1. Introduction
The contamination of soil with heavy metals, such as lead (Pb), poses significant environmental and health risks worldwide. Lead, a toxic metal commonly found in industrial areas, mining sites, and urban soils, can persist in the environment for extended periods, causing adverse effects on ecosystems and human health [
1]. Lead at shooting ranges is a significant environmental concern due to the amount of lead waste generated and accumulated at these sites due to its widespread use in ammunition. Bullets are primarily composed of lead because of its high density, which ensures effective energy transfer upon impact, and its relatively low cost [
1]. Lead azide is used in primers to activate a bullet when struck by the firing pin. This causes lead particles to be released into the air as dust when the firearm is discharged, contributing further to environmental contamination. The repeated discharge of firearms in these shooting ranges results in the accumulation of lead particles in the soil and dust, making the contamination substantial [
2]. For that reason, both commercial and private shooting ranges are considered major sources of environmental lead contamination [
3]. According to Mendes et al. [
4], activity at shooting ranges contributes millions of pounds of lead to the environment annually, surpassing most industries except for metal mining and manufacturing. This lead not only remains on site but can also leach into water sources and affect nearby wildlife and properties. For that reason, it is necessary to look for adequate management and mitigation strategies at shooting ranges to reduce lead exposure and protect both human health and the environment.
Traditional remediation methods for Pb-contaminated soils [
5,
6,
7] (electrokinetic remediation, soil excavation, soil washing, thermal desorption, chemical stabilization) often involve high costs and may have limited effectiveness in achieving long-term soil restoration [
8,
9]. For that reason, different approaches combining phytoremediation techniques with soil amendments have emerged as promising strategies for sustainable soil remediation [
10,
11].
Among the various soil amendments, biochar has garnered considerable attention due to its unique physicochemical properties and potential to improve soil quality and enhance plant growth [
12]. Biochar, a carbonaceous material produced from the pyrolysis of organic biomass, offers numerous benefits, including increased soil porosity, enhanced nutrient retention, and reduced bioavailability of contaminants [
13]. When integrated with phytoremediation, which utilizes plants to extract, degrade, or immobilize contaminants from soil and water [
11], biochar has been shown to augment the efficiency of metal uptake by plants, reduce metal leaching, and improve soil conditions, thereby facilitating the remediation of Pb-contaminated soils [
14]. Moreover, biochar can increase the uptake and sequestration of Pb by plants, accelerating the remediation process [
15]. Numerous studies have investigated its efficacy in diverse soil types, climatic conditions, and contaminant scenarios [
16,
17]. Research findings have consistently demonstrated the ability of biochar to enhance soil structure, water retention, nutrient availability, and microbial activity, thereby promoting plant growth and productivity [
18]. Moreover, biochar-mediated changes in soil’s physicochemical properties have been shown to influence the bioavailability and mobility of various contaminants, including heavy metals, organic pollutants, and excess nutrients, leading to their sequestration, degradation, or immobilization within the soil matrix [
15,
19,
20].
Phytoremediation studies have studied the efficacy of
Brassica rapa and
Lolium perenne.
Brassica species have been proven to be effective in the phytoextraction of various heavy metals like Pb, Cr, Cu, Cd, Ni, and Zn, utilizing defense mechanisms such as antioxidant enzymes and amino acids to alleviate metal stress without compromising plant growth and development [
21]. Asikin et al. [
22] studied the heavy metal tolerance of
Brassica species and found that
B. rapa species possess strong tolerance and accumulation capabilities for non-essential heavy metals (Cd, Cr and Pb), making them potential hyperaccumulators for green remediation techniques in toxic soil environments.
Lolium perenne is capable of absorbing metal into the root matrix, making it a potential candidate for the phytoremediation of landfill soil and the phytostabilization of Cu, Cr and Pb [
23]. Li et al. [
24] found that
Lolium perenne decreased the content of lead in soil by 44%.
