Nitrate and Nitrite Exposure Induces Visual Impairments in Adult Zebrafish
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Nitrate and Nitrite Exposure
2.3. Optomotor Response (OMR)
2.4. Avoidance of Visual Stimulus
2.5. Histological Analysis
2.6. Immunohistochemistry (IHC)
2.7. Real-Time PCR
2.8. Statistical Analysis
3. Results
3.1. Effects on Visual Behavior Responses
3.2. Effects on Retina Layers
3.3. Effect on the Gene Expression
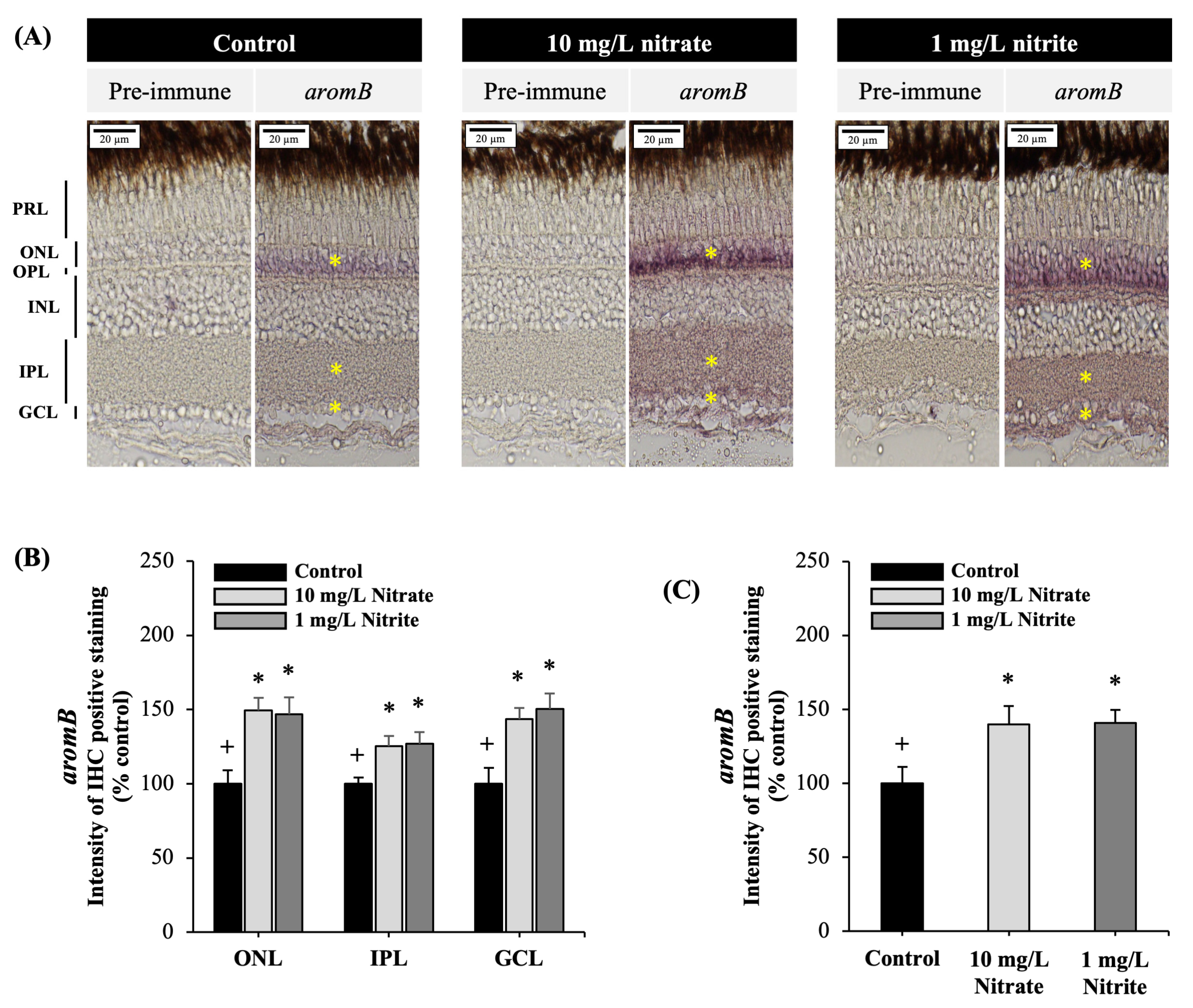
3.4. Effects on Brain Aromatase Protein in the Retina
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kazakis, N.; Voudouris, K.S. Groundwater vulnerability and pollution risk assessment of porous aquifers to nitrate: Modifying the DRASTIC method using quantitative parameters. J. Hydrol. 2015, 525, 13–25. [Google Scholar] [CrossRef]
- Qasemi, M.; Darvishian, M.; Nadimi, H.; Gholamzadeh, M.; Afsharnia, M.; Farhang, M.; Allahdadi, M.; Darvishian, M.; Zarei, A. Characteristics, water quality index and human health risk from nitrate and fluoride in Kakhk city and its rural areas, Iran. J. Food Compos. Anal. 2023, 115, 104870. [Google Scholar] [CrossRef]
- Back, J.O.; Rivett, M.O.; Hinz, L.B.; Mackay, N.; Wanangwa, G.J.; Phiri, O.L.; Songola, C.E.; Thomas, M.A.S.; Kumwenda, S.; Nhlema, M.; et al. Risk assessment to groundwater of pit latrine rural sanitation policy in developing country settings. Sci. Total Environ. 2018, 613–614, 592–610. [Google Scholar] [CrossRef] [PubMed]
- Zhai, Y.; Lei, Y.; Wu, J.; Teng, Y.; Wang, J.; Zhao, X.; Pan, X. Does the groundwater nitrate pollution in China pose a risk to human health? A critical review of published data. Environ. Sci. Pollut. Res. 2017, 24, 3640–3653. [Google Scholar] [CrossRef] [PubMed]
- Jin, Z.; Zhu, Y.; Li, X.; Dong, Y.; An, Z. Soil N retention and nitrate leaching in three types of dunes in the Mu Us desert of China. Sci. Rep. 2015, 5, 14222. [Google Scholar] [CrossRef] [PubMed]
- WHO. Nitrate and Nitrite in Drinking-Water. Available online: https://cdn.who.int/media/docs/default-source/wash-documents/wash-chemicals/nitrate-nitrite-background-jan17.pdf?sfvrsn=1c1e1502_4 (accessed on 25 February 2024).
- Ouedraogo, I.; Defourny, P.; Vanclooster, M. Mapping the groundwater vulnerability for pollution at the pan African scale. Sci. Total Environ. 2016, 544, 939–953. [Google Scholar] [CrossRef]
- Camargo, J.A.; Alonso, Á. Ecological and toxicological effects of inorganic nitrogen pollution in aquatic ecosystems: A global assessment. Environ. Int. 2006, 32, 831–849. [Google Scholar] [CrossRef] [PubMed]
- Ward, M.H.; Jones, R.R.; Brender, J.D.; de Kok, T.M.; Weyer, P.J.; Nolan, B.T.; Villanueva, C.M.; van Breda, S.G. Drinking Water Nitrate and Human Health: An Updated Review. Int. J. Environ. Res. Public Health 2018, 15, 1557. [Google Scholar] [CrossRef] [PubMed]
