Evaluation of THP-1 and Jurkat Cell Lines Coculture for the In Vitro Assessment of the Effects of Immunosuppressive Substances
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Cell Lines and Cell Culture
2.3. Metabolic Activity Assay
2.4. Cell Stimulations and Cytokine Measurements
3. Results
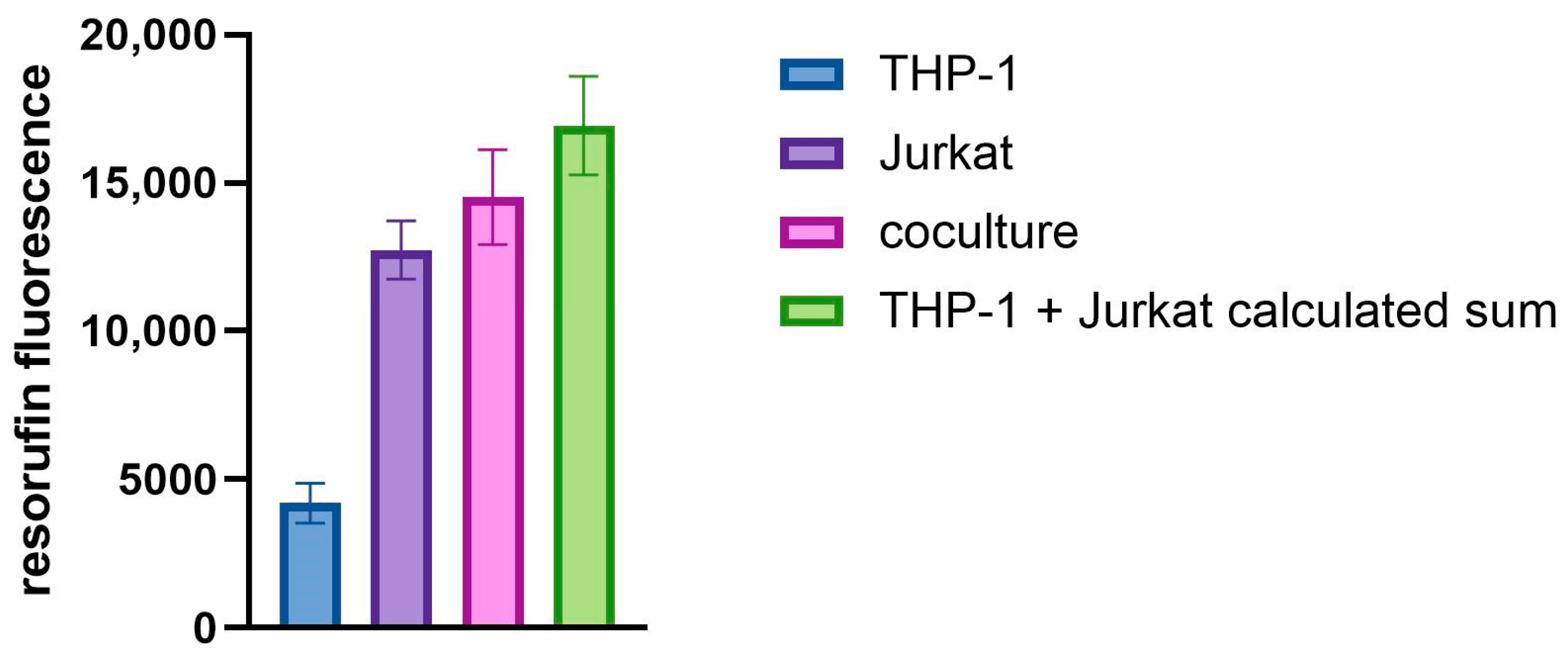
3.1. Cell Culture Viability and Activation
3.2. Effect of Immunosuppressive Drugs on Cytokine Release
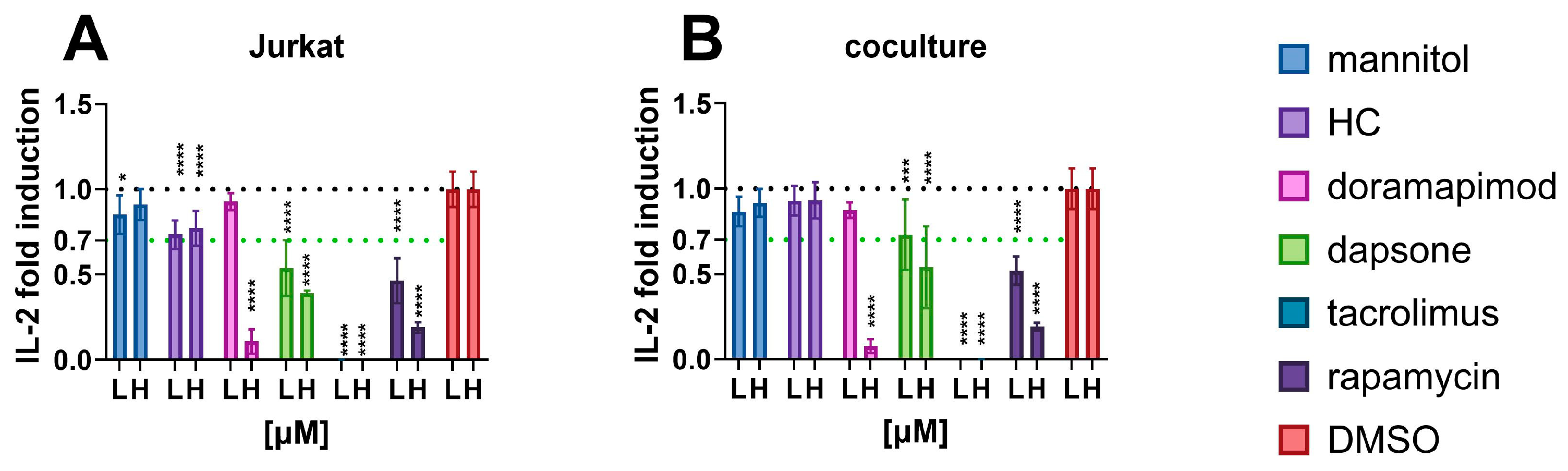
3.2.1. IL-2
3.2.2. TNFα
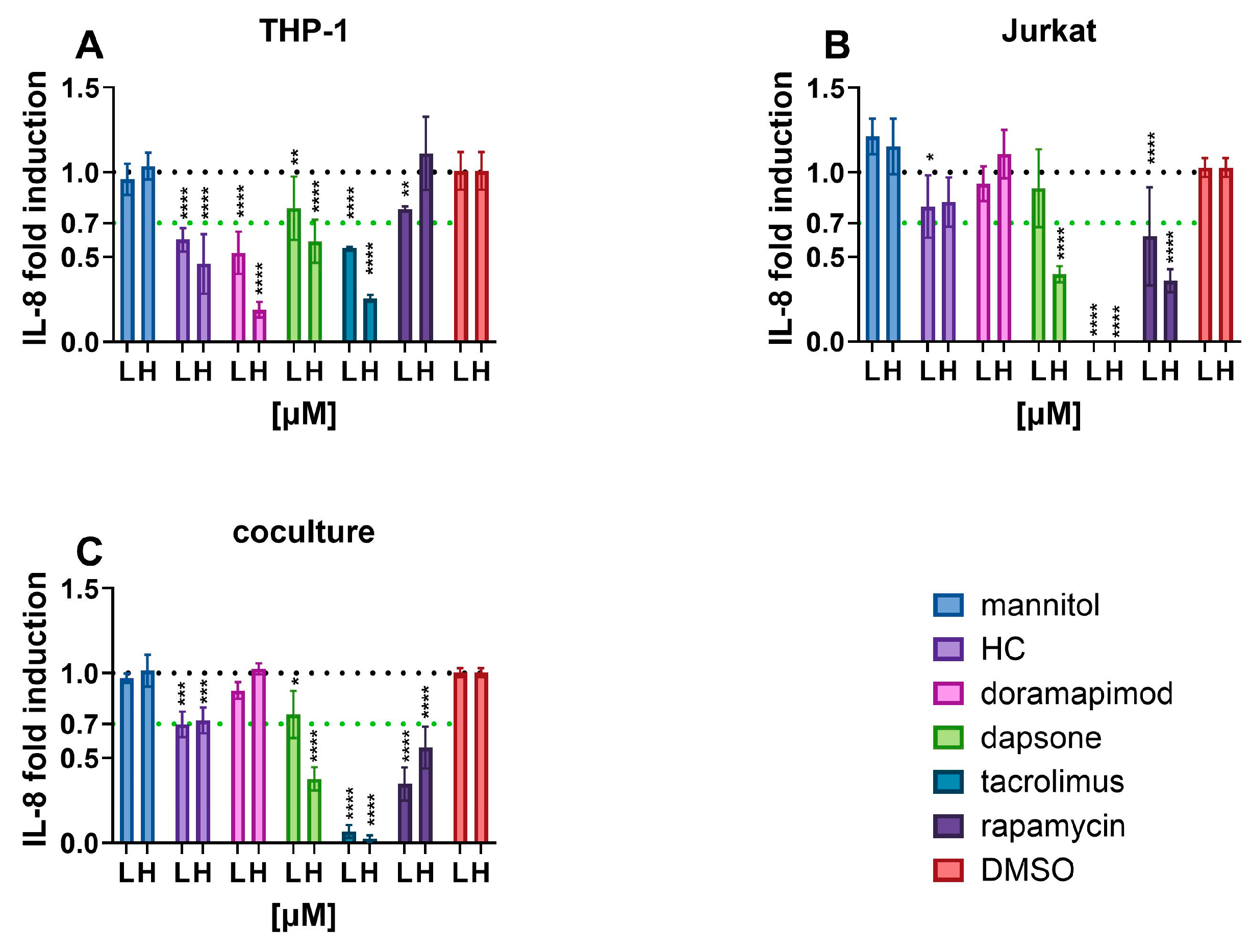
3.2.3. IL-8
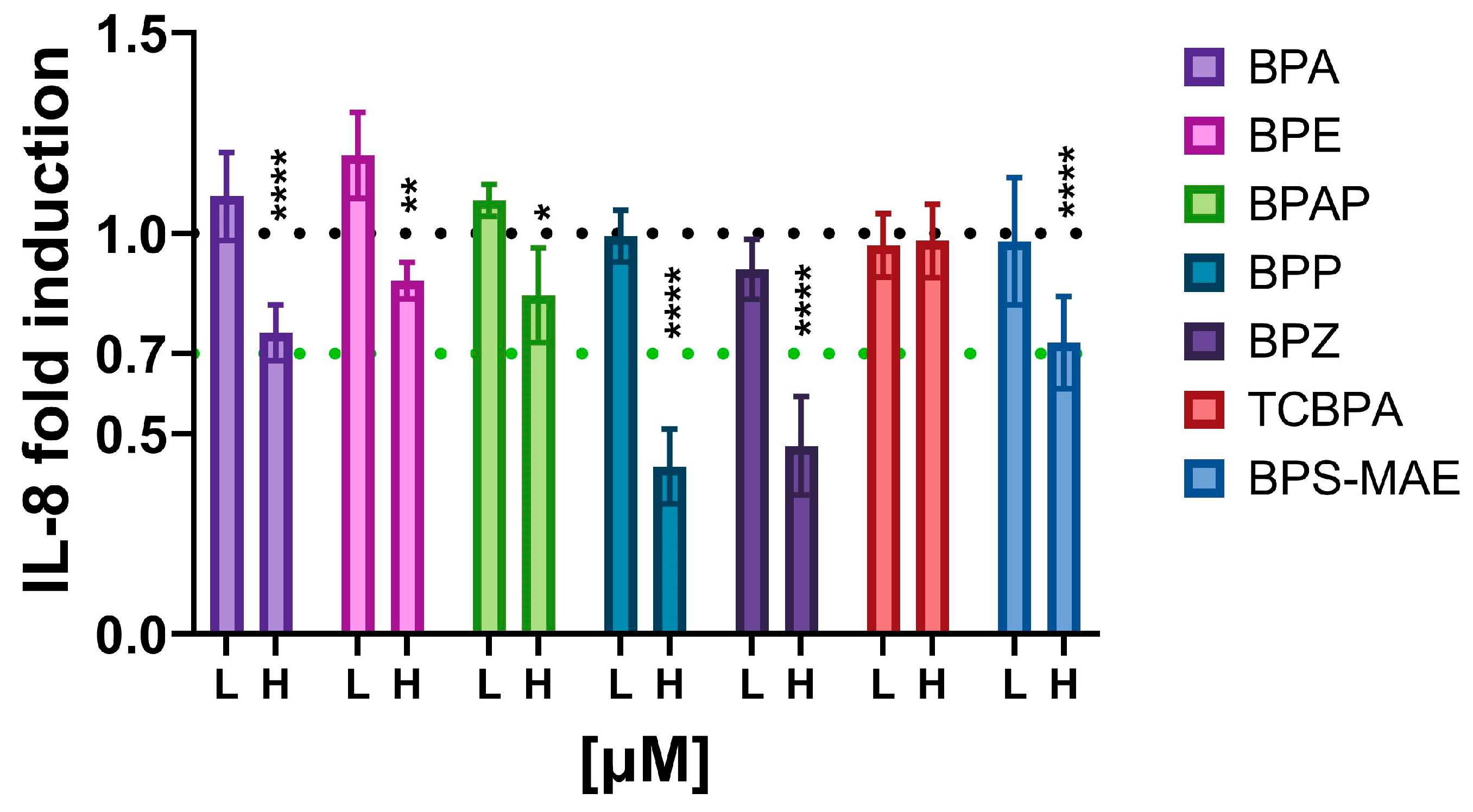
3.3. Evaluation of Immunosuppressive Action from Environmentally Occurring Bisphenols
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chaplin, D.D. Overview of the Immune Response. J. Allergy Clin. Immunol. 2010, 125, S3–S23. [Google Scholar] [CrossRef]