Despite growing interest in the combined use of biochar and phytoremediation, there remains a need to study the factors influencing their effectiveness in lead remediation. For this reason, the objective of this research is to investigate the combined effect of biochar and phytoremediation (Brassica rapa and Lolium perenne) on Pb remediation in shooting range soil and to identify the factors that contribute most significantly to reducing Pb content in soil.
2. Materials and Methods
2.1. Material Collection and Characterization
Soil samples were collected from the “General Morillo” military base in Pontevedra (42°23′9″ N and 8°39′15″ W), which is used as a shooting and maneuver range of the Organic Polyvalent Brigade “Galicia” VII (BRILAT). Samples were collected from tree zones: zone 0, or control (soil without contamination); Zone 1, located one meter from the firing line (named Z1); and Zone 2, located at the end of the shooting range behind the target area (named Z2). All samples were collected at a depth of approximately 20–30 cm.
The samples were dried at 25 °C and manually sieved at 2 mm. pH and conductivity were measured at a ratio of 1:10 (soil/ultrapure water). Pb and S concentrations were measured in aqueous extraction by inductively coupled mass spectrometry (ICP-MS) using an ICP-OES iCAP-pro (ThermoFisher, Karlsruhe, Germany). For this purpose, about 0.2 g of powdered soil samples were solubilized by acid digestion with 3 mL nitric acid, 3 mL hydrogen peroxide and 1 mL hydrochloric acid in Teflon digesters in a SYNTHOS 300 microwave oven (Anton Paar, Graz, Austria). The samples were then diluted to 50 mL with ultrapure water, shaken and filtered for subsequent analysis.
The soil fraction from zones 1 and 2 was divided into aliquots to analyze both the effect of the amount of biochar (5, 10 and 15% wt.) and its distribution (surface, named S, or mixed, named M). Biochar was purchased from LivingChar (Barcelona, Spain). This biochar is produced from wood residues by pyrolysis. This 100% organic product contains a high content of stable organic carbon. Its elevated stable organic matter content and high porosity are valuable properties that make this biochar an ideal material for improving the health and fertility of contaminated soils, as well as for inhibiting soil toxicity from heavy metals and toxic organic compounds. Its characteristics, provided by the supplier, are presented in
Table 1. Before mixing with the soil, biochar was manually sieved at 2 mm.
In all experiments, 500 g of each zone (1 and 2) was used to fill containers (12.5 cm × 19 cm × 4.5 cm) and subjected to an incubation period with biochar and subsequently to phytoremediation in two steps. The same amounts of uncontaminated soil (used as a control and taken from zone 0) and contaminated soil without biochar were subjected to the same process. Each experiment was carried out in duplicate. The scheme of the process is presented in
Figure 1, and
Table 2 gives the nomenclature of each soil sample.
2.2. Material Collection and Characterization
The incubation of amended soils was carried out over one week at 25 °C in closed containers. The containers were prepared with 500 g of contaminated soil and different amounts of biochar (5, 10 and 15% wt.) using two distributions: biochar mixed with soil (distribution 1) and biochar added to the soil surface (distribution 2). Water was added (150 mL) until the soil reached 70% humidity, measured with a moisture analyzer (MA 110.R, Radwag). pH was monitored at a ratio of 1:10 (soil/water) every two days, and Pb content was measured by mass spectrometry (ICP-MS) at the end of the period.
After one week of incubation, seeds of Brassica rapa and Lolium perenne were planted in each container. The containers were divided into two halves, allocating a half for each crop. The same number of seeds were planted, a total of 12 seeds of each type, distributing them into 3 rows and 4 columns, approximately 2 cm apart vertically and horizontally. The humidity was adjusted every two days by weighing. pH was monitored at a ratio of 1:10 (soil/water) every two days. The plants’ growth after two weeks was measured according to the number of germinated seeds, the length of the plants and the shoot length. Brassica rapa necessitates approximately 4 days to achieve initial growth, whereas Lolium perenne requires around 7 days to reach a similar developmental stage. A period of two weeks was selected to ensure complete and observable plant development.