- Kross, B.C.; Ayebo, A.D.; Fuortes, L.J. Methemoglobinemia: Nitrate toxicity in rural America. Am. Fam. Physician 1992, 46, 183–188. [Google Scholar]
- Cheng, S.Y.; Chen, J.C. Study on the oxyhemocyanin, deoxyhemocyanin, oxygen affinity and acid-base balance of Marsupenaeus japonicus following exposure to combined elevated nitrite and nitrate. Aquat. Toxicol. 2002, 61, 181–193. [Google Scholar] [CrossRef]
- Alonso, A.; Camargo, J.A. Short-term toxicity of ammonia, nitrite, and nitrate to the aquatic snail Potamopyrgus antipodarum (Hydrobiidae, Mollusca). Bull. Environ. Contam. Toxicol. 2003, 70, 1006–1012. [Google Scholar] [CrossRef] [PubMed]
- Simmons, A.E.; Karimi, I.; Talwar, M.; Simmons, T.W. Effects of nitrite on development of embryos and early larval stages of the zebrafish (Danio rerio). Zebrafish 2012, 9, 200–206. [Google Scholar] [CrossRef] [PubMed]
- Arbuckle, T.E.; Sherman, G.J.; Corey, P.N.; Walters, D.; Lo, B. Water nitrates and CNS birth defects: A population-based case-control study. Arch. Environ. Health 1988, 43, 162–167. [Google Scholar] [CrossRef] [PubMed]
- Croen, L.A.; Todoroff, K.; Shaw, G.M. Maternal exposure to nitrate from drinking water and diet and risk for neural tube defects. Am. J. Epidemiol. 2001, 153, 325–331. [Google Scholar] [CrossRef]
- Jannat, M.; Fatimah, R.; Kishida, M. Nitrate (NO3−) and nitrite (NO2−) are endocrine disruptors to downregulate expression of tyrosine hydroxylase and motor behavior through conversion to nitric oxide in early development of zebrafish. Biochem. Biophys. Res. Commun. 2014, 452, 608–613. [Google Scholar] [CrossRef] [PubMed]
- García-Jaramillo, M.; Beaver, L.M.; Truong, L.; Axton, E.R.; Keller, R.M.; Prater, M.C.; Magnusson, K.R.; Tanguay, R.L.; Stevens, J.F.; Hord, N.G. Nitrate and nitrite exposure leads to mild anxiogenic-like behavior and alters brain metabolomic profile in zebrafish. PLoS ONE 2021, 15, e0240070. [Google Scholar] [CrossRef] [PubMed]
- Emine, A.; Oguz, M. Nitric Oxide Synthase and Nitric Oxide Involvement in Different Toxicities. In Nitric Oxide Synthase; Seyed Soheil Saeedi, S., Ed.; IntechOpen: Rijeka, Croatia, 2017; Chapter 11. [Google Scholar]
- Lundberg, J.O.; Weitzberg, E.; Gladwin, M.T. The nitrate–nitrite–nitric oxide pathway in physiology and therapeutics. Nat. Rev. Drug Discov. 2008, 7, 156–167. [Google Scholar] [CrossRef] [PubMed]
- Moncada, S.; Higgs, A. The L-arginine-nitric oxide pathway. N. Engl. J. Med. 1993, 329, 2002–2012. [Google Scholar] [CrossRef]
- Förstermann, U.; Sessa, W.C. Nitric oxide synthases: Regulation and function. Eur. Heart J. 2012, 33, 829–837, 837a–837d. [Google Scholar] [CrossRef]
