonstrate a Hierarchical Oxidative Stress Response to Diesel Exhaust Particle Chemicals in a Macrophage Cell Line. J. Biol. Chem. 2003, 278, 50781–50790. [Google Scholar] [CrossRef] [PubMed]
- Biswas, S.K.; Rahman, I. Environmental toxicity, redox signaling and lung inflammation: The role of glutathione. Mol. Asp. Med. 2009, 30, 60–76. [Google Scholar] [CrossRef]
- Kooter, I.M.; Gerlofs-Nijland, M.E.; Boere, A.J.; Leseman, D.L.; Fokkens, P.H.; Spronk, H.M.; Frederix, K.; Ten Cate, H.; Knaapen, A.M.; Vreman, H.J.; et al. Diesel engine exhaust initiates a sequence of pulmonary and cardiovascular effects in rats. J. Toxicol. 2010, 2010, 206057. [Google Scholar] [CrossRef]
- Ribeiro Júnior, G.; de Souza Xavier Costa, N.; Belotti, L.; Dos Santos Alemany, A.A.; Amato-Lourenço, L.F.; da Cunha, P.G.; de Oliveira Duro, S.; Ribeiro, S.P.; Veras, M.M.; Quirino Dos Santos Lopes, F.D.T.; et al. Diesel exhaust exposure intensifies inflammatory and structural changes associated with lung aging in mice. Ecotoxicol. Environ. Saf. 2019, 170, 314–323. [Google Scholar] [CrossRef]
- Patel, H.; Eo, S.; Kwon, S. Effects of diesel particulate matters on inflammatory responses in static and dynamic culture of human alveolar epithelial cells. Toxicol. Lett. 2011, 200, 124–131. [Google Scholar] [CrossRef]
- Zhao, H.; Ma, J.K.; Barger, M.W.; Mercer, R.R.; Millecchia, L. Reactive oxygen species- and nitric oxide-mediated lung inflammation and mitochondrial dysfunction in wild-type and iNOS-deficient mice exposed to diesel exhaust particles. J. Toxicol. Environ. Health 2009, 72, 560–570. [Google Scholar] [CrossRef] [PubMed]
- Bouredji, A.; Pourchez, J.; Forest, V. Biological effects of Tire and Road Wear Particles (TRWP) assessed by in vitro and in vivo studies—A systematic review. Sci. Total Environ. 2023, 894, 164989. [Google Scholar] [CrossRef]
- Baulig, A.; Garlatti, M.; Bonvallot, V.; Marchand, A.; Barouki, R.; Marano, F.; Baeza-Squiban, A. Involvement of reative oxygen species in the metabolic pathways triggered by diesel exhaust particles in human airway epithelial cells. Am. J. Physiol. Lung Cell. Mol. Physiol. 2003, 285, 671–679. [Google Scholar] [CrossRef] [PubMed]
- Chaudhuri, N.; Paiva, C.; Donaldson, K.; Duffin, R.; Parker, L.C.; Sabroe, I. Diesel exhaust particles override natural injury limiting pathways in the lung. Am. J. Physiol. Lung Cell. Mol. Physiol. 2010, 299, 263–271. [Google Scholar] [CrossRef] [PubMed]
- Van Den Eeckhout, B.; Tavernier, J.; Gerlo, S. Interleukin-1 as Innate Mediator of T Cell Immunity. Front. Immunol. 2021, 11, 621931. [Google Scholar] [CrossRef] [PubMed]
- Rutella, S. Granulocyte colony-stimulating factor for the induction of T-cell tolerance. Transplantation 2007, 84 (Suppl. S1), S26–S30. [Google Scholar] [CrossRef] [PubMed]
- MacKall, C.L.; Fry, T.J.; Gress, R.E. Harnessing the biology of IL-7 for therapeutic application. Nat. Rev. Immunol. 2011, 11, 330–342. [Google Scholar] [CrossRef] [PubMed]
- Laddha, A.P.; Kulkarni, Y.A. VEGF and FGF-2: Promising targets for the treatment of respiratory disorders. Respir. Med. 2019, 156, 33–46. [Google Scholar] [CrossRef]
