Study on the Distribution Characteristics and Risk Assessment of Antibiotics and Resistance Genes in Water Sources of Wuhan
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemical Substances and Standards
2.2. Sampling Points and Sample Collection
2.3. Antibiotic Quantification
2.4. DNA Extraction and ARG Quantification
2.5. Ecological Risk Assessment
2.6. Statistical Analysis
3. Results and Discussion
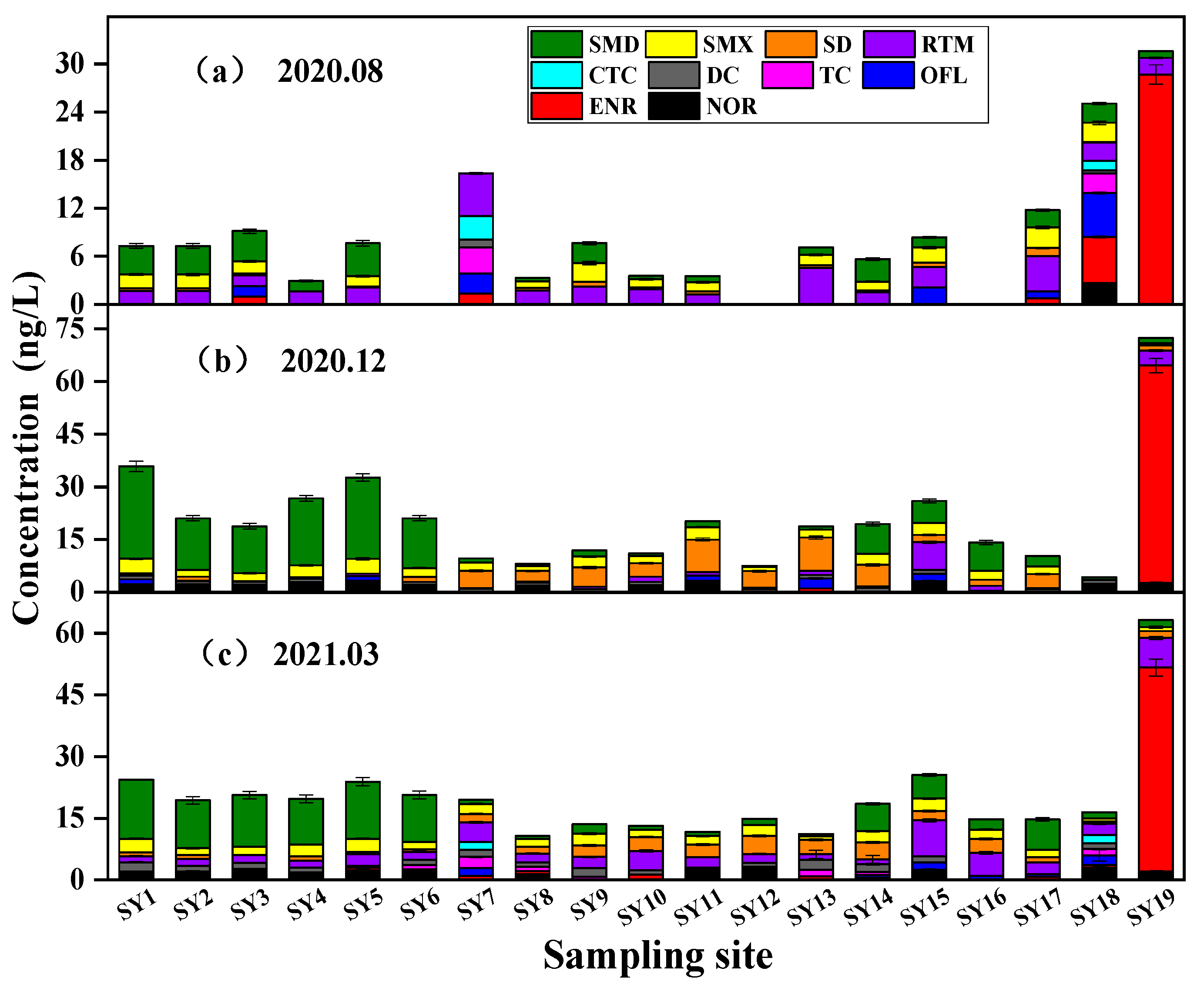
3.1. Spatiotemporal Distribution of Antibiotics
3.2. Antibiotic Risk Assessment
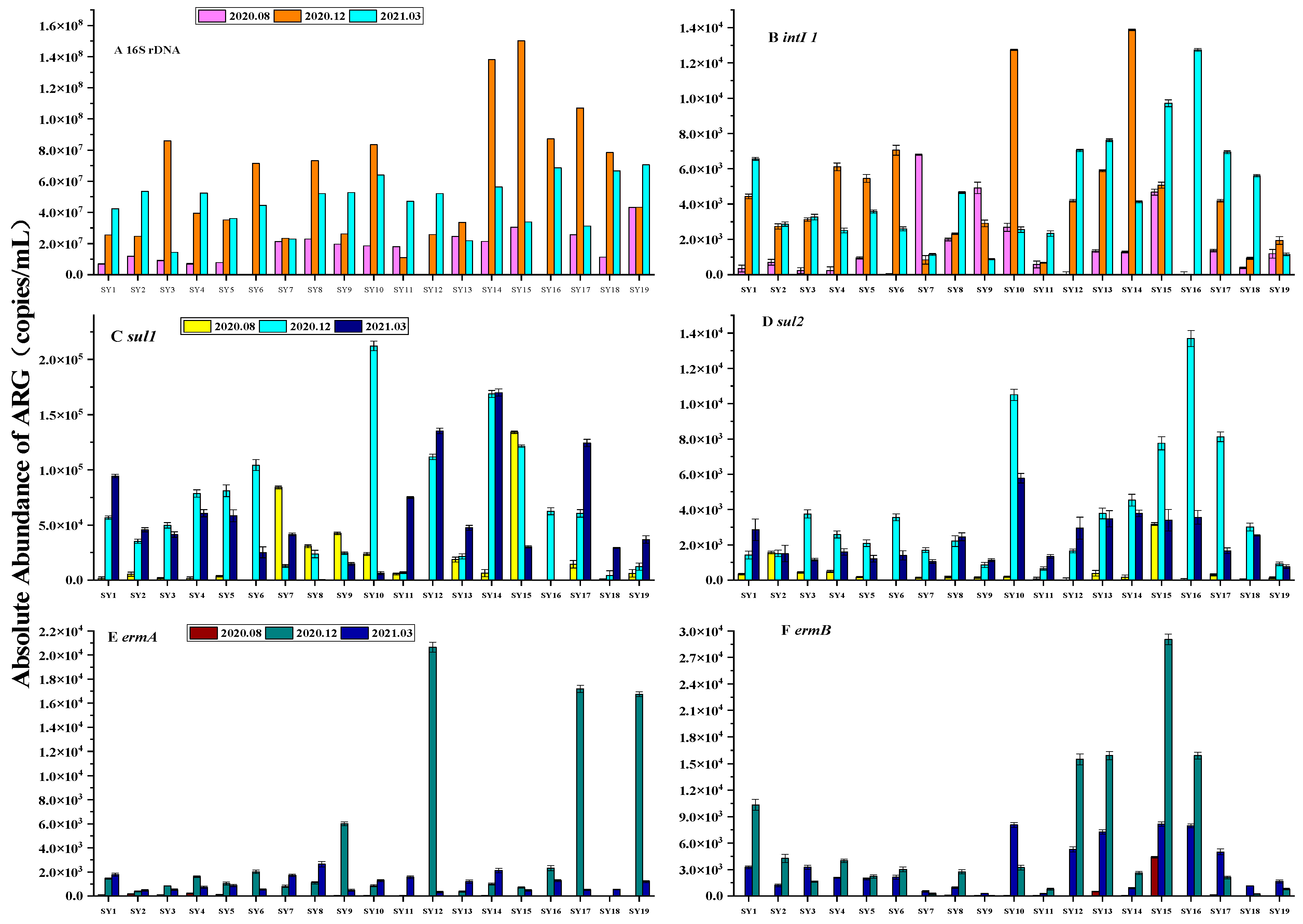
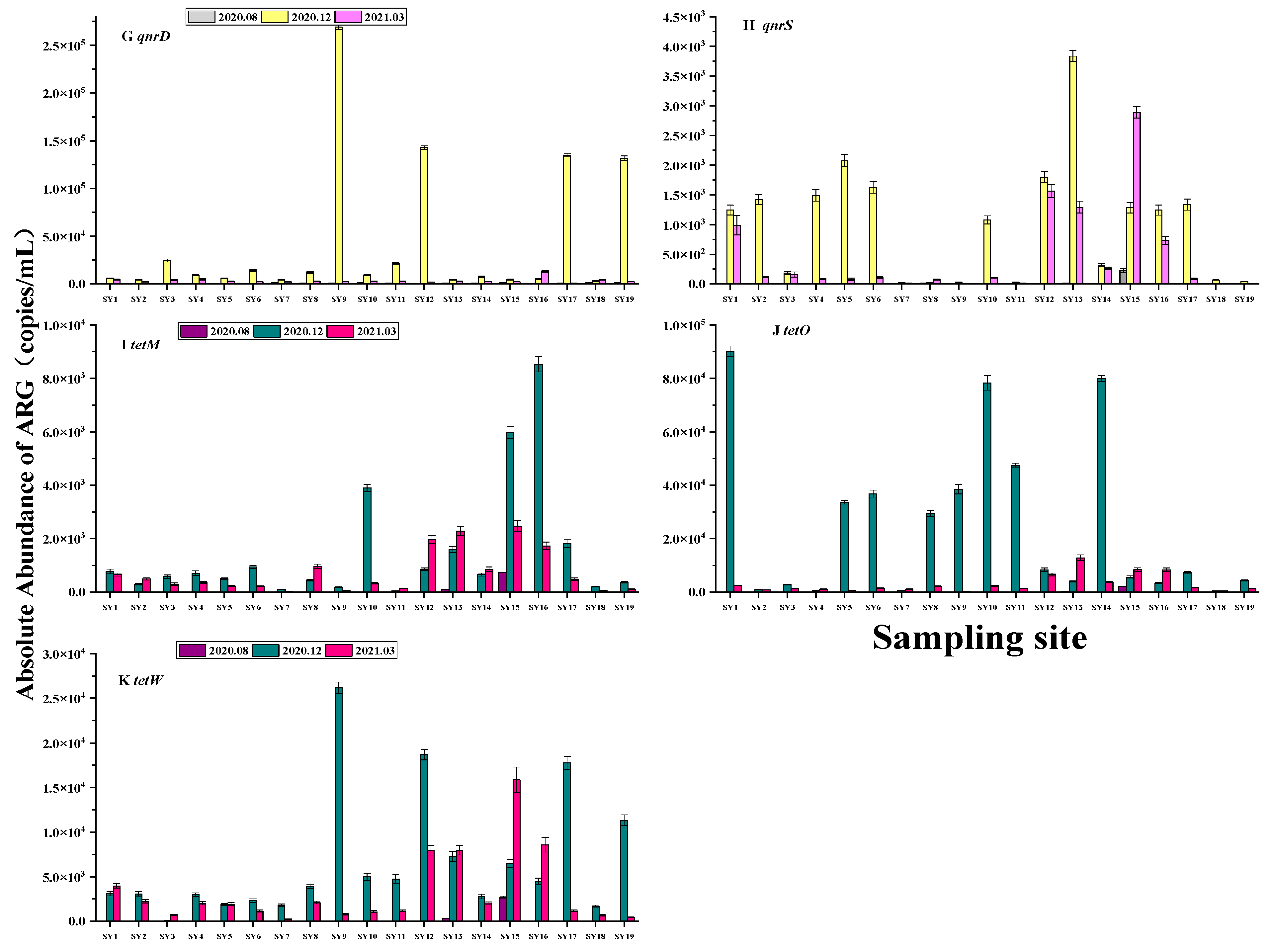
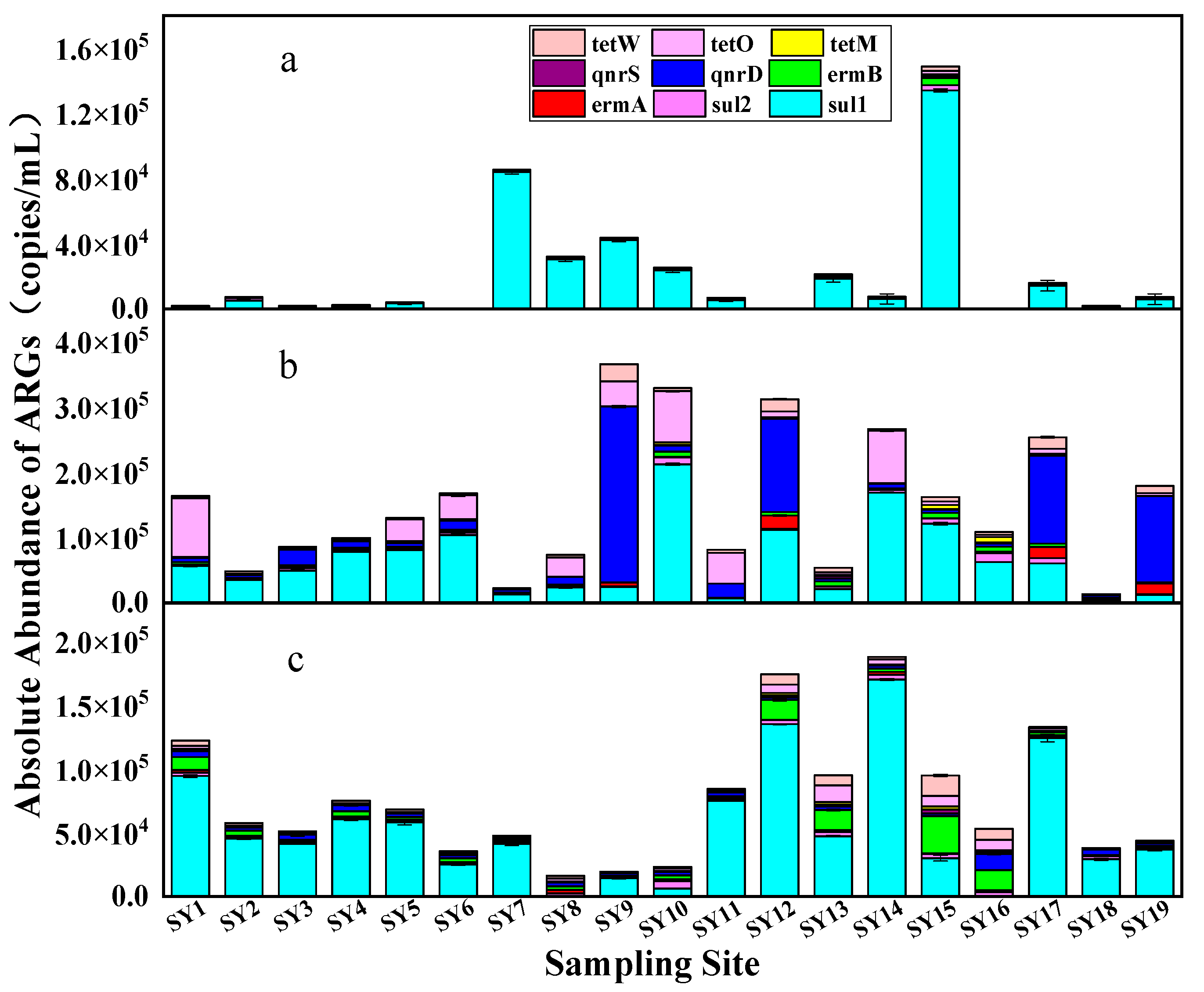
3.3. Spatial and Temporal Distribution of ARGs
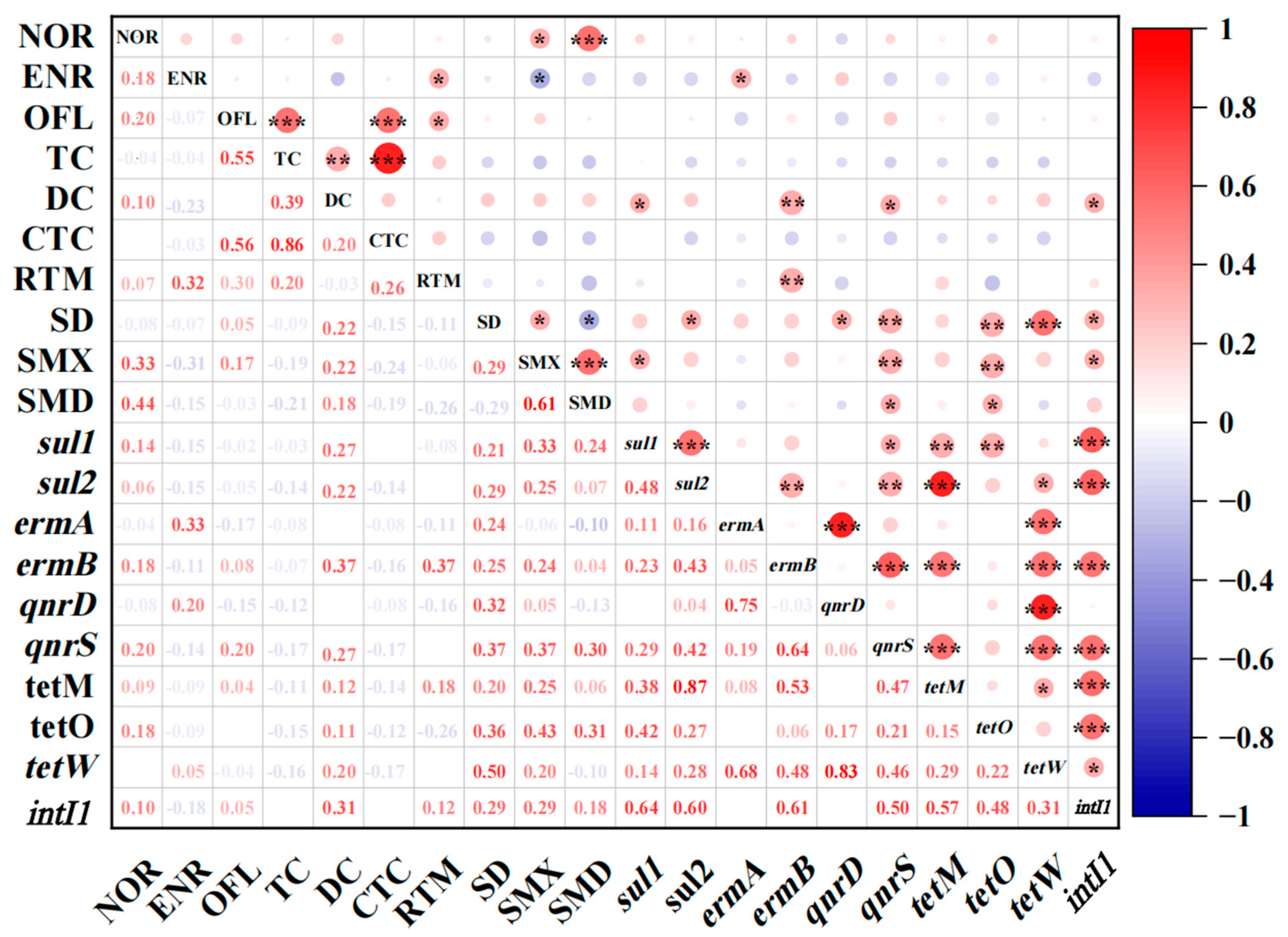
3.4. Correlation between ARGs and Antibiotics
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Liu, S.; Bekele, T.; Zhao, H.; Cai, X.; Chen, J. Bioaccumulation and tissue distribution of antibiotics in wild marine fish from Laizhou Bay, North China. Sci. Total Environ. 2018, 631–632, 1398–1405. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, G.; Liu, Y.; Lu, S.; Qin, P.; Guo, X.; Bi, B.; Wang, L.; Xi, B.; Wu, F.; et al. Occurrence and fate of antibiotics and antibiotic resistance genes in typical urban water of Beijing, China. Environ. Pollut. 2019, 246, 163–173. [Google Scholar] [CrossRef]
- Gao, H.; Zhao, F.; Li, R.; Jin, S.; Zhang, H.; Zhang, K.; Li, S.; Shu, Q.; Na, G. Occurrence and distribution of antibiotics and antibiotic resistance genes in water of Liaohe River Basin, China. J. Environ. Chem. Eng. 2022, 10, 108297. [Google Scholar] [CrossRef]
- Ding, D.; Wang, B.; Zhang, X.; Zhang, J.; Zhang, H.; Liu, X.; Gao, Z.; Yu, Z. The spread of antibiotic resistance to humans and potential protection strategies. Ecotoxicol. Environ. 2023, 254, 114734. [Google Scholar] [CrossRef]
- Zhang, S.; Zhang, Y.; Wang, Z.; Wang, Y.; Liu, X.; Li, M.; Fang, H.; Kong, M. Effect of antibiotics, antibiotic-resistant bacteria, and extracellular antibiotic resistance genes on the fate of ARGs in marine sediments. Sci. Total Environ. 2023, 891, 164305. [Google Scholar] [CrossRef] [PubMed]
- Inyinbor, A.A.; Bello, O.S.; Fadiji, A.E.; Inyinbor, H.E. Threats from antibiotics: A serious environmental concern. J. Environ. Chem. Eng. 2018, 6, 784–793. [Google Scholar] [CrossRef]
