Actin Dysregulation Mediates Nephrotoxicity of Cassiae Semen Aqueous Extracts
Abstract
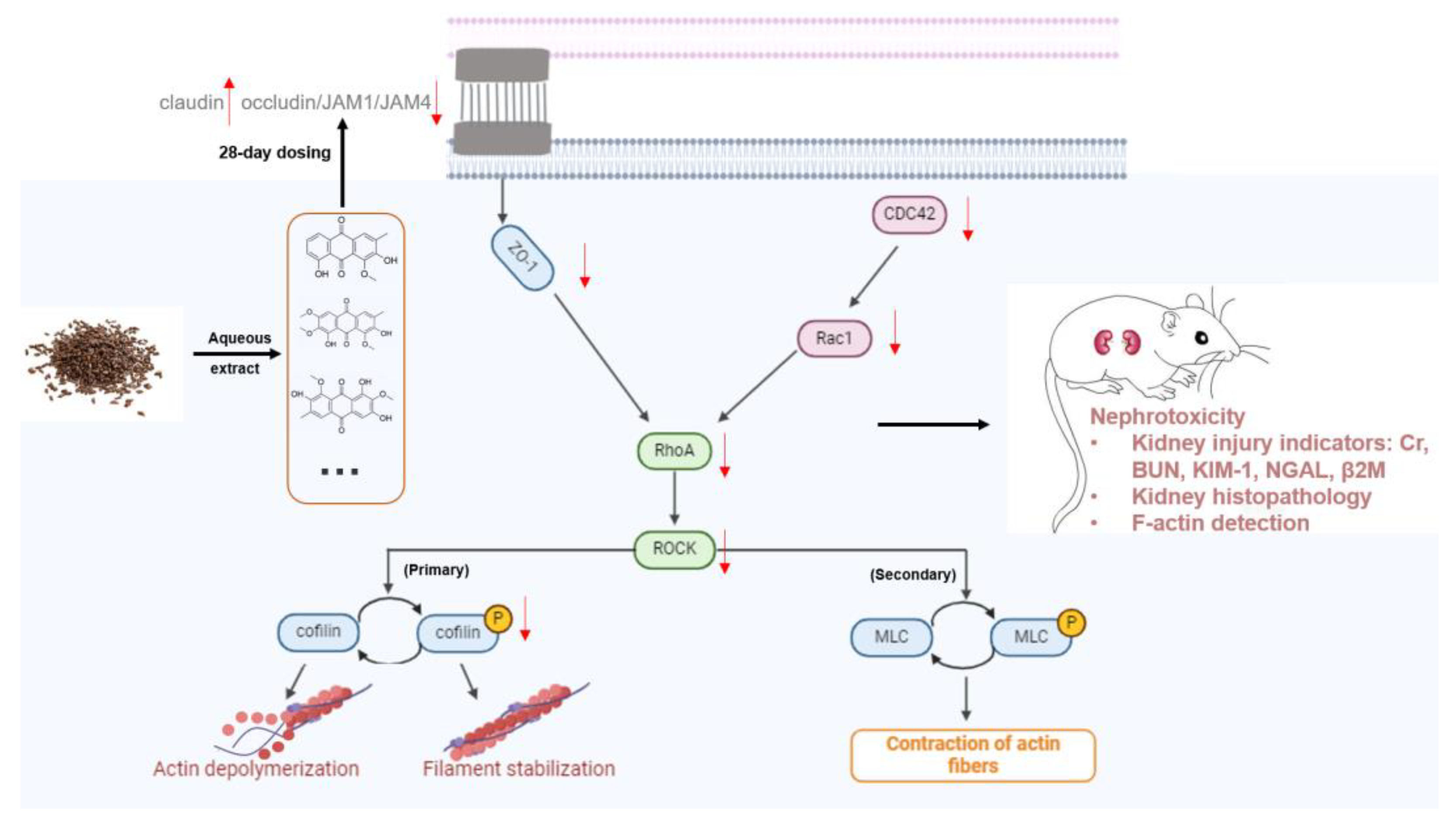
1. Introduction
2. Materials and Methods
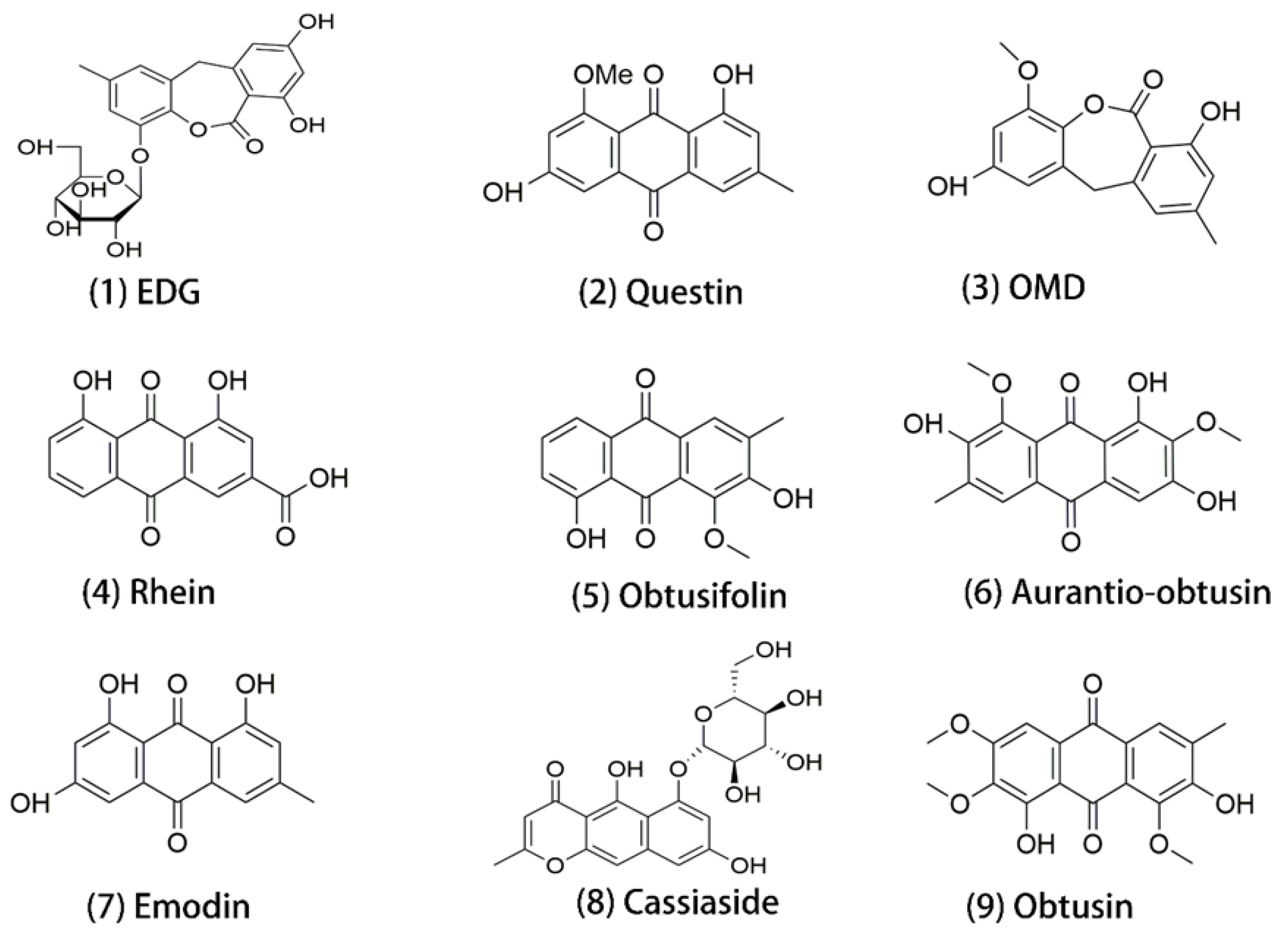
2.1. Chemicals and Materials
2.2. Preparation of Cassiae Semen Aqueous Extracts (CSAEs)
2.3. Animal Treatment
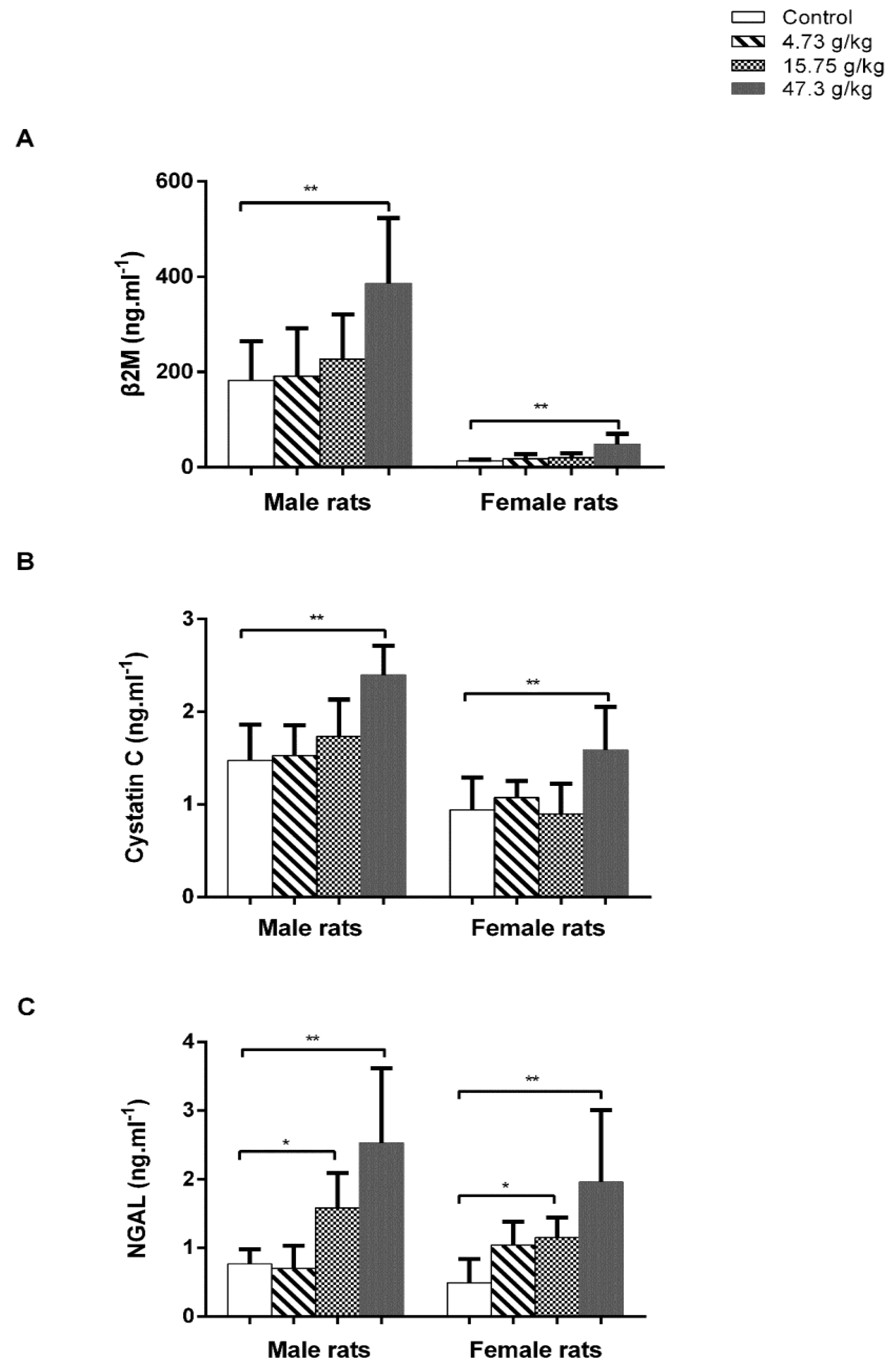
2.4. Serum Biochemical Parameters
2.5. Urinalysis
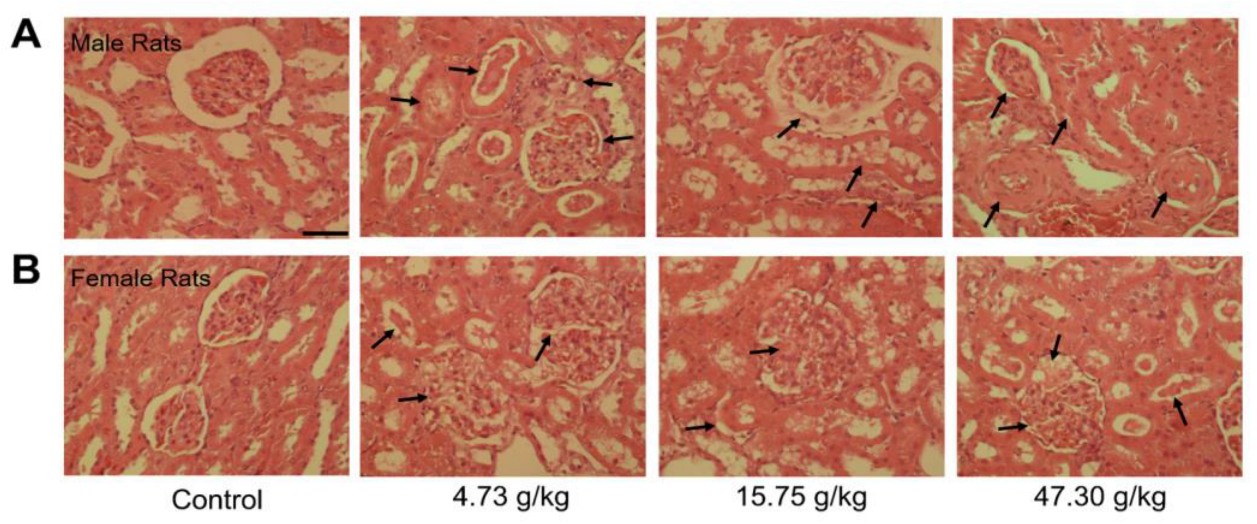
2.6. Histopathological Examinations
2.7. Fluorescent Staining of Cellular Cytoskeleton
2.8. Molecular Docking
2.9. RNA Isolation and RT-qPCR
2.10. Western Blotting
2.11. Statistical Analysis
3. Results
3.1. Administration of CSAEs Induces Nephrotoxicity in Rats
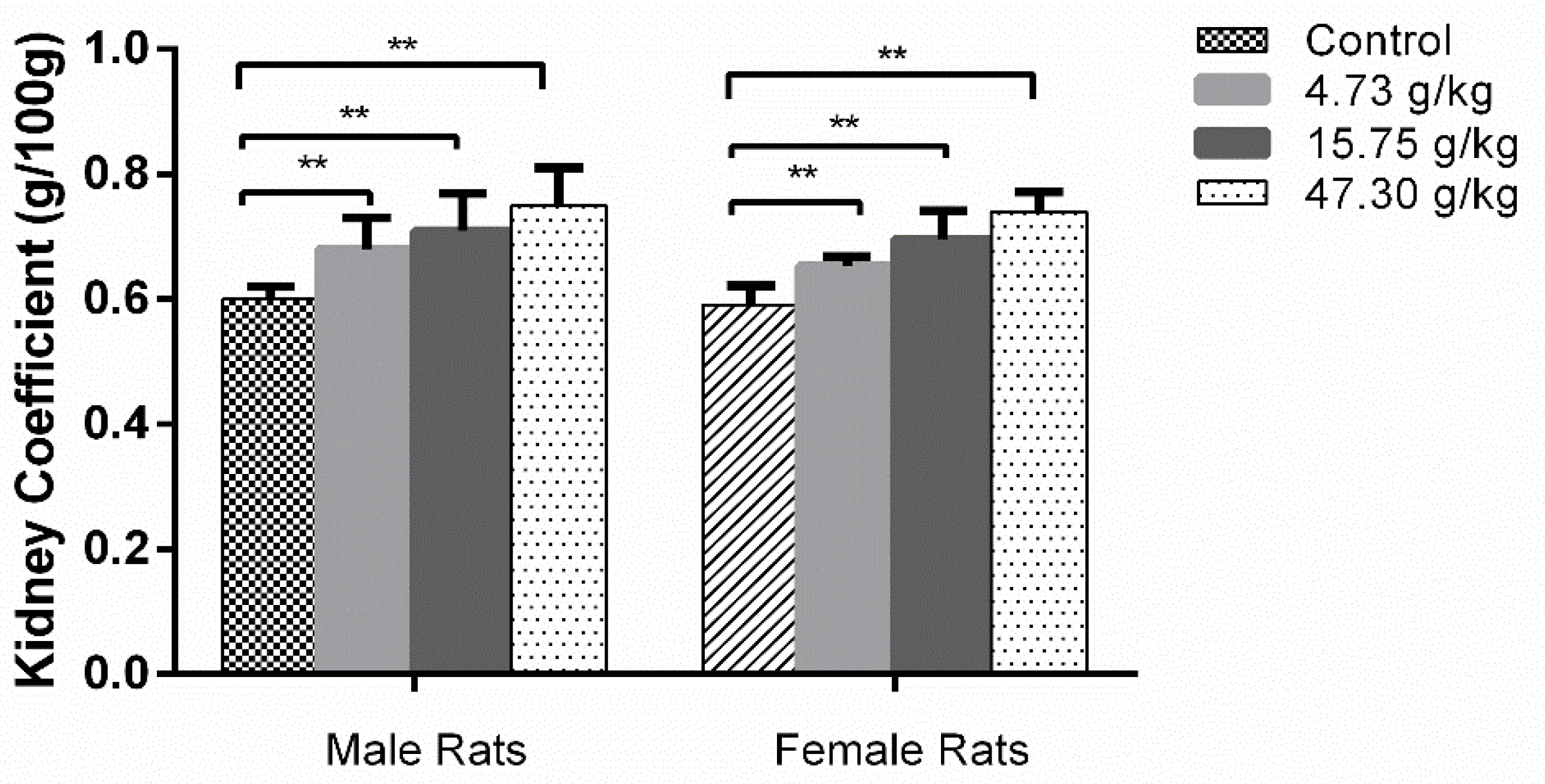
3.2. Changes in Organ Coefficients and Histopathology by CSAEs Administration
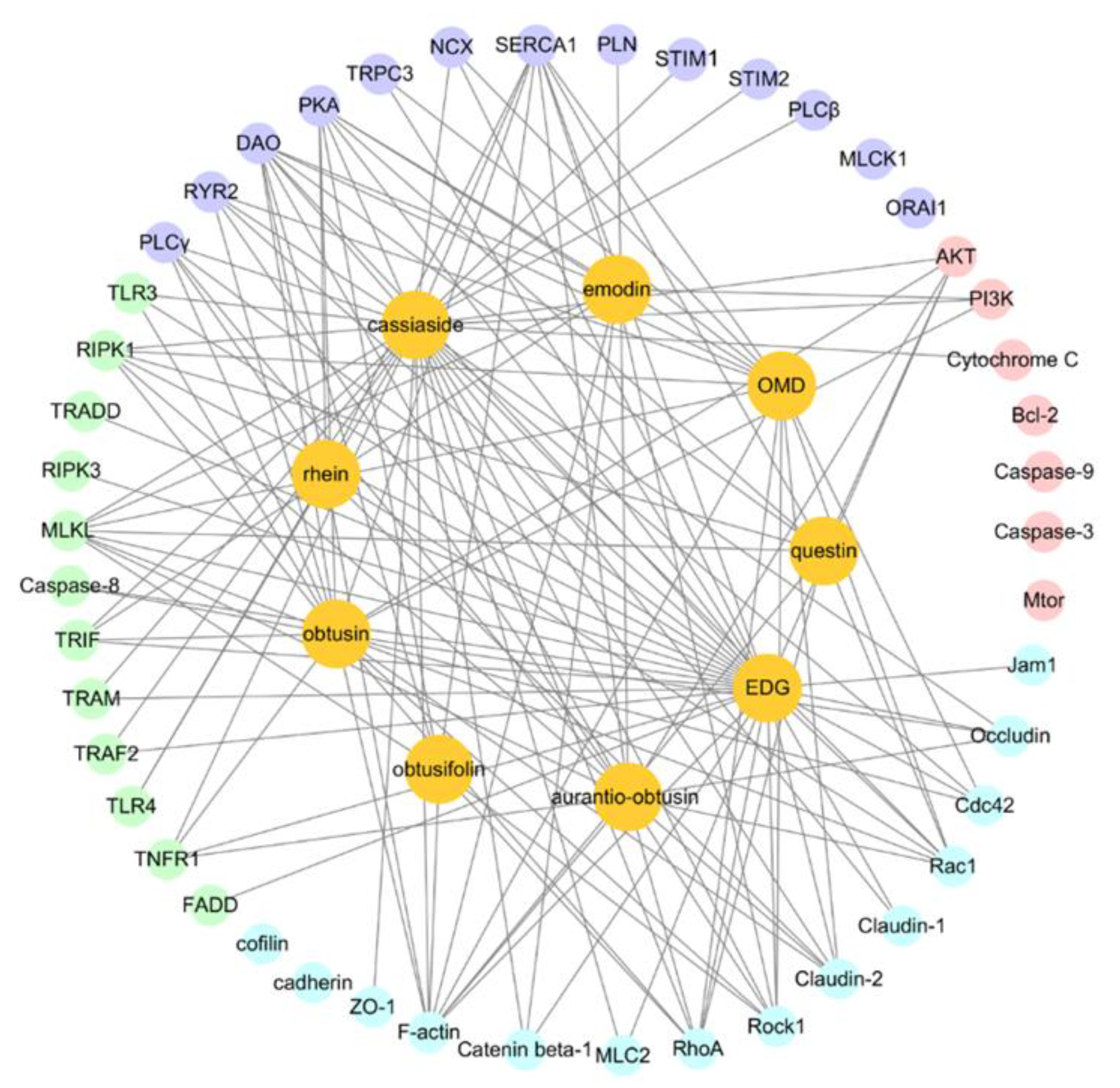
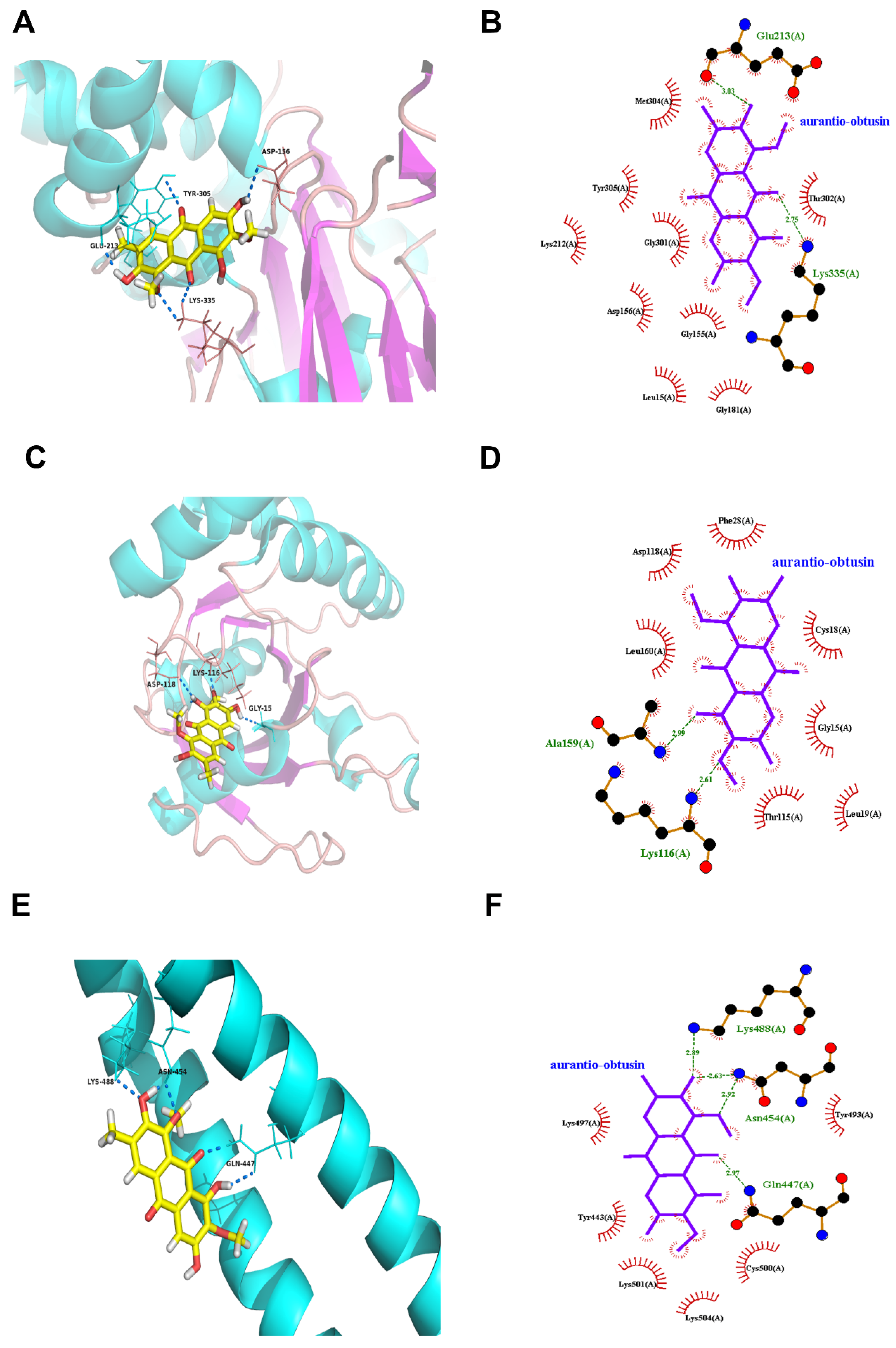
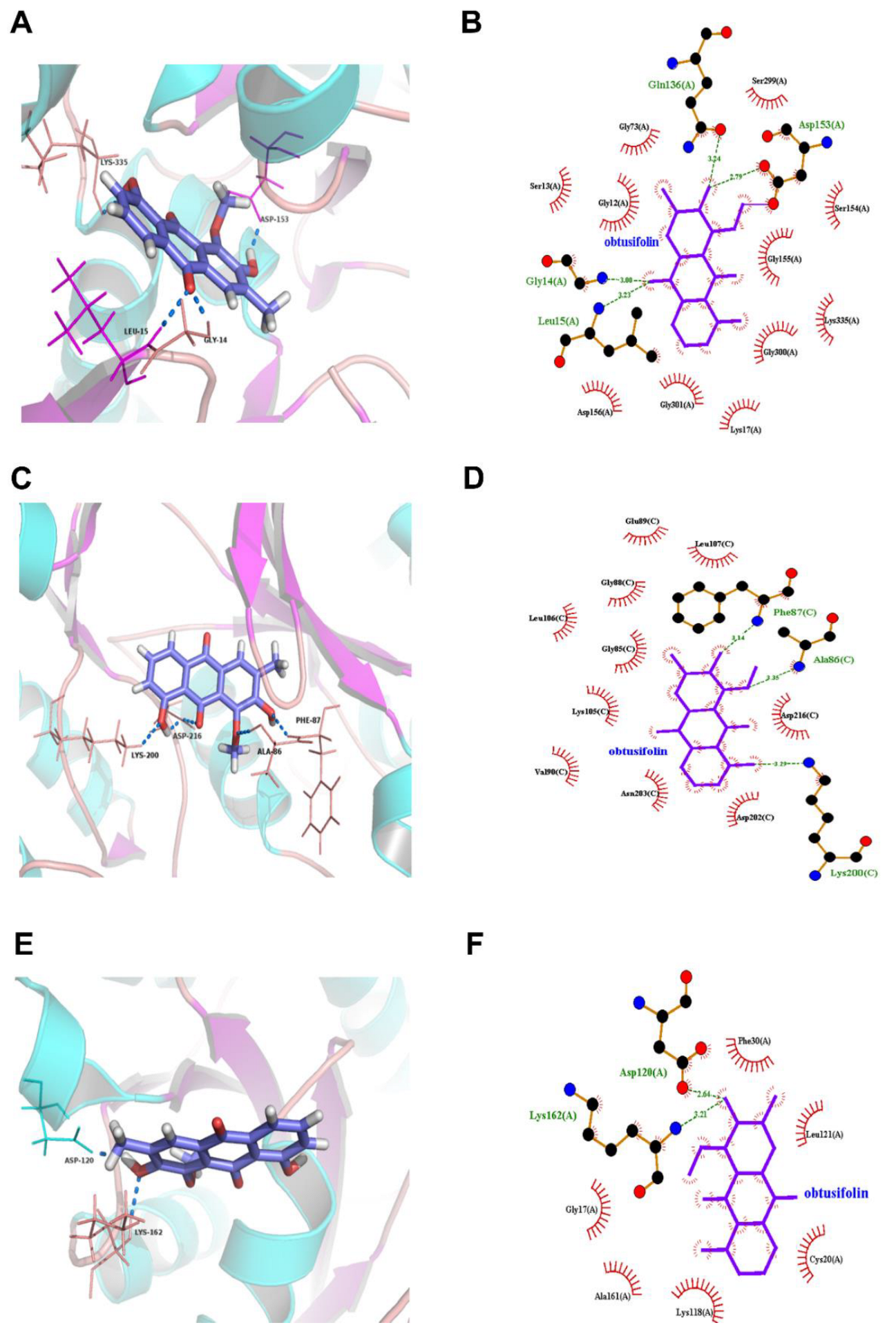
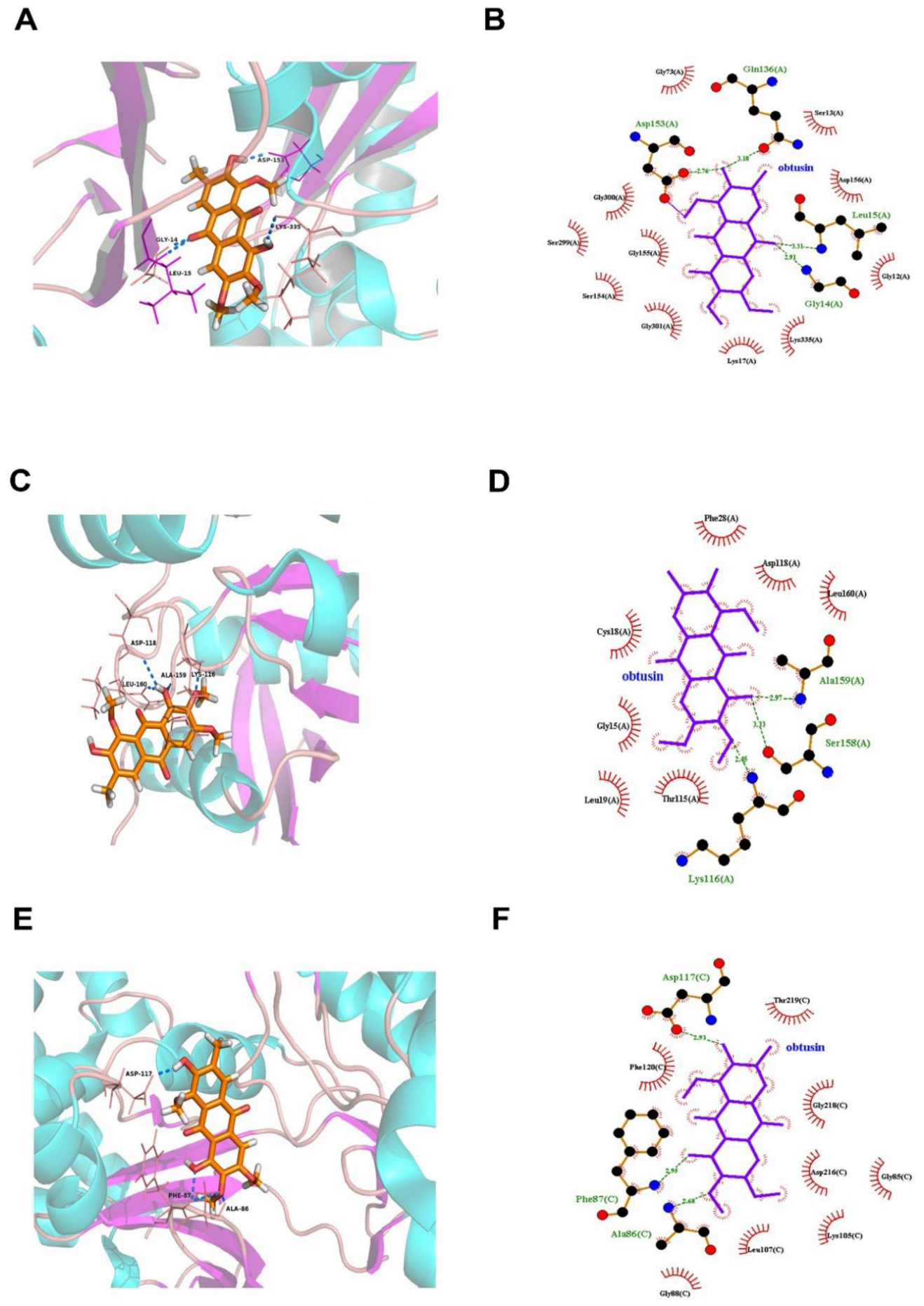
3.3. Molecular Docking Predicts the Potential Mechanisms of CSAEs-Induced Kidney Injury
3.4. F-Actin Protein Expression Was Inhibited in the Kidneys of CSAEs-Treated Rats
3.5. The RhoA–ROCK Pathway Is Inhibited by CSAEs
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dong, X.; Fu, J.; Yin, X.; Yang, C.; Zhang, X.; Wang, W.; Du, X.; Wang, Q.; Ni, J. Cassiae semen: A review of its phytochemistry and pharmacology (Review). Mol. Med. Rep. 2017, 16, 2331–2346. [Google Scholar] [CrossRef]