- Corsini, E.; Roggen, E.L. Overview of In Vitro Assessment of Immunotoxicity. Curr. Opin. Toxicol. 2017, 5, 13–18. [Google Scholar] [CrossRef]
- Meneghini, M.; Bestard, O.; Grinyo, J.M. Immunosuppressive Drugs Modes of Action. Best Pract. Res. Clin. Gastroenterol. 2021, 54–55, 101757. [Google Scholar] [CrossRef]
- Panackel, C.; Mathew, J.F.; Fawas, N.M.; Jacob, M. Immunosuppressive Drugs in Liver Transplant: An Insight. J. Clin. Exp. Hepatol. 2022, 12, 1557–1571. [Google Scholar] [CrossRef] [PubMed]
- Corsini, E.; Liesivuori, J.; Vergieva, T.; Loveren, V.; Colosio, C. Effects of Pesticide Exposure on the Human Immune System. Hum. Exp. Toxicol. 2008, 27, 671–680. [Google Scholar] [CrossRef] [PubMed]
- The Capacity of Toxic Agents to Compromise the Immune System (Biologic Markers of Immunosuppression)—Biologic Markers in Immunotoxicology—NCBI Bookshelf. Available online: https://www.ncbi.nlm.nih.gov/books/NBK235670/ (accessed on 1 February 2024).
- Fukuyama, T.; Tajima, Y.; Ueda, H.; Hayashi, K.; Shutoh, Y.; Harada, T.; Kosaka, T. Allergic Reaction Induced by Dermal and/or Respiratory Exposure to Low-Dose Phenoxyacetic Acid, Organophosphorus, and Carbamate Pesticides. Toxicology 2009, 261, 152–161. [Google Scholar] [CrossRef]
- Gennari, A.; Ban, M.; Braun, A.; Casati, S.; Corsini, E.; Dastych, J.; Descotes, J.; Hartung, T.; Hooghe-Peters, R.; House, R.; et al. The Use of In Vitro Systems for Evaluating Immunotoxicity: The Report and Recommendations of an ECVAM Workshop. J. Immunotoxicol. 2005, 2, 61–83. [Google Scholar] [CrossRef]
- OECD TG 444A Guideline for the Testing of Chemicals. Available online: http://www.oecd.org/termsandconditions/ (accessed on 31 January 2024).