- Kumar, A.; Pal, D. Antibiotic resistance and wastewater: Correlation, impact and critical human health challenges. J. Environ. Chem. Eng. 2018, 6, 52–58. [Google Scholar] [CrossRef]
- Agunbiade, F.; Moodley, B. Occurrence and distribution pattern of acidic pharmaceuticals in surface water, wastewater, and sediment of the Msunduzi River, Kwazulu-Natal, South Africa. Environ. Toxicol. Chem. 2016, 35, 36–46. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Zhang, J.; Wang, Z.; Wang, Y.; Liang, S.; Liu, C.; Wang, Z. Preliminary investigation on the occurrence of several sulfonamide antibiotics in the Haihe River Basin of China. IOP Conf. Ser. Earth Environ. Sci. 2017, 82, 012024. [Google Scholar] [CrossRef]
- Hu, Y.; Yan, X.; Shen, Y.; Di, M.; Wang, J. Antibiotics in surface water and sediments from Hanjiang River, Central China: Occurrence, behavior and risk assessment. Ecotox Environ. Safe 2018, 157, 150–158. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Shi, W.; You, M.; Zhang, R.; Kuang, Y.; Dang, C.; Sun, W.; Zhou, Y.; Wang, W.; Ni, J. Antibiotics in water and sediments of Danjiangkou Reservoir, China: Spatiotemporal distribution and indicator screening. Environ. Pollut. 2019, 246, 435–442. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.; Shi, T.; Wu, X.; Cao, H.; Li, X.; Hua, R.; Tang, F.; Yue, Y. The occurrence and distribution of antibiotics in Lake Chaohu, China: Seasonal variation, potential source and risk assessment. Chemosphere 2015, 122, 154–161. [Google Scholar] [CrossRef] [PubMed]
- Ding, H.; Wu, Y.; Zhang, W.; Zhong, J.; Lou, Q.; Yang, P.; Fang, Y. Occurrence, distribution, and risk assessment of antibiotics in the surface water of Poyang Lake, the largest freshwater lake in China. Chemosphere 2017, 184, 137–147. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Du, Y.; Yang, C.; Liu, X.; Zhang, J.; Li, E.; Zhang, Q.; Wang, X. Occurrence and ecological hazard assessment of selected antibiotics in the surface waters in and around Lake Honghu, China. Sci. Total Environ. 2017, 609, 1423–1432. [Google Scholar] [CrossRef] [PubMed]
- Yan, M.; Xu, C.; Huang, Y.; Nie, H.; Wang, J. Tetracyclines, sulfonamides and quinolones and their corresponding resistance genes in the Three Gorges Reservoir, China. Sci. Total Environ. 2018, 631, 840–848. [Google Scholar] [CrossRef] [PubMed]