- Yuen, H.; Hong Yang, A.W.; Hung, A.; Lenon, G.B. How does traditional knowledge of Cassiae semen shed light on weight management?—A classical and modern literature review. J. Ethnopharmacol. 2021, 268, 113572. [Google Scholar] [CrossRef]
- Xie, W.; Zhao, Y.; Du, L. Emerging approaches of traditional Chinese medicine formulas for the treatment of hyperlipidemia. J. Ethnopharmacol. 2012, 140, 345–367. [Google Scholar] [CrossRef]
- Chen, Y.; Chen, X.; Yang, X.; Gao, P.; Yue, C.; Wang, L.; Wu, T.; Jiang, T.; Wu, H.; Tang, L.; et al. Cassiae Semen: A comprehensive review of botany, traditional use, phytochemistry, pharmacology, toxicity, and quality control. J. Ethnopharmacol. 2023, 306, 116199. [Google Scholar] [CrossRef]
- Gao, P.; Sui, H.; Liu, H.B.; Zhi, Y.; Wang, H.L.; Yang, H.; Yu, Z.; Liu, C.X. A 90-day subchronic toxicity study on Semen Cassiae ethanol extract. Chin. J. Food Hyg. 2004, 16, 410–415. [Google Scholar]
- Pei, Y.; Wei, R.; Sun, J.; Gao, H. Safety assessment of freeze-dried powdered Cassiae Semen: Evaluation of chronic toxicity (26-week) in Sprague-Dawley rats. Toxicol. Rep. 2017, 4, 143–150. [Google Scholar] [CrossRef]