- Kimura, Y.; Terui, H.; Fujimura, C.; Amagai, R.; Takahashi, T.; Aiba, S. Optimization of the IL-2 Luc Assay for Immunosuppressive Drugs: A Novel in Vitro Immunotoxicity Test with High Sensitivity and Predictivity. Arch. Toxicol. 2021, 95, 2755–2768. [Google Scholar] [CrossRef]
- Kimura, Y.; Fujimura, C.; Ito, Y.; Takahashi, T.; Aiba, S. Evaluation of the Multi-ImmunoTox Assay Composed of 3 Human Cytokine Reporter Cells by Examining Immunological Effects of Drugs. Toxicol. Vitr. 2014, 28, 759–768. [Google Scholar] [CrossRef]
- Zhao, Y.; Hao, C.; Zhai, R.; Bao, L.; Wang, D.; Li, Y.; Yu, X.; Huang, R.; Yao, W. Effects of Cyclophosphamide on the Phenotypes and Functions of THP-1 Cells. Environ. Toxicol. Pharmacol. 2019, 70, 103201. [Google Scholar] [CrossRef]
- Pierzchalski, A.; Zenclussen, A.C.; Herberth, G. A Comprehensive Battery of Flow Cytometric Immunoassays for the In Vitro Testing of Chemical Effects in Human Blood Cells. Front. Immunol. 2023, 14, 1327960. [Google Scholar] [CrossRef]
- Wang, X.; Li, N.; Ma, M.; Han, Y.; Rao, K. Immunotoxicity In Vitro Assays for Environmental Pollutants under Paradigm Shift in Toxicity Tests. Int. J. Environ. Res. Public Health 2022, 20, 273. [Google Scholar] [CrossRef]
- Snapkow, I.; Smith, N.M.; Arnesdotter, E.; Beekmann, K.; Blanc, E.B.; Braeuning, A.; Corsini, E.; Sollner Dolenc, M.; Duivenvoorde, L.P.M.; Sundstøl Eriksen, G.; et al. New Approach Methodologies to Enhance Human Health Risk Assessment of Immunotoxic Properties of Chemicals—A PARC (Partnership for the Assessment of Risk from Chemicals) Project. Front. Toxicol. 2024, 6, 1339104. [Google Scholar] [CrossRef]
- Lebrec, H.; Roger, R.; Blot, C.; Burleson, G.R.; Bohuon, C.; Pallardy, M. Immunotoxicological Investigation Using Pharmaceutical Drugs. In Vitro Evaluation of Immune Effects Using Rodent or Human Immune Cells. Toxicology 1995, 96, 147–156. [Google Scholar] [CrossRef] [PubMed]
- Markovič, T.; Gobec, M.; Gurwitz, D.; Mlinarič-Raščan, I. Characterization of Human Lymphoblastoid Cell Lines as a Novel in Vitro Test System to Predict the Immunotoxicity of Xenobiotics. Toxicol. Lett. 2015, 233, 8–15. [Google Scholar] [CrossRef]
- Langezaal, I.; Hoffmann, S.; Hartung, T.; Coecke, S. Evaluation and Prevalidation of an Immunotoxicity Test Based on Human Whole-Blood Cytokine Release. Altern. Lab. Anim. 2002, 30, 581–595. [Google Scholar] [CrossRef]
- TSAR In Vitro Pyrogen Test Using Human Whole Blood/IL-1 Beta. Available online: https://tsar.jrc.ec.europa.eu/test-method/tm2002-01 (accessed on 31 January 2024).
- FDA Nonclinical Evaluation of the Immunotoxic Potential of Pharmaceuticals—Guidance for Industry. Available online: https://www.fda.gov/drugs/guidance-compliance-regulatory-information/guidances-drugs (accessed on 31 January 2024).
- Segre, E.; Fullerton, J.N. Stimulated Whole Blood Cytokine Release as a Biomarker of Immunosuppression in the Critically Ill: The Need for a Standardized Methodology. Shock 2016, 45, 490. [Google Scholar] [CrossRef] [PubMed]
- Kimura, Y.; Fujimura, C.; Ito, Y.; Takahashi, T.; Terui, H.; Aiba, S. Profiling the Immunotoxicity of Chemicals Based on in Vitro Evaluation by a Combination of the Multi-ImmunoTox Assay and the IL-8 Luc Assay. Arch. Toxicol. 2018, 92, 2043–2054. [Google Scholar] [CrossRef]
- Kaur, G.; Dufour, J.M. Cell Lines: Valuable Tools or Useless Artifacts. Spermatogenesis 2012, 2, 1–5. [Google Scholar] [CrossRef]
- ATCC THP-1. Available online: https://www.atcc.org/products/tib-202 (accessed on 27 June 2024).
- ATCC Jurkat. Available online: https://www.atcc.org/products/tib-152 (accessed on 27 June 2024).
- BD Biosciences BDTM Cytometric Bead Array (CBA) Human Inflammatory Cytokine Cytometric Bead Array (CBA). Available online: https://www.bdbiosciences.com/en-us/products/reagents/immunoassay-reagents/cba/cba-kits/human-inflammatory-cytokine-cytometric-bead-array-cba-i-kit.551811 (accessed on 27 June 2024).
