Abstract
Due to their robust migration capabilities, slow degradation, and propensity for adsorbing environmental pollutants, micro(nano)plastics (MNPs) are pervasive across diverse ecosystems. They infiltrate various organisms within different food chains through multiple pathways including inhalation and dermal contact, and pose a significant environmental challenge in the 21st century. Research indicates that MNPs pose health threats to a broad range of organisms, including humans. Currently, extensive detection data and studies using experimental animals and in vitro cell culture indicate that MNPs can trigger various forms of programmed cell death (PCD) and can induce various diseases. This review provides a comprehensive and systematic analysis of different MNP-induced PCD processes, including pyroptosis, ferroptosis, autophagy, necroptosis, and apoptosis, based on recent research findings and focuses on elucidating the links between PCD and diseases. Additionally, targeted therapeutic interventions for these diseases are described. This review provides original insights into the opportunities and challenges posed by current research findings. This review evaluates ways to mitigate various diseases resulting from cell death patterns. Moreover, this paper enhances the understanding of the biohazards associated with MNPs by providing a systematic reference for subsequent toxicological research and health risk mitigation efforts.
1. Introduction
Microplastics (MPs) are plastic fragments and particles with diameters less than 5 mm [1,2], and particles smaller than 1000 nm are classified as nanoplastics (NPs) [3]. MNPs are widely distributed in many ecosystems, including oceans, lakes, soil, and farmland [4,5]. However, MNPs are not confined to specific ecosystems; they can be found virtually anywhere in the world, influenced by numerous complex migration pathways such as wind currents, water currents, and biological activities [6,7]. For the past few years, increasing attention has been focused on the adverse impacts of MNPs on various organisms. Reports indicate that the toxicity of MNPs to various organisms is predominantly sublethal [8,9], and their toxic effects can be categorized into three primary forms: including the release of toxic chemical components, acting as carriers to adsorb harmful substances, and causing physical damage to biological organs through inhalation or ingestion [10,11]. Human activities have led to the widespread pollution of ecosystems with MNPs, resulting in inevitable human exposure to MNPs [12]. Bharath K et al. examined the type of MNPs in groundwater and found that the main components were polystyrene (PS) and polypropylene (PP), while small amounts of polyethylene (PE) and polyvinyl chloride (PVC) were also found [13]. Given that humans occupy the top of the food chain, they unavoidably consume food items, such as water and seafood, that are contaminated by MNPs in their daily lives. Additionally, humans might encounter MNPs through inhaling airborne particles and skin contact. Zhu et al. performed a systematic analysis of MPs larger than 20 μm in the human digestive and respiratory systems, and revealed their ubiquity in the human body [14]. Numerous studies have examined the detrimental effects of MNPs on the human body [15,16,17,18,19]. Lin et al. conducted a study on the impact of 80 nm PS-NPs on mitochondrial metabolic pathways and function in normal liver (L02) cells and lung (BEAS-2B) cells. They showed that exposure to NPs could not result in significant cell death; instead, it induced mitochondrial dysfunction [20]. Wu et al. assessed the low toxicity of MPs by examining alterations in cell survival, intracellular reactive oxygen species (ROS) levels, membrane integrity and fluidity, and mitochondrial depolarization in Caco-2 cells exposed to PS-MPs [21]. Weber et al. demonstrated that NPs induced inflammation in monocyte-derived dendritic cells and primary human monocytes [22]. Workers in the plastics and textile industries are chronically exposed to plastic fibers and have been reported to experience microplastic-induced lung damage, which is characterized by inflammation, allergies, and fibrosis [23,24]. Pulmonary inflammation is closely associated with disturbances in various modes of cell death, including pyroptosis, autophagy, and apoptosis [25,26,27,28,29]. Halimu et al. reported that PS-NPs induced apoptosis in the human lung epithelial cell A549 [30], Wang et al. reported that nanoparticles formed by silica induced inflammation by causing lysosome damage and dysfunction in autophagy through ROS/PARP/TRPM2 signaling pathways. [31], and Wu et al. reported that exposure to amine-polystyrene (APS) -NPs activated NLPR3 inflammasomes in MLE-12 cells, thereby inducing pyroptosis [32]. A recent study revealed that anionic NPs enhanced the aggregation of Parkinson’s disease-associated α-synuclein, thereby exacerbating the spread of α-synuclein pathology in interconnected vulnerable regions of the brain [33]. In summary, extensive exposure to MNPs can induce various types of cytotoxicity [34,35], and increase the risk of disease development. Marfella et al. were the first to demonstrate a link between MNPs and human health. Their research showed that MNPs infiltrate human arteries, significantly increasing the risk of serious diseases, including heart disease, stroke, and mortality [36]. However, the size and concentration of toxic MNPs vary among different species, influenced by the individual organism’s response to external stressors. A new study indicated that patterns of MPs in bronchoalveolar lavage fluid (BALF) in children with lung disease [37]. Consequently, MNP exposure is closely linked to human health and may result in damage to the reproductive system, nervous system, cardiovascular system, respiratory system, digestive system, immune system, endocrine system, and motor system [38,39] (Figure S1).
Numerous studies have shown that MNPs can induce varying degrees of toxicity in numerous species, including mice, zebrafish, oysters, chickens, earthworms, nematodes and sea urchins [40,41,42,43,44,45,46]. This toxicity induces liver toxicity, neurotoxicity, reproductive toxicity, and developmental toxicity. Li et al. demonstrated that PS-MPs could disrupt glycolytic flux and induce apoptosis through calcium overload in a mouse model [47]. Another study demonstrated that stress responses caused by exposure to PS-MPs exacerbated lipopolysaccharide (LPS)/d-galactosamine (d-GalN)-induced death in mice and increased the risk of liver damage [48]. In toxicology research, biological response pathways can be used to explain the pathway by which toxicity arises and how it ultimately leads to adverse health consequences for the organism. Thus, monitoring pre-disease events at the cellular level can effectively predict the harm caused by toxic substances to organisms. The physiological state of cells can reflect cellular responses to external substances [49]. Hence, it is crucial to assess the virulence of environmental exposure to MPs and the correlated metabolic pathways at the cellular level. A previous review delineated PCD induced by MNPs and scrutinized the various factors contributing to distinct forms of PCD triggered by MNPs, including particle size, functional group modification, surface potential, aging, and co-exposure. The particle size of MNPs, differences in surface-modified charge, aging, and co-exposure with other substances often influence changes in the mode of PCD [50]. Similarly, different exposure models produce varying toxic effects. Model animals, such as mice, zebrafish, and chickens, as well as specific cell lines, are often used to investigate MNP-induced PCD. To understand the potential biological impact of MNPs, we collected nearly five years of research papers on PCD caused by MNPs (Table 1) and were surprised to find that only two studies used PP-MPs, one study used PE-NPs, while the other studies used PS-MNPs. Additionally, long-term exposure at low doses is usually studied in animals, whereas acute exposure at higher doses is typically examined in cells. This review offers a comprehensive examination of microplastic-induced pyroptosis, ferroptosis, autophagy, necroptosis, and apoptosis, and sheds light on the pathways and mechanisms of MNP-induced cytotoxicity. Indeed, the various forms of PCD induced by MNPs are closely intertwined. Variations in environmental conditions, MNPs’ type and size, and species differences can prompt reciprocal conversions of PCD. Additionally, it summarizes the prospects and challenges of these processes in human diseases.

Table 1.
Cell death caused by MNPs, sorted by the type of PCD.
2. Research Status of PCD Caused by MNPs and the Association between PCD and Disease
This review provides a systematic analysis of the PCD induced by MNPs along with their close association with disease. Following the identification of MNP-PCD links, we conducted a comprehensive manual sea rch on Web of Science using predefined keywords related to MNPs and cell death, including (“microplastics” or “nanoplastics”) and (“pyroptosis”, “ferroptosis”, “autophagy”, “necroptosis”, or “apoptosis”). Additionally, we employed predefined keywords associated with cell death and disease, such as (“pyroptosis”, “ferroptosis”, “autophagy”, “necroptosis”, “apoptosis”, or “cuproptosis”) and (“disease”). Our database searches were restricted to articles published within the last decade.
We employed VOSviewer software (Version 1.6.20) to gain insights into these articles, conducting an analysis of terms, keywords, and categories. Prominent keywords shed light on PCD induced by MNPs and underscore the strong association between PCD and disease. This correlation not only delineates a significant avenue for research but also aids in organizing and summarizing subsequent findings. Initially, 423 papers were preliminarily identified over the past decade. Among them, there are 320 studies on MNPs, of which 121 studies focus on the generation and analysis of MNPs and pay little attention to the biological effects of MNPs. After checking the title, abstract, keywords, and abbreviations and considering their impact, 241 papers were included in this review. Utilizing VOSviewer, bibliometric analysis encompassed titles, abstracts, and keywords from 2015 to 2024. Additionally, we performed a keyword evolution analysis to pinpoint key research areas and identify recent trends (Figure S2). The analysis revealed that 62 keywords were cited at least 15 times each. Essential keywords such as “MNP”, “cell death”, and “disease” have emerged as focal points in current research, underscoring the imperative for continued investigation in these areas.
3. Molecular Regulatory Mechanisms of Pyroptosis Induced by MNPs and Associated Disease Risks
3.1. Pyroptosis Caused by Micro(nano)plastics
Pyroptosis represents a form of PCD facilitated by Gasdermin-D (GSDMD), typified by cellular swelling culminating in membrane rupture, thereby releasing cellular contents and eliciting a robust inflammatory reaction [86]. GSDMD is both the substrate of caspases-11/4/5 and the central executor of pyroptosis. The pore-forming activity of the Gasdermin-N domain drives pyroptosis, resulting in cell death [87,88,89]. Numerous studies have demonstrated that exposure to microplastics induces pyroptosis in cells in various organisms, including mice, chickens, and humans. Wei et al. demonstrated myocardial toxicity in mice exposed to PS-MPs and revealed that MPs induced pyroptosis through the GSDMD/Caspase-1/NLRP3 pathway [52]. Similarly, Hou et al. demonstrated that exposure to PS-MPs triggered pyroptosis in rat ovarian granulosa cells via activation of the NLRP3/Caspase-1 pathway [53]. Zhang et al. investigated the effects of various concentrations of 5 μm PS-MPs on chicken primary cardiomyocytes and discovered that PS-MPs induced pyroptosis via the NF-κB-NLRP3-GSDMD pathway (Figure S3) [54]. Zhong et al. established a mouse model of exposure to arsenic (As)-polystyrene microplastics and found that combined exposure to MPs and As triggered pyroptosis in liver cells via the Caspase-1/NLPR3 pathway by targeting the expression of ASC, NLPR3, GSDMD, and Caspase-1 [90]. Importantly, several studies have reported that PS-MPs could induce toxicity in human (micro) vascular sites, which was characterized by hemolysis, thrombosis, coagulation, and damage to vascular endothelial cells. The primary causes of these effects include oxidative stress, apoptosis, and pyroptosis [91,92]. These findings suggest that MPs are a potential cause of some cardiovascular diseases, such as myocarditis, atherosclerosis, and myocardial infarction.
3.2. Regulatory Mechanism
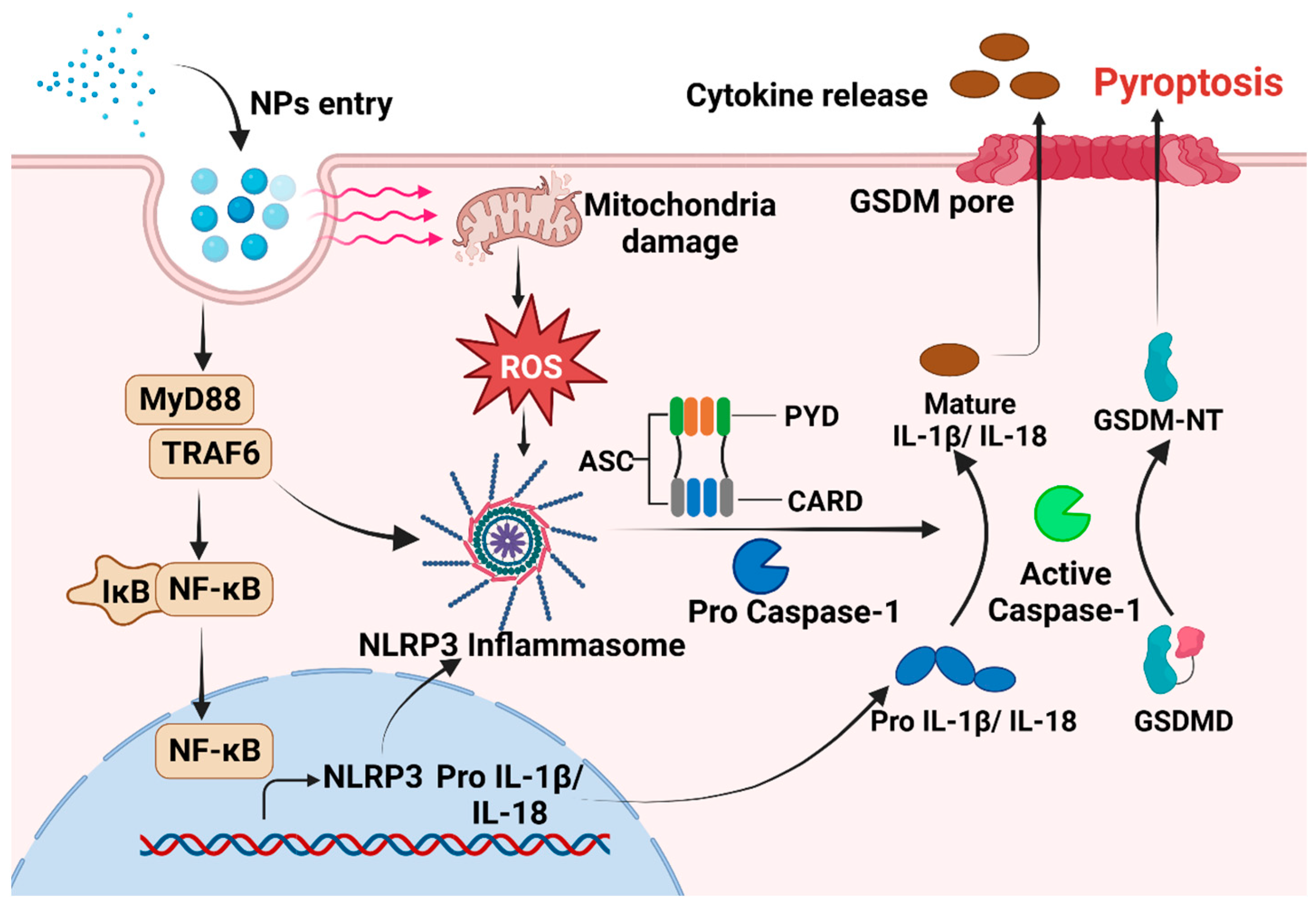
Exposure to MNPs can result in the excessive accumulation of ROS within organisms and imbalance between oxidation and antioxidant effects, consequently inducing oxidative stress [93,94]. The toxic impact of MNPs on organisms in vivo and in vitro are mediated by the induction of oxidative stress [93,95]. When cells exposed to MPs encounter oxidative stress, NF-κB can undergo [96,97]. Phosphorylated NF-κB plays a role in activating the NLRP3 inflammasome [97]. Furthermore, there is evidence to suggest that MNPs can trigger the inflammatory NF-κB/MyD88/NLRP3 pathway [98]. The close relationship between oxidative stress and proinflammatory processes has been acknowledged for an extended period. A number of studies have proved that oxidative stress can directly trigger inflammation, thereby initiating inflammatory responses and the release of chemokines [99]. The intracellular pattern recognition receptor NLRP3 forms oligomers and exposes its clustered pyrrole ring domain, facilitating signal recognition. It then binds to the precursor of Caspase-1 via the adaptor protein ASC, forming a multiprotein complex that activates Caspase-1 [100]. Activated Caspase-1 performs dual roles: First, it cleaves GSDMD, leading to the formation of peptides containing the active domain of GSDM-NT [101,102]. This induces cell membrane perforation, disrupts osmotic potential, causes cell rupture and content release, and triggers an inflammatory response [103,104]. The Gasdermin-N domain of GSDMD alone is adequate to induce pyroptosis in any cell [105]. Second, the IL-18 and IL-1β precursors are cleaved by activated Caspase-1, generating active IL-18 and IL-1β, and then released from the cell [106]. This release recruits inflammatory cells, amplifying the inflammatory response (Figure 1). However, in certain conditions, macrophages, dendritic cells, and neutrophils may withstand lysis induced by inflammasome-activated GSDMD and do not undergo membrane rupture or pyroptosis, which is termed hyperactivation [86]. Given that hyperactivated cells can release increased amounts of inflammatory mediators, it is feasible to differentiate between hyperactivation and pyroptosis by assessing lactate dehydrogenase levels in the cell culture supernatant [107]. However, the precise mechanism that determines whether GSDMD cleavage results in pyroptosis or hyperactivation remains unclear. NPs can activate various signaling pathways, including the NLRP3, Caspase-1, and NF-κB pathways, via oxidative stress and inflammation [32,90]. This activation significantly upregulates the expression of IL-18 and IL-1β, facilitates the cleavage of GSDMD, induces pyroptosis, and ultimately results in tissue damage in vivo. Hence, pyroptosis may serve as the cellular mechanism through which MPs exert adverse effects on organisms via ROS production and NF-κB activation. Targeting inflammation-dependent pyroptosis could be a novel strategy for mitigating the adverse effects of MPs. Studies have shown that ASC can augment the recruitment and phosphorylation of Caspase-1, and treatments targeting ASC can effectively decrease the activation of Caspase-1 and IL-1β, consequently reducing tissue damage [108,109].
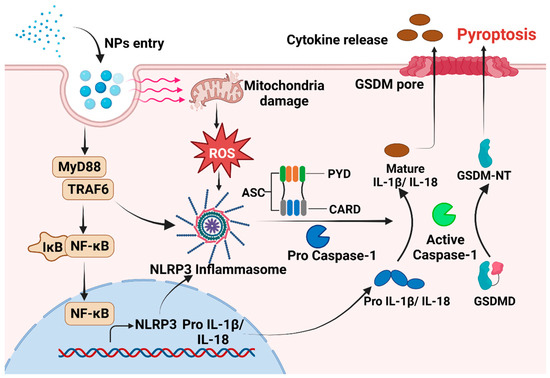
Figure 1.