- Yang, J.; Zhu, A.; Xiao, S.; Zhang, T.; Wang, L.; Wang, Q.; Han, L. Anthraquinones in the aqueous extract of Cassiae semen cause liver injury in rats through lipid metabolism disorder. Phytomedicine 2019, 64, 153059. [Google Scholar] [CrossRef]
- Hu, M.; Zhong, Y.; Liu, J.; Zheng, S.; Lin, L.; Lin, X.; Liang, B.; Huang, Y.; Xian, H.; Li, Z.; et al. An adverse outcome pathway-based approach to assess aurantio-obtusin-induced hepatotoxicity. Toxicology 2022, 478, 153293. [Google Scholar] [CrossRef]
- Qin, S.-H.; Xu, Y.; Li, K.-L.; Gong, K.-Y.; Peng, J.; Shi, S.-L.; Yan, F.; Cai, W. Identification of Metabolites of Aurantio-Obtusin in Rats Using Ultra-High-Performance Liquid Chromatography-Q-Exactive Orbitrap Mass Spectrometry with Parallel Reaction Monitoring. J. Anal. Methods Chem. 2021, 2021, 6630604. [Google Scholar] [CrossRef]
- Huang, Z.; Sun, Q.; Hao, W.; Zhao, J. Pharmacokinetics and tissue distribution study of obtusifolin in rats by liquid chromatography-tandem mass spectrometry. Biomed. Chromatogr. 2021, 35, e5009. [Google Scholar] [CrossRef]
- Yang, C.; Wang, S.; Guo, X.; Sun, J.; Liu, L.; Wu, L. Simultaneous determination of seven anthraquinones in rat plasma by Ultra High Performance Liquid Chromatography-tandem Mass Spectrometry and pharmacokinetic study after oral administration of Semen Cassiae extract. J. Ethnopharmacol. 2015, 169, 305–313. [Google Scholar] [CrossRef]