- Buttle, T.S.; Hummerstone, C.Y.; Billahalli, T.; Ward, R.J.B.; Barnes, K.E.; Marshall, N.J.; Spong, V.C.; Bothamley, G.H. The Monocyte-to-Lymphocyte Ratio: Sex-Specific Differences in the Tuberculosis Disease Spectrum, Diagnostic Indices and Defining Normal Ranges. PLoS ONE 2021, 16, e0247745. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Xu, Y.; Lu, P.; Zhou, H.; Yang, M.; Xiang, L. The Prognostic Value of Monocyte-to-Lymphocyte Ratio in Peritoneal Dialysis Patients. Eur. J. Med. Res. 2023, 28, 152. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Zhang, J.; Zhong, Y.; Mai, Y.; Huang, D.; Wei, W.; Huang, J.; Zhao, P.; Lin, F.; Jin, J. Predictive Value of the Monocyte-to-Lymphocyte Ratio in the Diagnosis of Prostate Cancer. Medicine 2021, 100, e27244. [Google Scholar] [CrossRef]
- Selim, Z.I.; Mostafa, N.M.; Ismael, E.O.; Kamal, D. Platelet Lymphocyte Ratio, Lymphocyte Monocyte Ratio, Mean Platelet Volume, and Neutrophil Lymphocyte Ratio in Behcet’s Disease and Their Relation to Disease Activity. Egypt. Rheumatol. Rehabil. 2023, 50, 1–8. [Google Scholar] [CrossRef]
- Mirna, M.; Schmutzler, L.; Topf, A.; Hoppe, U.C.; Lichtenauer, M. Neutrophil-to-Lymphocyte Ratio and Monocyte-to-Lymphocyte Ratio Predict Length of Hospital Stay in Myocarditis. Sci. Rep. 2021, 11, 18101. [Google Scholar] [CrossRef]
- Gawiński, C.; Michalski, W.; Mróz, A.; Wyrwicz, L. Correlation between Lymphocyte-to-Monocyte Ratio (LMR), Neutrophil-to-Lymphocyte Ratio (NLR), Platelet-to-Lymphocyte Ratio (PLR) and Tumor-Infiltrating Lymphocytes (TILs) in Left-Sided Colorectal Cancer Patients. Biology 2022, 11, 385. [Google Scholar] [CrossRef]
- Moosazadeh, M.; Maleki, I.; Alizadeh-Navaei, R.; Kheradmand, M.; Hedayatizadeh-Omran, A.; Shamshirian, A.; Barzegar, A. Normal Values of Neutrophil-to-Lymphocyte Ratio, Lymphocyte-to-Monocyte Ratio and Platelet-to-Lymphocyte Ratio among Iranian Population: Results of Tabari Cohort. Casp. J. Intern. Med. 2019, 10, 320. [Google Scholar] [CrossRef]
- Goldinger, D.M.; Demierre, A.L.; Zoller, O.; Rupp, H.; Reinhard, H.; Magnin, R.; Becker, T.W.; Bourqui-Pittet, M. Endocrine Activity of Alternatives to BPA Found in Thermal Paper in Switzerland. Regul. Toxicol. Pharmacol. 2015, 71, 453–462. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Chen, M.; Shan, G.; Chen, P.; Cui, S.; Yi, S.; Zhu, L. Bioaccumulation and Biomagnification of Emerging Bisphenol Analogues in Aquatic Organisms from Taihu Lake, China. Sci. Total Environ. 2017, 598, 814–820. [Google Scholar] [CrossRef] [PubMed]
- Siddique, S.; Zhang, G.; Coleman, K.; Kubwabo, C. Investigation of the Migration of Bisphenols from Baby Bottles and Sippy Cups. Curr. Res. Food Sci. 2021, 4, 619–626. [Google Scholar] [CrossRef]
- ECHA. Available online: https://echa.europa.eu/sl/-/new-approach-methodologies-workshop-towards-an-animal-free-regulatory-system-for-industrial-chemicals (accessed on 6 February 2024).
- New Approach Methods Work Plan|US EPA. Available online: https://www.epa.gov/chemical-research/new-approach-methods-work-plan (accessed on 6 February 2024).