The mechanism underlying pyroptosis induced by NPs. After NPs enter the cell, there is an increase in intracellular ROS levels. This increase in ROS activates NLRP3, which then binds to procaspase via the adaptor protein ASC. In addition, MNPs can trigger the inflammatory NF-κB /MyD88/NLRP3 pathway. Subsequently, activated Caspase-1 is formed, leading to the initiation of pyroptosis.
3.3. Therapeutic Strategies Targeting Pyroptosis in Cancer, Neurodegenerative Diseases and Immune Diseases
Pyroptosis represents a critical innate immune response, serving a pivotal role in responding to endogenous danger signals and counteracting infections [110]. It is extensively implicated in the onset and progression of immune disorders, nervous ailments, and infectious diseases [111,112,113,114]. Despite existing knowledge gaps regarding the precise involvement of pyroptosis in diverse pathological conditions, conducting thorough investigations into its signaling pathways, regulatory mechanisms, and pathological implications holds immense potential for the development of novel therapeutic strategies aimed at preventing and treating a wide array of human diseases.
GSDMD knockdown has been shown to activate Caspase-3 cleavage in lung cancer cells of mouse, inducing the inhibition of tumor growth in mice [88]. Currently, numerous GSDMD inhibitors including disulfiram, necrosulfonamide, dimethyl fumarate, LDC7559, and angpomegranate have been identified [115]. During dendritic cell treatment, the absence of NLRP3 has been shown to significantly decrease melanoma metastasis in the lungs [115]. N-acetylcysteine (NAC), which is a common antioxidant, inhibits ROS production, suggesting its potential for preventing NLRP3 inflammasome activation [116]. These findings indicate that inhibiting GSDMD and the inflammasome may be a promising strategy for tumor treatment. Moreover, various components of the NLRP3 inflammasome, such as Caspase-1, NLRP3 and ASC, are markedly upregulated in microglia within the substantia nigra of Parkinson’s disease patients, suggesting a link between pyroptosis and Parkinson’s disease [117]. Compounds such as flufenac and mefenac have been shown to inhibit NLRP3 activation, thereby alleviating the symptoms of Parkinson’s disease [118]. Therefore, targeting the NLRP3 inflammasome and its associated molecules could be a therapeutic strategy for neuroinflammatory diseases, including Parkinson’s disease. The gradual depletion of CD4 T cells in individuals infected with HIV is a primary factor that contributes to AIDS [119]. Caspase-1-mediated pyroptosis occurs when CD4 T cells fail to infect with the virus. However, caspase-1 inhibitors such as VX-765 and AC-YVAD-CMK may alter this process [120,121], suggesting the possibility of a novel therapy that targets the host rather than the virus to treat AIDS [122]. In summary, targeting GSDMD, NLRP3, Caspase-1, and related molecules is a promising approach for regulating PCD. However, significant advancements are needed to translate these therapeutic methods into clinical practice.
4. Molecular Regulatory Mechanisms of Ferroptosis Caused by MNPs and Associated Disease Risks
4.1. Ferroptosis Caused by Micro(nano)plastics
Ferroptosis, a recently identified type of iron-dependent PCD, is characterized by pronounced lipid peroxidation and iron accumulation. Ferroptosis inducers can indirectly or directly impact glutathione peroxidase, leading to a reduction in the cellular antioxidant capacity and the accumulation of lipid ROS, ultimately resulting in oxidative cell death [123,124]. Mu et al. demonstrated that PS-MPs induced lipid peroxidation in the livers of mice, which upregulated transferrin receptor (TFRC) expression and downregulated ferritin heavy chain 1 (FTH1), the XCT system, glutathione peroxidase 4 (Gpx4), and ACSL4, thereby inducing ferroptosis [51]. Tang et al. observed that exposing mice during pregnancy to PS-NPs caused ferroptosis in the small intestine cells of their offspring [57]. Chen et al. showed that PS-NPs disrupted lipid peroxidation and iron metabolism in zebrafish larvae, thereby inducing ferroptosis [80]. Yin et al. reported that chickens exposed to PS-MPs exhibited increases in hepatic glutamine and glutamate synthesis, which promoted autophagy-dependent ferroptosis via the liver–brain axis [56]. Carbo et al. observed ferroptosis in microalgae exposed to bisphenol A (BPA), which is a major component of MPs, due to the disruption of iron dynamics and reoxidation balance. The reversal of microalgal growth by the classic ferroptosis inhibitor Fer-1 further confirmed that microplastics induced ferroptosis [125]. Moreover, studies have indicated that combined exposure to microplastics and other substances can induce ferroptosis in cells, such as PE-imidacloprid-induced ferroptosis in earthworm cells and PE/PS-polybrominated diphenyl ether-induced alterations in ferroptosis pathways factors in grouper [126,127]. Importantly, excessive cell death due to ferroptosis is related to the pathogenesis of numerous diseases, suggesting that MPs may be potential pathogenic factors associated with disease development.
4.2. Regulatory Mechanisms
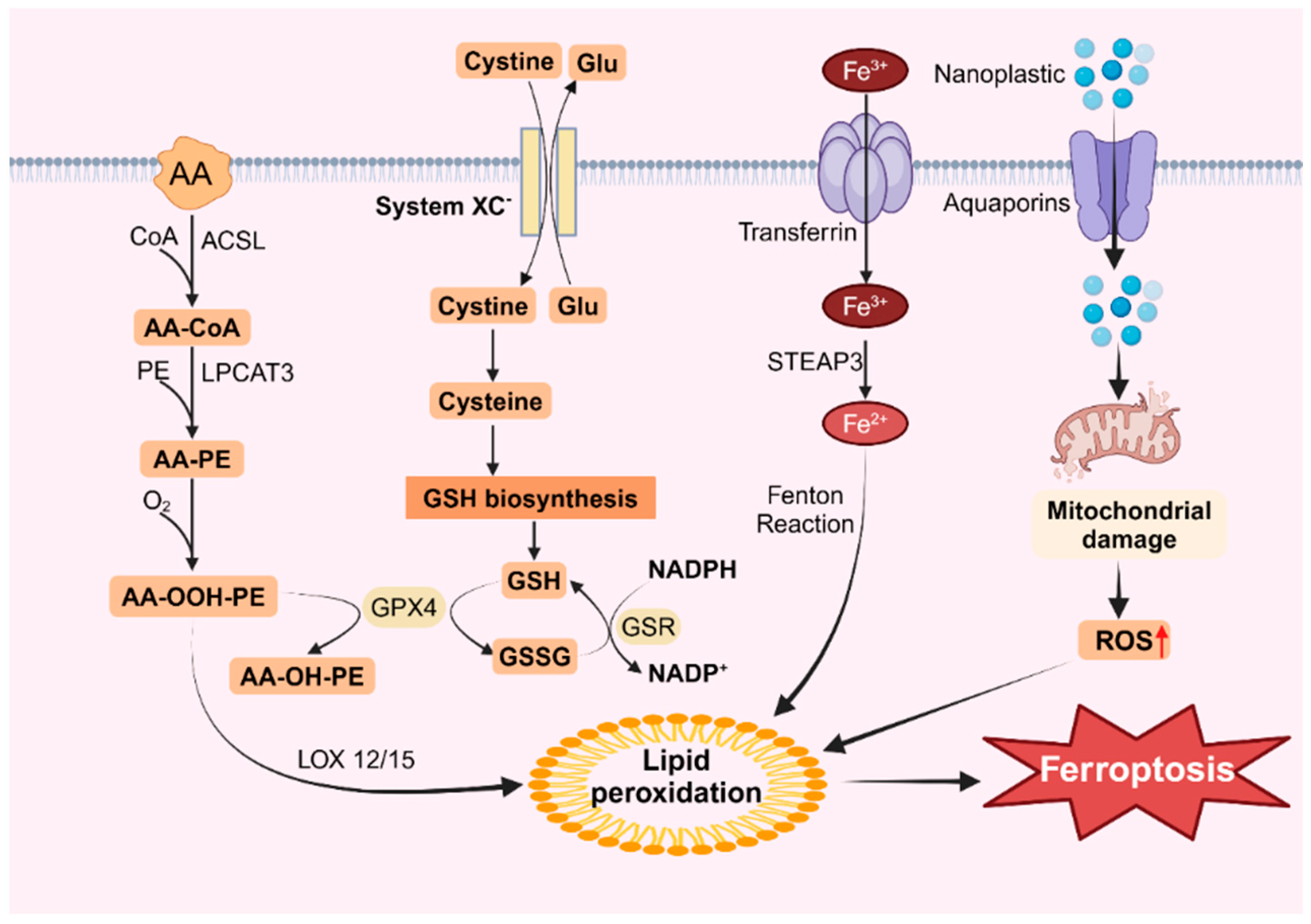
Exposure to MNPs can trigger ROS accumulation in organisms, and ROS-mediated lipid peroxidation is a primary driver of ferroptosis [128,129]. Lipid peroxidation in cells involves two main processes, one of which is catalyzed by fatty acid enzymes [130]. Polyunsaturated fatty acids (PUFAs) can be converted into highly reactive lipid peroxides by a series of enzymes. Research indicates that in addition to the cellular membrane systems, the primary sources of these PUFAs are arachidonic acid (AA) and linoleic acid, which are widely present within cells [131]. Experimental evidence has confirmed that the addition of AA and linoleic acid during cell culture can expedite ferroptosis [132]. Mu et al. demonstrated that exposure to microplastics induced the expression of ACSL4, which promoted lipid synthesis and subsequent lipid peroxidation [51]. The oxidation of AA is primarily regulated by three enzymes: AA is converted to AA-CoA by acyl CoA synthetase long-chain protein 4 (ACSL4) and then esterified with phosphatidylcholine to form AA-PE by LPCAT3, followed by lipid peroxidation by the lipoxygenase protein family (LOXs) [133]. These enzymes, particularly ACSL4, play critical roles in ferroptosis, and the LOX family members ALOX5 and ALOX12 are targeted by various ferroptosis inducers [133]. Additionally, exposure to MNPs increases the activity of the XC system and GSH system and downregulates Gpx4 expression [134,135]. The main function of the XC system is to transport cystine and glutamate into and out of cells, and while cysteine is involved in the synthesis of GSH via the XC system [136]. Gpx4 acts as a key intracellular lipid peroxidation reducer that regulates ferroptosis by controlling lipid peroxidation. Gpx4 converts GSH to GSSG, thereby inhibiting ROS accumulation [137]. Several studies have reported decreased Gpx4 expression in response to microplastics, suggesting that reduced Gpx4 levels may inhibit the conversion of lipid hydrogen peroxide to lipid alcohol, thereby promoting lipid peroxidation [138,139].
An additional intracellular route for lipid peroxidation involves the Fenton reaction, which is triggered by free iron ions. Iron within the cell is primarily transported by transporters containing trivalent iron, which enter the cell via the transferrin receptor TFR on the cytomembrane [140]. Sun et al. demonstrated that exposure of BV2 cells to microplastics led to a dose-dependent increase in intracellular ferritin levels and TFRC protein expression [55]. Moreover, the pH in the cell is acidic, and free trivalent iron is reduced to bivalent iron by the iron reductase STEAP3 [141]. Iron ions within the cell react with peroxides to generate iron ions and peroxy radicals. These peroxy radicals then attack lipid molecules, oxidizing them into lipid peroxides (Figure 2). Under normal conditions, lipid peroxides are maintained in homeostasis because the concentration of iron ions remains stable. However, a sudden increase in intracellular iron ions significantly intensifies the Fenton reaction, resulting in the excessive accumulation of lipid peroxides, thereby inducing ferroptosis [142]. This process is characterized by reduced mitochondrial mass, increased membrane density, and decreased ridges [143].
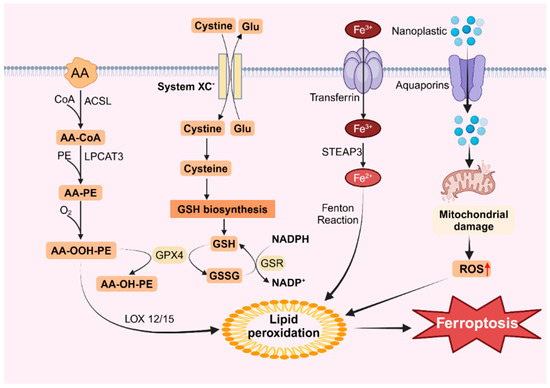
Figure 2.
Ferroptosis caused by nanoplastics. Nanoplastics can enter cells through aquaporins, damage mitochondria and increase ROS levels, leading to lipid peroxidation and inducing ferroptosis.
4.3. The Role of Ferroptosis in Targeted Treatments for Cancer, IRI, and Inflammatory Enteritis
The role of ferroptosis in human diseases has been extensively investigated (Table 2), and researchers have examined treatments targeting ferroptosis-induced pathologies. Notably, cancer is a primary focus due to the ubiquitous expression of Gpx4, which is crucial for cancer cell survival [144]. Additionally, studies have shown that inhibiting the XC system markedly suppresses tumor growth and metastasis in murine models [145]. The XC system functions as a reverse transporter to facilitate the uptake of cystine, a vital component of glutathione (GSH) that counteracts oxidative stress induced by oxidants such as hydrogen peroxide [146]. Ferroptosis has also been implicated in neuropathic disorders. Dixon et al. reported that ferrostatin 1 effectively mitigated glutamate-induced cell death and ferroptosis in cancer cells in mouse brain slices cultured in vitro [147]. Furthermore, Gao et al. provided evidence linking ferroptosis to ischemia reperfusion-induced injury (IRI) through in vitro experiments with mouse embryonic fibroblasts [148]. Given their pivotal role in glutamine metabolism in ferroptosis, these enzymes are potential targets for IRI treatment. Moreover, ferroptosis is closely associated with Crohn’s disease [149]. ROS have been implicated in enteritis pathogenesis, and oral iron supplementation can exacerbate inflammatory bowel disease. Conversely, the iron chelator desferriamine significantly ameliorates IBD symptoms [150]. Chen et al. demonstrated that ferrostatin 1 could alleviate DSS-induced Crohn’s disease in rats by inhibiting the HO-1/Nrf2 signaling pathway [151]. The modulation of ferroptosis to inhibit the onset and progression of diseases has become a prominent area of research and a therapeutic focus, although the functional alterations and specific molecular mechanisms warrant further examination.

Table 2.
The link between ferroptosis and disease.
5. Molecular Mechanisms of Autophagy Induced by MNPs and Associated Disease Risks
5.1. Autophagy Caused by Micro(nano)plastics
Autophagy, an evolutionarily conserved process, is responsible for intracellular material turnover in eukaryotes, serving as a mechanism of PCD. During autophagy, damaged organelles and proteins are sequestered within autophagic vesicles featuring bilayer membranes, and subsequently transported to vacuoles or lysosomes for degradation and recycling [161,162]. A number of researchers have documented the induction of autophagy by MNPs in organisms. Shaoyong et al. administered environmental concentrations of PS-MPs to mice via gavage, and revealed that PS-MPs induced autophagy in mouse cells and facilitated the accumulation of autophagic vacuoles [58]. Lin et al. showed that exposure to PS-NPs activated the AMPK/mTOR/ULK1 signaling pathway in mice, and exacerbated lipopolysaccharide (LPS)-induced autophagy [163]. Nie et al. exposed SH-5Y5Y cells to PS-NPs, and observed an increase in the number of autophagosomes. Western blot analysis confirmed increased expression of LC3B-II/LC3B-I, ATG7, and ATG5, indicating that MPs activated autophagy in SH-5Y5Y cells [59]. Similarly, Ding et al. noted a noteworthy rise in the number of autophagosomes and autolysosomes in GES-1 cells treated with PS-NPs, indicating NPs activated autophagy [62]. A study examined the autophagy effect of PS-NPs with different particle sizes, and small NPs showed a higher likelihood to induce autophagy and autophagosomes formations [60]. Conversely, a study revealed that exposing human nasal epithelial cells to 50 nm PS-NPs reduced autolysosomes [61], suggesting impaired autophagic flux, that was due to severe damage and ultimately led to cell death.
5.2. Regulatory Mechanism
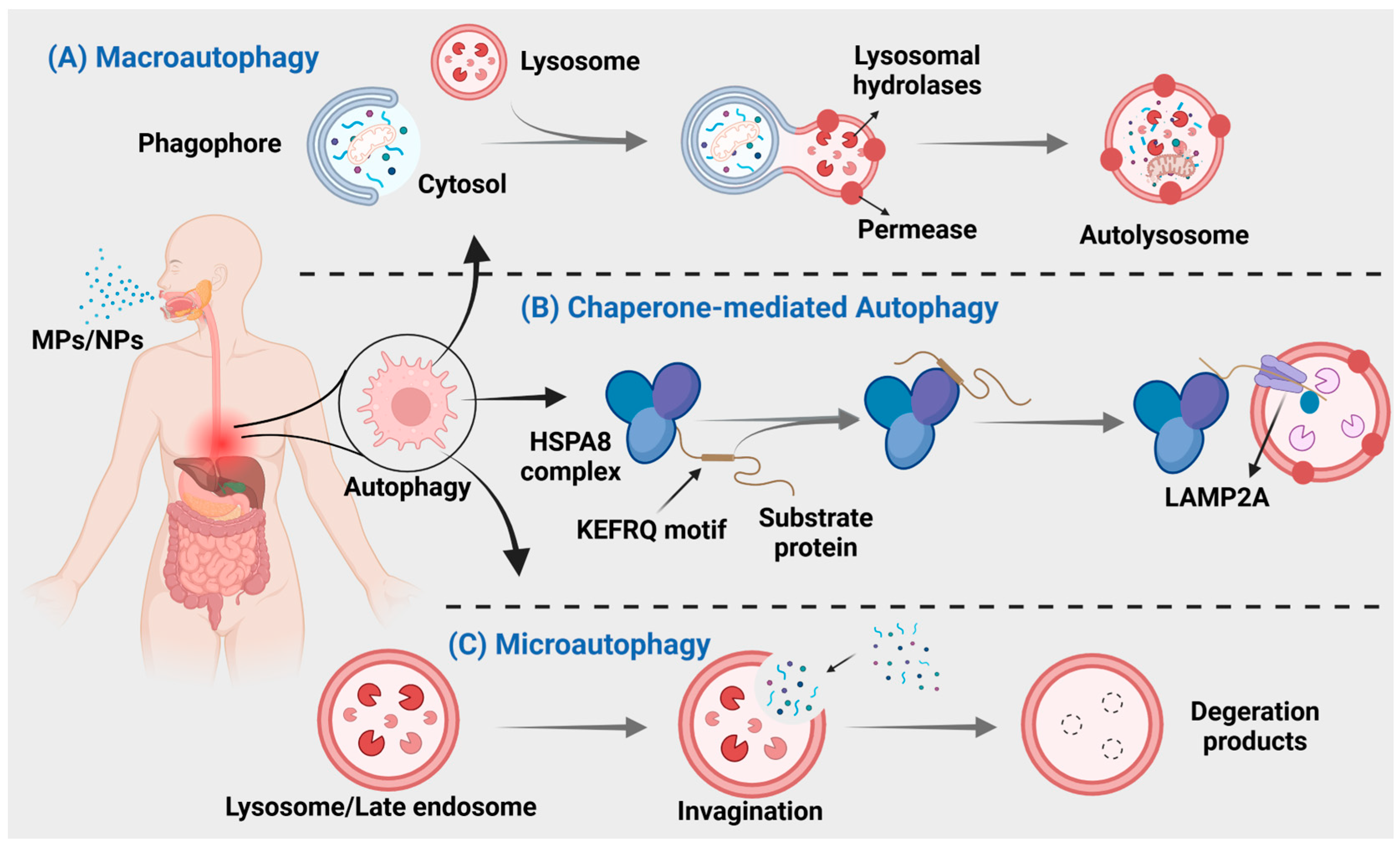
Autophagy is a crucial process for intracellular material transfer in eukaryotes that is evolutionarily conserved. Nevertheless, the role of autophagy extends beyond mere substance elimination; it is a dynamic process that generates energy and new building blocks, contributing to homeostasis and cell renewal [164,165]. Autophagy can be classified into three main types: macroautophagy, microautophagy, and chaperone-mediated autophagy (Figure 3), based on distinct substances and transport mechanisms [166].