After plant extraction, new seeds of Brassica rapa and Lolium perenne were planted again in the same soil for another two weeks. In both cultures, the containers’ position was changed every two days to avoid any possible influence of microclimatic conditions (25 ± 1 °C). pH was monitored at a ratio of 1:10 (soil/water) every two days, and Pb content was measured by mass spectrometry (ICP-MS) at the end of the period. The plants’ growth after two weeks was measured according to the number of germinated seeds, the length of the plants, and the shoot length.
2.3. Data Analysis
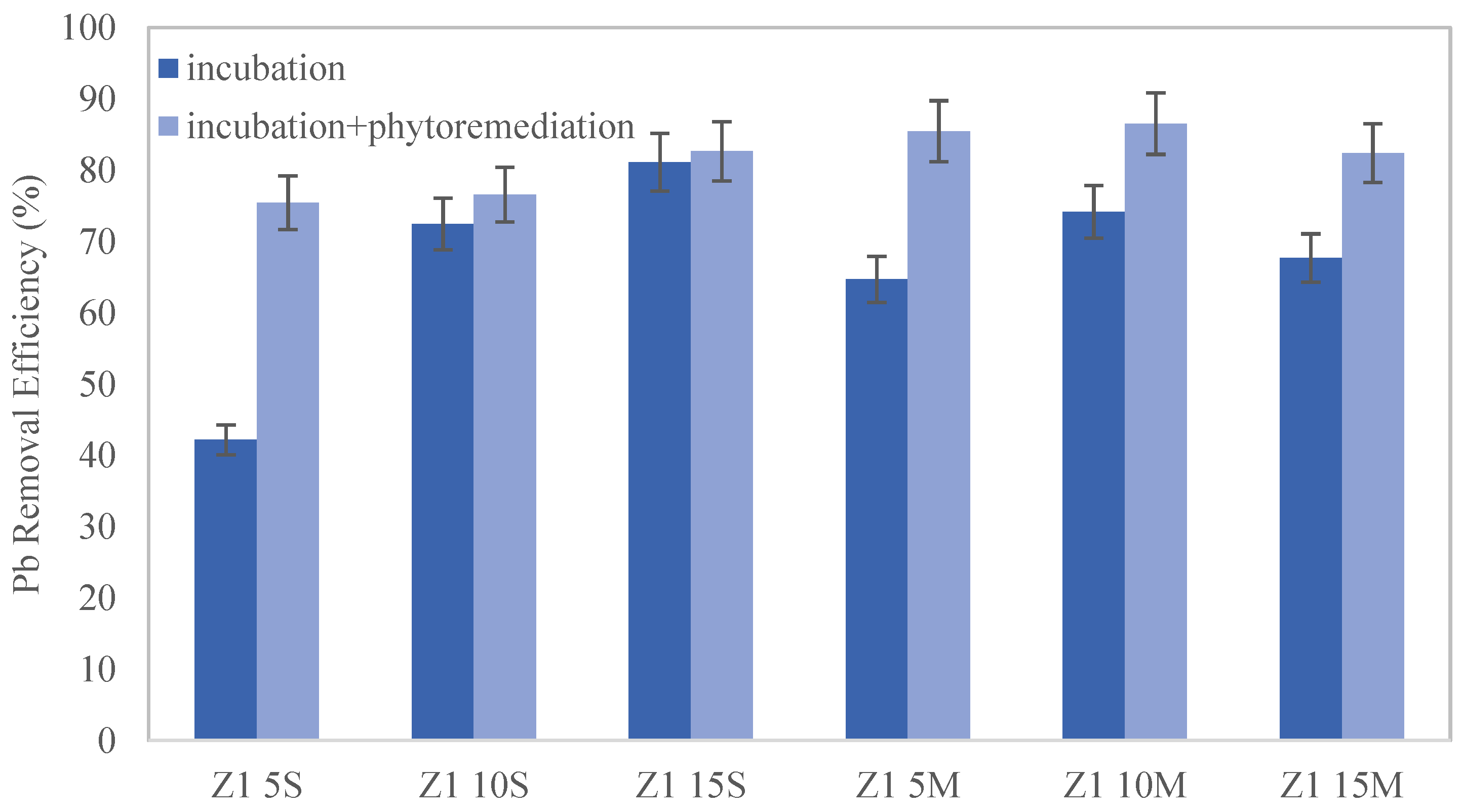
The removal efficiency (%) of each sample was calculated using Equation (1) at the end of incubation and cultivation. Moreover, in distribution 2 (biochar added to the soil surface), the biochar fraction and the soil fraction were analyzed separately, using the latter to determine the removal efficiency (%). However, when biochar was mixed with soil (distribution 1), a homogeneous sample was analyzed due to the impossibility of separating soil and biochar.