- Stucki, A.O.; Barton-Maclaren, T.S.; Bhuller, Y.; Henriquez, J.E.; Henry, T.R.; Hirn, C.; Miller-Holt, J.; Nagy, E.G.; Perron, M.M.; Ratzlaff, D.E.; et al. Use of New Approach Methodologies (NAMs) to Meet Regulatory Requirements for the Assessment of Industrial Chemicals and Pesticides for Effects on Human Health. Front. Toxicol. 2022, 4, 964553. [Google Scholar] [CrossRef]
- Sonnenburg, A.; Stahlmann, R.; Kreutz, R.; Peiser, M. A New Cell Line Based Coculture System for Skin Sensitisation Testing in One Single Assay Using T Cells, Aryl Hydrocarbon Receptor Knockout, and Co-Inhibitory Blockage. Arch. Toxicol. 2023, 97, 1677–1689. [Google Scholar] [CrossRef]
- Lacrazs, S.; Isler, P.; Vey, E.; Welguss, H.G.; Dayem, J.-M. Direct Contact between T Lymphocytes and Monocytes Is a Major Pathway for Induction of Metalloproteinase Expression. J. Biol. Chem. 1994, 269, 22027–22033. [Google Scholar] [CrossRef]
- Park, S.J.; Choi, S.H.; Cho, Y.D.; Kim, J.Y.; Cho, H.J.; Kim, K.H.; Kim, W.Y. Protective Effects of Pentoxifylline on T-Cell Viability under Inflammatory Conditions. Eur. J. Inflamm. 2022, 20, 1721727X221120753. [Google Scholar] [CrossRef]
- Bremner, T.A.; Chatterjee, D.; Han, Z.; Tsan, M.F.; Wyche, J.H. THP-1 Monocytic Leukemia Cells Express Fas Ligand Constitutively and Kill Fas-Positive Jurkat Cells. Leuk. Res. 1999, 23, 865–870. [Google Scholar] [CrossRef]
- Ai, W.; Li, H.; Song, N.; Li, L.; Chen, H. Optimal Method to Stimulate Cytokine Production and Its Use in Immunotoxicity Assessment. Int. J. Environ. Res. Public Health 2013, 10, 3834. [Google Scholar] [CrossRef]
- Fujihara, M.; Muroi, M.; Tanamoto, K.I.; Suzuki, T.; Azuma, H.; Ikeda, H. Molecular Mechanisms of Macrophage Activation and Deactivation by Lipopolysaccharide: Roles of the Receptor Complex. Pharmacol. Ther. 2003, 100, 171–194. [Google Scholar] [CrossRef]
- Kim, Y.K.; Hwang, J.H.; Lee, H.T. Differential Susceptibility to Lipopolysaccharide Affects the Activation of Toll-like-Receptor 4 Signaling in THP-1 Cells and PMA-Differentiated THP-1 Cells. Innate Immun. 2022, 28, 122–129. [Google Scholar] [CrossRef] [PubMed]
- Wolf, B.; Morgan, H.; Krieg, J.; Gani, Z.; Milicov, A.; Warncke, M.; Brennan, F.; Jones, S.; Sims, J.; Kiessling, A. A Whole Blood in Vitro Cytokine Release Assay with Aqueous Monoclonal Antibody Presentation for the Prediction of Therapeutic Protein Induced Cytokine Release Syndrome in Humans. Cytokine 2012, 60, 828–837. [Google Scholar] [CrossRef]
- Duffy, D.; Rouilly, V.; Braudeau, C.; Corbière, V.; Djebali, R.; Ungeheuer, M.N.; Josien, R.; LaBrie, S.T.; Lantz, O.; Louis, D.; et al. Standardized Whole Blood Stimulation Improves Immunomonitoring of Induced Immune Responses in Multi-Center Study. Clin. Immunol. 2017, 183, 325–335. [Google Scholar] [CrossRef]
- Hydrocortisone. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Hydrocortisone#section=Drug-Warnings (accessed on 27 June 2024).
- FDA Dapsone. Available online: https://www.fda.gov/media/131969/download (accessed on 27 June 2024).
- PubChem Sirolimus. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/5284616 (accessed on 27 June 2024).
- Park, Y.J.; Yoo, S.A.; Kim, M.; Kim, W.U. The Role of Calcium–Calcineurin–NFAT Signaling Pathway in Health and Autoimmune Diseases. Front. Immunol. 2020, 11, 195. [Google Scholar] [CrossRef] [PubMed]
- Kaunisto, A.; Henry, W.S.; Montaser-Kouhsari, L.; Jaminet, S.C.; Oh, E.Y.; Zhao, L.; Luo, H.R.; Beck, A.H.; Toker, A. NFAT1 Promotes Intratumoral Neutrophil Infiltration by Regulating IL8 Expression in Breast Cancer. Mol. Oncol. 2015, 9, 1140–1154. [Google Scholar] [CrossRef]
- Falvo, J.V.; Tsytsykova, A.V.; Goldfeld, A.E. Transcriptional Control of the TNF Gene. Curr. Dir. Autoimmun. 2010, 11, 27–60. [Google Scholar] [CrossRef] [PubMed]
- Vitiello, M.; D’Isanto, M.; Galdiero, M.; Raieta, K.; Tortora, A.; Rotondo, P.; Peluso, L.; Galdiero, M. Interleukin-8 Production by THP-1 Cells Stimulated by Salmonella Enterica Serovar Typhimurium Porins Is Mediated by AP-1, NF-ΚB and MAPK Pathways. Cytokine 2004, 27, 15–24. [Google Scholar] [CrossRef] [PubMed]
- He, W.; Qu, T.; Yu, Q.; Wang, Z.; Lv, H.; Zhang, J.; Zhao, X.; Wang, P. LPS Induces IL-8 Expression through TLR4, MyD88, NF-KappaB and MAPK Pathways in Human Dental Pulp Stem Cells. Int. Endod. J. 2013, 46, 128–136. [Google Scholar] [CrossRef] [PubMed]