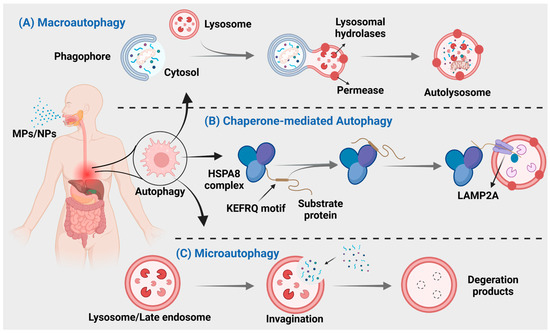
Figure 3.
MNPs can cause three different types of autophagy. (A) Autophagosomes derived from the endoplasmic reticulum, Golgi apparatus, or plasma membrane, engulf the material and subsequently merge with lysosomes for degradation. (B) Direct encapsulation of long-lived proteins by lysosomal membranes, leading to their degradation within lysosomes. (C) Intracytoplasmic proteins bind to chaperones for transport into lysosomal cavities, followed by digestion by lysosomal enzymes.
Several studies have demonstrated that smaller NPs can trigger autophagy in cells (Figure S4). High levels of autophagy can indicate that cells are trying to survive and that death occurs only when this effort fails [167]. Cells exposed to NPs typically internalize them via vesicle-mediated endocytosis, leading to the formation of autophagosomes, which is a critical aspect of subsequent cell death [168]. Given the intimate relationship between autophagy and apoptosis, excessive autophagic activation can culminate in apoptosis, which shares common regulatory signaling pathways governing cell survival and growth. Recent studies have revealed that autophagosomes can induce apoptosis by serving for a platform for caspase activation, particularly caspase-8 [169,170]. In response to NP exposure, autophagy serves as a specific target of the negative regulator of caspase activity through degradation, inducing the increase in caspase activity and thus promoting cell death.
Although autophagy has been traditionally considered as a mechanism of cell death, the current consensus is that its primary role is protective, and it is crucial for maintaining intracellular homeostasis. However, severe damage can disrupt autophagic flux, leading to inadequate autophagy [171]. Zhou et al. used earthworms and observed the internalization of nanoplastics by these organisms. The nanoplastics predominantly accumulated in lysosomes, causing lysosomal swelling and an abnormal increase in lysosomal membrane permeability, which has been identified as a potential factor contributing to cell death. In addition, the positively charged nanoplastics were 83% more cytotoxic than the negatively charged nanoplastics [71]. These stimuli destabilize the lysosomal membrane, leading to its rupture and hindering autophagy and cellular clearance processes, ultimately culminating in cell death. Moreover, the distribution of MNPs varied depending on the dose. At low doses, MNPs accumulated in lysosomes and lysosomal vacuoles, and at high doses, they predominantly accumulated in autolysosomes [172]. Autolysosomes, which are composed of autophagosomes and lysosomes, contain endogenous substrates such as senescent organelles or local cytoplasm [173]. The infiltration of MNPs significantly disrupts cells. Although high doses of MNPs initially triggered autophagy, prolonged exposure suppressed this process [63]. Additionally, the increased presence of autolysin due to MNP absorption inhibited the ability of the cell to eliminate them, and the exocytosis pathway was obstructed, impairing the expulsion of a large quantity of MNPs and ultimately leading to cell death [174].
5.3. Targeting Autophagy to Treat Diseases
Dysregulation of autophagy significantly contributes to diseases, including encompassing neurodegenerative disorders, cancers, and metabolic conditions. Currently, mutations in ATG, which is a core gene within the autophagy pathway, are considered primary causes of some neurodegenerative diseases including Parkinson’s disease and spinocerebellar ataxia [161,162]. Furthermore, in Alzheimer’s disease, neuronal dysfunction can be exacerbated by factors that promote amyloid formation, such as amyloid precursor proteins and presenilin, which impair lysosomal function and autophagosome clearance [175,176]. However, the inhibition of autophagy is considered advantageous in cancer treatment, because cancer cells rely more heavily on autophagy than normal cells [177,178]. Hydroxychloroquine and chloroquine can be used to treat various cancers, including multiple myeloma, glioblastoma, and melanoma by inhibiting the overall function of lysosomes and the final degradation phase of autophagy [179]. Autophagy fulfills essential metabolic roles in major organs, contributing to energy equilibrium, and complete inhibition directly impacts the composition of the extracellular metabolome, thereby affecting metabolic connections between different tissues [180]. Consequently, autophagy acts a pivotal role in responding to both short-term and long-term metabolic stresses. Alterations in autophagy influence the development of metabolic disorders like nonalcoholic fatty liver disease, type 2 diabetes, and obesity [181].
Although much work remains to be conducted, regulating autophagy for therapeutic purposes remains a promising strategy for treating a wide range of human diseases. Interventions targeting autophagy regulation also hold promise for preventing or mitigating phenotypic abnormalities in the most common human diseases.
6. Regulatory Mechanisms of Necroptosis Induced by MNPs and Associated Disease Risks
6.1. Necroptosis Caused by Micro(nano)plastics
Necroptosis is a process of PCD that relies on the activation of RIPK1, RIPK3, and MLKL and is mediated by inflammatory cell death mechanisms. When apoptosis is inhibited, both intracellular and extracellular signals may trigger necroptosis [182,183]. Previous studies have shown that MPs can upregulate the expression of RIPK3 and MLKL in mice, leading to necroptosis. Shan et al. used immortalized human brain microvascular endothelial cells, indicating that PS-NPs could be internalized by cells and induce necroptosis [74]. Similarly, Meng et al. observed necroptosis induction in the kidney following oral administration of PS-MPs to chickens [76]. Wang et al. revealed that the cytotoxicity of MPs was size-dependent. Small-sized MPs increased necroptosis in cells; PS-MPs significantly increased the protein expression levels of p-MLKL, RIPK3 and RIPK1 in MBECs and decreased caspase-8 protein expression. Treatment with N-acetyl-L-cysteine (NAC) remarkably reduced the cellular changes associated with necroptosis regulators, indicating that PS-MPs could induce necroptosis through oxidative stress [75]. Additionally, Tang et al. demonstrated that PS-NPs activated the MAPK/ROS pathway to increase lipopolysaccharide-induced necroptosis in RAW264.7 cells [184], and Wu et al. confirmed that simultaneous exposure to PS-MPs and DEHP increased necroptosis in the ovarian granulosa cells of GRM02 mice [185].
6.2. Regulatory Mechanisms
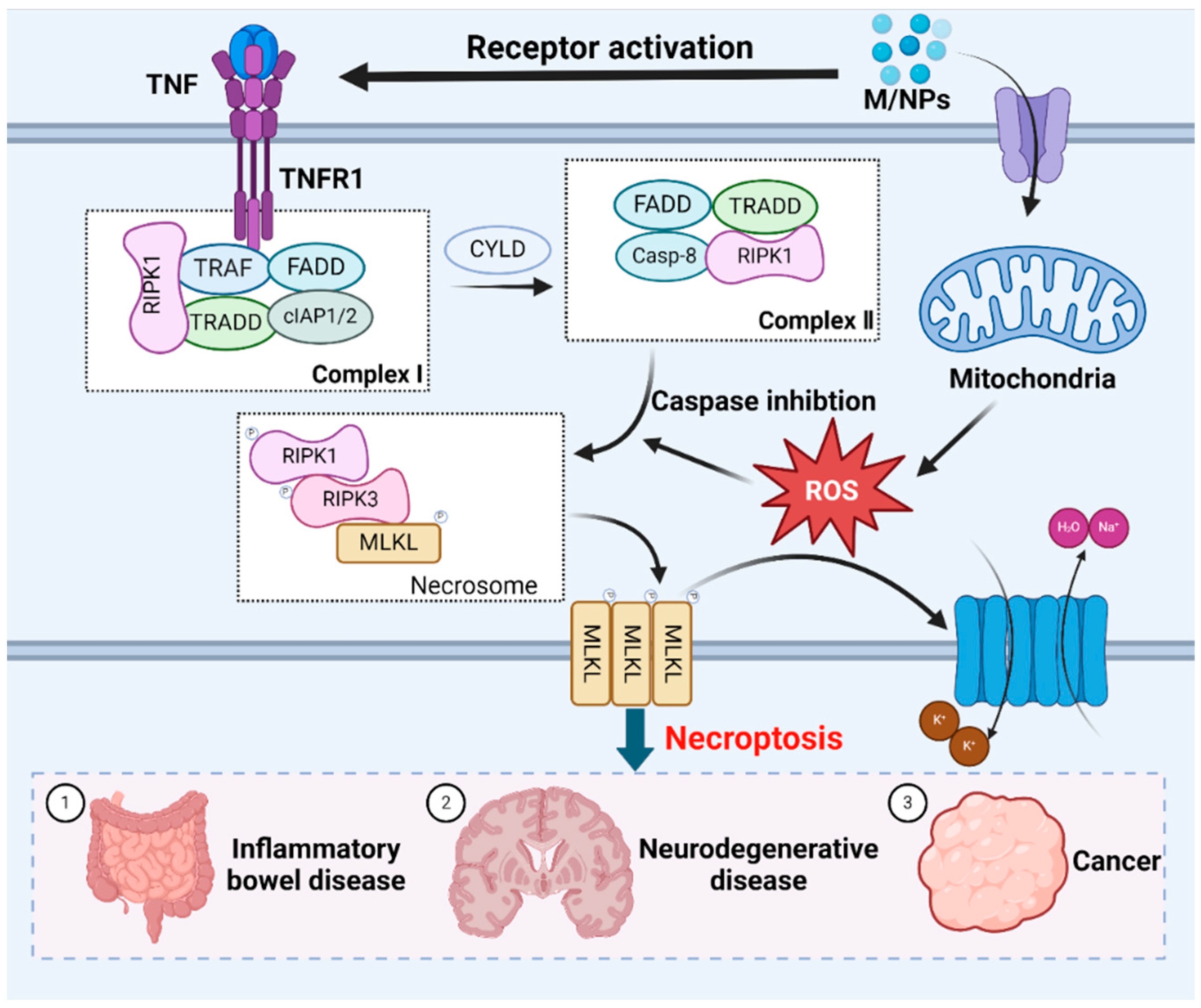
ROS play a crucial role in necroptosis [186]. Mitochondrial ROS production alters RIPK1 residues, facilitating RIPK1 autophosphorylation, the recruitment of RIPK3 to form amyloid-like structures, and subsequent necroptosis [187]. Additionally, MPs exacerbate intracellular ROS generation and oxidative stress after entering and accumulating within organisms, and necroptosis can be induced by stimuli such as MPs, which are known to trigger cell death [188]. RIP1 kinase is a pivotal upstream regulator in this pathway, exerting control through multiple phosphorylation and ubiquitination events. While RIPK1 is deubiquitinated and the activity of caspase-8 is inhibited, necroptosis occurs [189]. Tumor necrosis factor receptor 1 (TNFR1) can recognize the tumor necrosis factor (TNF), this process prompts the formation of membrane-associated complex I. The membrane-associated complex I is composed of cellular inhibitor of apoptosis protein 1 (cIAP1) and cIAP2, TNF-associated factor (TRAF), TNFRSF1A-associated death domain (TRADD) and receptor-interacting serine-threonine kinase 1 (RIPK1) [190]. In this complexus, RIPK1 undergoes ubiquitination by cIAP1/2 or deubiquitination by cylindromatosis protein (CYLD) [191]. RIPK1 deubiquitination leads to the dissociation of RIPK1 and TRADD from complex I, which triggers necroptosis or apoptosis [192,193]. During apoptosis, caspase-8 and FAS death domain-associated protein (FADD) are recruited to RIPK1 and TRADD, thereby activating caspase-8 to induce apoptosis [194]. Conversely, caspase-8 is absent during necroptosis, leading to RIPK1 phosphorylation and the subsequent activation of RIPK3. Activated RIPK3 phosphorylates the mixed lineage kinase domain (MLKL), inducing conformational changes and the oligomerization of MLKL [195,196]. These changes allow MLKL to disrupt the plasma membrane [197]. The resulting influx of Na+ and water, and efflux of K+, cause cell swelling, interrupt membrane potential, ultimately leading to cell death [198] (Figure 4).
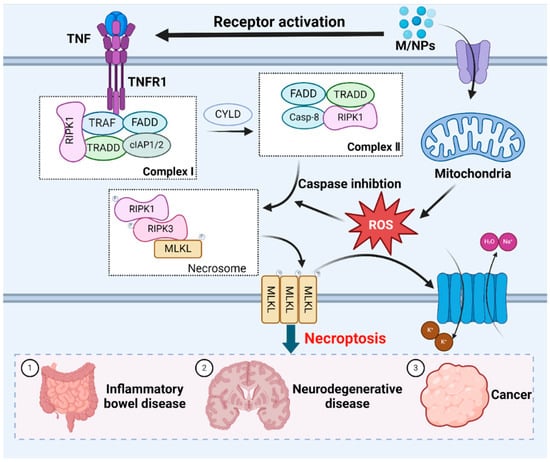
Figure 4.
The molecular mechanisms underlying necroptotic cell death induced by MNPs. Exposure to MNPs triggers necroptosis mediated by RIPK1, which requires RIPK3-dependent phosphorylation of MLKL. Oligomerization of MLKL culminates in plasma membrane disruption, which is accompanied by the efflux K+, and influx of Na+ and H2O, resulting in cell swelling. Necroptosis is closely related to inflammatory bowel disease, neurodegenerative disease and cancer.
6.3. Targeting Necroptosis to Treat Inflammation, Parkinson’s Diseases and Cancer
Necroptosis, which is a PCD pathway, significantly contributes to various human diseases due to its pivotal role in initiating inflammation [199,200]. Kang et al. reported that the RIPK1/RIPK3/MLKL/caspase-8 axis enhanced the assembly and function of the lipopolysaccharide-induced NLRP3 inflammasome in dendritic cells in the absence of caspase-8, underscoring the association of necroptosis with specific inflammatory conditions [201]. Necroptosis is an inflammatory trigger. In an in vivo mouse genetic model, Welz et al. demonstrated a link between the regulatory mechanism of necroptosis and the pathogenesis of chronic intestinal inflammation in humans [202]. Moreover, epithelial necroptosis has been observed in rectal biopsy specimens from individuals with Crohn’s disease [203]. Necroptosis is also closely associated with neurodegenerative disorders. Following spinal cord injury in mice, RIPK1, RIPK3, and MLKL have been detected in the cytoplasm and lysosomes [204]. In a Parkinson’s disease model, pharmacological inhibition of RIPK1 mitigated mitochondrial morphological alterations and dysfunction in dopaminergic neurons, indicating the beneficial impact of blocking necroptosis on Parkinson’s disease and suggesting its potential for therapeutic target [205]. In the context of cancer therapy, necroptosis holds promise as an antitumor mechanism. Given that cancer often evades apoptosis, triggering necroptosis, which is an alternative cell death pathway, is a potential treatment strategy [206]. While numerous necroptosis-targeting drugs have shown antitumor efficacy, their clinical therapeutic effects require validation [207,208,209]. Although the expression of key necroptosis pathway regulators is typically downregulated in most cancer cells, their expression is upregulated in certain cancer types [210,211]. Considering the intricate interplay between necroptosis and various diseases, further research should focus on elucidating the molecular mechanisms and characteristics of necroptosis, as well as its interactions with other cell death modalities, to identify potential cancer treatment strategies.
7. Regulatory Mechanisms of Apoptosis Induced by MNPs and Associated Disease Risks
7.1. Apoptosis Caused by Micro(nano)plastics
Apoptosis is a regulated cell death (RCD) process in which cells respond to physiological and pathological signals from the environment, alterations in environmental conditions, or injurious stimuli in an organized manner. It is morphologically characterized by cell shrinkage, the loss of cellular connections, detachment from neighboring cells, and the eventual formation of apoptotic bodies without the leakage of cellular contents, thereby preventing an inflammatory response [212,213]. Various researchers have documented that MPs can induce apoptosis in various organisms. Yang et al. demonstrated that PS-NPs induced apoptosis in N2A and JEG-3 cells through ROS production, subsequently leading to damage to GABAergic neurons in the mouse thalamus [81]. Similarly, PS-MPs exhibit phagocytic activity in human microglia HMC-3 cells, resulting in alterations in cell morphology, the immune response, and the induction of microglial apoptosis [59]. Several investigations have reported the occurrence of apoptosis in zebrafish cells following exposure to MPs. Chen et al. reported that PS-NPs exposure triggered the mitochondria-dependent apoptosis pathway in juvenile zebrafish cells [80]. Umamaheswari et al. revealed that exposure to PS-MPs upregulated the expression of p53, gadd45ba and casp3b, leading to apoptosis in zebrafish gills [83]. Santos et al. reported increased expression of apoptosis-related genes (casp8, casp9, and casp3) in zebrafish exposed to MPs [214].