- Zou, S.; Xu, W.; Zhang, R.; Tang, J.; Chen, Y.; Zhang, G. Occurrence and distribution of antibiotics in coastal water of the Bohai Bay, China: Impacts of river discharge and aquaculture activities. Environ. Pollut. 2011, 159, 2913–2920. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Liu, S.; Xu, X.; Zhou, G.; Liu, S.; Yue, W.; Sun, K.; Ying, G. Antibiotics in the coastal environment of the Hailing Bay region, South China Sea: Spatial distribution, source analysis and ecological risks. Mar. Pollut. Bull. 2015, 95, 365–373. [Google Scholar] [CrossRef] [PubMed]
- Aydin, S.; Aydin, M.; Ulvi, A.; Kilic, H. Antibiotics in hospital effluents: Occurrence, contribution to urban wastewater, removal in a wastewater treatment plant, and environmental risk assessment. Environ. Sci. Pollut. R. 2019, 26, 544–558. [Google Scholar] [CrossRef]
- Hu, X.; Zhou, Q.; Luo, Y. Occurrence and source analysis of typical veterinary antibiotics in manure, soil, vegetables and groundwater from organic vegetable bases, northern China. Environ. Pollut. 2010, 158, 2992–2998. [Google Scholar] [CrossRef] [PubMed]
- Oyedeji, A.; Msagati, T.; Williams, A.; Benson, N. Determination of antibiotic residues in frozen poultry by a solid-phase dispersion method using liquid chromatography-triple quadrupole mass spectrometry. Toxicol. Rep. 2019, 6, 951–956. [Google Scholar] [CrossRef] [PubMed]
- Jo, S.; Shin, C.; Shin, Y.; Kim, P.; So, J. Heavy metal and antibiotic co-resistance in Vibrio parahaemolyticus isolated from shellfish. Mar. Pollut. Bull. 2020, 156, 111246. [Google Scholar] [CrossRef] [PubMed]
- Gullberg, E.; Cao, S.; Berg, O.; Ilbäck, C.; Sandegren, L.; Hughes, D.; Andersson, D. Selection of resistant bacteria at very low antibiotic concentrations. PLoS Pathog. 2011, 7, e1002158. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Mao, D.; Mu, Q.; Luo, Y. Enhanced Horizontal Transfer of Antibiotic Resistance Genes in Freshwater Microcosms Induced by an Ionic Liquid. PLoS ONE 2015, 10, e0126784. [Google Scholar] [CrossRef]
