Immunological Effects of Diesel Particles in a Murine Model of Healthy Mice
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Diesel Exhaust Particles (DEP)
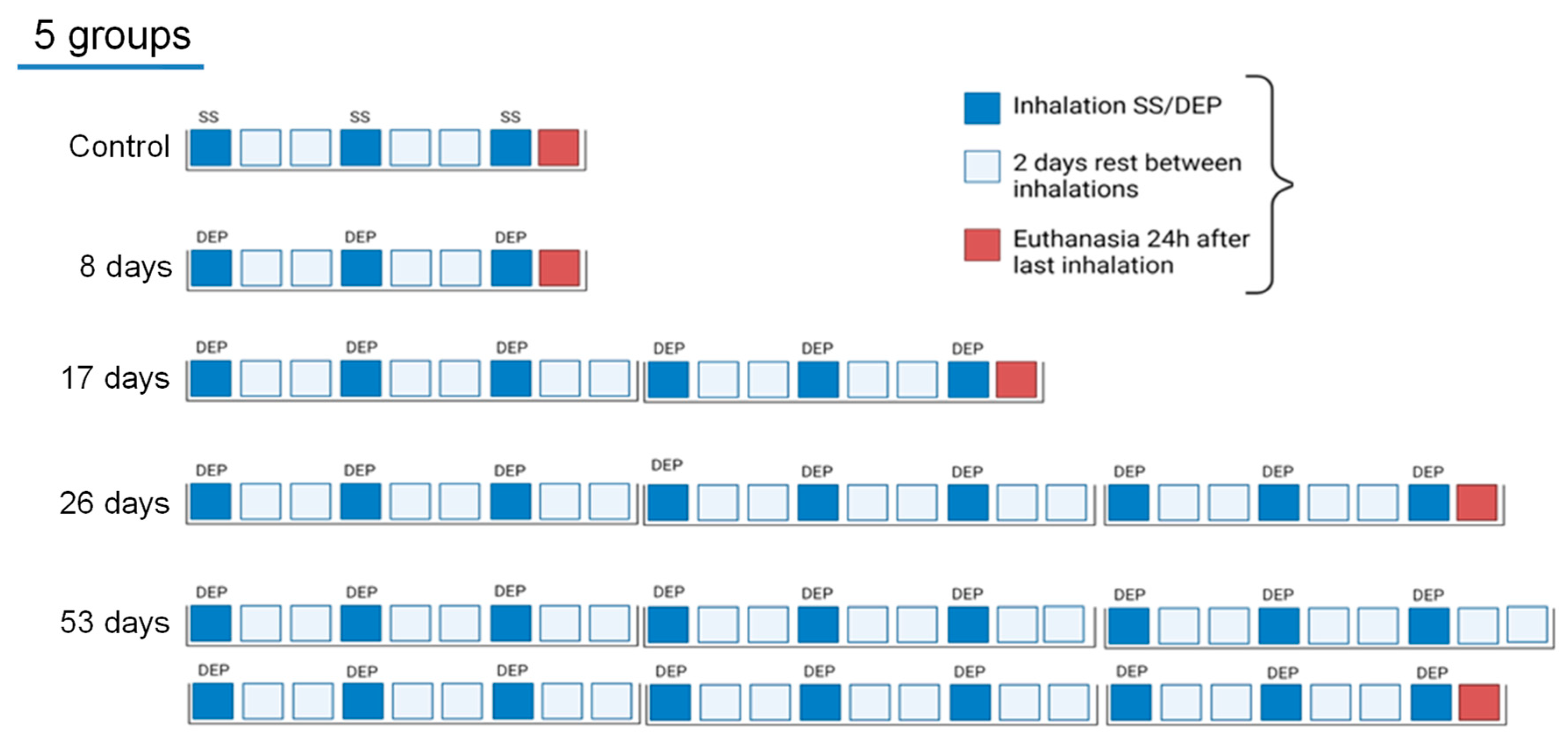
2.3. Experimental Design
2.4. Lung Function Assessment
2.5. Bronchoalveolar Lavage
2.6. DEP-Uptake Analysis
2.7. Flow Cytometry
2.8. Data Analysis
3. Results
3.1. Lung Function Assessment
3.2. Bronchoalveolar Lavage
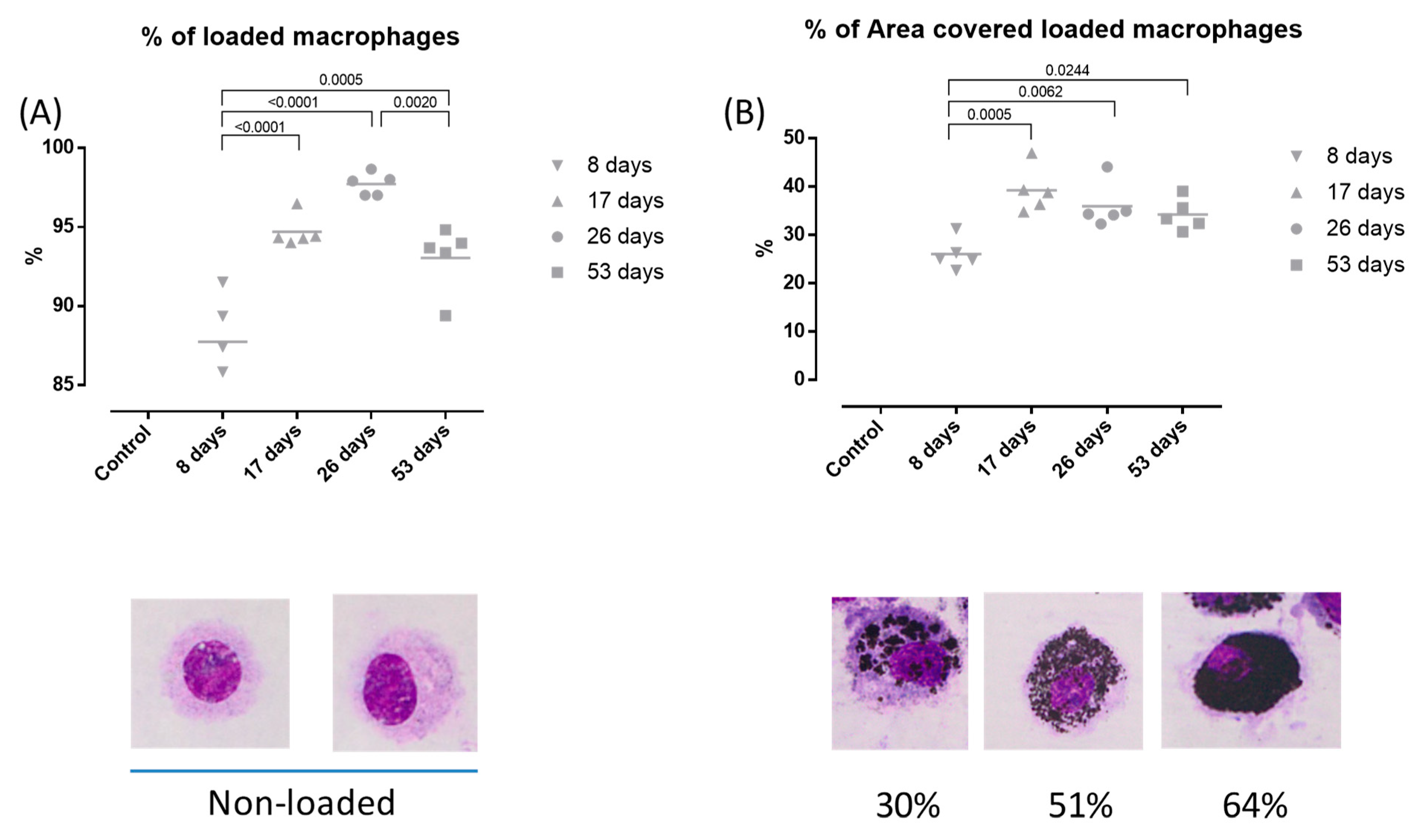
3.3. DEP-Uptake Analysis
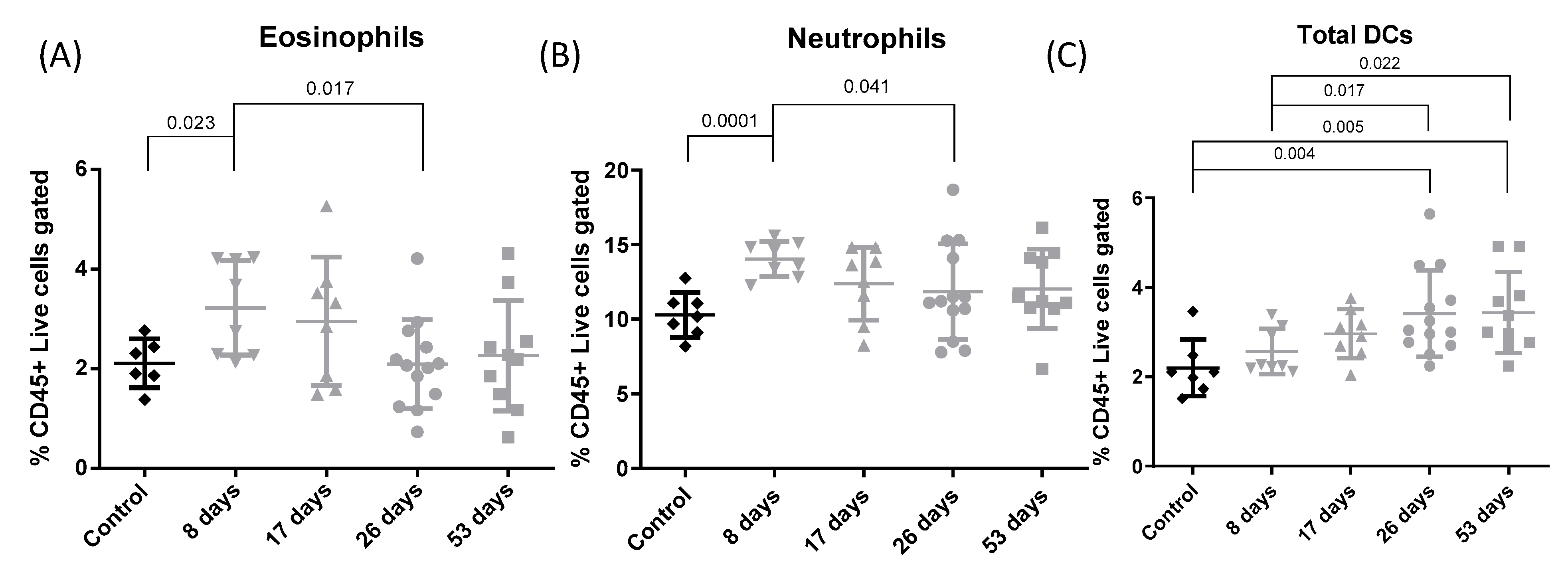
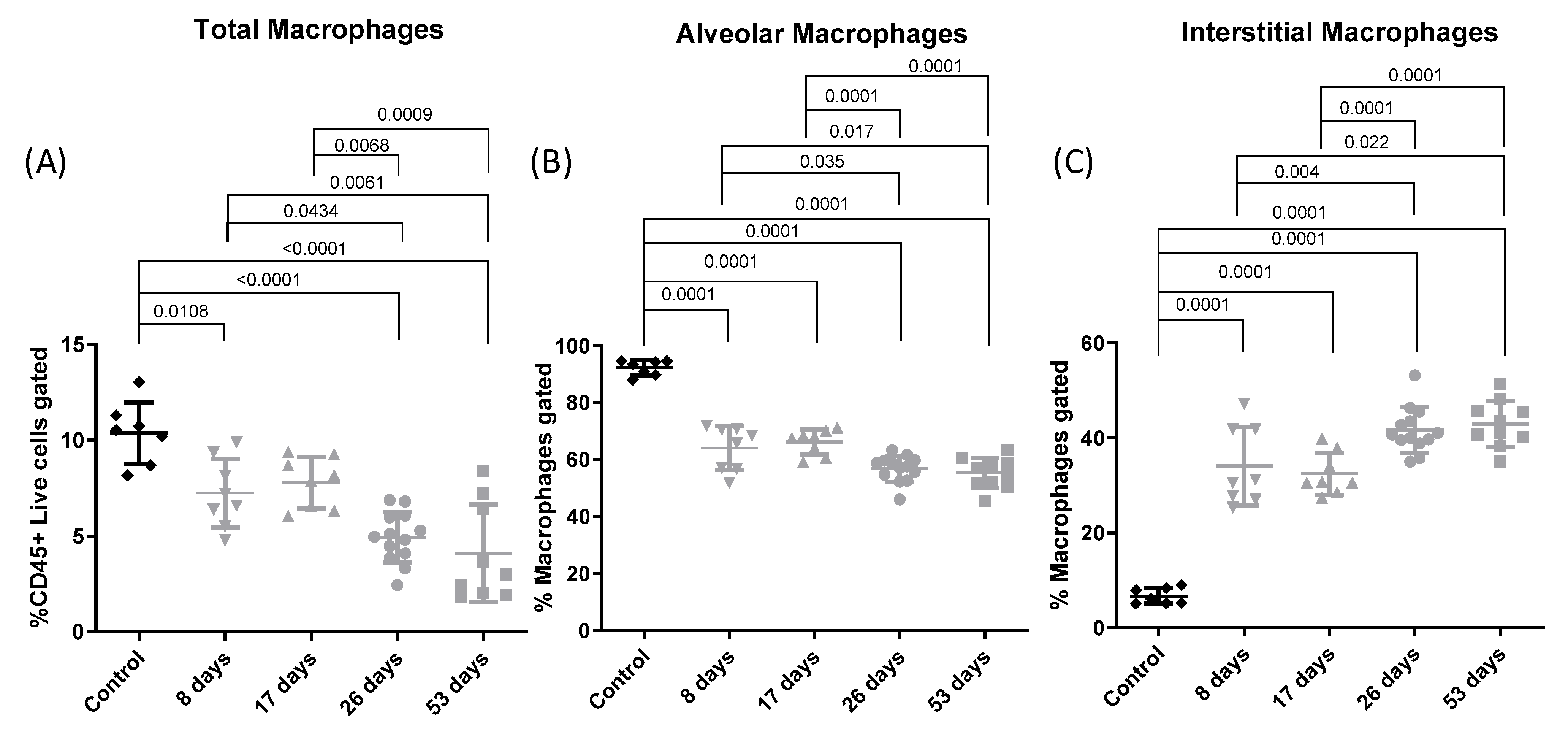
3.4. Flow Cytometry
4. Discussions
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Health Impacts of Air Pollution in Europe, 2022—European Environment Agency [Internet]. Available online: https://www.eea.europa.eu/publications/air-quality-in-europe-2022/health-impacts-of-air-pollution (accessed on 3 March 2023).
- Liu, C.; Chen, R.; Sera, F.; Vicedo-Cabrera, A.M.; Guo, Y.; Tong, S.; Coelho, M.S.Z.S.; Saldiva, P.H.N.; Lavigne, E.; Matus, P.; et al. Ambient Particulate Air Pollution and Daily Mortality in 652 Cities. N. Engl. J. Med. 2019, 381, 705–715. [Google Scholar] [CrossRef] [PubMed]
- Bendtsen, K.M.; Gren, L.; Malmborg, V.B.; Shukla, P.C.; Tunér, M.; Essig, Y.J.; Krais, A.M.; Clausen, P.A.; Berthing, T.; Loeschner, K.; et al. Particle characterization and toxicity in C57BL/6 mice following instillation of five different diesel exhaust particles designed to differ in physicochemical properties. Part. Fibre Toxicol. 2020, 17, 38. [Google Scholar] [CrossRef] [PubMed]
- Schraufnagel, D.E.; Balmes, J.R.; Cowl, C.T.; De Matteis, S.; Jung, S.H.; Mortimer, K.; Perez-Padilla, R.; Rice, M.B.; Riojas-Rodriguez, H.; Sood, A.; et al. Air Pollution and Noncommunicable Diseases: A Review by the Forum of International Respiratory Societies’ Environmental Committee, Part 1: The Damaging Effects of Air Pollution. Chest 2019, 155, 409–416. [Google Scholar] [CrossRef] [PubMed]