- Luyckx, V.A. Nephrotoxicity of alternative medicine practice. Adv. Chronic Kidney Dis. 2012, 19, 129–141. [Google Scholar] [CrossRef]
- Yang, B.; Xie, Y.; Guo, M.; Rosner, M.H.; Yang, H.; Ronco, C. Nephrotoxicity and Chinese Herbal Medicine. Clin. J. Am. Soc. Nephrol. 2018, 13, 1605–1611. [Google Scholar] [CrossRef]
- Mally, A.; Jarzina, S. Mapping Adverse Outcome Pathways for Kidney Injury as a Basis for the Development of Mechanism-Based Animal-Sparing Approaches to Assessment of Nephrotoxicity. Front. Toxicol. 2022, 4, 863643. [Google Scholar] [CrossRef]
- Dominguez, R.; Holmes, K.C. Actin structure and function. Annu. Rev. Biophys. 2011, 40, 169–186. [Google Scholar] [CrossRef]
- Descazeaud, V.; Mestre, E.; Marquet, P.; Essig, M. Calcineurin regulation of cytoskeleton organization: A new paradigm to analyse the effects of calcineurin inhibitors on the kidney. J. Cell. Mol. Med. 2012, 16, 218–227. [Google Scholar] [CrossRef]
- Burridge, K.; Wennerberg, K. Rho and Rac take center stage. Cell 2004, 116, 167–179. [Google Scholar] [CrossRef]
- Cai, R.; Wang, Y.; Huang, Z.; Zou, Q.; Pu, Y.; Yu, C.; Cai, Z. Role of RhoA/ROCK signaling in Alzheimer’s disease. Behav. Brain Res. 2021, 414, 113481. [Google Scholar] [CrossRef]
- Shi, J.; Wei, L. Rho kinase in the regulation of cell death and survival. Arch. Immunol. Ther. Exp. 2007, 55, 61–75. [Google Scholar] [CrossRef]