7.2. Regulatory Mechanisms
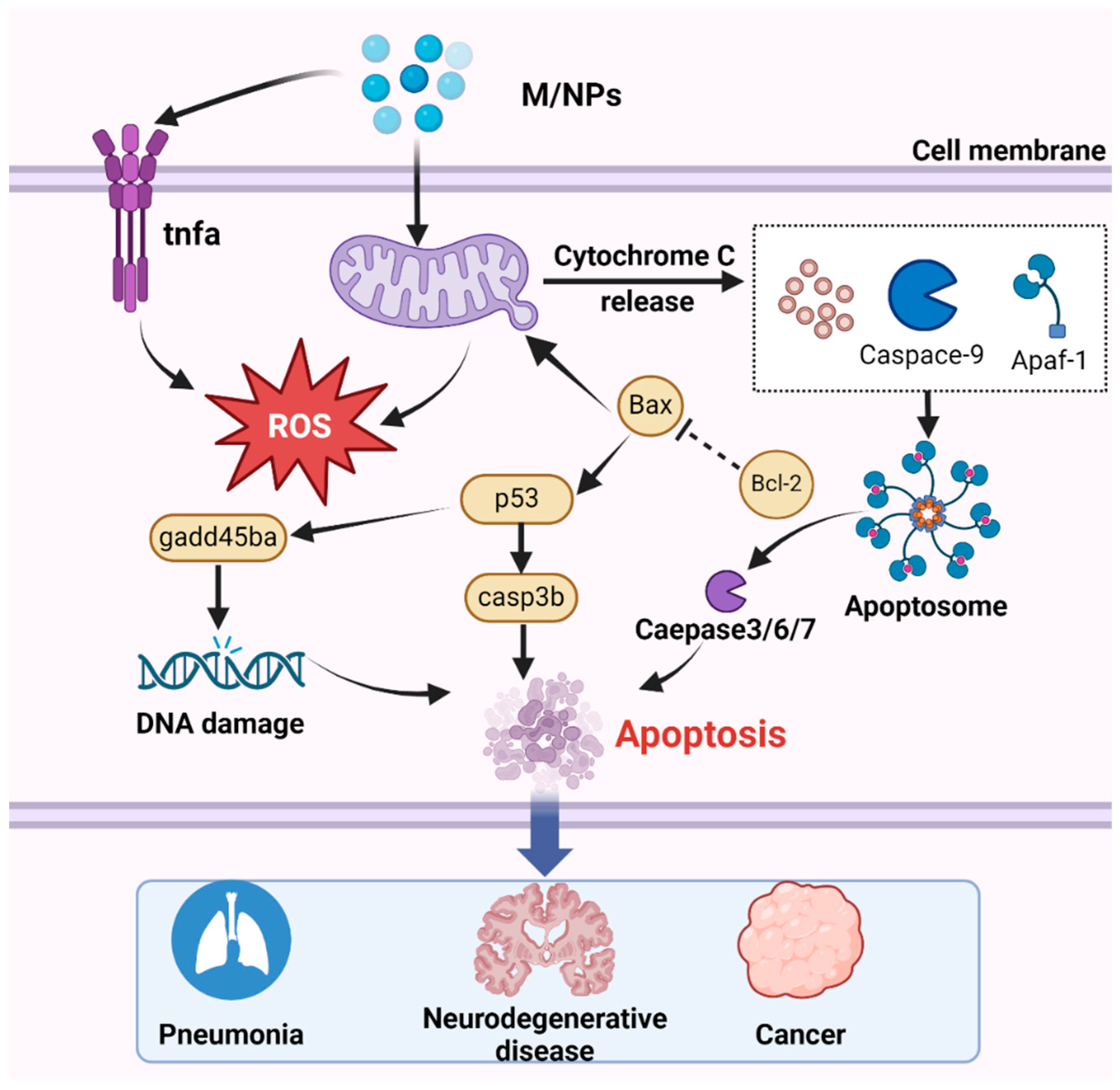
Numerous studies have demonstrated that exposure to MNPs leads to excessive ROS production [215,216]. While ROS serve as critical cell signaling molecules, their overabundance can induce apoptosis, thereby causing tissue damage and adverse effects [217]. Among the various apoptosis pathway, mitochondria-dependent apoptosis is particularly important and has been extensively investigated [218]. Notably, mitochondria not only serve as a source of ROS but also as targets of ROS-mediated damage [219]. ROS produced by MNP exposure can upregulate the transcription of the p53 gene, and subsequently activate casp3b, which promotes the transcription of gadd45ba, leading to DNA damage and apoptosis [83]. In the mitochondrial apoptosis pathway, the caspase family and Bcl-2 play indispensable roles [220]. Although only small-sized nanoparticles can be internalized by cells, larger MPs can also induce apoptosis. Exposure to MNPs triggers the generation of apoptotic signals, leading to the downregulation of the antiapoptotic gene bcl-2 and translocation of the proapoptotic protein Bax to the mitochondrial membrane [90,221]. Consequently, mitochondrial permeability increases, resulting in the release of cytochrome c (Cyt c) into the cytoplasm, where it binds to apoptotic protease-activating factor 1 (Apaf-1) and recruits procaspase-9 to form apoptosomes [212]. During apoptosis, caspase-9 undergoes self-proteolytic cleavage, which initiates the caspase processing cascade (Figure 5). Moreover, MPs influence the concentration of calcium ions (Ca2+) in mitochondria by inducing changes in mitochondrial membrane potential [222], thereby impairing adenosine triphosphate (ATP) production, activating downstream caspase-9 and caspase-3, and ultimately triggering the mitochondrial apoptosis pathway [223].
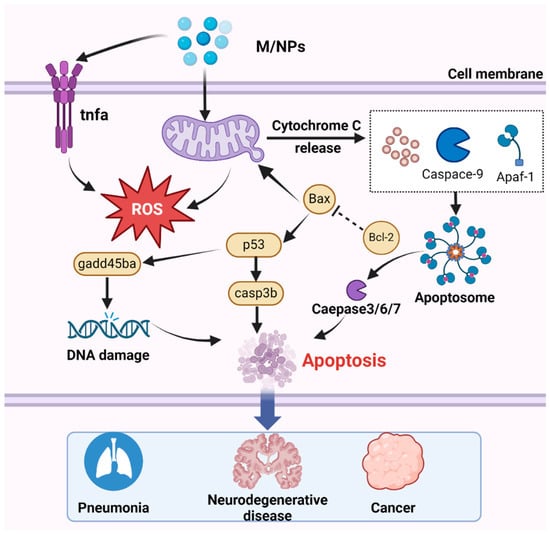
Figure 5.
The mechanism by which MNPs induce apoptosis involves several key steps. After entering the cell, with the accumulation of MNPs, MNPs trigger mitochondrial detoxification, leading to the generation of ROS. Subsequently, the increase in ROS levels upregulates the transcription of the p53 gene, thereby activating casp3b. Activated caspase-3 promotes the transcription of gadd45ba, ultimately resulting in DNA damage and apoptosis. Additionally, Cyt c is released by the mitochondria, binds to Apaf-1, and facilitates the recruitment of procaspase-9 and the formation of apoptotic bodies, then activated effector caspase, thus inducing apoptosis. Apoptosis is associated with numerous diseases, including pneumonia, neurodegenerative disorders, and cancer.
7.3. Targeting Apoptosis to Treat Lung Diseases, Neurodegenerative Diseases, Cancer and Other Diseases
Apoptosis plays a crucial role in chronic obstructive pulmonary disease (COPD), and there is a notable increase in lung apoptosis that is often attributed to cigarette smoke exposure [224]. Animal experiments further showed the involvement of apoptosis in COPD pathogenesis. Mice exposed to cigarette smoke exhibited lung cell apoptosis and subsequently developed emphysema after tracheal perfusion of caspase. Conversely, mice treated with Caspase-3 inhibitors or subjected to Caspase gene deletion showed reduced lung damage [225,226]. As a neurodegenerative disease that may lead to brain death, stroke is also associated with apoptosis. Given its energy-dependent nature, apoptosis is notably prevalent in neurons in the ischemic penumbra with compromised energy production [227]. Studies have shown that organisms can influence pathways such as LRRK2 and GSK-3 beta/Tau hyperphosphorylation/Nrf2, as well as the aggregation of alpha-synuclein, through apoptotic cell death [228,229]. The deletion of a single proapoptotic Bax gene plays a neuroprotective role [230]. Too little apoptosis is one of the pathogenic factors associated with cancer because a reduction in apoptosis can allow malignant cells to continue to survive [231]. Although apoptosis is one of the causes of cancer, it is also a potential target for cancer treatment. Recent research has identified various effective substances that can inhibit apoptosis. Chen et al. reported that exogenous NO significantly mitigated NP-induced apoptosis by targeting mitochondria [80], and Yang et al. discovered that FGFR4 and EZH2 inhibitors synergistically induced HCC cell apoptosis by inhibiting YAP signaling [232]. The potential of targeting apoptosis to treat diseases is substantial, indicating promising avenues for future clinical research.
8. Cuproptosis
In addition to classic PCD, a recent research by Tsvetkov et al. revealed that copper inside cells induced a novel form of regulated cell death that was unlike oxidative stress-related cell death: cuproptosis [233]. Three mechanisms are known to trigger cuproptosis. First, FDX1 reduces Cu2+ to Cu+ and promotes the lipylation (LA) and accumulation of enzymes involved in the regulation of the mitochondrial TCA cycle, especially DLAT. FDX1 causes an imbalance in Fe-S cluster proteins to trigger cuproptosis [234]. Second, excess copper induces cell death through the oligomerization of mitochondrial lipidated proteins [235]. Although copper is essential for cellular metabolism, excess copper can be deleterious. The third mechanism involves inhibiting enzymes that are necessary for apoptosis. Copper acts as both an endoplasmic reticulum (ER) stress inducer and a caspase-3 inhibitor, forcing cells to undergo caspase-independent cell death [236,237]. Several studies have shown that MPs can be used as carriers for adsorbing Cu2+ [238], and MPs exposed to coastal seawater can form biofilms, thereby promoting the adsorption and transport of Cu2+. Studies have shown that exposure to microplastics and Cu2+ can increase the accumulation of Cu2+ in the body. Qiao’s study compared zebrafish exposed to PS-MPs and Cu2+ with those exposed to Cu2+ alone and revealed an increase in the toxic effect of MPs on Cu2+ [239]. However, whether cuproptosis occurs in these contexts warrants further investigation.
Copper is implicated in certain diseases, notably Wilson’s disease, which is an autosomal recessive genetic disease typical of various mutations in the ATP7B gene. ATP7B dysfunction impairs copper excretion, resulting in the accumulation of copper in the liver and brain. Cuproptosis has emerged as a potential therapeutic target for Wilson’s disease, and copper chelators such as trientine and D-penicillamine are considered effective treatments [240]. Furthermore, Liu et al. suggested that targeting cuproptosis could serve as a potential therapeutic approach for kidney cancer and brain cancer, based on pancancer analysis of genomic and transcriptomic data associated with cuproptosis [241].
9. Conclusions
This review highlighted the adverse effects of MNPs on organisms and elucidated the various mechanisms leading to cell death and resulting in a threat to normal organismal development. Numerous researchers have demonstrated the detrimental effects of MNPs on various species, including humans, fish, mice, chickens, and nematodes, which are characterized by tissue damage, neurotoxicity, developmental toxicity, and oxidative stress. These data indicate that MNPs in in vivo studies are typically exposed to low doses over prolonged periods, whereas in vitro studies employ higher exposure concentrations but for shorter durations. Most research predominantly examines the impact of PS on organisms, neglecting the effects of other plastics such as PE, PP, and PVC. Moreover, the heightened toxicity of MNPs modified by functional groups (-NH3, -COOH and -SO3H) warrants attention, as they often exhibit greater toxicity than unmodified MNPs. Additionally, environmental endocrine disruptor chemicals (EDCs) capable of interacting with MNPs in the environment should be a concern. Given the detection of MNPs in human placentas, genetic variability has emerged as a significant factor influencing the impact of MNPs on cellular health. Consequently, there is a pressing need for further research on microplastic pollution to comprehensively assess the interaction between microplastics and human health. Genetic and pathological investigations have provided compelling evidence linking genetic mutations in various forms of PCD (including pyroptosis, ferroptosis, autophagy, necroptosis, apoptosis, and cuproptosis) and the onset of numerous human diseases, underscoring the significance of programmed death and associated cellular functions in disease pathogenesis. However, due to limited direct evidence regarding the health risks posed by MNP exposure to humans, significant knowledge gaps persist concerning the fate of MNPs and their adverse influence on human health. Currently, research about the impact of MNPs on human health has predominantly been confined to in vitro studies, indicating that investigations are still in the nascent stages. Therefore, there is an urgent need to perform systematic and comprehensive studies to determine the actual human exposure concentrations, distribution patterns, metabolic pathways and the size and concentration of toxic MNPs in humans and to develop a standard, if possible, to elucidate their effects on human health.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/toxics12070493/s1, Figure S1: The distribution of MNPs in the human body and potential health risks associated with exposure to MNPs; Figure S2: VOS viewer schematic for bibliometric analysis concerning PCD induced by MNPs, and its association with disease; Figure S3: PS-MPs induce pyroptosis in vitro; Figure S4: NPs can induce autophagy in cells.