- Shears, R.K.; Jacques, L.C.; Naylor, G.; Miyashita, L.; Khandaker, S.; Lebre, F.; Lavelle, E.C.; Grigg, J.; French, N.; Neill, D.R.; et al. Exposure to diesel exhaust particles increases susceptibility to invasive pneumococcal disease. J. Allergy Clin. Immunol. 2020, 145, 1272–1284.e6. [Google Scholar] [CrossRef] [PubMed]
- Lee, P.H.; Kim, B.G.; Park, M.K.; Hong, J.; Lee, Y.G.; Jang, A.S. The impact of diesel exhaust particles on tight junctional proteins on nose and lung in a mouse model. Allergy Asthma Immunol. Res. 2020, 13, 350–352. [Google Scholar] [CrossRef] [PubMed]
- Chatkin, J.; Correa, L.; Santos, U. External Environmental Pollution as a Risk Factor for Asthma. Clin. Rev. Allergy Immunol. 2021, 62, 72–89. [Google Scholar] [CrossRef] [PubMed]
- Epstein, T.G.; Kesavalu, B.; Bernstein, C.K.; Ryan, P.H.; Bernstein, J.A.; Zimmermann, N.; Lummus, Z.; Villareal, M.S.; Smith, A.M.; Lenz, P.H.; et al. Chronic traffic pollution exposure is associated with eosinophilic, but not neutrophilic inflammation in older adult asthmatics. J. Asthma 2013, 50, 983–989. [Google Scholar] [CrossRef] [PubMed]
- Whitehouse, A.L.; Mushtaq, N.; Miyashita, L.; Barratt, B.; Khan, A.; Kalsi, H.; Koh, L.; Padovan, M.G.; Brugha, R.; Balkwill, F.R.; et al. Airway dendritic cell maturation in children exposed to air pollution. PLoS ONE 2020, 15, e0232040. [Google Scholar] [CrossRef] [PubMed]
- Long, E.; Carlsten, C. Controlled human exposure to diesel exhaust: Results illuminate health effects of traffic-related air pollution and inform future directions. Part. Fibre Toxicol. 2022, 19, 11. [Google Scholar] [CrossRef]