- Zhao, W.; Chen, Y.; Hu, N.; Long, D.; Cao, Y. The uses of zebrafish (Danio rerio) as an in vivo model for toxicological studies: A review based on bibliometrics. Ecotoxicol. Environ. Saf. 2024, 272, 116023. [Google Scholar] [CrossRef]
- Howe, K.; Clark, M.D.; Torroja, C.F.; Torrance, J.; Berthelot, C.; Muffato, M.; Collins, J.E.; Humphray, S.; McLaren, K.; Matthews, L.; et al. The zebrafish reference genome sequence and its relationship to the human genome. Nature 2013, 496, 498–503. [Google Scholar] [CrossRef] [PubMed]
- Bibliowicz, J.; Tittle, R.K.; Gross, J.M. Toward a better understanding of human eye disease insights from the zebrafish, Danio rerio. Prog. Mol. Biol. Transl. Sci. 2011, 100, 287–330. [Google Scholar] [CrossRef] [PubMed]
- Peachey, N.S.; Ray, T.A.; Florijn, R.; Rowe, L.B.; Sjoerdsma, T.; Contreras-Alcantara, S.; Baba, K.; Tosini, G.; Pozdeyev, N.; Iuvone, P.M.; et al. GPR179 is required for depolarizing bipolar cell function and is mutated in autosomal-recessive complete congenital stationary night blindness. Am. J. Hum. Genet. 2012, 90, 331–339. [Google Scholar] [CrossRef] [PubMed]
- Najafian, M.; Alerasool, N.; Moshtaghian, J. The effect of motion aftereffect on optomotor response in larva and adult zebrafish. Neurosci. Lett. 2014, 559, 179–183. [Google Scholar] [CrossRef] [PubMed]
- Saputra, F.; Kishida, M.; Hu, S.-Y. Oxidative stress induced by hydrogen peroxide disrupts zebrafish visual development by altering apoptosis, antioxidant and estrogen related genes. Sci. Rep. 2024, 14, 14454. [Google Scholar] [CrossRef] [PubMed]
- McNeil, P.L.; Nebot, C.; Cepeda, A.; Sloman, K.A. Environmental concentrations of prednisolone alter visually mediated responses during early life stages of zebrafish (Danio rerio). Environ. Pollut. 2016, 218, 981–987. [Google Scholar] [CrossRef] [PubMed]
- Sullivan-Brown, J.; Bisher, M.E.; Burdine, R.D. Embedding, serial sectioning and staining of zebrafish embryos using JB-4 resin. Nat. Protoc. 2011, 6, 46–55. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Li, F.; Zhang, Q.; Li, J.; Liu, Q. Tracing nitrate pollution sources and transformation in surface- and ground-waters using environmental isotopes. Sci. Total Environ. 2014, 490, 213–222. [Google Scholar] [CrossRef] [PubMed]
- Secondi, J.; Hinot, E.; Djalout, Z.; Sourice, S.; Jadas-Hécart, A. Realistic nitrate concentration alters the expression of sexual traits and olfactory male attractiveness in newts. Funct. Ecol. 2009, 23, 800–808. [Google Scholar] [CrossRef]
- Weber, D.N.; Connaughton, V.P.; Dellinger, J.A.; Klemer, D.; Udvadia, A.; Carvan, M.J., 3rd. Selenomethionine reduces visual deficits due to developmental methylmercury exposures. Physiol. Behav. 2008, 93, 250–260. [Google Scholar] [CrossRef]