Author Contributions
H.L. (Huanpeng Liu) reviewed the published studies and composed the draft of the manuscript. H.L. (Huiqi Li), T.C. and F.Y. offered critical feedback on drafts of the manuscript. Q.L., H.Z. and L.J. performed project administration. R.P. initiated the idea, guided the article structure, obtained financial support, and improved the final manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Construction project of Dominant Characteristic Discipline (Ecology) in 2022 (No. 2022217001).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Wu, P.; Huang, J.; Zheng, Y.; Yang, Y.; Zhang, Y.; He, F.; Chen, H.; Quan, G.; Yan, J.; Li, T.; et al. Environmental occurrences, fate, and impacts of microplastics. Ecotoxicol. Environ. Saf. 2019, 184, 109612. [Google Scholar] [CrossRef] [PubMed]
- Schwabl, P.; Köppel, S.; Königshofer, P.; Bucsics, T.; Trauner, M.; Reiberger, T.; Liebmann, B. Detection of Various Microplastics in Human Stool: A Prospective Case Series. Ann. Intern. Med. 2019, 171, 453–457. [Google Scholar] [CrossRef] [PubMed]
- Qian, N.; Gao, X.; Lang, X.; Deng, H.; Bratu, T.M.; Chen, Q.; Stapleton, P.; Yan, B.; Min, W. Rapid single-particle chemical imaging of nanoplastics by SRS microscopy. Proc. Natl. Acad. Sci. USA 2024, 121, e2300582121. [Google Scholar] [CrossRef] [PubMed]
- Blunt, J.W.; Carroll, A.R.; Copp, B.R.; Davis, R.A.; Keyzers, R.A.; Prinsep, M.R. Marine natural products. Nat. Prod. Rep. 2021, 38, 8–53. [Google Scholar] [CrossRef]
- Gao, D.; Liu, X.; Junaid, M.; Liao, H.; Chen, G.; Wu, Y.; Wang, J. Toxicological impacts of micro(nano)plastics in the benthic environment. Sci. Total Environ. 2022, 836, 155620. [Google Scholar] [CrossRef]
- Zhang, S.; Wang, W.; Yan, P.; Wang, J.; Yan, S.; Liu, X.; Aurangzeib, M. Microplastic migration and distribution in the terrestrial and aquatic environments: A threat to biotic safety. J. Environ. Manag. 2023, 333, 117412. [Google Scholar] [CrossRef]
- Kumar, M.; Chen, H.; Sarsaiya, S.; Qin, S.; Liu, H.; Awasthi, M.K.; Kumar, S.; Singh, L.; Zhang, Z.; Bolan, N.S.; et al. Current research trends on micro- and nano-plastics as an emerging threat to global environment: A review. J. Hazard. Mater. 2021, 409, 124967. [Google Scholar] [CrossRef]
- Venâncio, C.; Gabriel, A.; Oliveira, M.; Lopes, I. Feeding exposure and feeding behaviour as relevant approaches in the assessment of the effects of micro(nano)plastics to early life stages of amphibians. Environ. Res. 2022, 212 Pt D, 113476. [Google Scholar] [CrossRef]
- Pelegrini, K.; Pereira, T.C.B.; Maraschin, T.G.; Teodoro, L.D.S.; Basso, N.R.D.S.; De Galland, G.L.B.; Ligabue, R.A.; Bogo, M.R. Micro- and nanoplastic toxicity: A review on size, type, source, and test-organism implications. Sci. Total Environ. 2023, 878, 162954. [Google Scholar] [CrossRef]
- Blackburn, K.; Green, D. The potential effects of microplastics on human health: What is known and what is unknown. Ambio 2022, 51, 518–530. [Google Scholar] [CrossRef]
- Xu, J.-L.; Lin, X.; Wang, J.J.; Gowen, A.A. A review of potential human health impacts of micro- and nanoplastics exposure. Sci. Total Environ. 2022, 851 Pt 1, 158111. [Google Scholar] [CrossRef] [PubMed]
- Rillig, M.C.; Lehmann, A. Microplastic in terrestrial ecosystems. Science 2020, 368, 1430–1431. [Google Scholar] [CrossRef] [PubMed]
- Natesan, U.; Vaikunth, R.; Kumar, P.; Ruthra, R.; Srinivasalu, S. Spatial distribution of microplastic concentration around landfill sites and its potential risk on groundwater. Chemosphere 2021, 277, 130263. [Google Scholar] [CrossRef]
- Zhu, L.; Kang, Y.; Ma, M.; Wu, Z.; Zhang, L.; Hu, R.; Xu, Q.; Zhu, J.; Gu, X.; An, L. Tissue accumulation of microplastics and potential health risks in human. Sci. Total Environ. 2024, 915, 170004. [Google Scholar] [CrossRef] [PubMed]
- Song, X.; Du, L.; Sima, L.; Zou, D.; Qiu, X. Effects of micro(nano)plastics on the reproductive system: A review. Chemosphere 2023, 336, 139138. [Google Scholar] [CrossRef]
- Dusza, H.M.; van Boxel, J.; van Duursen, M.B.M.; Forsberg, M.M.; Legler, J.; Vähäkangas, K.H. Experimental human placental models for studying uptake, transport and toxicity of micro- and nanoplastics. Sci. Total Environ. 2023, 860, 160403. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Wang, C.; Duan, X.; Liang, B.; Genbo Xu, E.; Huang, Z. Micro- and nanoplastics: A new cardiovascular risk factor? Environ. Int. 2023, 171, 107662. [Google Scholar] [CrossRef]
- Ali, N.; Katsouli, J.; Marczylo, E.L.; Gant, T.W.; Wright, S.; de la Serna, J.B. The potential impacts of micro-and-nano plastics on various organ systems in humans. eBioMedicine 2024, 99, 104901. [Google Scholar] [CrossRef]
- Vethaak, A.D.; Legler, J. Microplastics and human health. Science 2021, 371, 672–674. [Google Scholar] [CrossRef]
- Lin, S.; Zhang, H.; Wang, C.; Su, X.-L.; Song, Y.; Wu, P.; Yang, Z.; Wong, M.-H.; Cai, Z.; Zheng, C. Metabolomics Reveal Nanoplastic-Induced Mitochondrial Damage in Human Liver and Lung Cells. Environ. Sci. Technol. 2022, 56, 12483–12493. [Google Scholar] [CrossRef]
- Wu, B.; Wu, X.; Liu, S.; Wang, Z.; Chen, L. Size-dependent effects of polystyrene microplastics on cytotoxicity and efflux pump inhibition in human Caco-2 cells. Chemosphere 2019, 221, 333–341. [Google Scholar] [CrossRef] [PubMed]
- Weber, A.; Schwiebs, A.; Solhaug, H.; Stenvik, J.; Nilsen, A.M.; Wagner, M.; Relja, B.; Radeke, H.H. Nanoplastics affect the inflammatory cytokine release by primary human monocytes and dendritic cells. Environ. Int. 2022, 163, 107173. [Google Scholar] [CrossRef] [PubMed]
- Landrigan, P.J.; Raps, H.; Cropper, M.; Bald, C.; Brunner, M.; Canonizado, E.M.; Charles, D.; Chiles, T.C.; Donohue, M.J.; Enck, J.; et al. The Minderoo-Monaco Commission on Plastics and Human Health. Ann. Glob. Health 2023, 89, 23. [Google Scholar] [CrossRef] [PubMed]
- Jenner, L.C.; Rotchell, J.M.; Bennett, R.T.; Cowen, M.; Tentzeris, V.; Sadofsky, L.R. Detection of microplastics in human lung tissue using μFTIR spectroscopy. Sci. Total Environ. 2022, 831, 154907. [Google Scholar] [CrossRef] [PubMed]
- Yin, H.; Fang, L.; Wang, L.; Xia, Y.; Tian, J.; Ma, L.; Zhang, J.; Li, N.; Li, W.; Yao, S.; et al. Acute Silica Exposure Triggers Pulmonary Inflammation Through Macrophage Pyroptosis: An Experimental Simulation. Front. Immunol. 2022, 13, 874459. [Google Scholar] [CrossRef] [PubMed]
- Song, M.; Wang, J.; Sun, Y.; Pang, J.; Li, X.; Liu, Y.; Zhou, Y.; Yang, P.; Fan, T.; Liu, Y.; et al. Inhibition of gasdermin D-dependent pyroptosis attenuates the progression of silica-induced pulmonary inflammation and fibrosis. Acta Pharm. Sin. B 2022, 12, 1213–1224. [Google Scholar] [CrossRef] [PubMed]
- Du, S.; Li, C.; Lu, Y.; Lei, X.; Zhang, Y.; Li, S.; Liu, F.; Chen, Y.; Weng, D.; Chen, J. Dioscin Alleviates Crystalline Silica-Induced Pulmonary Inflammation and Fibrosis through Promoting Alveolar Macrophage Autophagy. Theranostics 2019, 9, 1878. [Google Scholar] [CrossRef]
- Klionsky, D.J.; Petroni, G.; Amaravadi, R.K.; Baehrecke, E.H.; Ballabio, A.; Boya, P.; Bravo-San Pedro, J.M.; Cadwell, K.; Cecconi, F.; Choi, A.M.; et al. Autophagy in major human diseases. EMBO J. 2021, 40, ee108863. [Google Scholar] [CrossRef]
- El Kebir, D.; Gjorstrup, P.; Filep, J.G. Resolvin E1 promotes phagocytosis-induced neutrophil apoptosis and accelerates resolution of pulmonary inflammation. Proc. Natl. Acad. Sci. USA 2012, 109, 14983–14988. [Google Scholar] [CrossRef]
- Halimu, G.; Zhang, Q.; Liu, L.; Zhang, Z.; Wang, X.; Gu, W.; Zhang, B.; Dai, Y.; Zhang, H.; Zhang, C.; et al. Toxic effects of nanoplastics with different sizes and surface charges on epithelial-to-mesenchymal transition in A549 cells and the potential toxicological mechanism. J. Hazard. Mater. 2022, 430, 128485. [Google Scholar] [CrossRef]
- Wang, M.; Li, J.; Dong, S.; Cai, X.; Simaiti, A.; Yang, X.; Zhu, X.; Luo, J.; Jiang, L.H.; Du, B.; et al. Silica nanoparticles induce lung inflammation in mice via ROS/PARP/TRPM2 signaling-mediated lysosome impairment and autophagy dysfunction. Part. Fibre Toxicol. 2020, 17, 23. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Yao, Y.; Bai, H.; Shimizu, K.; Li, R.; Zhang, C. Investigation of pulmonary toxicity evaluation on mice exposed to polystyrene nanoplastics: The potential protective role of the antioxidant N-acetylcysteine. Sci. Total Environ. 2023, 855, 158851. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Sokratian, A.; Duda, A.M.; Xu, E.; Stanhope, C.; Fu, A.; Strader, S.; Li, H.; Yuan, Y.; Bobay, B.G.; et al. Anionic nanoplastic contaminants promote Parkinson’s disease-associated α-synuclein aggregation. Sci. Adv. 2023, 9, eadi8716. [Google Scholar] [CrossRef] [PubMed]
- Bredeck, G.; Halamoda-Kenzaoui, B.; Bogni, A.; Lipsa, D.; Bremer-Hoffmann, S. Tiered testing of micro- and nanoplastics using intestinal in vitro models to support hazard assessments. Environ. Int. 2022, 158, 106921. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.; Wang, X.; Huang, R.; Tang, C.; Hu, C.; Ning, P.; Wang, F. Cytotoxicity and Genotoxicity of Polystyrene Micro- and Nanoplastics with Different Size and Surface Modification in A549 Cells. Int. J. Nanomed. 2022, 17, 4509–4523. [Google Scholar] [CrossRef] [PubMed]
- Marfella, R.; Prattichizzo, F.; Sardu, C.; Fulgenzi, G.; Graciotti, L.; Spadoni, T.; D’Onofrio, N.; Scisciola, L.; La Grotta, R.; Frigé, C.; et al. Microplastics and Nanoplastics in Atheromas and Cardiovascular Events. N. Engl. J. Med. 2024, 390, 900–910. [Google Scholar] [CrossRef]
- Chen, C.; Liu, F.; Quan, S.; Chen, L.; Shen, A.; Jiao, A.; Qi, H.; Yu, G. Microplastics in the Bronchoalveolar Lavage Fluid of Chinese Children: Associations with Age, City Development, and Disease Features. Environ. Sci. Technol. 2023, 57, 12594–12601. [Google Scholar] [CrossRef] [PubMed]
- Prata, J.C.; da Costa, J.P.; Lopes, I.; Duarte, A.C.; Rocha-Santos, T. Environmental exposure to microplastics: An overview on possible human health effects. Sci. Total Environ. 2020, 702, 134455. [Google Scholar] [CrossRef] [PubMed]
- Das, A. The emerging role of microplastics in systemic toxicity: Involvement of reactive oxygen species (ROS). Sci. Total Environ. 2023, 895, 165076. [Google Scholar] [CrossRef]
- Jin, H.; Ma, T.; Sha, X.; Liu, Z.; Zhou, Y.; Meng, X.; Chen, Y.; Han, X.; Ding, J. Polystyrene microplastics induced male reproductive toxicity in mice. J. Hazard. Mater. 2021, 401, 123430. [Google Scholar] [CrossRef]
- Lu, Y.; Zhang, Y.; Deng, Y.; Jiang, W.; Zhao, Y.; Geng, J.; Ding, L.; Ren, H. Uptake and Accumulation of Polystyrene Microplastics in Zebrafish (Danio rerio) and Toxic Effects in Liver. Environ. Sci. Technol. 2016, 50, 4054–4060. [Google Scholar] [CrossRef]
- Teng, J.; Zhao, J.; Zhu, X.; Shan, E.; Zhang, C.; Zhang, W.; Wang, Q. Toxic effects of exposure to microplastics with environmentally relevant shapes and concentrations: Accumulation, energy metabolism and tissue damage in oyster Crassostrea gigas. Environ. Pollut. 2021, 269, 116169. [Google Scholar] [CrossRef]
- Wang, M.; Rücklin, M.; Poelmann, R.E.; de Mooij, C.L.; Fokkema, M.; Lamers, G.E.M.; de Bakker, M.A.G.; Chin, E.; Bakos, L.J.; Marone, F.; et al. Nanoplastics causes extensive congenital malformations during embryonic development by passively targeting neural crest cells. Environ. Int. 2023, 173, 107865. [Google Scholar] [CrossRef]
- Lahive, E.; Cross, R.; Saarloos, A.I.; Horton, A.A.; Svendsen, C.; Hufenus, R.; Mitrano, D.M. Earthworms ingest microplastic fibres and nanoplastics with effects on egestion rate and long-term retention. Sci. Total Environ. 2022, 807 Pt 3, 151022. [Google Scholar] [CrossRef] [PubMed]
- Hua, X.; Cao, C.; Zhang, L.; Wang, D. Activation of FGF signal in germline mediates transgenerational toxicity of polystyrene nanoparticles at predicted environmental concentrations in Caenorhabditis elegans. J. Hazard. Mater. 2023, 451, 131174. [Google Scholar] [CrossRef] [PubMed]
- Zeng, F.; Wang, L.; Zhen, H.; Guo, C.; Liu, A.; Xia, X.; Pei, H.; Dong, C.; Ding, J. Nanoplastics affect the growth of sea urchins (Strongylocentrotus intermedius) and damage gut health. Sci. Total Environ. 2023, 869, 161576. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Ma, Y.; Ye, S.; Tang, S.; Liang, N.; Liang, Y.; Xiao, F. Polystyrene microplastics trigger hepatocyte apoptosis and abnormal glycolytic flux via ROS-driven calcium overload. J. Hazard. Mater. 2021, 417, 126025. [Google Scholar] [CrossRef]
- Ma, S.; Xiao, Y.; Zhang, X.; Xu, Y.; Zhu, K.; Zhang, K.; Li, X.; Zhou, H.; Chen, G.; Guo, X. Dietary exposure to polystyrene microplastics exacerbates liver damage in fulminant hepatic failure via ROS production and neutrophil extracellular trap formation. Sci. Total Environ. 2024, 907, 167403. [Google Scholar] [CrossRef]
- McKnight, S.L. On getting there from here. Science 2010, 330, 1338–1339. [Google Scholar] [CrossRef]
- Wu, Q.; Cao, J.; Liu, X.; Zhu, X.; Huang, C.; Wang, X.; Song, Y. Micro(nano)-plastics exposure induced programmed cell death and corresponding influence factors. Sci. Total Environ. 2024, 921, 171230. [Google Scholar] [CrossRef]
- Mu, Y.; Sun, J.; Li, Z.; Zhang, W.; Liu, Z.; Li, C.; Peng, C.; Cui, G.; Shao, H.; Du, Z. Activation of pyroptosis and ferroptosis is involved in the hepatotoxicity induced by polystyrene microplastics in mice. Chemosphere 2022, 291 Pt 2, 132944. [Google Scholar] [CrossRef] [PubMed]
- Wei, J.; Wang, X.; Liu, Q.; Zhou, N.; Zhu, S.; Li, Z.; Li, X.; Yao, J.; Zhang, L. The impact of polystyrene microplastics on cardiomyocytes pyroptosis through NLRP3/Caspase-1 signaling pathway and oxidative stress in Wistar rats. Environ. Toxicol. 2021, 36, 935–944. [Google Scholar] [CrossRef] [PubMed]
- Hou, J.; Lei, Z.; Cui, L.; Hou, Y.; Yang, L.; An, R.; Wang, Q.; Li, S.; Zhang, H.; Zhang, L. Polystyrene microplastics lead to pyroptosis and apoptosis of ovarian granulosa cells via NLRP3/Caspase-1 signaling pathway in rats. Ecotoxicol. Environ. Saf. 2021, 212, 112012. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Yin, K.; Wang, D.; Wang, Y.; Lu, H.; Zhao, H.; Xing, M. Polystyrene microplastics-induced cardiotoxicity in chickens via the ROS-driven NF-κB-NLRP3-GSDMD and AMPK-PGC-1α axes. Sci. Total Environ. 2022, 840, 156727. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Wang, Y.; Du, Y.; Zhang, W.; Liu, Z.; Bai, J.; Cui, G.; Du, Z. Involvement of the JNK/HO-1/FTH1 signaling pathway in nanoplastic-induced inflammation and ferroptosis of BV2 microglia cells. Int. J. Mol. Med. 2023, 52, 61. [Google Scholar] [CrossRef] [PubMed]
- Yin, K.; Wang, D.; Zhao, H.; Wang, Y.; Zhang, Y.; Liu, Y.; Li, B.; Xing, M. Polystyrene microplastics up-regulates liver glutamine and glutamate synthesis and promotes autophagy-dependent ferroptosis and apoptosis in the cerebellum through the liver-brain axis. Environ. Pollut. 2022, 307, 119449. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.; Bu, W.; Hu, W.; Zhao, Z.; Liu, L.; Luo, C.; Wang, R.; Fan, S.; Yu, S.; Wu, Q.; et al. Ferroptosis Is Involved in Sex-Specific Small Intestinal Toxicity in the Offspring of Adult Mice Exposed to Polystyrene Nanoplastics during Pregnancy. ACS Nano 2023, 17, 2440–2449. [Google Scholar] [CrossRef] [PubMed]