- Muñoz, X.; Barreiro, E.; Bustamante, V.; Lopez-Campos, J.L.; González-Barcala, F.J.; Cruz, M.J. Diesel exhausts particles: Their role in increasing the incidence of asthma. Reviewing the evidence of a causal link. Sci. Total Environ. 2019, 652, 1129–1138. [Google Scholar] [CrossRef]
- Olsson, D.; Forsberg, B.; Bråbäck, L.; Geels, C.; Brandt, J.; Christensen, J.H.; Frohn, L.M.; Oudin, A. Early childhood exposure to ambient air pollution is associated with increased risk of paediatric asthma: An administrative cohort study from Stockholm, Sweden. Environ. Int. 2021, 155, 106667. [Google Scholar] [CrossRef] [PubMed]
- Ryan, P.; Bernstein, D.; Levin, L.; Burkle, J.; Villareal, M.; Kalra, H.; Lockey, J.; Khuranahershey, G.; Lemasters, G. Exposure to Diesel Exhaust Particles and Indoor Endotoxin During Early Childhood Increases the Risk for Persistent Wheeze at Age Three. J. Allergy Clin. Immunol. 2008, 121, S65. [Google Scholar] [CrossRef]
- Yang, S.I. Particulate matter and childhood allergic diseases. Korean J. Pediatr. 2019, 62, 22–29. [Google Scholar] [CrossRef] [PubMed]
- Stenfors, N.; Nordenhäll, C.; Salvi, S.S.; Mudway, I.; Söderberg, M.; Blomberg, A.; Helleday, R.; Levin, J.-O.; Holgate, S.; Kelly, F.; et al. Different airway inflammatory responses in asthmatic and healthy humans exposed to diesel. Eur. Respir. J. 2004, 23, 82–86. [Google Scholar] [CrossRef] [PubMed]
- Behndig, A.F.; Larsson, N.; Brown, J.L.; Stenfors, N.; Helleday, R.; Duggan, S.T.; Dove, R.E.; Wilson, S.J.; Sandstrom, T.; Kelly, F.J.; et al. Proinflammatory doses of diesel exhaust in healthy subjects fail to elicit equivalent or augmented airway inflammation in subjects with asthma. Thorax 2011, 66, 12–19. [Google Scholar] [CrossRef] [PubMed]