- Zaman, M.M.; Gelrud, A.; Junaidi, O.; Regan, M.M.; Warny, M.; Shea, J.C.; Kelly, C.; O’Sullivan, B.P.; Freedman, S.D. Interleukin 8 Secretion from Monocytes of Subjects Heterozygous for the ΔF508 Cystic Fibrosis Transmembrane Conductance Regulator Gene Mutation Is Altered. Clin. Diagn. Lab. Immunol. 2004, 11, 819–824. [Google Scholar] [CrossRef] [PubMed]
- Del Arco, P.G.; Martínez-Martínez, S.; Maldonado, J.L.; Ortega-Pérez, I.; Redondo, J.M. A Role for the P38 MAP Kinase Pathway in the Nuclear Shuttling of NFATp. J. Biol. Chem. 2000, 275, 13872–13878. [Google Scholar] [CrossRef] [PubMed]
- Allan, S. Seeing MTOR in a New Light. Nat. Rev. Immunol. 2008, 8, 904. [Google Scholar] [CrossRef]
- Lin, H.Y.H.; Chang, K.T.; Hung, C.C.; Kuo, C.H.; Hwang, S.J.; Chen, H.C.; Hung, C.H.; Lin, S.F. Effects of the MTOR Inhibitor Rapamycin on Monocyte-Secreted Chemokines. BMC Immunol. 2014, 15, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Kanoh, S.; Tanabe, T.; Rubin, B.K. Dapsone Inhibits IL-8 Secretion From Human Bronchial Epithelial Cells Stimulated With Lipopolysaccharide and Resolves Airway Inflammation in the Ferret. Chest 2011, 140, 980–990. [Google Scholar] [CrossRef]
- Schmidt, E.; Reimer, S.; Kruse, N.; Bröcker, E.B.; Zillikens, D. The IL-8 Release from Cultured Human Keratinocytes, Mediated by Antibodies to Bullous Pemphigoid Autoantigen 180, Is Inhibited by Dapsone. Clin. Exp. Immunol. 2002, 124, 157–162. [Google Scholar] [CrossRef]
- Kwon, M.J.; Joo, H.G. Dapsone Modulates Lipopolysaccharide-Activated Bone Marrow Cells by Inducing Cell Death and down-Regulating Tumor Necrosis Factor-α Production. J. Vet. Sci. 2018, 19, 744–749. [Google Scholar] [CrossRef] [PubMed]
- Mohammad Jafari, R.; Shayesteh, S.; Ala, M.; Yousefi-Manesh, H.; Rashidian, A.; Hashemian, S.M.; Sorouri, M.; Dehpour, A.R. Dapsone Ameliorates Colitis through TLR4/NF-KB Pathway in TNBS Induced Colitis Model in Rat. Arch. Med. Res. 2021, 52, 595–602. [Google Scholar] [CrossRef] [PubMed]
- Northrop, J.P.; Crabtree, G.R.; Mattila, P.S. Negative Regulation of Interleukin 2 Transcription by the Glucocorticoid Receptor. J. Exp. Med. 1992, 175, 1235–1245. [Google Scholar] [CrossRef] [PubMed]
- Vacca, A.; Martinottit, S.; Screpanti, I.; Maroder, M.; Fellit, M.P.; Farina, A.R.; Gismondiz, A.; Santoni, A.; Frati, L.; Gulino, A. Transcriptional Regulation of the Interleukin 2 Gene by Glucocorticoid Hormones. Role of Steroid Receptor and Antigen-Responsive 5’-Flanking Sequences. J. Biol. Chem. 1990, 265, 8075–8080. [Google Scholar] [CrossRef] [PubMed]
- Riml, S.; Schmidt, S.; Ausserlechner, M.J.; Geley, S.; Kofler, R. Glucocorticoid Receptor Heterozygosity Combined with Lack of Receptor Auto-Induction Causes Glucocorticoid Resistance in Jurkat Acute Lymphoblastic Leukemia Cells. Cell Death Differ. 2004, 11, S65–S72. [Google Scholar] [CrossRef] [PubMed]
- Fantuzzi, G.; Galli, G.; Zinetti, M.; Fratelli, M.; Ghezzi, P. The Upregulating Effect of Dexamethasone on Tumor Necrosis Factor Production Is Mediated by a Nitric Oxide-Producing Cytochrome P450. Cell. Immunol. 1995, 160, 305–308. [Google Scholar] [CrossRef] [PubMed]
- Busillo, J.M.; Azzams, K.M.; Cidlowski, J.A. Glucocorticoids Sensitize the Innate Immune System through Regulation of the NLRP3 Inflammasome. J. Biol. Chem. 2011, 286, 38703–38713. [Google Scholar] [CrossRef] [PubMed]
- Skov, L.; Beurskens, F.J.; Zachariae, C.O.C.; Reitamo, S.; Teeling, J.; Satijn, D.; Knudsen, K.M.; Boot, E.P.J.; Hudson, D.; Baadsgaard, O.; et al. IL-8 as Antibody Therapeutic Target in Inflammatory Diseases: Reduction of Clinical Activity in Palmoplantar Pustulosis. J. Immunol. 2008, 181, 669–679. [Google Scholar] [CrossRef]
- Gesser, B.; Deleuran, B.; Lund, M.; Vestergård, C.; Lohse, N.; Deleuran, M.; Jensen, S.L.; Pedersen, S.S.; Thestrup-Pedersen, K.; Larsen, C.G. Interleukin-8 Induces Its Own Production in CD4+ T Lymphocytes: A Process Regulated by Interleukin 10. Biochem. Biophys. Res. Commun. 1995, 210, 660–669. [Google Scholar] [CrossRef]
- Baggiolini, M.; Clark-Lewis, I. Interleukin-8, a Chemotactic and Inflammatory Cytokine. FEBS Lett. 1992, 307, 97–101. [Google Scholar] [CrossRef]
- Tsai, Y.C.; Tsai, T.F. Anti-Interleukin and Interleukin Therapies for Psoriasis: Current Evidence and Clinical Usefulness. Ther. Adv. Musculoskelet. Dis. 2017, 9, 277–294. [Google Scholar] [CrossRef]
- Commission Directive 2011/8/EU. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX%3A32011L0008 (accessed on 4 November 2022).