- Shaoyong, W.; Jin, H.; Jiang, X.; Xu, B.; Liu, Y.; Wang, Y.; Jin, M. Benzo [a] pyrene-loaded aged polystyrene microplastics promote colonic barrier injury via oxidative stress-mediated notch signalling. J. Hazard. Mater. 2023, 457, 131820. [Google Scholar] [CrossRef] [PubMed]
- Nie, J.-H.; Shen, Y.; Roshdy, M.; Cheng, X.; Wang, G.; Yang, X. Polystyrene nanoplastics exposure caused defective neural tube morphogenesis through caveolae-mediated endocytosis and faulty apoptosis. Nanotoxicology 2021, 15, 885–904. [Google Scholar] [CrossRef]
- Lu, Y.-Y.; Li, H.; Ren, H.; Zhang, X.; Huang, F.; Zhang, D.; Huang, Q.; Zhang, X. Size-dependent effects of polystyrene nanoplastics on autophagy response in human umbilical vein endothelial cells. J. Hazard. Mater. 2022, 421, 126770. [Google Scholar] [CrossRef]
- Annangi, B.; Villacorta, A.; López-Mesas, M.; Fuentes-Cebrian, V.; Marcos, R.; Hernández, A. Hazard Assessment of Polystyrene Nanoplastics in Primary Human Nasal Epithelial Cells, Focusing on the Autophagic Effects. Biomolecules 2023, 13, 220. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.; Zhang, R.; Li, B.; Du, Y.; Li, J.; Tong, X.; Wu, Y.; Ji, X.; Zhang, Y. Tissue distribution of polystyrene nanoplastics in mice and their entry, transport, and cytotoxicity to GES-1 cells. Environ. Pollut. 2021, 280, 116974. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.-L.; Lee, Y.-H.; Hsu, Y.-H.; Chiu, I.-J.; Huang, C.C.-Y.; Huang, C.-C.; Chia, Z.-C.; Lee, C.-P.; Lin, Y.-F.; Chiu, H.-W. The Kidney-Related Effects of Polystyrene Microplastics on Human Kidney Proximal Tubular Epithelial Cells HK-2 and Male C57BL/6 Mice. Environ. Health Perspect. 2021, 129, 57003. [Google Scholar] [CrossRef]
- Jeon, M.S.; Kim, J.W.; Han, Y.B.; Jeong, M.H.; Kim, H.R.; Sik Kim, H.; Park, Y.J.; Chung, K.H. Polystyrene microplastic particles induce autophagic cell death in BEAS-2B human bronchial epithelial cells. Environ. Toxicol. 2023, 38, 359–367. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.; Yin, K.; Su, H.; Wang, D.; Zhang, Y.; Hou, L.; Li, J.B.; Wang, Y.; Xing, M. Polystyrene microplastics induce autophagy and apoptosis in birds lungs via PTEN/PI3K/AKT/mTOR. Environ. Toxicol. 2023, 38, 78–89. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.-C.; Chen, K.-F.; Lin, K.-Y.A.; Chen, J.-K.; Jiang, X.-Y.; Lin, C.-H. The nephrotoxic potential of polystyrene microplastics at realistic environmental concentrations. J. Hazard. Mater. 2022, 427, 127871. [Google Scholar] [CrossRef]
- Bobori, D.C.; Feidantsis, K.; Dimitriadi, A.; Datsi, N.; Ripis, P.; Kalogiannis, S.; Sampsonidis, I.; Kastrinaki, G.; Ainali, N.M.; Lambropoulou, D.A.; et al. Dose-Dependent Cytotoxicity of Polypropylene Microplastics (PP-MPs) in Two Freshwater Fishes. Int. J. Mol. Sci. 2022, 23, 13878. [Google Scholar] [CrossRef]
- Yang, M.; Wang, W.-X. Differential cascading cellular and subcellular toxicity induced by two sizes of nanoplastics. Sci. Total Environ. 2022, 829, 154593. [Google Scholar] [CrossRef] [PubMed]
- Yu, C.-W.; Wu, Y.-C.; Liao, V.H.-C. Nanoplastics exposure disrupts circadian rhythm associated with dysfunction of the endolysosomal pathway and autophagy in Caenorhabditis elegans. J. Hazard. Mater. 2023, 452, 131308. [Google Scholar] [CrossRef]
- Gopinath, P.M.; Twayana, K.S.; Ravanan, P.; Thomas, J.; Mukherjee, A.; Jenkins, D.F.; Chandrasekaran, N. Prospects on the nano-plastic particles internalization and induction of cellular response in human keratinocytes. Part. Fibre Toxicol. 2021, 18, 35. [Google Scholar] [CrossRef]
- Zhou, Y.; He, G.; Jiang, H.; Pan, K.; Liu, W. Nanoplastics induces oxidative stress and triggers lysosome-associated immune-defensive cell death in the earthworm Eisenia fetida. Environ. Int. 2023, 174, 107899. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wang, D.; Yin, K.; Zhao, H.; Lu, H.; Meng, X.; Hou, L.; Li, J.; Xing, M. Endoplasmic reticulum stress-controlled autophagic pathway promotes polystyrene microplastics-induced myocardial dysplasia in birds. Environ. Pollut. 2022, 311, 119963. [Google Scholar] [CrossRef] [PubMed]
- Xu, D.; Ma, Y.; Peng, C.; Gan, Y.; Wang, Y.; Chen, Z.; Han, X.; Chen, Y. Differently surface-labeled polystyrene nanoplastics at an environmentally relevant concentration induced Crohn’s ileitis-like features via triggering intestinal epithelial cell necroptosis. Environ. Int. 2023, 176, 107968. [Google Scholar] [CrossRef] [PubMed]
- Shan, S.; Zhang, Y.; Zhao, H.; Zeng, T.; Zhao, X. Polystyrene nanoplastics penetrate across the blood-brain barrier and induce activation of microglia in the brain of mice. Chemosphere 2022, 298, 134261. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wang, S.; Xu, T.; Cui, W.; Shi, X.; Xu, S. A new discovery of polystyrene microplastics toxicity: The injury difference on bladder epithelium of mice is correlated with the size of exposed particles. Sci. Total Environ. 2022, 821, 153413. [Google Scholar] [CrossRef] [PubMed]
- Meng, X.; Yin, K.; Zhang, Y.; Wang, D.; Lu, H.; Hou, L.; Zhao, H.; Xing, M. Polystyrene microplastics induced oxidative stress, inflammation and necroptosis via NF-κB and RIP1/RIP3/MLKL pathway in chicken kidney. Toxicology 2022, 478, 153296. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhang, X.; Sun, K.; Wang, S.; Gong, D. Polystyrene microplastics induce apoptosis and necroptosis in swine testis cells via ROS/MAPK/HIF1α pathway. Environ. Toxicol. 2022, 37, 2483–2492. [Google Scholar] [CrossRef] [PubMed]
- Xu, D.; Ma, Y.; Han, X.; Chen, Y. Systematic toxicity evaluation of polystyrene nanoplastics on mice and molecular mechanism investigation about their internalization into Caco-2 cells. J. Hazard. Mater. 2021, 417, 126092. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Guo, J.; Yao, Y.; Xu, S. Polystyrene nanoplastics induced cardiomyocyte apoptosis and myocardial inflammation in carp by promoting ROS production. Fish. Shellfish. Immunol. 2022, 125, 1–8. [Google Scholar] [CrossRef]
- Chen, Q.; Cao, Y.; Li, H.; Liu, H.; Liu, Y.; Bi, L.; Zhao, H.; Jin, L.; Peng, R. Sodium nitroprusside alleviates nanoplastics-induced developmental toxicity by suppressing apoptosis, ferroptosis and inflammation. J. Environ. Manag. 2023, 345, 118702. [Google Scholar] [CrossRef]
- Yang, D.; Zhu, J.; Zhou, X.; Pan, D.; Nan, S.; Yin, R.; Lei, Q.; Ma, N.; Zhu, H.; Chen, J.; et al. Polystyrene micro- and nano-particle coexposure injures fetal thalamus by inducing ROS-mediated cell apoptosis. Environ. Int. 2022, 166, 107362. [Google Scholar] [CrossRef]
- Kwon, W.; Kim, D.; Kim, H.-Y.; Jeong, S.W.; Lee, S.-G.; Kim, H.-C.; Lee, Y.-J.; Kwon, M.K.; Hwang, J.-S.; Han, J.E.; et al. Microglial phagocytosis of polystyrene microplastics results in immune alteration and apoptosis in vitro and in vivo. Sci. Total Environ. 2022, 807 Pt 2, 150817. [Google Scholar] [CrossRef]
- Umamaheswari, S.; Priyadarshinee, S.; Kadirvelu, K.; Ramesh, M. Polystyrene microplastics induce apoptosis via ROS-mediated p53 signaling pathway in zebrafish. Chem.-Biol. Interact. 2021, 345, 109550. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.; Halimu, G.; Zhang, Q.; Song, Y.; Fu, X.; Li, Y.; Li, Y.; Zhang, H. Internalization and toxicity: A preliminary study of effects of nanoplastic particles on human lung epithelial cell. Sci. Total Environ. 2019, 694, 133794. [Google Scholar] [CrossRef]
- Liang, B.; Zhong, Y.; Huang, Y.; Lin, X.; Liu, J.; Lin, L.; Hu, M.; Jiang, J.; Dai, M.; Wang, B.; et al. Underestimated health risks: Polystyrene micro- and nanoplastics jointly induce intestinal barrier dysfunction by ROS-mediated epithelial cell apoptosis. Part. Fibre Toxicol. 2021, 18, 20. [Google Scholar] [CrossRef] [PubMed]
- Hsu, S.-K.; Li, C.-Y.; Lin, I.-L.; Syue, W.-J.; Chen, Y.-F.; Cheng, K.-C.; Teng, Y.-N.; Lin, Y.-H.; Yen, C.-H.; Chiu, C.-C. Inflammation-related pyroptosis, a novel programmed cell death pathway, and its crosstalk with immune therapy in cancer treatment. Theranostics 2021, 11, 8813–8835. [Google Scholar] [CrossRef]
- Shi, J.; Zhao, Y.; Wang, K.; Shi, X.; Wang, Y.; Huang, H.; Zhuang, Y.; Cai, T.; Wang, F.; Shao, F. Cleavage of GSDMD by inflammatory caspases determines pyroptotic cell death. Nature 2015, 526, 660–665. [Google Scholar] [CrossRef]
- Liu, X.; Xia, S.; Zhang, Z.; Wu, H.; Lieberman, J. Channelling inflammation: Gasdermins in physiology and disease. Nat. Rev. Drug Discov. 2021, 20, 384–405. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Gao, W.; Shao, F. Pyroptosis: Gasdermin-Mediated Programmed Necrotic Cell Death. Trends Biochem. Sci. 2017, 42, 245–254. [Google Scholar] [CrossRef]
- Zhong, G.; Rao, G.; Tang, L.; Wu, S.; Tang, Z.; Huang, R.; Ruan, Z.; Hu, L. Combined effect of arsenic and polystyrene-nanoplastics at environmentally relevant concentrations in mice liver: Activation of apoptosis, pyroptosis and excessive autophagy. Chemosphere 2022, 300, 134566. [Google Scholar] [CrossRef]
- Zhaolin, Z.; Guohua, L.; Shiyuan, W.; Zuo, W. Role of pyroptosis in cardiovascular disease. Cell Prolif. 2019, 52, e12563. [Google Scholar] [CrossRef] [PubMed]
- Shengchen, W.; Jing, L.; Yujie, Y.; Yue, W.; Shiwen, X. Polystyrene microplastics-induced ROS overproduction disrupts the skeletal muscle regeneration by converting myoblasts into adipocytes. J. Hazard. Mater. 2021, 417, 125962. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Chen, L.; Shi, X.; Wang, Y.; Xu, S. Polystyrene microplastics-induced macrophage extracellular traps contributes to liver fibrotic injury by activating ROS/TGF-β/Smad2/3 signaling axis. Environ. Pollut. 2023, 324, 121388. [Google Scholar] [CrossRef]
- Wu, H.; Xu, T.; Chen, T.; Liu, J.; Xu, S. Oxidative stress mediated by the TLR4/NOX2 signalling axis is involved in polystyrene microplastic-induced uterine fibrosis in mice. Sci. Total Environ. 2022, 838 Pt 2, 155825. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Wang, Y.; Kong, Y.; Zhang, X.; Zhang, H.; Gang, Y.; Bai, L. Carnosine Prevents Type 2 Diabetes-Induced Osteoarthritis Through the ROS/NF-κB Pathway. Front. Pharmacol. 2018, 9, 598. [Google Scholar] [CrossRef] [PubMed]
- Lianxu, C.; Hongti, J.; Changlong, Y. NF-kappaBp65-specific siRNA inhibits expression of genes of COX-2, NOS-2 and MMP-9 in rat IL-1beta-induced and TNF-alpha-induced chondrocytes. Osteoarthr. Cartil. 2006, 14, 367–376. [Google Scholar] [CrossRef] [PubMed]
- Zeng, J.; Chen, Y.; Ding, R.; Feng, L.; Fu, Z.; Yang, S.; Deng, X.; Xie, Z.; Zheng, S. Isoliquiritigenin alleviates early brain injury after experimental intracerebral hemorrhage via suppressing ROS- and/or NF-κB-mediated NLRP3 inflammasome activation by promoting Nrf2 antioxidant pathway. J. Neuroinflamm. 2017, 14, 119. [Google Scholar] [CrossRef]
- Caputi, S.; Diomede, F.; Lanuti, P.; Marconi, G.D.; Di Carlo, P.; Sinjari, B.; Trubiani, O. Microplastics Affect the Inflammation Pathway in Human Gingival Fibroblasts: A Study in the Adriatic Sea. Int. J. Environ. Res. Public Health 2022, 19, 7782. [Google Scholar] [CrossRef]
- Kim, J.; Montagnani, M.; Koh, K.K.; Quon, M.J. Reciprocal relationships between insulin resistance and endothelial dysfunction: Molecular and pathophysiological mechanisms. Circulation 2006, 113, 1888–1904. [Google Scholar] [CrossRef]
- Yu, P.; Zhang, X.; Liu, N.; Tang, L.; Peng, C.; Chen, X. Pyroptosis: Mechanisms and diseases. Signal Transduct. Target. Ther. 2021, 6, 128. [Google Scholar] [CrossRef]
- Abe, J.-I.; Morrell, C. Pyroptosis as a Regulated Form of Necrosis: PI+/Annexin V-/High Caspase 1/Low Caspase 9 Activity in Cells = Pyroptosis? Circ. Res. 2016, 118, 1457–1460. [Google Scholar] [CrossRef]
- Li, S.; Sun, Y.; Song, M.; Song, Y.; Fang, Y.; Zhang, Q.; Li, X.; Song, N.; Ding, J.; Lu, M.; et al. NLRP3/caspase-1/GSDMD-mediated pyroptosis exerts a crucial role in astrocyte pathological injury in mouse model of depression. JCI Insight 2021, 6, e146852. [Google Scholar] [CrossRef]
- Wang, Y.; Gao, W.; Shi, X.; Ding, J.; Liu, W.; He, H.; Wang, K.; Shao, F. Chemotherapy drugs induce pyroptosis through caspase-3 cleavage of a gasdermin. Nature 2017, 547, 99–103. [Google Scholar] [CrossRef]
- Zhao, W.; Ma, L.; Cai, C.; Gong, X. Caffeine Inhibits NLRP3 Inflammasome Activation by Suppressing MAPK/NF-κB and A2aR Signaling in LPS-Induced THP-1 Macrophages. Int. J. Biol. Sci. 2019, 15, 1571–1581. [Google Scholar] [CrossRef] [PubMed]
- Zhivaki, D.; Kagan, J.C. Innate immune detection of lipid oxidation as a threat assessment strategy. Nat. Rev. Immunol. 2022, 22, 322–330. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Jiang, Q. Uncoupled pyroptosis and IL-1β secretion downstream of inflammasome signaling. Front. Immunol. 2023, 14, 1128358. [Google Scholar] [CrossRef]
- Li, Y.; Sheng, H.; Yan, Z.; Guan, B.; Qiang, S.; Qian, J.; Wang, Y. Bilirubin stabilizes the mitochondrial membranes during NLRP3 inflammasome activation. Biochem. Pharmacol. 2022, 203, 115204. [Google Scholar] [CrossRef]
- Martín-Sánchez, F.; Compan, V.; Peñín-Franch, A.; Tapia-Abellán, A.; Gómez, A.I.; Baños-Gregori, M.C.; Schmidt, F.I.; Pelegrin, P. ASC oligomer favors caspase-1CARD domain recruitment after intracellular potassium efflux. J. Cell Biol. 2023, 222, e202003053. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wang, J.; Huang, D.; Yu, C. Baicalin Alleviates Contrast-Induced Acute Kidney Injury Through ROS/NLRP3/Caspase-1/GSDMD Pathway-Mediated Proptosis in vitro. Drug Des. Devel Ther. 2022, 16, 3353–3364. [Google Scholar] [CrossRef]
- Kesavardhana, S.; Malireddi, R.K.S.; Kanneganti, T.-D. Caspases in Cell Death, Inflammation, and Pyroptosis. Annu. Rev. Immunol. 2020, 38, 567–595. [Google Scholar] [CrossRef]
- McKenzie, B.A.; Dixit, V.M.; Power, C. Fiery Cell Death: Pyroptosis in the Central Nervous System. Trends Neurosci. 2020, 43, 55–73. [Google Scholar] [CrossRef] [PubMed]
- Rao, Z.; Zhu, Y.; Yang, P.; Chen, Z.; Xia, Y.; Qiao, C.; Liu, W.; Deng, H.; Li, J.; Ning, P.; et al. Pyroptosis in inflammatory diseases and cancer. Theranostics 2022, 12, 4310–4329. [Google Scholar] [CrossRef] [PubMed]
- You, R.; Xiao, R. Pyroptosis and Its Role in Autoimmune Disease: A Potential Therapeutic Target. Front. Immunol. 2022, 13, 841732. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Qiu, X.; Xi, G.; Liu, H.; Zhang, F.; Lv, T.; Song, Y. Downregulation of GSDMD attenuates tumor proliferation via the intrinsic mitochondrial apoptotic pathway and inhibition of EGFR/Akt signaling and predicts a good prognosis in non-small cell lung cancer. Oncol. Rep. 2018, 40, 1971–1984. [Google Scholar] [CrossRef] [PubMed]
- Janneh, A.H.; Kassir, M.F.; Atilgan, F.C.; Lee, H.G.; Sheridan, M.; Oleinik, N.; Szulc, Z.; Voelkel-Johnson, C.; Nguyen, H.; Li, H.; et al. Crosstalk between pro-survival sphingolipid metabolism and complement signaling induces inflammasome-mediated tumor metastasis. Cell Rep. 2022, 41, 111742. [Google Scholar] [CrossRef] [PubMed]
- Sayin, V.I.; Ibrahim, M.X.; Larsson, E.; Nilsson, J.A.; Lindahl, P.; Bergo, M.O. Antioxidants accelerate lung cancer progression in mice. Sci. Transl. Med. 2014, 6, 221ra15. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Sun, S.; Sun, Y.; Song, Q.; Zhu, J.; Song, N.; Chen, M.; Sun, T.; Xia, M.; Ding, J.; et al. Small molecule-driven NLRP3 inflammation inhibition via interplay between ubiquitination and autophagy: Implications for Parkinson disease. Autophagy 2019, 15, 1860–1881. [Google Scholar] [CrossRef]