- Dauchet, L.; Hulo, S.; Cherot-Kornobis, N.; Matran, R.; Amouyel, P.; Edmé, J.L.; Giovannelli, J. Short-term exposure to air pollution: Associations with lung function and inflammatory markers in non-smoking, healthy adults. Environ. Int. 2018, 121, 610–619. [Google Scholar] [CrossRef] [PubMed]
- Rice, M.B.; Ljungman, P.L.; Wilker, E.H.; Gold, D.R.; Schwartz, J.D.; Koutrakis, P.; Washko, G.R.; O’connor, G.T.; Mittleman, M.A. Short-term exposure to air pollution and lung function in the framingham heart study. Am. J. Respir. Crit. Care Med. 2013, 188, 1351–1357. [Google Scholar] [CrossRef] [PubMed]
- Shalaby, K.H.; Gold, L.G.; Schuessler, T.F.; Martin, J.G.; Robichaud, A. Combined forced oscillation and forced expiration measurements in mice for the assessment of airway hyperresponsiveness. Respir. Res. 2010, 11, 82. [Google Scholar] [CrossRef] [PubMed]
- Decaesteker, T.; Vanhoffelen, E.; Trekels, K.; Jonckheere, A.C.; Cremer, J.; Vanstapel, A.; Dilissen, E.; Bullens, D.; Dupont, L.J.; Vanoirbeek, J.A. Differential effects of intense exercise and pollution on the airways in a murine model. Part. Fibre Toxicol. 2021, 18, 12. [Google Scholar] [CrossRef]
- de Homdedeu, M.; Cruz, M.; Sánchez-Díez, S.; Gómez-Ollés, S.; Ojanguren, I.; Ma, D.; Muñoz, X. Role of diesel exhaust particles in the induction of allergic asthma to low doses of soybean. Environ. Res. 2021, 196, 110337. [Google Scholar] [CrossRef]
- Gie, A.G.; Regin, Y.; Salaets, T.; Casiraghi, C.; Salomone, F.; Deprest, J.; Muñoz, X. Intratracheal budesonide/surfactant attenuates hyperoxia-induced lung injury in preterm rabbits. Am. J. Physiol.—Lung Cell. Mol. Physiol. 2020, 319, L949–L956. [Google Scholar] [CrossRef]
- Miyata, R.; van Eeden, S.F. The innate and adaptive immune response induced by alveolar macrophages exposed to ambient particulate matter. Toxicol. Appl. Pharmacol. 2011, 257, 209–226. [Google Scholar] [CrossRef] [PubMed]