- Gould, C.J.; Wiegand, J.L.; Connaughton, V.P. Acute developmental exposure to 4-hydroxyandrostenedione has a long-term effect on visually-guided behaviors. Neurotoxicol. Teratol. 2017, 64, 45–49. [Google Scholar] [CrossRef] [PubMed]
- Loiseau, A.; Raîche-Marcoux, G.; Maranda, C.; Bertrand, N.; Boisselier, E. Animal Models in Eye Research: Focus on Corneal Pathologies. Int. J. Mol. Sci. 2023, 24, 16661. [Google Scholar] [CrossRef] [PubMed]
- Pelkowski, S.D.; Kapoor, M.; Richendrfer, H.A.; Wang, X.; Colwill, R.M.; Creton, R. A novel high-throughput imaging system for automated analyses of avoidance behavior in zebrafish larvae. Behav. Brain Res. 2011, 223, 135–144. [Google Scholar] [CrossRef] [PubMed]
- Richendrfer, H.; Créton, R. Automated high-throughput behavioral analyses in zebrafish larvae. J. Vis. Exp. 2013, e50622. [Google Scholar] [CrossRef]
- Creton, R. Automated analysis of behavior in zebrafish larvae. Behav. Brain Res. 2009, 203, 127–136. [Google Scholar] [CrossRef] [PubMed]
- Richardson, R.; Tracey-White, D.; Webster, A.; Moosajee, M. The zebrafish eye—A paradigm for investigating human ocular genetics. Eye 2017, 31, 68–86. [Google Scholar] [CrossRef] [PubMed]
- Angueyra, J.M.; Kindt, K.S. Leveraging Zebrafish to Study Retinal Degenerations. Front. Cell Dev. Biol. 2018, 6, 110. [Google Scholar] [CrossRef] [PubMed]
- Gollisch, T.; Meister, M. Eye smarter than scientists believed: Neural computations in circuits of the retina. Neuron 2010, 65, 150–164. [Google Scholar] [CrossRef]
- Caioni, G.; Merola, C.; Bertolucci, C.; Lucon-Xiccato, T.; Savaşçı, B.B.; Massimi, M.; Colasante, M.; Fioravanti, G.; Cacciola, N.A.; Ippoliti, R.; et al. Early-life exposure to environmentally relevant concentrations of triclocarban impairs ocular development in zebrafish larvae. Chemosphere 2023, 324, 138348. [Google Scholar] [CrossRef]
- Hanovice, N.J.; Leach, L.L.; Slater, K.; Gabriel, A.E.; Romanovicz, D.; Shao, E.; Collery, R.; Burton, E.A.; Lathrop, K.L.; Link, B.A.; et al. Regeneration of the zebrafish retinal pigment epithelium after widespread genetic ablation. PLoS Genet. 2019, 15, e1007939. [Google Scholar] [CrossRef]
- Lima Cunha, D.; Arno, G.; Corton, M.; Moosajee, M. The Spectrum of PAX6 Mutations and Genotype-Phenotype Correlations in the Eye. Genes 2019, 10, 1050. [Google Scholar] [CrossRef] [PubMed]
- Richardson, R.; Hingorani, M.; Van Heyningen, V.; Gregory-Evans, C.; Moosajee, M. Clinical utility gene card for: Aniridia. Eur. J. Hum. Genet. 2016, 24, 4. [Google Scholar] [CrossRef]
- Uttley, K.; Papanastasiou, A.S.; Lahne, M.; Brisbane, J.M.; MacDonald, R.B.; Bickmore, W.A.; Bhatia, S. Unique activities of two overlapping PAX6 retinal enhancers. Life Sci. Alliance 2023, 6, e202302126. [Google Scholar] [CrossRef]