- Daniels, M.J.D.; Rivers-Auty, J.; Schilling, T.; Spencer, N.G.; Watremez, W.; Fasolino, V.; Booth, S.J.; White, C.S.; Baldwin, A.G.; Freeman, S.; et al. Fenamate NSAIDs inhibit the NLRP3 inflammasome and protect against Alzheimer’s disease in rodent models. Nat. Commun. 2016, 7, 12504. [Google Scholar] [CrossRef] [PubMed]
- Thomas, C. Roadblocks in HIV research: Five questions. Nat. Med. 2009, 15, 855–859. [Google Scholar] [CrossRef]
- Pan, P.; Shen, M.; Yu, Z.; Ge, W.; Chen, K.; Tian, M.; Xiao, F.; Wang, Z.; Wang, J.; Jia, Y.; et al. SARS-CoV-2 N protein promotes NLRP3 inflammasome activation to induce hyperinflammation. Nat. Commun. 2021, 12, 4664. [Google Scholar] [CrossRef]
- Stack, J.H.; Beaumont, K.; Larsen, P.D.; Straley, K.S.; Henkel, G.W.; Randle, J.C.R.; Hoffman, H.M. IL-converting enzyme/caspase-1 inhibitor VX-765 blocks the hypersensitive response to an inflammatory stimulus in monocytes from familial cold autoinflammatory syndrome patients. J. Immunol. 2005, 175, 2630–2634. [Google Scholar] [CrossRef] [PubMed]
- Doitsh, G.; Galloway, N.L.K.; Geng, X.; Yang, Z.; Monroe, K.M.; Zepeda, O.; Hunt, P.W.; Hatano, H.; Sowinski, S.; Muñoz-Arias, I.; et al. Cell death by pyroptosis drives CD4 T-cell depletion in HIV-1 infection. Nature 2014, 505, 509–514. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Li, J.; Kang, R.; Klionsky, D.J.; Tang, D. Ferroptosis: Machinery and regulation. Autophagy 2021, 17, 2054–2081. [Google Scholar] [CrossRef] [PubMed]
- Stockwell, B.R. Ferroptosis turns 10: Emerging mechanisms, physiological functions, and therapeutic applications. Cell 2022, 185, 2401–2421. [Google Scholar] [CrossRef]
- Carbó, M.; Chaturvedi, P.; Álvarez, A.; Pineda-Cevallos, D.; Ghatak, A.; González, P.R.; Cañal, M.J.; Weckwerth, W.; Valledor, L. Ferroptosis is the key cellular process mediating Bisphenol A responses in Chlamydomonas and a promising target for enhancing microalgae-based bioremediation. J. Hazard. Mater. 2023, 448, 130997. [Google Scholar] [CrossRef] [PubMed]
- Fu, H.; Zhu, L.; Mao, L.; Zhang, L.; Zhang, Y.; Chang, Y.; Liu, X.; Jiang, H. Combined ecotoxicological effects of different-sized polyethylene microplastics and imidacloprid on the earthworms (Eisenia fetida). Sci. Total Environ. 2023, 870, 161795. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Li, Y.; Maryam, B.; Ji, Z.; Sun, J.; Liu, X. Polybrominated diphenyl ethers as hitchhikers on microplastics: Sorption behaviors and combined toxicities to Epinephelus moara. Aquat. Toxicol. 2022, 252, 106317. [Google Scholar] [CrossRef] [PubMed]
- Lyamzaev, K.G.; Panteleeva, A.A.; Simonyan, R.A.; Avetisyan, A.V.; Chernyak, B.V. Mitochondrial Lipid Peroxidation Is Responsible for Ferroptosis. Cells 2023, 12, 611. [Google Scholar] [CrossRef]
- Barrera, G.; Pizzimenti, S.; Dianzani, M.U. Lipid peroxidation: Control of cell proliferation, cell differentiation and cell death. Mol. Asp. Med. 2008, 29, 1–8. [Google Scholar] [CrossRef]
- Lee, J.-Y.; Nam, M.; Son, H.Y.; Hyun, K.; Jang, S.Y.; Kim, J.W.; Kim, M.W.; Jung, Y.; Jang, E.; Yoon, S.-J.; et al. Polyunsaturated fatty acid biosynthesis pathway determines ferroptosis sensitivity in gastric cancer. Proc. Natl. Acad. Sci. USA 2020, 117, 32433–32442. [Google Scholar] [CrossRef]
- Suda, A.; Umaru, B.A.; Yamamoto, Y.; Shima, H.; Saiki, Y.; Pan, Y.; Jin, L.; Sun, J.; Low, Y.L.C.; Suzuki, C.; et al. Polyunsaturated fatty acids-induced ferroptosis suppresses pancreatic cancer growth. Sci. Rep. 2024, 14, 4409. [Google Scholar] [CrossRef]
- Kagan, V.E.; Mao, G.; Qu, F.; Angeli, J.P.F.; Doll, S.; Croix, C.S.; Dar, H.H.; Liu, B.; Tyurin, V.A.; Ritov, V.B.; et al. Oxidized arachidonic and adrenic PEs navigate cells to ferroptosis. Nat. Chem. Biol. 2017, 13, 81–90. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Wei, X.; Hu, F.; Dong, W.; Sun, L. Development and validation of a novel 3-gene prognostic model for pancreatic adenocarcinoma based on ferroptosis-related genes. Cancer Cell Int. 2022, 22, 21. [Google Scholar] [CrossRef] [PubMed]
- Ge, Y.; Yang, S.; Zhang, T.; Gong, S.; Wan, X.; Zhu, Y.; Fang, Y.; Hu, C.; Yang, F.; Yin, L.; et al. Ferroptosis participated in inhaled polystyrene nanoplastics-induced liver injury and fibrosis. Sci. Total Environ. 2024, 916, 170342. [Google Scholar] [CrossRef] [PubMed]
- Jeyavani, J.; Sibiya, A.; Bhavaniramya, S.; Mahboob, S.; Al-Ghanim, K.A.; Nisa, Z.-U.; Riaz, M.N.; Nicoletti, M.; Govindarajan, M.; Vaseeharan, B. Toxicity evaluation of polypropylene microplastic on marine microcrustacean Artemia salina: An analysis of implications and vulnerability. Chemosphere 2022, 296, 133990. [Google Scholar] [CrossRef]
- Dixon, S.J.; Patel, D.N.; Welsch, M.; Skouta, R.; Lee, E.D.; Hayano, M.; Thomas, A.G.; Gleason, C.E.; Tatonetti, N.P.; Slusher, B.S.; et al. Pharmacological inhibition of cystine-glutamate exchange induces endoplasmic reticulum stress and ferroptosis. eLife 2014, 3, e02523. [Google Scholar] [CrossRef]
- Liang, D.; Feng, Y.; Zandkarimi, F.; Wang, H.; Zhang, Z.; Kim, J.; Cai, Y.; Gu, W.; Stockwell, B.R.; Jiang, X. Ferroptosis surveillance independent of GPX4 and differentially regulated by sex hormones. Cell 2023, 186, 2748–2764.e22. [Google Scholar] [CrossRef] [PubMed]
- Cheng, W.; Zhou, Y.; Chen, H.; Wu, Q.; Li, Y.; Wang, H.; Feng, Y.; Wang, Y. The iron matters: Aged microplastics disrupted the iron homeostasis in the liver organoids. Sci. Total Environ. 2024, 906, 167529. [Google Scholar] [CrossRef]
- Xu, T.; Cui, J.; Xu, R.; Cao, J.; Guo, M.-Y. Microplastics induced inflammation and apoptosis via ferroptosis and the NF-κB pathway in carp. Aquat. Toxicol. 2023, 262, 106659. [Google Scholar] [CrossRef]
- Henning, Y.; Blind, U.S.; Larafa, S.; Matschke, J.; Fandrey, J. Hypoxia aggravates ferroptosis in RPE cells by promoting the Fenton reaction. Cell Death Dis. 2022, 13, 662. [Google Scholar] [CrossRef]
- Roos, A.; Boron, W.F. Intracellular pH. Physiol. Rev. 1981, 61, 296–434. [Google Scholar] [CrossRef] [PubMed]
- He, Y.-J.; Liu, X.-Y.; Xing, L.; Wan, X.; Chang, X.; Jiang, H.-L. Fenton reaction-independent ferroptosis therapy via glutathione and iron redox couple sequentially triggered lipid peroxide generator. Biomaterials 2020, 241, 119911. [Google Scholar] [CrossRef] [PubMed]
- Gao, M.; Yi, J.; Zhu, J.; Minikes, A.M.; Monian, P.; Thompson, C.B.; Jiang, X. Role of Mitochondria in Ferroptosis. Mol. Cell 2019, 73, 354–363.e3. [Google Scholar] [CrossRef] [PubMed]
- Hangauer, M.J.; Viswanathan, V.S.; Ryan, M.J.; Bole, D.; Eaton, J.K.; Matov, A.; Galeas, J.; Dhruv, H.D.; Berens, M.E.; Schreiber, S.L.; et al. Drug-tolerant persister cancer cells are vulnerable to GPX4 inhibition. Nature 2017, 551, 247–250. [Google Scholar] [CrossRef] [PubMed]
- Tang, B.; Wang, Y.; Xu, W.; Zhu, J.; Weng, Q.; Chen, W.; Fang, S.; Yang, Y.; Qiu, R.; Chen, M.; et al. Macrophage xCT deficiency drives immune activation and boosts responses to immune checkpoint blockade in lung cancer. Cancer Lett. 2023, 554, 216021. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Liu, Y.; Du, T.; Yang, H.; Lei, L.; Guo, M.; Ding, H.-F.; Zhang, J.; Wang, H.; Chen, X.; et al. ATF3 promotes erastin-induced ferroptosis by suppressing system Xc. Cell Death Differ. 2020, 27, 662–675. [Google Scholar] [CrossRef] [PubMed]
- Dixon, S.J.; Lemberg, K.M.; Lamprecht, M.R.; Skouta, R.; Zaitsev, E.M.; Gleason, C.E.; Patel, D.N.; Bauer, A.J.; Cantley, A.M.; Yang, W.S.; et al. Ferroptosis: An iron-dependent form of nonapoptotic cell death. Cell 2012, 149, 1060–1072. [Google Scholar] [CrossRef] [PubMed]
- Gao, M.; Monian, P.; Quadri, N.; Ramasamy, R.; Jiang, X. Glutaminolysis and Transferrin Regulate Ferroptosis. Mol. Cell 2015, 59, 298–308. [Google Scholar] [CrossRef] [PubMed]
- Mayr, L.; Grabherr, F.; Schwärzler, J.; Reitmeier, I.; Sommer, F.; Gehmacher, T.; Niederreiter, L.; He, G.-W.; Ruder, B.; Kunz, K.T.R.; et al. Dietary lipids fuel GPX4-restricted enteritis resembling Crohn’s disease. Nat. Commun. 2020, 11, 1775. [Google Scholar] [CrossRef]
- Xue, X.; Bredell, B.X.; Anderson, E.R.; Martin, A.; Mays, C.; Nagao-Kitamoto, H.; Huang, S.; Győrffy, B.; Greenson, J.K.; Hardiman, K.; et al. Quantitative proteomics identifies STEAP4 as a critical regulator of mitochondrial dysfunction linking inflammation and colon cancer. Proc. Natl. Acad. Sci. USA 2017, 114, E9608–E9617. [Google Scholar] [CrossRef]
- Chen, Y.-E.; Xu, S.-J.; Lu, Y.-Y.; Chen, S.-X.; Du, X.-H.; Hou, S.-Z.; Huang, H.-Y.; Liang, J. Asperuloside suppressing oxidative stress and inflammation in DSS-induced chronic colitis and RAW 264.7 macrophages via Nrf2/HO-1 and NF-κB pathways. Chem. Biol. Interact. 2021, 344, 109512. [Google Scholar] [CrossRef]
- Fang, X.; Wang, H.; Han, D.; Xie, E.; Yang, X.; Wei, J.; Gu, S.; Gao, F.; Zhu, N.; Yin, X.; et al. Ferroptosis as a target for protection against cardiomyopathy. Proc. Natl. Acad. Sci. USA 2019, 116, 2672–2680. [Google Scholar] [CrossRef]
- Li, Y.; Feng, D.; Wang, Z.; Zhao, Y.; Sun, R.; Tian, D.; Liu, D.; Zhang, F.; Ning, S.; Yao, J.; et al. Ischemia-induced ACSL4 activation contributes to ferroptosis-mediated tissue injury in intestinal ischemia/reperfusion. Cell Death Differ. 2019, 26, 2284–2299. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Zhang, X.; Wei, C.; Zheng, D.; Lu, X.; Yang, Y.; Luo, A.; Zhang, K.; Duan, X.; Wang, Y. Targeting SLC7A11 specifically suppresses the progression of colorectal cancer stem cells via inducing ferroptosis. Eur. J. Pharm. Sci. 2020, 152, 105450. [Google Scholar] [CrossRef]
- Xu, M.; Tao, J.; Yang, Y.; Tan, S.; Liu, H.; Jiang, J.; Zheng, F.; Wu, B. Ferroptosis involves in intestinal epithelial cell death in ulcerative colitis. Cell Death Dis. 2020, 11, 86. [Google Scholar] [CrossRef] [PubMed]
- Doll, S.; Freitas, F.P.; Shah, R.; Aldrovandi, M.; Da Silva, M.C.; Ingold, I.; Goya Grocin, A.; Xavier Da Silva, T.N.; Panzilius, E.; Scheel, C.H.; et al. FSP1 is a glutathione-independent ferroptosis suppressor. Nature 2019, 575, 693–698. [Google Scholar] [CrossRef] [PubMed]
- Louandre, C.; Ezzoukhry, Z.; Godin, C.; Barbare, J.-C.; Mazière, J.-C.; Chauffert, B.; Galmiche, A. Iron-dependent cell death of hepatocellular carcinoma cells exposed to sorafenib: Iron-dependent cytotoxicity of sorafenib. Int. J. Cancer 2013, 133, 1732–1742. [Google Scholar] [CrossRef]
- Zhu, S.; Zhang, Q.; Sun, X.; Zeh, H.J.; Lotze, M.T.; Kang, R.; Tang, D. HSPA5 Regulates Ferroptotic Cell Death in Cancer Cells. Cancer Res. 2017, 77, 2064–2077. [Google Scholar] [CrossRef] [PubMed]
- Mahoney-Sánchez, L.; Bouchaoui, H.; Ayton, S.; Devos, D.; Duce, J.A.; Devedjian, J.-C. Ferroptosis and its potential role in the physiopathology of Parkinson’s Disease. Prog. Neurobiol. 2021, 196, 101890. [Google Scholar] [CrossRef]
- Bao, W.-D.; Pang, P.; Zhou, X.-T.; Hu, F.; Xiong, W.; Chen, K.; Wang, J.; Wang, F.; Xie, D.; Hu, Y.-Z.; et al. Loss of ferroportin induces memory impairment by promoting ferroptosis in Alzheimer’s disease. Cell Death Differ. 2021, 28, 1548–1562. [Google Scholar] [CrossRef]
- Kim, K.H.; Lee, M.-S. Autophagy--a key player in cellular and body metabolism. Nat. Rev. Endocrinol. 2014, 10, 322–337. [Google Scholar] [CrossRef]
- Mizushima, N.; Komatsu, M. Autophagy: Renovation of cells and tissues. Cell 2011, 147, 728–741. [Google Scholar] [CrossRef] [PubMed]
- Lin, P.; Tong, X.; Xue, F.; Qianru, C.; Xinyu, T.; Zhe, L.; Zhikun, B.; Shu, L. Polystyrene nanoplastics exacerbate lipopolysaccharide-induced myocardial fibrosis and autophagy in mice via ROS/TGF-β1/Smad. Toxicology 2022, 480, 153338. [Google Scholar] [CrossRef]
- Parzych, K.R.; Klionsky, D.J. An overview of autophagy: Morphology, mechanism, and regulation. Antioxid. Redox Signal. 2014, 20, 460–473. [Google Scholar] [CrossRef]
- Yonekawa, T.; Thorburn, A. Autophagy and cell death. Essays Biochem. 2013, 55, 105–117. [Google Scholar] [CrossRef]
- Liu, S.; Yao, S.; Yang, H.; Liu, S.; Wang, Y. Autophagy: Regulator of cell death. Cell Death Dis. 2023, 14, 648. [Google Scholar] [CrossRef] [PubMed]
- Dikic, I.; Elazar, Z. Mechanism and medical implications of mammalian autophagy. Nat. Rev. Mol. Cell Biol. 2018, 19, 349–364. [Google Scholar] [CrossRef]
- Sorice, M. Crosstalk of Autophagy and Apoptosis. Cells 2022, 11, 1479. [Google Scholar] [CrossRef] [PubMed]
- Luo, Z.; Xu, X.; Sho, T.; Zhang, J.; Xu, W.; Yao, J.; Xu, J. ROS-induced autophagy regulates porcine trophectoderm cell apoptosis, proliferation, and differentiation. Am. J. Physiol. Cell Physiol. 2019, 316, C198–C209. [Google Scholar] [CrossRef]
- Hattori, T.; Fundora, K.A.; Hamamoto, K.; Opozda, D.M.; Liang, X.; Liu, X.; Zhang, J.; Uzun, Y.; Takahashi, Y.; Wang, H.-G. ER stress elicits non-canonical CASP8 (caspase 8) activation on autophagosomal membranes to induce apoptosis. Autophagy 2024, 20, 349–364. [Google Scholar] [CrossRef]
- Yin, Y.; Sun, G.; Li, E.; Kiselyov, K.; Sun, D. ER stress and impaired autophagy flux in neuronal degeneration and brain injury. Ageing Res. Rev. 2017, 34, 3–14. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.G.; Codogno, P.; Zhang, H. Machinery, regulation and pathophysiological implications of autophagosome maturation. Nat. Rev. Mol. Cell Biol. 2021, 22, 733–750. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Ma, Y.; Ye, S.; Su, Y.; Hu, D.; Xiao, F. Endogenous hydrogen sulfide counteracts polystyrene nanoplastics-induced mitochondrial apoptosis and excessive autophagy via regulating Nrf2 and PGC-1α signaling pathway in mouse spermatocyte-derived GC-2spd(ts) cells. Food Chem. Toxicol. 2022, 164, 113071. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Chu, Q.; Ye, X.; Sun, Y.; Liu, Y.; Jia, R.; Li, Y.; Tu, P.; Tang, Q.; Yu, T.; et al. Canidin-3-glucoside prevents nano-plastics induced toxicity via activating autophagy and promoting discharge. Environ. Pollut. 2021, 274, 116524. [Google Scholar] [CrossRef] [PubMed]
- Kocak, M.; Ezazi Erdi, S.; Jorba, G.; Maestro, I.; Farrés, J.; Kirkin, V.; Martinez, A.; Pless, O. Targeting autophagy in disease: Established and new strategies. Autophagy 2022, 18, 473–495. [Google Scholar] [CrossRef] [PubMed]
- Deneubourg, C.; Ramm, M.; Smith, L.J.; Baron, O.; Singh, K.; Byrne, S.C.; Duchen, M.R.; Gautel, M.; Eskelinen, E.-L.; Fanto, M.; et al. The spectrum of neurodevelopmental, neuromuscular and neurodegenerative disorders due to defective autophagy. Autophagy 2022, 18, 496–517. [Google Scholar] [CrossRef] [PubMed]
- Mustaly-Kalimi, S.; Gallegos, W.; Marr, R.A.; Gilman-Sachs, A.; Peterson, D.A.; Sekler, I.; Stutzmann, G.E. Protein mishandling and impaired lysosomal proteolysis generated through calcium dysregulation in Alzheimer’s disease. Proc. Natl. Acad. Sci. USA 2022, 119, e2211999119. [Google Scholar] [CrossRef] [PubMed]
- Levy, J.M.M.; Towers, C.G.; Thorburn, A. Targeting autophagy in cancer. Nat. Rev. Cancer 2017, 17, 528–542. [Google Scholar] [CrossRef] [PubMed]
- Debnath, J.; Gammoh, N.; Ryan, K.M. Autophagy and autophagy-related pathways in cancer. Nat. Rev. Mol. Cell Biol. 2023, 24, 560–575. [Google Scholar] [CrossRef]