- Mazzarella, G.; Ferraraccio, F.; Prati, M.V.; Annunziata, S.; Bianco, A.; Mezzogiorno, A.; Liguori, G.; Angelillo, I.; Cazzola, M. Effects of diesel exhaust particles on human lung epithelial cells: An in vitro study. Respir. Med. 2007, 101, 1155–1162. [Google Scholar] [CrossRef] [PubMed]
- Champion, J.A.; Mitragotri, S. Shape induced inhibition of phagocytosis of polymer particles. Pharm. Res. 2009, 26, 244–249. [Google Scholar] [CrossRef] [PubMed]
- Jaguin, M.; Fardel, O.; Lecureur, V. Exposure to diesel exhaust particle extracts (DEPe) impairs some polarization markers and functions of human macrophages through activation of AhR and Nrf2. PLoS ONE 2015, 10, e0116560. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.M.; Antonini, J.M.; Barger, M.W.; Butterworth, L.; Roberts, J.R.; Ma, J.K.H.; Castranova, V.; Ma, J.Y. Diesel exhaust particles suppress macrophage function and slow the pulmonary clearance of Listeria monocytogenes in rats. Environ. Health Perspect. 2001, 109, 515–521. [Google Scholar] [CrossRef] [PubMed]
- Lawal, A.O. Diesel Exhaust Particles and the Induction of Macrophage Activation and Dysfunction. Inflammation 2018, 41, 356–363. [Google Scholar] [CrossRef] [PubMed]
- Dewhurst, J.A.; Lea, S.; Hardaker, E.; Dungwa, J.V.; Ravi, A.K.; Singh, D. Characterisation of lung macrophage subpopulations in COPD patients and controls. Sci. Rep. 2017, 7, 7143. [Google Scholar] [CrossRef]
- Desch, A.N.; Gibbings, S.L.; Goyal, R.; Kolde, R.; Bednarek, J.; Bruno, T.; Slansky, J.E.; Jacobelli, J.; Mason, R.; Ito, Y.; et al. Flow cytometric analysis of mononuclear phagocytes in nondiseased human lung and lung-draining lymph nodes. Am. J. Respir. Crit. Care Med. 2016, 193, 614–626. [Google Scholar] [CrossRef]