- Bertolla, F.; Kay, E.; Simonet, P. Potential dissemination of antibiotic resistance genes from transgenic plants to microorganisms. Infect. Control. Hosp. Epidemiol. 2000, 21, 390–393. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Ying, G.; Zhao, J.; Tao, R.; Su, H.; Liu, Y. Spatial and seasonal distribution of selected antibiotics in surface waters of the Pearl Rivers, China. J. Environ. Sci. Heal. B 2011, 46, 272–280. [Google Scholar] [CrossRef] [PubMed]
- Qiao, M.; Ying, G.; Singer, A.; Zhu, Y. Review of antibiotic resistance in China and its environment. Environ. Int. 2018, 110, 160–172. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Feng, M.; Wang, B.; Zhao, X.; Chen, J. Distribution and potential risk assessment of antibiotic pollution in the main drinking water sources of Nanjing, China. Environ. Sci. Pollut. R 2020, 27, 21429–21441. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Chen, Y.; Feng, M.; Chen, J.; Zhang, S. Occurrence of antibiotics and antibiotic resistance genes and their correlations in river-type drinking water source, China. Environ. Sci. Pollut. R 2021, 28, 42339–42352. [Google Scholar] [CrossRef] [PubMed]
- Tong, L.; Qin, L.; Xie, C.; Liu, H.; Wang, Y.; Guan, C.; Huang, S. Distribution of antibiotics in alluvial sediment near animal breeding areas at the Jianghan Plain, Central China. Chemosphere 2017, 186, 100–107. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Lu, S.; Wang, Y.; Liu, X.; Liu, Y.; Xu, J.; Zhang, T.; Wang, Z. Occurrence of antibiotics and antibiotic resistance genes and their correlations in lower Yangtze River, China. Environ. Pollut. 2020, 257, 113365. [Google Scholar] [CrossRef]
- Wang, N.; Wang, N.; Qi, D.; Kang, G.; Wang, W.; Zhang, C.; Zhang, Z.; Zhang, Y.; Zhang, H.; Zhang, S.; et al. Comprehensive overview of antibiotic distribution, risk and priority: A study of large-scale drinking water sources from the lower Yangtze River. J. Environ. Manag. 2023, 344, 118705. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.; Zhai, W.; Wang, J.; Li, G.; Zhou, Z.; Li, B.; Zhuo, H. Antibiotics and antibiotic resistance genes in the water sources of the Wuhan stretch of the Yangtze River: Occurrence, distribution, and ecological risks. Environ. Res. 2023, 239, 117295. [Google Scholar] [CrossRef] [PubMed]