- Ferreira, P.M.P.; Sousa, R.W.R.d.; Ferreira, J.R.d.O.; Militão, G.C.G.; Bezerra, D.P. Chloroquine and hydroxychloroquine in antitumor therapies based on autophagy-related mechanisms. Pharmacol. Res. 2021, 168, 105582. [Google Scholar] [CrossRef]
- Boya, P.; Reggiori, F.; Codogno, P. Emerging regulation and functions of autophagy. Nat. Cell Biol. 2013, 15, 713–720. [Google Scholar] [CrossRef]
- Weinlich, R.; Oberst, A.; Beere, H.M.; Green, D.R. Necroptosis in development, inflammation and disease. Nat. Rev. Mol. Cell Biol. 2017, 18, 127–136. [Google Scholar] [CrossRef] [PubMed]
- Yuan, J.; Amin, P.; Ofengeim, D. Necroptosis and RIPK1-mediated neuroinflammation in CNS diseases. Nat. Rev. Neurosci. 2019, 20, 19–33. [Google Scholar] [CrossRef] [PubMed]
- Tang, X.; Fan, X.; Xu, T.; He, Y.; Chi, Q.; Li, Z.; Li, S. Polystyrene nanoplastics exacerbated lipopolysaccharide-induced necroptosis and inflammation via the ROS/MAPK pathway in mice spleen. Environ. Toxicol. 2022, 37, 2552–2565. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Liu, Q.; Yang, N.; Xu, S. Polystyrene-microplastics and DEHP co-exposure induced DNA damage, cell cycle arrest and necroptosis of ovarian granulosa cells in mice by promoting ROS production. Sci. Total Environ. 2023, 871, 161962. [Google Scholar] [CrossRef] [PubMed]
- Basit, F.; van Oppen, L.M.; Schöckel, L.; Bossenbroek, H.M.; van Emst-de Vries, S.E.; Hermeling, J.C.; Grefte, S.; Kopitz, C.; Heroult, M.; Hgm Willems, P.; et al. Mitochondrial complex I inhibition triggers a mitophagy-dependent ROS increase leading to necroptosis and ferroptosis in melanoma cells. Cell Death Dis. 2017, 8, e2716. [Google Scholar] [CrossRef] [PubMed]
- Jia, Y.; Wang, F.; Guo, Q.; Li, M.; Wang, L.; Zhang, Z.; Jiang, S.; Jin, H.; Chen, A.; Tan, S.; et al. Curcumol induces RIPK1/RIPK3 complex-dependent necroptosis via JNK1/2-ROS signaling in hepatic stellate cells. Redox Biol. 2018, 19, 375–387. [Google Scholar] [CrossRef] [PubMed]
- Newton, K.; Wickliffe, K.E.; Dugger, D.L.; Maltzman, A.; Roose-Girma, M.; Dohse, M.; Kőműves, L.; Webster, J.D.; Dixit, V.M. Cleavage of RIPK1 by caspase-8 is crucial for limiting apoptosis and necroptosis. Nature 2019, 574, 428–431. [Google Scholar] [CrossRef] [PubMed]
- Tummers, B.; Mari, L.; Guy, C.S.; Heckmann, B.L.; Rodriguez, D.A.; Rühl, S.; Moretti, J.; Crawford, J.C.; Fitzgerald, P.; Kanneganti, T.-D.; et al. Caspase-8-Dependent Inflammatory Responses Are Controlled by Its Adaptor, FADD, and Necroptosis. Immunity 2020, 52, 994–1006.e8. [Google Scholar] [CrossRef]
- Dowling, J.P.; Alsabbagh, M.; Del Casale, C.; Liu, Z.-G.; Zhang, J. TRADD regulates perinatal development and adulthood survival in mice lacking RIPK1 and RIPK3. Nat. Commun. 2019, 10, 705. [Google Scholar] [CrossRef]
- Anderton, H.; Bandala-Sanchez, E.; Simpson, D.S.; Rickard, J.A.; Ng, A.P.; Di Rago, L.; Hall, C.; Vince, J.E.; Silke, J.; Liccardi, G.; et al. RIPK1 prevents TRADD-driven, but TNFR1 independent, apoptosis during development. Cell Death Differ. 2019, 26, 877–889. [Google Scholar] [CrossRef] [PubMed]
- Schorn, F.; Werthenbach, J.P.; Hoffmann, M.; Daoud, M.; Stachelscheid, J.; Schiffmann, L.M.; Hildebrandt, X.; Lyu, S.I.; Peltzer, N.; Quaas, A.; et al. cIAPs control RIPK1 kinase activity-dependent and -independent cell death and tissue inflammation. EMBO J. 2023, 42, e113614. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Kalac, M.; Markson, M.; Chan, M.; Brody, J.D.; Bhagat, G.; Ang, R.L.; Legarda, D.; Justus, S.J.; Liu, F.; et al. Reversal of CYLD phosphorylation as a novel therapeutic approach for adult T-cell leukemia/lymphoma (ATLL). Cell Death Dis. 2020, 11, 94. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Liu, Y.; Xu, C.; Zhao, Q.; Liu, J.; Xing, M.; Li, X.; Zhang, H.; Wu, X.; Wang, L.; et al. Ubiquitin-binding domain in ABIN1 is critical for regulating cell death and inflammation during development. Cell Death Differ. 2022, 29, 2034–2045. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Ran, Q.; Zhou, Y.; Liu, S.; Zhao, C.; Yu, X.; Zhu, F.; Ji, Y.; Du, Q.; Yang, T.; et al. Doxorubicin sensitizes cancer cells to Smac mimetic via synergistic activation of the CYLD/RIPK1/FADD/caspase-8-dependent apoptosis. Apoptosis 2020, 25, 441–455. [Google Scholar] [CrossRef] [PubMed]
- Deng, X.-X.; Li, S.-S.; Sun, F.-Y. Necrostatin-1 Prevents Necroptosis in Brains after Ischemic Stroke via Inhibition of RIPK1-Mediated RIPK3/MLKL Signaling. Aging Dis. 2019, 10, 807–817. [Google Scholar] [CrossRef] [PubMed]
- Duan, X.; Liu, X.; Liu, N.; Huang, Y.; Jin, Z.; Zhang, S.; Ming, Z.; Chen, H. Inhibition of keratinocyte necroptosis mediated by RIPK1/RIPK3/MLKL provides a protective effect against psoriatic inflammation. Cell Death Dis. 2020, 11, 134. [Google Scholar] [CrossRef]
- Lawlor, K.E.; Khan, N.; Mildenhall, A.; Gerlic, M.; Croker, B.A.; D’Cruz, A.A.; Hall, C.; Kaur Spall, S.; Anderton, H.; Masters, S.L.; et al. RIPK3 promotes cell death and NLRP3 inflammasome activation in the absence of MLKL. Nat. Commun. 2015, 6, 6282. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Tong, A.; Zhang, Q.; Wei, Y.; Wei, X. The molecular mechanisms of MLKL-dependent and MLKL-independent necrosis. J. Mol. Cell Biol. 2021, 13, 3–14. [Google Scholar] [CrossRef]
- Wang, R.; Li, H.; Wu, J.; Cai, Z.-Y.; Li, B.; Ni, H.; Qiu, X.; Chen, H.; Liu, W.; Yang, Z.-H.; et al. Gut stem cell necroptosis by genome instability triggers bowel inflammation. Nature 2020, 580, 386–390. [Google Scholar] [CrossRef]
- Kang, T.-B.; Yang, S.-H.; Toth, B.; Kovalenko, A.; Wallach, D. Caspase-8 blocks kinase RIPK3-mediated activation of the NLRP3 inflammasome. Immunity 2013, 38, 27–40. [Google Scholar] [CrossRef]
- Welz, P.-S.; Wullaert, A.; Vlantis, K.; Kondylis, V.; Fernández-Majada, V.; Ermolaeva, M.; Kirsch, P.; Sterner-Kock, A.; van Loo, G.; Pasparakis, M. FADD prevents RIP3-mediated epithelial cell necrosis and chronic intestinal inflammation. Nature 2011, 477, 330–334. [Google Scholar] [CrossRef] [PubMed]
- Pierdomenico, M.; Negroni, A.; Stronati, L.; Vitali, R.; Prete, E.; Bertin, J.; Gough, P.J.; Aloi, M.; Cucchiara, S. Necroptosis is active in children with inflammatory bowel disease and contributes to heighten intestinal inflammation. Am. J. Gastroenterol. 2014, 109, 279–287. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Li, Y.; Choi, H.M.C.; Sarkar, C.; Koh, E.Y.; Wu, J.; Lipinski, M.M. Lysosomal damage after spinal cord injury causes accumulation of RIPK1 and RIPK3 proteins and potentiation of necroptosis. Cell Death Dis. 2018, 9, 476. [Google Scholar] [CrossRef] [PubMed]
- Iannielli, A.; Bido, S.; Folladori, L.; Segnali, A.; Cancellieri, C.; Maresca, A.; Massimino, L.; Rubio, A.; Morabito, G.; Caporali, L.; et al. Pharmacological Inhibition of Necroptosis Protects from Dopaminergic Neuronal Cell Death in Parkinson’s Disease Models. Cell Rep. 2018, 22, 2066–2079. [Google Scholar] [CrossRef] [PubMed]
- Gong, Y.; Fan, Z.; Luo, G.; Yang, C.; Huang, Q.; Fan, K.; Cheng, H.; Jin, K.; Ni, Q.; Yu, X.; et al. The role of necroptosis in cancer biology and therapy. Mol. Cancer 2019, 18, 100. [Google Scholar] [CrossRef] [PubMed]
- Su, Z.; Yang, Z.; Xie, L.; DeWitt, J.P.; Chen, Y. Cancer therapy in the necroptosis era. Cell Death Differ. 2016, 23, 748–756. [Google Scholar] [CrossRef] [PubMed]
- Baik, J.Y.; Liu, Z.; Jiao, D.; Kwon, H.-J.; Yan, J.; Kadigamuwa, C.; Choe, M.; Lake, R.; Kruhlak, M.; Tandon, M.; et al. ZBP1 not RIPK1 mediates tumor necroptosis in breast cancer. Nat. Commun. 2021, 12, 2666. [Google Scholar] [CrossRef]
- Tong, X.; Tang, R.; Xiao, M.; Xu, J.; Wang, W.; Zhang, B.; Liu, J.; Yu, X.; Shi, S. Targeting cell death pathways for cancer therapy: Recent developments in necroptosis, pyroptosis, ferroptosis, and cuproptosis research. J. Hematol. Oncol. 2022, 15, 174. [Google Scholar] [CrossRef]
- Tan, H.Y.; Wang, N.; Chan, Y.T.; Zhang, C.; Guo, W.; Chen, F.; Zhong, Z.; Li, S.; Feng, Y. ID1 overexpression increases gefitinib sensitivity in non-small cell lung cancer by activating RIP3/MLKL-dependent necroptosis. Cancer Lett. 2020, 475, 109–118. [Google Scholar] [CrossRef]
- Mohanty, S.; Yadav, P.; Lakshminarayanan, H.; Sharma, P.; Vivekanandhan, A.; Karunagaran, D. RETRA induces necroptosis in cervical cancer cells through RIPK1, RIPK3, MLKL and increased ROS production. Eur. J. Pharmacol. 2022, 920, 174840. [Google Scholar] [CrossRef] [PubMed]
- Bredesen, D.E. Neural apoptosis. Ann. Neurol. 1995, 38, 839–851. [Google Scholar] [CrossRef] [PubMed]
- D’Arcy, M.S. Cell death: A review of the major forms of apoptosis, necrosis and autophagy. Cell Biol. Int. 2019, 43, 582–592. [Google Scholar] [CrossRef] [PubMed]
- Santos, D.; Luzio, A.; Félix, L.; Cabecinha, E.; Bellas, J.; Monteiro, S.M. Microplastics and copper induce apoptosis, alter neurocircuits, and cause behavioral changes in zebrafish (Danio rerio) brain. Ecotoxicol. Environ. Saf. 2022, 242, 113926. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Zhuan, Q.; Zhang, L.; Meng, L.; Fu, X.; Hou, Y. Polystyrene microplastics induced female reproductive toxicity in mice. J. Hazard. Mater. 2022, 424 Pt C, 127629. [Google Scholar] [CrossRef] [PubMed]
- Cheng, W.; Li, X.; Zhou, Y.; Yu, H.; Xie, Y.; Guo, H.; Wang, H.; Li, Y.; Feng, Y.; Wang, Y. Polystyrene microplastics induce hepatotoxicity and disrupt lipid metabolism in the liver organoids. Sci. Total Environ. 2022, 806 Pt 1, 150328. [Google Scholar] [CrossRef] [PubMed]
- Simon, H.U.; Haj-Yehia, A.; Levi-Schaffer, F. Role of reactive oxygen species (ROS) in apoptosis induction. Apoptosis 2000, 5, 415–418. [Google Scholar] [CrossRef] [PubMed]
- Matsuyama, S.; Reed, J.C. Mitochondria-dependent apoptosis and cellular pH regulation. Cell Death Differ. 2000, 7, 1155–1165. [Google Scholar] [CrossRef] [PubMed]
- Willems, P.H.G.M.; Rossignol, R.; Dieteren, C.E.J.; Murphy, M.P.; Koopman, W.J.H. Redox Homeostasis and Mitochondrial Dynamics. Cell Metab. 2015, 22, 207–218. [Google Scholar] [CrossRef]
- Czabotar, P.E.; Garcia-Saez, A.J. Mechanisms of BCL-2 family proteins in mitochondrial apoptosis. Nat. Rev. Mol. Cell Biol. 2023, 24, 732–748. [Google Scholar] [CrossRef]
- Li, Y.; Guo, M.; Niu, S.; Shang, M.; Chang, X.; Sun, Z.; Zhang, R.; Shen, X.; Xue, Y. ROS and DRP1 interactions accelerate the mitochondrial injury induced by polystyrene nanoplastics in human liver HepG2 cells. Chem. Biol. Interact. 2023, 379, 110502. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Wu, Z.; Lu, Z.; Yan, L.; Dong, X.; Dai, Z.; Sun, R.; Hong, P.; Zhou, C.; Li, C. Differences in toxicity induced by the various polymer types of nanoplastics on HepG2 cells. Sci. Total Environ. 2024, 918, 170664. [Google Scholar] [CrossRef] [PubMed]
- Sakthivel, R.; Malar, D.S.; Devi, K.P. Phytol shows anti-angiogenic activity and induces apoptosis in A549 cells by depolarizing the mitochondrial membrane potential. Biomed. Pharmacother. 2018, 105, 742–752. [Google Scholar] [CrossRef] [PubMed]
- Sauler, M.; Bazan, I.S.; Lee, P.J. Cell Death in the Lung: The Apoptosis-Necroptosis Axis. Annu. Rev. Physiol. 2019, 81, 375–402. [Google Scholar] [CrossRef] [PubMed]
- Clauss, M.; Voswinckel, R.; Rajashekhar, G.; Sigua, N.L.; Fehrenbach, H.; Rush, N.I.; Schweitzer, K.S.; Yildirim, A.Ö.; Kamocki, K.; Fisher, A.J.; et al. Lung endothelial monocyte-activating protein 2 is a mediator of cigarette smoke-induced emphysema in mice. J. Clin. Investig. 2011, 121, 2470–2479. [Google Scholar] [CrossRef] [PubMed]
- Aoshiba, K.; Yokohori, N.; Nagai, A. Alveolar wall apoptosis causes lung destruction and emphysematous changes. Am. J. Respir. Cell Mol. Biol. 2003, 28, 555–562. [Google Scholar] [CrossRef] [PubMed]
- Sekerdag, E.; Solaroglu, I.; Gursoy-Ozdemir, Y. Cell Death Mechanisms in Stroke and Novel Molecular and Cellular Treatment Options. Curr. Neuropharmacol. 2018, 16, 1396–1415. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Liu, Y.; Zhu, J.; Lei, S.; Dong, Y.; Li, L.; Jiang, B.; Tan, L.; Wu, J.; Yu, S.; et al. GSK-3β downregulates Nrf2 in cultured cortical neurons and in a rat model of cerebral ischemia-reperfusion. Sci. Rep. 2016, 6, 20196. [Google Scholar] [CrossRef]
- Wen, Y.; Yang, S.; Liu, R.; Simpkins, J.W. Transient cerebral ischemia induces site-specific hyperphosphorylation of tau protein. Brain Res. 2004, 1022, 30–38. [Google Scholar] [CrossRef]
- Wang, X.; Han, W.; Du, X.; Zhu, C.; Carlsson, Y.; Mallard, C.; Jacotot, E.; Hagberg, H. Neuroprotective effect of Bax-inhibiting peptide on neonatal brain injury. Stroke 2010, 41, 2050–2055. [Google Scholar] [CrossRef]
- Wong, R.S.Y. Apoptosis in cancer: From pathogenesis to treatment. J. Exp. Clin. Cancer Res. 2011, 30, 87. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Zhang, Y.; Cao, J.; Su, Z.; Li, F.; Zhang, P.; Zhang, B.; Liu, R.; Zhang, L.; Xie, J.; et al. FGFR4 and EZH2 inhibitors synergistically induce hepatocellular carcinoma apoptosis via repressing YAP signaling. J. Exp. Clin. Cancer Res. 2023, 42, 96. [Google Scholar] [CrossRef]
- Tsvetkov, P.; Coy, S.; Petrova, B.; Dreishpoon, M.; Verma, A.; Abdusamad, M.; Rossen, J.; Joesch-Cohen, L.; Humeidi, R.; Spangler, R.D.; et al. Copper induces cell death by targeting lipoylated TCA cycle proteins. Science 2022, 375, 1254–1261. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.-Y.; Zhang, X.-Y.; Li, S.-L.; Jiang, F.-L.; Jiang, P.; Liu, Y. AuPt-Loaded Cu-Doped Polydopamine Nanocomposites with Multienzyme-Mimic Activities for Dual-Modal Imaging-Guided and Cuproptosis-Enhanced Photothermal/Nanocatalytic Therapy. Anal. Chem. 2023, 95, 14025–14035. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Tian, Z.; Zhang, P.; Zhen, L.; Meng, Q.; Sun, B.; Xu, X.; Jia, T.; Li, S. The molecular mechanisms of cuproptosis and its relevance to cardiovascular disease. Biomed. Pharmacother. 2023, 163, 114830. [Google Scholar] [CrossRef]
- Xue, Q.; Kang, R.; Klionsky, D.J.; Tang, D.; Liu, J.; Chen, X. Copper metabolism in cell death and autophagy. Autophagy 2023, 19, 2175–2195. [Google Scholar] [CrossRef]
- Tardito, S.; Bassanetti, I.; Bignardi, C.; Elviri, L.; Tegoni, M.; Mucchino, C.; Bussolati, O.; Franchi-Gazzola, R.; Marchiò, L. Copper binding agents acting as copper ionophores lead to caspase inhibition and paraptotic cell death in human cancer cells. J. Am. Chem. Soc. 2011, 133, 6235–6242. [Google Scholar] [CrossRef]
- Domenech, J.; Cortés, C.; Vela, L.; Marcos, R.; Hernández, A. Polystyrene Nanoplastics as Carriers of Metals. Interactions of Polystyrene Nanoparticles with Silver Nanoparticles and Silver Nitrate, and Their Effects on Human Intestinal Caco-2 Cells. Biomolecules 2021, 11, 859. [Google Scholar] [CrossRef]
- Qiao, R.; Lu, K.; Deng, Y.; Ren, H.; Zhang, Y. Combined effects of polystyrene microplastics and natural organic matter on the accumulation and toxicity of copper in zebrafish. Sci. Total Environ. 2019, 682, 128–137. [Google Scholar] [CrossRef]
- Chen, L.; Min, J.; Wang, F. Copper homeostasis and cuproptosis in health and disease. Signal Transduct. Target. Ther. 2022, 7, 378. [Google Scholar] [CrossRef]
- Liu, H.; Tang, T. Pan-cancer genetic analysis of cuproptosis and copper metabolism-related gene set. Front. Oncol. 2022, 12, 952290. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).