- Hoeffel, G.; Chen, J.; Lavin, Y.; Low, D.; Almeida, F.F.; See, P.; Beaudin, A.E.; Lum, J.; Low, I.; Forsberg, E.C.; et al. C-Myb+ Erythro-Myeloid Progenitor-Derived Fetal Monocytes Give Rise to Adult Tissue-Resident Macrophages. Immunity 2015, 42, 665–678. [Google Scholar] [CrossRef]
- Zasłona, Z.; Przybranowski, S.; Wilke, C.; van Rooijen, N.; Teitz-Tennenbaum, S.; Osterholzer, J.J.; Wilkinson, J.E.; Moore, B.B.; Peters-Golden, M. Resident Alveolar Macrophages Suppress, whereas Recruited Monocytes Promote, Allergic Lung Inflammation in Murine Models of Asthma. J. Immunol. 2014, 193, 4245–4253. [Google Scholar] [CrossRef] [PubMed]
- Hou, F.; Xiao, K.; Tang, L.; Xie, L. Diversity of Macrophages in Lung Homeostasis and Diseases. Front. Immunol. 2021, 12, 753940. [Google Scholar] [CrossRef] [PubMed]
- Coillard, A.; Segura, E. In vivo differentiation of human monocytes. Front. Immunol. 2019, 10, 1907. [Google Scholar] [CrossRef] [PubMed]
- Wynn, T.A.; Vannella, K.M. Macrophages in Tissue Repair, Regeneration, and Fibrosis. Immunity 2016, 44, 450–462. [Google Scholar] [CrossRef]
- Eguíluz-Gracia, I.; Bosco, A.; Dollner, R.; Melum, G.R.; Lexberg, M.H.; Jones, A.C.; Dheyauldeen, S.A.; Holt, P.G.; Bækkevold, E.S.; Jahnsen, F.L. Rapid recruitment of CD14+ monocytes in experimentally induced allergic rhinitis in human subjects. J. Allergy Clin. Immunol. 2016, 137, 1872–1881.e12. [Google Scholar] [CrossRef] [PubMed]
- Jones, G.R.; Bain, C.C.; Fenton, T.M.; Kelly, A.; Brown, S.L.; Ivens, A.C.; Travis, M.A.; Cook, P.C.; MacDonald, A.S. Dynamics of colon monocyte and macrophage activation during colitis. Front. Immunol. 2018, 9, 2764. [Google Scholar] [CrossRef] [PubMed]