where
C0 and
Cn are the metal concentrations at the beginning and the end of the process for each sample, respectively.
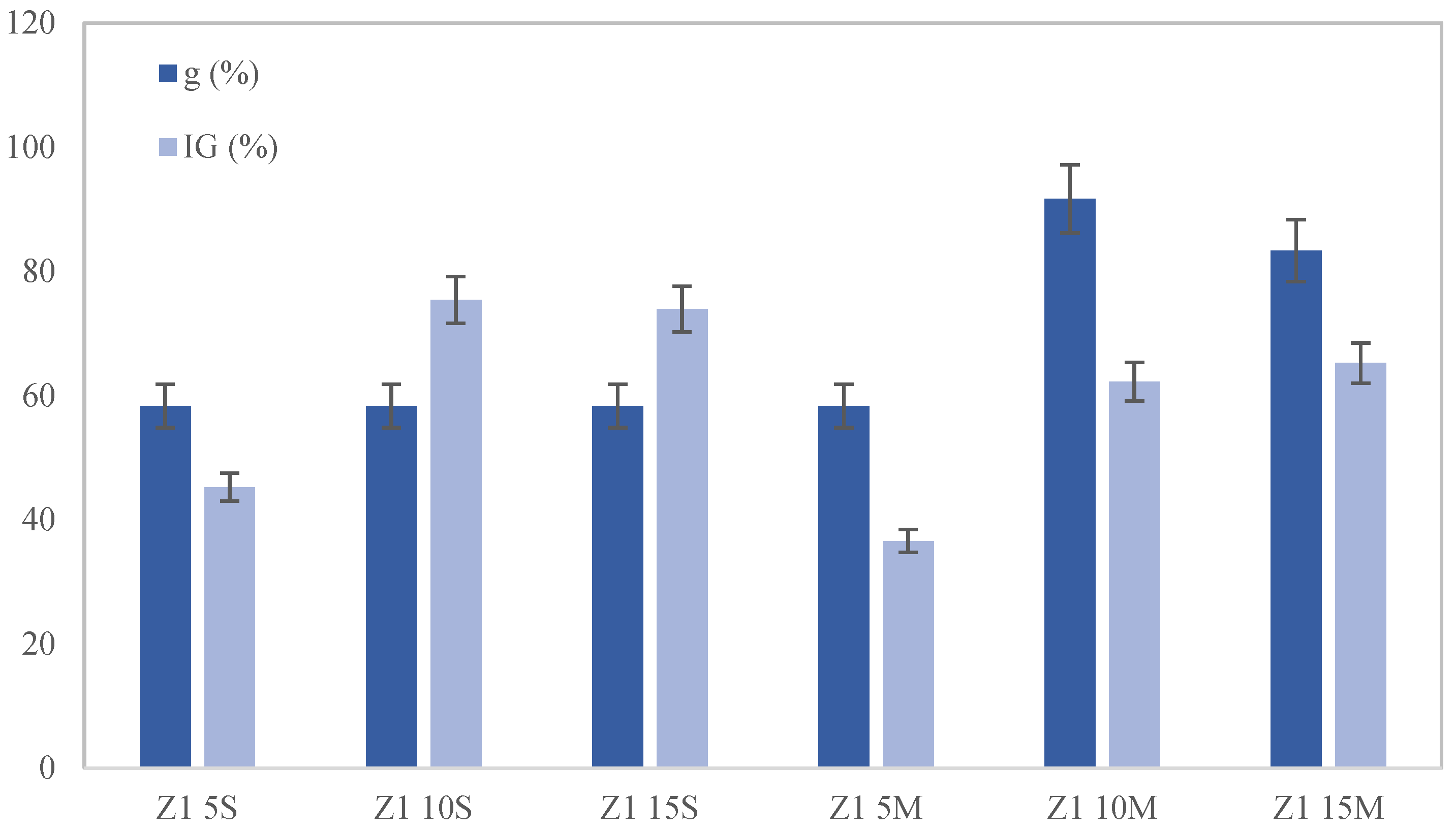
The toxicity of the soil after incubation and after the cultures was studied for two species:
Brassica rapa and
Lolium perenne. In each container, the germination speed (GS), germination percentage (G), germination coefficient (g), germination index (IG), root length stress tolerance index (RLSTI) and shoot length stress tolerance index (SLSTI) were evaluated according to the following equations [
25,
26]:
where
S0 is the total number of planted seeds;
S1,
S2 and
Sn are the numbers of germinated seeds on the first, second and
nth day, respectively, for each sample;
D1,
D2 and
Dn are the numbers of days after sowing;
Sc is the number of seeds germinated in the uncontaminated soil (control);
Gn and
Gc are the germination percentages in each sample and in the uncontaminated soil (control);
RLn and
RLc are the root lengths of the stressed and control plants, respectively; and
SLn and
SLc are the shoot lengths of the stressed and control plants.
2.4. Statistical Analysis
A two-way ANOVA was used to carry out a statistical analysis on the effect of the amount of biochar and its distribution on different soil and plant properties. In the statistical tests, a p-value of 0.05 was considered significant, and less than 0.01 was considered very significant. The analyses were conducted using Excel 2016 for Windows.
4. Conclusions and Perspectives for Future Studies
This study highlights the significant impact of biochar on the remediation of lead-contaminated soils. Moreover, the combined application of biochar and phytoremediation techniques has shown promising results. The results indicate that seed germination and plant development were notably superior in soil samples where biochar was mixed rather than applied superficially, with the optimal results