- Klann, M.; Seaver, E.C. Functional role of pax6 during eye and nervous system development in the annelid Capitella teleta. Dev. Biol. 2019, 456, 86–103. [Google Scholar] [CrossRef] [PubMed]
- Cabiscol, E.; Tamarit, J.; Ros, J. Oxidative stress in bacteria and protein damage by reactive oxygen species. Int. Microbiol. 2000, 3, 3–8. [Google Scholar] [PubMed]
- Muthumani, M.; Prabu, S.M. Silibinin potentially protects arsenic-induced oxidative hepatic dysfunction in rats. Toxicol. Mech. Methods 2012, 22, 277–288. [Google Scholar] [CrossRef] [PubMed]
- Handy, D.E.; Loscalzo, J. The role of glutathione peroxidase-1 in health and disease. Free Radic. Biol. Med. 2022, 188, 146–161. [Google Scholar] [CrossRef] [PubMed]
- Moreno-Vivián, C.; Cabello, P.; Martínez-Luque, M.; Blasco, R.; Castillo, F. Prokaryotic Nitrate Reduction: Molecular Properties and Functional Distinction among Bacterial Nitrate Reductases. J. Bacteriol. 1999, 181, 6573–6584. [Google Scholar] [CrossRef] [PubMed]
- Jansson, E.A.; Huang, L.; Malkey, R.; Govoni, M.; Nihlén, C.; Olsson, A.; Stensdotter, M.; Petersson, J.; Holm, L.; Weitzberg, E.; et al. A mammalian functional nitrate reductase that regulates nitrite and nitric oxide homeostasis. Nat. Chem. Biol. 2008, 4, 411–417. [Google Scholar] [CrossRef]
- Pacher, P.; Beckman, J.S.; Liaudet, L. Nitric oxide and peroxynitrite in health and disease. Physiol. Rev. 2007, 87, 315–424. [Google Scholar] [CrossRef]
- Kaur, C.; Foulds, W.S.; Ling, E.A. Hypoxia-ischemia and retinal ganglion cell damage. Clin. Ophthalmol. 2008, 2, 879–889. [Google Scholar] [CrossRef] [PubMed]
- Nathan, C.; Xie, Q.W. Nitric oxide synthases: Roles, tolls, and controls. Cell 1994, 78, 915–918. [Google Scholar] [CrossRef]
- Kowluru, R.A.; Koppolu, P.; Chakrabarti, S.; Chen, S. Diabetes-induced activation of nuclear transcriptional factor in the retina, and its inhibition by antioxidants. Free Radic. Res. 2003, 37, 1169–1180. [Google Scholar] [CrossRef]
- Yabu, T.; Kishi, S.; Okazaki, T.; Yamashita, M. Characterization of zebrafish caspase-3 and induction of apoptosis through ceramide generation in fish fathead minnow tailbud cells and zebrafish embryo. Biochem. J. 2001, 360, 39–47. [Google Scholar] [CrossRef] [PubMed]
- Norbury, C.J.; Hickson, I.D. Cellular responses to DNA damage. Annu. Rev. Pharmacol. Toxicol. 2001, 41, 367–401. [Google Scholar] [CrossRef] [PubMed]
- Hardwick, J.M.; Soane, L. Multiple functions of BCL-2 family proteins. Cold Spring Harb. Perspect. Biol. 2013, 5, a008722. [Google Scholar] [CrossRef]
- Storer, N.Y.; Zon, L.I. Zebrafish models of p53 functions. Cold Spring Harb. Perspect. Biol. 2010, 2, a001123. [Google Scholar] [CrossRef]
- Chen, J.; Ng, S.M.; Chang, C.; Zhang, Z.; Bourdon, J.C.; Lane, D.P.; Peng, J. p53 isoform Δ113p53 is a p53 target gene that antagonizes p53 apoptotic activity via BclxL activation in zebrafish. Genes Dev. 2009, 23, 278–290. [Google Scholar] [CrossRef]