- Bonato, M.; Gallo, E.; Bazzan, E.; Marson, G.; Zagolin, L.; Cosio, M.G.; Barbato, A.; Saetta, M.; Gregori, D.; Baraldo, S. Air pollution relates to airway pathology in children with wheezing. Ann. Am. Thorac. Soc. 2021, 18, 2033–2040. [Google Scholar] [CrossRef] [PubMed]
- Mellman, I. Dendritic cells: Master regulators of the immune response. Cancer Immunol. Res. 2013, 1, 145–149. [Google Scholar] [CrossRef] [PubMed]
- Fucikova, J.; Palova-Jelinkova, L.; Bartunkova, J.; Spisek, R. Induction of tolerance and immunity by dendritic cells: Mechanisms and clinical applications. Front. Immunol. 2019, 10, 2393. [Google Scholar] [CrossRef]
- Castañeda, A.; Pinkerton, K.; Bein, K.; Magaña-Méndez, A.; Yang, H.; Ashwood, P.; Vogel, C.F.A. Ambient particulate matter activates the aryl hydrocarbon receptor in dendritic cells and enhances Th17 polarization Alejandro. Physiol. Behav. 2018, 292, 85–96. [Google Scholar]
- Brandt, E.B.; Kovacic, M.B.; Lee, G.B.; Aaron, M.; Acciani, T.H.; Le Cras, T.D.; Ryan, P.H.; Budelsky, A.L.; Hershey, G.K.K. Diesel exhaust particle induction of IL17A contributes to severe asthma. J. Allergy Clin. Immunol. 2013, 132, 1194–1204.e2. [Google Scholar] [CrossRef] [PubMed]
- Collin, M.; Bigley, V. Human dendritic cell subsets: An update. Immunology 2018, 154, 3–20. [Google Scholar] [CrossRef] [PubMed]
- Kubesch, N.J.; De Nazelle, A.; Westerdahl, D.; Martinez, D.; Carrasco-Turigas, G.; Bouso, L.; Guerra, S.; Nieuwenhuijsen, M.J. Respiratory and inflammatory responses to short-term exposure to traffic-related air pollution with and without moderate Physical activity. Occup. Environ. Med. 2015, 72, 284–293. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.P.; Zhang, X.; Duan, H.W.; Meng, T.; Niu, Y. Long-term exposure to diesel engine exhaust induced lung function decline in a cross sectional study. Ind. Health 2017, 55, 13–26. [Google Scholar] [CrossRef] [PubMed]