- Kasprzyk-Hordern, B.; Dinsdale, R.; Guwy, A. The occurrence of pharmaceuticals, personal care products, endocrine disruptors and illicit drugs in surface water in South Wales, UK. Water Res. 2008, 42, 3498–3518. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Huang, X.; Witter, J.; Spongberg, A.; Wang, K.; Wang, D.; Liu, J. Occurrence of pharmaceuticals and personal care products and associated environmental risks in the central and lower Yangtze river, China. Ecotox Environ. Safe 2014, 106, 19–26. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Ouyang, W.; Qian, Y.; Su, C.; Su, J.; Chen, H. High-throughput profiling of antibiotic resistance genes in drinking water treatment plants and distribution systems. Environ. Pollut. 2016, 213, 119–126. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Feng, C.; Gu, E.; Tian, C.; Shen, Z. Spatial distribution, source apportionment and risk assessment of antibiotics in the surface water and sediments of the Yangtze Estuary. Sci. Total Environ. 2019, 671, 548–557. [Google Scholar] [CrossRef] [PubMed]
- Burns, E.; Carter, L.; Kolpin, D.; Thomas-Oates, J.; Boxall, A. Temporal and spatial variation in pharmaceutical concentrations in an urban river system. Water Res. 2018, 137, 72–85. [Google Scholar] [CrossRef] [PubMed]
- Ben, W.; Wang, J.; Pan, X.; Qiang, Z. Dissemination of antibiotic resistance genes and their potential removal by on-farm treatment processes in nine swine feedlots in Shandong Province, China. Chemosphere 2017, 167, 262–268. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Xu, L.; Rysz, M.; Wang, Y.; Zhang, H.; Alvarez, P. Occurrence and transport of tetracycline, sulfonamide, quinolone, and macrolide antibiotics in the Haihe River Basin, China. Environ. Sci. Technol. 2011, 45, 1827–1833. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Cao, Z.; Liu, X.; Zhan, Y.; Zhang, J.; Xiao, X.; Yang, Y.; Zhou, J.; Xu, J. Seasonal variation, flux estimation, and source analysis of dissolved emerging organic contaminants in the Yangtze Estuary, China. Mar. Pollut. Bull. 2017, 125, 208–215. [Google Scholar] [CrossRef] [PubMed]
- Bradley, J.; Langford; So, M.; Raybardhan, S.; Leung, V.; Soucy, J.; Westwood, D.; Daneman, N.; MacFadden, D. Antibiotic prescribing in patients with COVID-19: Rapid review and meta-analysis. Clin. Microbiol. Infect. 2021, 27, 1622–1629. [Google Scholar] [CrossRef]