- Tomasella, A.; Blangy, A.; Brancolini, C. A receptor-interacting protein 1 (RIP1)-independent necrotic death under the control of protein phosphatase PP2A that involves the reorganization of actin cytoskeleton and the action of cofilin-1. J. Biol. Chem. 2014, 289, 25699–25710. [Google Scholar] [CrossRef]
- Lee, J.Y.; Lee, J.; Lee, S.H.; Hwang, J.H.; Suh, H.N. Pelargonium sidoides extract mediates nephrotoxicity through mitochondrial malfunction and cytoskeleton destabilization. Toxicol. Res. 2023, 39, 601–609. [Google Scholar] [CrossRef]
- Bellomo, R.; Ronco, C.; Kellum, J.A.; Mehta, R.L.; Palevsky, P. Acute renal failure—Definition, outcome measures, animal models, fluid therapy and information technology needs: The Second International Consensus Conference of the Acute Dialysis Quality Initiative (ADQI) Group. Crit. Care 2004, 8, R204–R212. [Google Scholar] [CrossRef]
- Tsuji, S.; Sugiura, M.; Tsutsumi, S.; Yamada, H. Sex differences in the excretion levels of traditional and novel urinary biomarkers of nephrotoxicity in rats. J. Toxicol. Sci. 2017, 42, 615–627. [Google Scholar] [CrossRef]
- Bamburg, J.R.; Bernstein, B.W. Roles of ADF/cofilin in actin polymerization and beyond. F1000 Biol. Rep. 2010, 2, 62. [Google Scholar] [CrossRef]
- Hartsock, A.; Nelson, W.J. Adherens and tight junctions: Structure, function and connections to the actin cytoskeleton. Biochim. Biophys. Acta 2008, 1778, 660–669. [Google Scholar] [CrossRef]