- Paulin, L.; Hansel, N. Particulate air pollution and impaired lung function. F1000Research 2016, 5, F1000 Faculty Rev-201. [Google Scholar] [CrossRef] [PubMed]
- Egger, C.; Gerard, C.; Vidotto, N.; Accart, N.; Cannet, C.; Dunbar, A.; Tigani, B.; Piaia, A.; Jarai, G.; Jarman, E.; et al. Lung volume quantified by MRI reflects extracellular-matrix deposition and altered pulmonary function in bleomycin models of fibrosis: Effects of SOM230. Am. J. Physiol.—Lung Cell. Mol. Physiol. 2014, 306, L1064–L1077. [Google Scholar] [CrossRef]
- Southam, D.S.; Dolovich, M.; O’Byrne, P.M.; Inman, M.D. Distribution of intranasal instillations in mice: Effects of volume, time, body position, and anesthesia. Am. J. Physiol.—Lung Cell. Mol. Physiol. 2002, 282, 833–839. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Soler-Segovia, D.; de Homdedeu, M.; Sánchez-Díez, S.; Romero-Mesones, C.; Espejo, D.; Marain, F.; Vanoirbeek, J.; Munoz, X.; Cruz, M.-J. Immunological Effects of Diesel Particles in a Murine Model of Healthy Mice. Toxics 2024, 12, 530. https://doi.org/10.3390/toxics12080530
Soler-Segovia D, de Homdedeu M, Sánchez-Díez S, Romero-Mesones C, Espejo D, Marain F, Vanoirbeek J, Munoz X, Cruz M-J. Immunological Effects of Diesel Particles in a Murine Model of Healthy Mice. Toxics. 2024; 12(8):530. https://doi.org/10.3390/toxics12080530
Chicago/Turabian StyleSoler-Segovia, David, Miquel de Homdedeu, Silvia Sánchez-Díez, Christian Romero-Mesones, David Espejo, Fopke Marain, Jeroen Vanoirbeek, Xavier Munoz, and María-Jesús Cruz. 2024. "Immunological Effects of Diesel Particles in a Murine Model of Healthy Mice" Toxics 12, no. 8: 530. https://doi.org/10.3390/toxics12080530