observed at a 10% wt. biochar amendment. When biochar is mixed into the soil, its contact with lead particles is maximized, significantly enhancing adsorption efficiency and reducing the mobility and bioavailability of lead. In addition, it should be noted that the contact time between the biochar and the soil allows for an increased amount of lead removal.
This study showed that integrating biochar with phytoremediation can lead to improved growth rates and higher metal uptake in shooting range soils, making it a promising strategy for sustainable soil management and remediation. However, there are certain limitations that need to be addressed. Firstly, the study’s scope was limited to a specific type of biochar and plant species, which may not fully represent the wide variety of potential biochar–plant combinations available for remediation purposes. Future research should explore a broader range of biochar types derived from different feedstocks and diverse plant species to identify the most effective combinations for various soil conditions and contaminants.
Additionally, the long-term effects of biochar on soil health and heavy metal dynamics were not thoroughly examined. Longitudinal studies are necessary to understand the sustainability and potential cumulative impacts of biochar applications over extended periods of time. The interaction between biochar and soil microbial communities, which play an important role in bioremediation, also warrants further investigation.
Finally, our study primarily focused on the laboratory scale, and field trials are essential to validate these findings under real-world conditions. Environmental variables such as climate, soil type, and contamination levels can significantly influence the outcomes of biochar-assisted phytoremediation. Thus, large-scale field studies will help in assessing the practical applicability and scalability of this remediation approach.