- Merino, D.; Lok, S.W.; Visvader, J.E.; Lindeman, G.J. Targeting BCL-2 to enhance vulnerability to therapy in estrogen receptor-positive breast cancer. Oncogene 2016, 35, 1877–1887. [Google Scholar] [CrossRef]
- Van de Craen, M.; Vandenabeele, P.; Declercq, W.; Van den Brande, I.; Van Loo, G.; Molemans, F.; Schotte, P.; Van Criekinge, W.; Beyaert, R.; Fiers, W. Characterization of seven murine caspase family members. FEBS Lett. 1997, 403, 61–69. [Google Scholar] [CrossRef]
- Nakajima, K.; Takahashi, A.; Yaoita, Y. Structure, expression, and function of the Xenopus laevis caspase family. J. Biol. Chem. 2000, 275, 10484–10491. [Google Scholar] [CrossRef] [PubMed]
- Dorstyn, L.; Read, S.H.; Quinn, L.M.; Richardson, H.; Kumar, S. DECAY, a novel Drosophila caspase related to mammalian caspase-3 and caspase-7. J. Biol. Chem. 1999, 274, 30778–30783. [Google Scholar] [CrossRef] [PubMed]
- Callard, G.V.; Tchoudakova, A.V.; Kishida, M.; Wood, E. Differential tissue distribution, developmental programming, estrogen regulation and promoter characteristics of cyp19 genes in teleost fish. J. Steroid Biochem. Mol. Biol. 2001, 79, 305–314. [Google Scholar] [CrossRef] [PubMed]
- Gelinas, D.; Callard, G.V. Immunocytochemical and biochemical evidence for aromatase in neurons of the retina, optic tectum and retinotectal pathways in goldfish. J. Neuroendocr. 1993, 5, 635–641. [Google Scholar] [CrossRef] [PubMed]
- Cascio, C.; Russo, D.; Drago, G.; Galizzi, G.; Passantino, R.; Guarneri, R.; Guarneri, P. 17β-Estradiol synthesis in the adult male rat retina. Exp. Eye Res. 2007, 85, 166–172. [Google Scholar] [CrossRef] [PubMed]
- McCarthy, M.M. Estradiol and the developing brain. Physiol. Rev. 2008, 88, 91–124. [Google Scholar] [CrossRef] [PubMed]
- Menuet, A.; Pellegrini, E.; Brion, F.; Gueguen, M.M.; Anglade, I.; Pakdel, F.; Kah, O. Expression and estrogen-dependent regulation of the zebrafish brain aromatase gene. J. Comp. Neurol. 2005, 485, 304–320. [Google Scholar] [CrossRef] [PubMed]
- Menuet, A.; Pellegrini, E.; Anglade, I.; Blaise, O.; Laudet, V.; Kah, O.; Pakdel, F. Molecular Characterization of Three Estrogen Receptor Forms in Zebrafish: Binding Characteristics, Transactivation Properties, and Tissue Distributions1. Biol. Reprod. 2002, 66, 1881–1892. [Google Scholar] [CrossRef]
- Guarneri, P.; Guarneri, R.; Cascio, C.; Pavasant, P.; Piccoli, F.; Papadopoulos, V. Neurosteroidogenesis in rat retinas. J. Neurochem. 1994, 63, 86–96. [Google Scholar] [CrossRef]
- Cascio, C.; Deidda, I.; Russo, D.; Guarneri, P. The estrogenic retina: The potential contribution to healthy aging and age-related neurodegenerative diseases of the retina. Steroids 2015, 103, 31–41. [Google Scholar] [CrossRef]