- Ren, M.; Guo, Q.; Guo, L.; Lenz, M.; Qian, F.; Koenen, R.R.; Xu, H.; Schilling, A.B.; Weber, C.; Ye, R.D.; et al. Polymerization of MIP-1 chemokine (CCL3 and CCL4) and clearance of MIP-1 by insulin-degrading enzyme. EMBO J. 2010, 29, 3952–3966. [Google Scholar] [CrossRef]
- Dai, Y.; Ren, D.; Bassig, B.A.; Vermeulen, R.; Hu, W.; Niu, Y.; Duan, H.; Ye, M.; Meng, T.; Xu, J.; et al. Occupational exposure to diesel engine exhaust and serum cytokine levels. Environ. Mol. Mutagen. 2018, 59, 144–150. [Google Scholar] [CrossRef]
- Nel, A.E.; Diaz-Sanchez, D.; Ng, D.; Hiura, T.; Saxon, A. Enhancement of allergic inflammation by the interaction between diesel exhaust particles and the immune system. J. Allergy Clin. Immunol. 1998, 102, 539. [Google Scholar] [CrossRef] [PubMed]
- Diaz-Sanchez, D.; Tsien, A.; Fleming, J.; Saxon, A. Combined diesel exhaust particulate and ragweed allergen challenge markedly enhances human in vivo nasal ragweed-specific IgE and skews cytokine production to a T helper cell 2-type pattern. J. Immunol. 1997, 158, 2406. [Google Scholar] [CrossRef] [PubMed]
- Provoost, S.; Maes, T.; Joos, G.F.; Tournoy, K.G. Monocyte-derived dendritic cell recruitment and allergic Th2 responses after exposure to diesel particles are CCR2 dependent. J. Allergy Clin. Immunol. 2012, 129, 483–491. [Google Scholar] [CrossRef] [PubMed]
- Sauty, A.; Dziejman, M.; Taha, R.A.; Iarossi, A.S.; Neote, K.; Garcia-Zepeda, E.A.; Hamid, Q.; Luster, A.D. The T cell-specific CXC chemokines IP-10, Mig, and I-TAC are expressed by activated human bronchial epithelial cells. J. Immunol. 1999, 162, 3549. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Romero-Mesones, C.; de Homdedeu, M.; Soler-Segovia, D.; Gómez-Ollés, C.; Espejo-Castellanos, D.; Ojanguren, I.; Saez-Gimenez, B.; Cruz, M.-J.; Munoz, X. The Impact of the Reduction in Environmental Pollution during COVID-19 Lockdown on Healthy Individuals. Toxics 2024, 12, 492. https://doi.org/10.3390/toxics12070492
Romero-Mesones C, de Homdedeu M, Soler-Segovia D, Gómez-Ollés C, Espejo-Castellanos D, Ojanguren I, Saez-Gimenez B, Cruz M-J, Munoz X. The Impact of the Reduction in Environmental Pollution during COVID-19 Lockdown on Healthy Individuals. Toxics. 2024; 12(7):492. https://doi.org/10.3390/toxics12070492
Chicago/Turabian StyleRomero-Mesones, Christian, Miquel de Homdedeu, David Soler-Segovia, Carlos Gómez-Ollés, David Espejo-Castellanos, Inigo Ojanguren, Berta Saez-Gimenez, María-Jesús Cruz, and Xavier Munoz. 2024. "The Impact of the Reduction in Environmental Pollution during COVID-19 Lockdown on Healthy Individuals" Toxics 12, no. 7: 492. https://doi.org/10.3390/toxics12070492
APA StyleRomero-Mesones, C., de Homdedeu, M., Soler-Segovia, D., Gómez-Ollés, C., Espejo-Castellanos, D., Ojanguren, I., Saez-Gimenez, B., Cruz, M.-J., & Munoz, X. (2024). The Impact of the Reduction in Environmental Pollution during COVID-19 Lockdown on Healthy Individuals. Toxics, 12(7), 492. https://doi.org/10.3390/toxics12070492