- Lee, M.-J.; Nho, J.-H.; Yang, B.-D.; Park, H.; Lee, H.-J.; Lee, K.-H.; Jang, J.-H.; Jung, H.-K.; Kim, S.-R.; Cho, H.-W.; et al. Subchronic toxicity evaluation of ethanol extract of Cassia tora L. seeds in rats. Regul. Toxicol. Pharmacol. 2019, 109, 104487. [Google Scholar] [CrossRef]
- Brown, J.W.; McKnight, C.J. Molecular model of the microvillar cytoskeleton and organization of the brush border. PLoS ONE 2010, 5, e9406. [Google Scholar] [CrossRef]
- Huang, Q.; Fan, M.; Ji, F.; Wang, Y.; Ding, H.; Xu, J.; Wang, X.; Liu, B.; Wang, B.; Yu, X.; et al. The safety evaluation of Shenze Shugan capsule and mechanism of apoptosis induced by five potentially nephrotoxic components. J. Ethnopharmacol. 2024, 324, 117777. [Google Scholar] [CrossRef]
- Silva, J.P.; Araújo, A.M.; de Pinho, P.G.; Carmo, H.; Carvalho, F. Synthetic Cannabinoids JWH-122 and THJ-2201 Disrupt Endocannabinoid-Regulated Mitochondrial Function and Activate Apoptotic Pathways as a Primary Mechanism of In Vitro Nephrotoxicity at In Vivo Relevant Concentrations. Toxicol. Sci. 2019, 169, 422–435. [Google Scholar] [CrossRef]
- Chen, K.; Zhang, W.; Chen, J.; Li, S.; Guo, G. Rho-associated protein kinase modulates neurite extension by regulating microtubule remodeling and vinculin distribution. Neural Regen. Res. 2013, 8, 3027–3035. [Google Scholar] [CrossRef]