| Treatments | Temperature (°C) | Ammonia (mg/L) | Nitrate (mg/L) | Nitrite (mg/L) | pH |
|---|---|---|---|---|---|
| Morning (Pre-feeding) | |||||
| Control | 27.0 ± 0.46 | 0.04 ± 0.01 | 0.05 ± 0.02 | 0.05 ± 0.02 | 7,3 ± 0.33 |
| 10 mg/L nitrate | 27.0 ± 0.41 | 0.04 ± 0.01 | 10.2 ± 0.70 | 0.07 ± 0.02 | 7.3 ± 0.39 |
| 1 mg/L nitrite | 27.3 ± 0.60 | 0.05 ± 0.01 | 0.05 ± 0.02 | 1.1 ± 0.12 | 7.1 ± 0.49 |
| Afternoon (Post-feeding) | |||||
| Control | 26.6 ± 0.39 | 0.05 ± 0.02 | 0.06 ± 0.02 | 0.05 ± 0.02 | 7.3 ± 0.40 |
| 10 mg/L nitrate | 27.0 ± 0.63 | 0.06 ± 0.02 | 10.3 ± 0.34 | 0.07 ± 0.02 | 7.2 ± 0.49 |
| 1 mg/L nitrite | 26.9 ± 0.56 | 0.06 ± 0.01 | 0.06 ± 0.02 | 1.19 ± 0.24 | 7.1 ± 0.42 |
| Gene Name | Primer Sequence (5′-3′) | Accession Number |
|---|---|---|
| pax6a (paired box 6a) | F: CTCAAACAGAAGAGCGAAATGGA R: GAAGCTGCTGCTGATGGGTAT | XM_009297889.3 |
| pax6b (paired box 6b) | F: CCTCCAGTCACATTCCCATCA R: AGCATTGAGCCTGTCGTGAA | NM_131641.1 |
| sod1 (superoxide dismutase 1) | F: GTCGTCTGGCTTGTGGAGTG R: TGTCAGCGGGCTAGTGCTT | NM_131294.1 |
| gpx1a (glutathione peroxidase 1a) | F: GGCACAACAGTCAGGGATTA R: CAGGACGGACGTATTTCAGA | NM_001007281.2 |
| nos2a (nitric oxide synthase 2a) | F: GGAGATGCAAGGTCAGCTTC R: GGCAAAGCTCAGTGACTTCC | XM005165296 |
| casp3 (caspase 3) | F: CCGCTGCCCATCACTA R: ATCCTTTCACGACCATCT | NM_131877.3 |
| tp53 (tumor protein p53) | F: GGGCAATCAGCGAGCAAA R: ACTGACCTTCCTGAGTCTCCA | NM_131327.2 |
| bcl2a (BCL2 apoptosis regulator a) | F: AGGAAAATGGAGGTTGGGATG R: TGTTAGGTATGAAAACGGGTGGA | NM_001030253.2 |
| esr1 (estrogen receptor 1) | F: CCGGCCCTACACAGAGATCA R: AGCCAAGAGCTCTCCAACAACT | NM_152959.1 |
| esr2a (estrogen receptor 2a) | F: CTGTGCCGTCTGCAGTGATT R: CGGCGGTTCTTGTCGATAGT | NM_180966.2 |
| esr2b (estrogen receptor 2b) | F: TCCGACACCTCAGCAACAAA R: TTTCTGGGCTCTGTTGTCTGTCT | NM_174862.3 |
| cyp19a1a (ovarian aromatase) | F: AGATGTCGAGTTAAAGATCC R: ACTCGTTGATAAAACTCTCC | NM_131154.3 |
| cyp12a1b (brain aromatase) | F: GCAAATCGTACAGGAGATAC R: CGTCCAATGTTCAGGATTAG | NM_131642.2 |
| eef1a1 (elongation factor 1 alpha 1) | F: TGGTGGTGTCGGTGAGTTTG R: AAACGAGCCTGGCTGTAAGG | AY422992.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saputra, F.; Kishida, M.; Hu, S.-Y. Nitrate and Nitrite Exposure Induces Visual Impairments in Adult Zebrafish. Toxics 2024, 12, 518. https://doi.org/10.3390/toxics12070518
Saputra F, Kishida M, Hu S-Y. Nitrate and Nitrite Exposure Induces Visual Impairments in Adult Zebrafish. Toxics. 2024; 12(7):518. https://doi.org/10.3390/toxics12070518
Chicago/Turabian StyleSaputra, Febriyansyah, Mitsuyo Kishida, and Shao-Yang Hu. 2024. "Nitrate and Nitrite Exposure Induces Visual Impairments in Adult Zebrafish" Toxics 12, no. 7: 518. https://doi.org/10.3390/toxics12070518