- Zhan, H.; Zhou, Q. Detection levels and contamination diffusion of tetracycline antibiotics in different medium. Chin. J. Ecol. 2020, 39, 4206–4215. [Google Scholar] [CrossRef]
- Chen, Y.; Cui, K.; Huang, Q.; Guo, Z.; Huang, Y.; Yu, K.; He, Y. Comprehensive insights into the occurrence, distribution, risk assessment and indicator screening of antibiotics in a large drinking reservoir system. Sci. Total Environ. 2020, 716, 137060. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Chen, H.; Jing, L.; Teng, Y. Ecotoxicological risk assessment and source apportionment of antibiotics in the waters and sediments of a peri-urban river. Sci. Total Environ. 2020, 731, 139128. [Google Scholar] [CrossRef] [PubMed]
- Soda, S.; Otsuki, H.; Inoue, D.; Tsutsui, H.; Sei, K.; Ike, M. Transfer of antibiotic multiresistant plasmid RP4 from Escherichia coli to activated sludge bacteria. J. Biosci. Bioeng. 2008, 106, 292–296. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.; Yuan, K.; Liang, X.; Chen, X.; Zhao, Z.; Yang, Y.; Zou, S.; Luan, T.; Chen, B. Occurrences and distribution of sulfonamide and tetracycline resistance genes in the Yangtze River Estuary and nearby coastal area. Mar. Pollut. Bull. 2015, 100, 304–310. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Niu, Z.; Zhang, Y.; Zhang, K. Occurrence of intracellular and extracellular antibiotic resistance genes in coastal areas of Bohai Bay (China) and the factors affecting them. Environ. Pollut. 2018, 236, 126–136. [Google Scholar] [CrossRef] [PubMed]
- Dang, B.; Mao, D.; Xu, Y.; Luo, Y. Conjugative multi-resistant plasmids in Haihe River and their impacts on the abundance and spatial distribution of antibiotic resistance genes. Water Res. 2017, 111, 81–91. [Google Scholar] [CrossRef] [PubMed]
- Dong, P.; Cui, Q.; Fang, T.; Huang, Y.; Wang, H. Occurrence of antibiotic resistance genes and bacterial pathogens in water and sediment in urban recreational water. J. Environ. Sci. 2019, 77, 65–74. [Google Scholar] [CrossRef] [PubMed]


| Category | Compound | Abbreviation | Molecular Weight | Molecular Formula | Internal Standard |
|---|---|---|---|---|---|
| SAs | Sulfamethazine | SMZ | 278.33 | C12H14N4O2S | Sulfamethazine-13C6 Sulfamethoxazole-d4 |
| Sulfadiazine | SD | 250.28 | C10H10N4O2S | ||
| Sulfapyridine | SP | 249.29 | C11H11N3O2S | ||