- Strassheim, D.; Gerasimovskaya, E.; Irwin, D.; Dempsey, E.C.; Stenmark, K.; Karoor, V. RhoGTPase in Vascular Disease. Cells 2019, 8, 551. [Google Scholar] [CrossRef]
- Budzyn, K.; Marley, P.D.; Sobey, C.G. Targeting Rho and Rho-kinase in the treatment of cardiovascular disease. Trends Pharmacol. Sci. 2006, 27, 97–104. [Google Scholar] [CrossRef]
- Riento, K.; Ridley, A.J. Rocks: Multifunctional kinases in cell behaviour. Nat. Rev. Mol. Cell Biol. 2003, 4, 446–456. [Google Scholar] [CrossRef]
- Williams, J.M.; Johnson, A.C.; Stelloh, C.; Dreisbach, A.W.; Franceschini, N.; Regner, K.R.; Townsend, R.R.; Roman, R.J.; Garrett, M.R. Genetic variants in Arhgef11 are associated with kidney injury in the Dahl salt-sensitive rat. Hypertension 2012, 60, 1157–1168. [Google Scholar] [CrossRef]
- Moreno, C.A.; Sobreira, N.; Pugh, E.; Zhang, P.; Steel, G.; Torres, F.R.; Cavalcanti, D.P. Homozygous deletion in MYL9 expands the molecular basis of megacystis-microcolon-intestinal hypoperistalsis syndrome. Eur. J. Hum. Genet. 2018, 26, 669–675. [Google Scholar] [CrossRef]
- Andrianantoandro, E.; Pollard, T.D. Mechanism of actin filament turnover by severing and nucleation at different concentrations of ADF/cofilin. Mol. Cell 2006, 24, 13–23. [Google Scholar] [CrossRef]