- Commission Regulation (EU) 2016/2235 Annex XVII. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX%3A32016R2235 (accessed on 4 November 2022).
- Lambré, C.; Barat Baviera, J.M.; Bolognesi, C.; Chesson, A.; Cocconcelli, P.S.; Crebelli, R.; Gott, D.M.; Grob, K.; Lampi, E.; Mengelers, M.; et al. Re-evaluation of the Risks to Public Health Related to the Presence of Bisphenol A (BPA) in Foodstuffs. EFSA J. 2023, 21, 6857–7959. [Google Scholar] [CrossRef]
- EPA: Bisphenol A Alternatives in Thermal Paper. Available online: https://www.epa.gov/sites/default/files/2015-08/documents/bpa_final.pdf (accessed on 4 November 2022).
- ECHA: Assessment of Regulatory Needs. Available online: https://echa.europa.eu/documents/10162/3448017/GMT_109_Bisphenols_Report_public_23502_en.pdf/1bd5525c-432c-495d-9dab-d7806bf34312?t=1647590013566 (accessed on 4 November 2022).
- BPA Being Replaced by BPS in Thermal Paper, ECHA Survey Finds. Available online: https://echa.europa.eu/sl/-/bpa-being-replaced-by-bps-in-thermal-paper-echa-survey-finds (accessed on 30 November 2023).
- Kodila, A.; Franko, N.; Sollner Dolenc, M. A Review on Immunomodulatory Effects of BPA Analogues. Arch. Toxicol. 2023, 97, 1831–1846. [Google Scholar] [CrossRef]
- Chen, Y.; Xu, H.S.; Guo, T.L. Modulation of Cytokine/Chemokine Production in Human Macrophages by Bisphenol A: A Comparison to Analogues and Interactions with Genistein. J. Immunotoxicol. 2018, 15, 96–103. [Google Scholar] [CrossRef]
- Peillex, C.; Kerever, A.; Lachhab, A.; Pelletier, M. Bisphenol A, Bisphenol S and Their Glucuronidated Metabolites Modulate Glycolysis and Functional Responses of Human Neutrophils. Environ. Res. 2021, 196, 110336. [Google Scholar] [CrossRef] [PubMed]
- Buoso, E.; Kenda, M.; Masi, M.; Linciano, P.; Galbiati, V.; Racchi, M.; Sollner Dolenc, M.; Corsini, E. Effects of Bisphenols on RACK1 Expression and Their Immunological Implications in THP-1 Cells. Front. Pharmacol. 2021, 12, 743991. [Google Scholar] [CrossRef]
- Ndebele, K.; Tchounwou, P.B.; McMurray, R.W. Coumestrol, Bisphenol-A, DDT, and TCDD Modulation of Interleukin-2 Expression in Activated CD+4 Jurkat T Cells. Int. J. Environ. Res. Public Health 2004, 1, 3–11. [Google Scholar] [CrossRef]
- Ma, N.; Ma, D.; Liu, X.; Zhao, L.; Ma, L.; Ma, D.; Dong, S. Bisphenol P Exposure in C57BL/6 Mice Caused Gut Microbiota Dysbiosis and Induced Intestinal Barrier Disruption via LPS/TLR4/NF-ΚB Signaling Pathway. Environ. Int. 2023, 175, 107949. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Li, S.; Zhang, M.; Bai, S.; Ni, Y.; Xu, Q.; Fan, Y.; Lu, C.; Xu, Z.; Ji, C.; et al. Early-Life Bisphenol AP Exposure Impacted Neurobehaviors in Adulthood through Microglial Activation in Mice. Chemosphere 2023, 317, 137935. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, W.; Li, A.; Song, M. Tetrachlorobisphenol A Induced Immunosuppression and Uterine Injury in Mice. Ecotoxicol. Environ. Saf. 2021, 207, 111527. [Google Scholar] [CrossRef]
- Durcik, M.; Hiti, L.; Tomašič, T.; Peterlin Mašič, L. New Bisphenol A and Bisphenol S Analogs: Evaluation of Their HERα Agonistic and Antagonistic Activities Using the OECD 455 in-Vitro Assay and Molecular Modeling. Chem.-Biol. Interact. 2022, 354, 109820. [Google Scholar] [CrossRef] [PubMed]
- Grimaldi, M.; Boulahtouf, A.; Toporova, L.; Balaguer, P. Functional Profiling of Bisphenols for Nuclear Receptors. Toxicology 2019, 420, 39–45. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Franko, N.; Sollner Dolenc, M. Evaluation of THP-1 and Jurkat Cell Lines Coculture for the In Vitro Assessment of the Effects of Immunosuppressive Substances. Toxics 2024, 12, 607. https://doi.org/10.3390/toxics12080607
Franko N, Sollner Dolenc M. Evaluation of THP-1 and Jurkat Cell Lines Coculture for the In Vitro Assessment of the Effects of Immunosuppressive Substances. Toxics. 2024; 12(8):607. https://doi.org/10.3390/toxics12080607
Chicago/Turabian StyleFranko, Nina, and Marija Sollner Dolenc. 2024. "Evaluation of THP-1 and Jurkat Cell Lines Coculture for the In Vitro Assessment of the Effects of Immunosuppressive Substances" Toxics 12, no. 8: 607. https://doi.org/10.3390/toxics12080607