| Sulfamethoxazole | SMX | 253.27 | C10H11N3O3S | ||
| Sulfameter | SMD | 280.3 | C11H12N4O3S | ||
| Sulfamerazine | SM | 264.30 | C11H12N4O2S | ||
| FQs | Norfloxacin | NOR | 319.33 | C16H18FN3O3 | Ciprofloxacin-d8 |
| Ofloxacin | OFL | 331.34 | C18H20FN3O4 | ||
| Enrofloxacin | ENR | 359.4 | C19H22FN3O3 | ||
| TCs | Tetracycline | TC | 444.43 | C22H24N2O8 | Thiabendazole-d4 |
| Oxytetracycline | OTC | 496.90 | C22H28N2O11 | ||
| Chlorotetracycline | CTC | 515.34 | C22H23ClN2O8 | ||
| Doxycyclinehyclate | DC | 444.44 | C22H24N2O8 | ||
| MLs | Erythromycin | ETM | 731.95 | C38H69NO12 | Erythromycin-13C-d3 |
| Roxithromycin | RTM | 837.05 | C41H76N2O15 |
| Category | Nomenclature |
|---|---|
| Tetracycline | tetM |
| tetO | |
| tetW | |
| Quinolone | qnrS |
| qnrD | |
| Sulfa | sul1 |
| sul2 | |
| Cyclolactone | ermA |
| ermB | |
| Bacterial reference gene | 16S rDNA |
| I integrons | intI1 |
| Antibiotic Name | August 2020 (Wet Season) | December 2020 (Dry Season) | March 2021 (Normal Water Season) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| The Detection Rate % | Average ng/L | Max ng/L | The Detection Rate % | Average ng/L | Max ng/L | The Detection Rate % | Average ng/L | Max ng/L | |
| SMD | 93.75 | 1.871 | 4.110 | 100 | 7.685 | 26.317 | 94.74 | 5.738 | 14.357 |
| SMX | 75 | 1.597 | 2.566 | 94.74 | 2.576 | 4.190 | 100 | 2.148 | 3.361 |
| SD | 75 | 0.361 | 1.008 | 73.68 | 4.202 | 9.331 | 94.97 | 2.123 | 4.373 |
| RTM | 100 | 2.381 | 5.302 | 94.74 | 1.217 | 7.903 | 100 | 3.167 | 8.746 |
| ENR | 31.25 | 7.509 | 28.686 | 73.68 | 4.633 | 61.883 | 73.68 | 4.184 | 49.883 |
| NOR | 6.25 | 2.65 | 2.65 | 63.16 | 2.376 | 3.082 | 63.16 | 2.157 | 2.965 |
| OFL | 31.25 | 2.448 | 5.472 | 26.32 | 1.840 | 3.415 | 42.11 | 1.147 | 2.331 |
| DC | 12.50 | 0.699 | 1.002 | 78.95 | 0.917 | 1.128 | 73.68 | 1.538 | 2.406 |
| TC | 12.50 | 2.852 | 3.260 | 0 | — | — | 36.84 | 1.389 | 2.762 |
| CTC | 12.50 | 2.066 | 2.968 | 0 | — | — | 10.53 | 1.970 | 2.010 |
| Compound | CAS | Acute Toxicity Value (ng/L) | Chronic Toxicity Value (ng/L) | Compound Toxicity | ||||
|---|---|---|---|---|---|---|---|---|
| Fish (LC50) | Daphnia (LC50) | Algae (EC50) | Fish (ChV) | Daphnia (ChV) | Algae (ChV) | |||
| SD | 68-35-9 | 907 | 10.3 | 40.4 | 23.8 | 0.101 | 29 | Harmful |