- Qiao, Y.; Chen, J.; Lim, Y.B.; Finch-Edmondson, M.L.; Seshachalam, V.P.; Qin, L.; Jiang, T.; Low, B.C.; Singh, H.; Lim, C.T.; et al. YAP Regulates Actin Dynamics through ARHGAP29 and Promotes Metastasis. Cell Rep. 2017, 19, 1495–1502. [Google Scholar] [CrossRef]
- Chen, B.; Lin, W.; Qi, W.; Li, S.; Hong, Z.; Zhao, H. Cofilin Inhibition by Limk1 Reduces Rod Formation and Cell Apoptosis after Ischemic Stroke. Neuroscience 2020, 444, 64–75. [Google Scholar] [CrossRef]
- Bernard, O. Lim kinases, regulators of actin dynamics. Int. J. Biochem. Cell Biol. 2007, 39, 1071–1076. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, J.; Xiao, S.; Li, L.; Zhu, A.; Xiao, W.; Wang, Q. Actin Dysregulation Mediates Nephrotoxicity of Cassiae Semen Aqueous Extracts. Toxics 2024, 12, 556. https://doi.org/10.3390/toxics12080556
Yang J, Xiao S, Li L, Zhu A, Xiao W, Wang Q. Actin Dysregulation Mediates Nephrotoxicity of Cassiae Semen Aqueous Extracts. Toxics. 2024; 12(8):556. https://doi.org/10.3390/toxics12080556
Chicago/Turabian StyleYang, Jinlan, Sheng Xiao, Ludi Li, An Zhu, Wusheng Xiao, and Qi Wang. 2024. "Actin Dysregulation Mediates Nephrotoxicity of Cassiae Semen Aqueous Extracts" Toxics 12, no. 8: 556. https://doi.org/10.3390/toxics12080556
APA StyleYang, J., Xiao, S., Li, L., Zhu, A., Xiao, W., & Wang, Q. (2024). Actin Dysregulation Mediates Nephrotoxicity of Cassiae Semen Aqueous Extracts. Toxics, 12(8), 556. https://doi.org/10.3390/toxics12080556