| SP | 144-83-2 | 246 | 6.17 | 20.8 | 4.54 | 0.066 | 10.4 | Toxic |
| SMX | 723-46-6 | 267 | 6.43 | 21.8 | 5 | 0.068 | 11.1 | Toxic |
| SMD | 651-06-9 | 899 | 11.1 | 42.5 | 22.9 | 0.109 | 29.5 | Harmful |
| SM | 127-79-7 | 421 | 7.89 | 28.1 | 8.83 | 0.082 | 16.1 | Toxic |
| SMZ | 57-68-1 | 195 | 6.02 | 15.9 | 3.26 | 0.065 | 8.88 | Toxic |
| OFL | 82419-36-1 | 19,400 | 1790 | 2440 | 2460 | 114 | 675 | Non-toxic |
| NOR | 70458-96-7 | 20,100 | 1830 | 2570 | 2650 | 116 | 703 | Non-toxic |
| ENR | 93106-60-6 | 4920 | 505 | 561 | 454 | 35.8 | 167 | Non-toxic |
| TC | 60-54-8 | 13,100 | 1060 | 1890 | 2490 | 59.9 | 474 | Non-toxic |
| OTC | 79-57-2 | 139,000 | 9440 | 23,800 | 45,500 | 447 | 5270 | Non-toxic |
| CTC | 57-62-5 | 5340 | 446 | 712 | 804 | 28.2 | 189 | Non-toxic |
| DC | 564-25-0 | 13,900 | 1120 | 2000 | 2660 | 62.7 | 501 | Non-toxic |
| ETM | 114-07-8 | 68.4 | 8.62 | 6.37 | 3.36 | 0.747 | 2.2 | Harmful |
| RTM | 80214-83-1 | 51.6 | 6.72 | 4.66 | 2.3 | 0.601 | 1.65 | Harmful |
| RQ (The Han River) | RQ (The Yangtze River) | RQ (SheShui) | RQ (JuShui) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Fish | Daphnia | Algae | Fish | Daphnia | Algae | Fish | Daphnia | Algae | Fish | Daphnia | Algae | |
| SD | ||||||||||||
| SMX | ||||||||||||
| SMD | ||||||||||||
| OFL | ||||||||||||
| NOR | ||||||||||||
| ENR | ||||||||||||
| TC | ||||||||||||
| CTC | ||||||||||||
| DC | ||||||||||||
| RTM | ||||||||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, J.; Yu, Y.; Jiang, J.; Li, B.; Xie, W.; Li, G.; Song, H.; Zhai, W.; Li, Y. Study on the Distribution Characteristics and Risk Assessment of Antibiotics and Resistance Genes in Water Sources of Wuhan. Toxics 2024, 12, 507. https://doi.org/10.3390/toxics12070507
Wang J, Yu Y, Jiang J, Li B, Xie W, Li G, Song H, Zhai W, Li Y. Study on the Distribution Characteristics and Risk Assessment of Antibiotics and Resistance Genes in Water Sources of Wuhan. Toxics. 2024; 12(7):507. https://doi.org/10.3390/toxics12070507
Chicago/Turabian StyleWang, Jun, Ying Yu, Jiayi Jiang, Bolin Li, Weimin Xie, Gezi Li, Huanjie Song, Wanying Zhai, and Ye Li. 2024. "Study on the Distribution Characteristics and Risk Assessment of Antibiotics and Resistance Genes in Water Sources of Wuhan" Toxics 12, no. 7: 507. https://doi.org/10.3390/toxics12070507





