The Impact of Ambient Air Pollution on Allergic Rhinitis Symptoms: A Prospective Follow-Up Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Participants
2.2. Measurement of Ambient Air Pollution Data
2.3. Health Effect Measurements
2.4. Statistical Analysis
3. Results
3.1. Participants
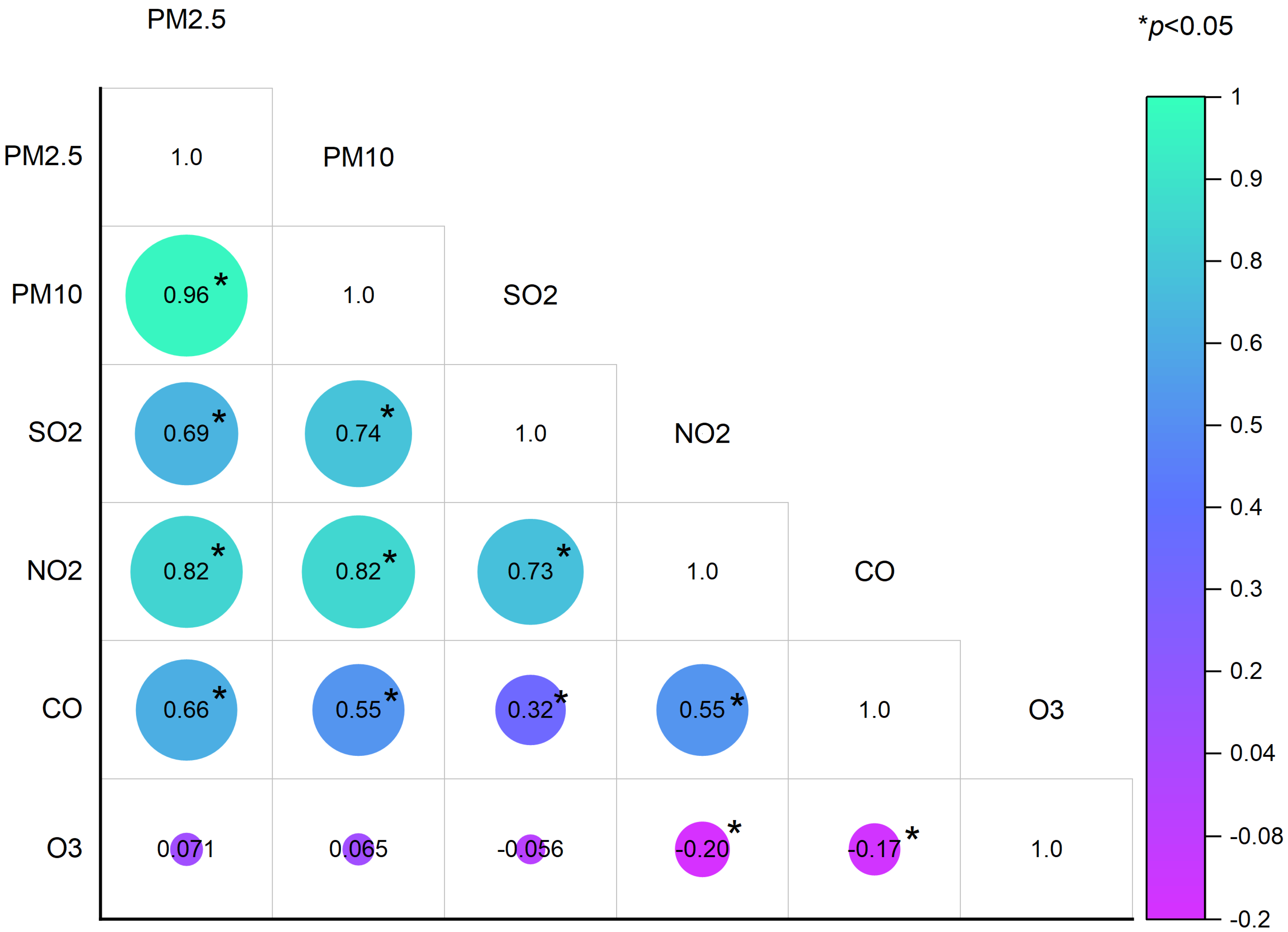
3.2. Ambient Air Pollution
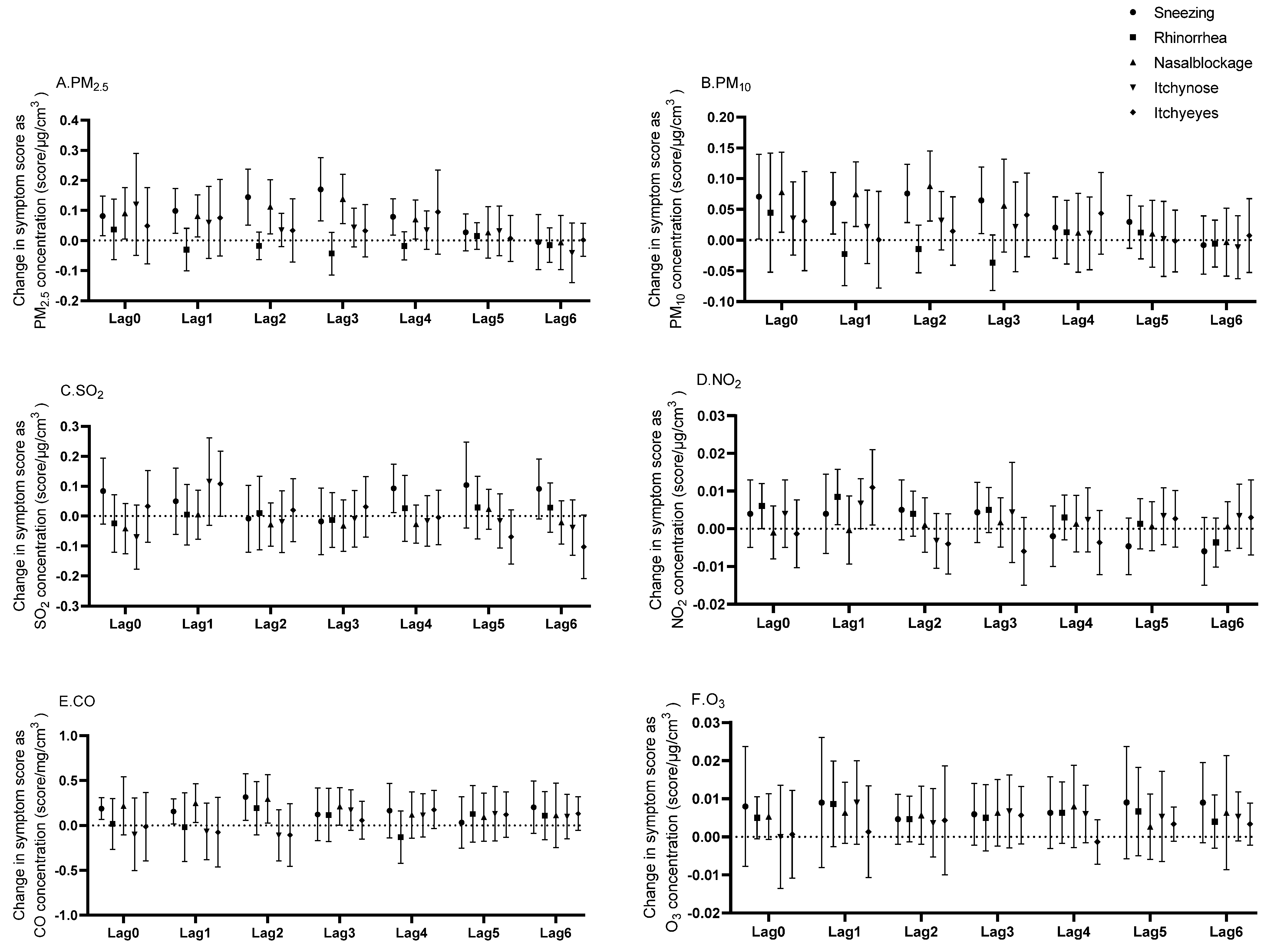
3.3. Lagged Effects of Ambient Air Pollutants on Allergic Rhinitis Symptoms
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization. Ambient (Outdoor) Air Pollution. Available online: https://www.who.int/news-room/fact-sheets/detail/ambient-(outdoor)-air-quality-and-health (accessed on 24 April 2024).
- Adamkiewicz, G.; Liddie, J.; Gaffin, J.M. The Respiratory Risks of Ambient/Outdoor Air Pollution. Clin. Chest Med. 2020, 41, 809–824. [Google Scholar] [CrossRef] [PubMed]
- Tran, H.M.; Tsai, F.; Lee, Y.; Chang, J.; Chang, L.; Chang, T.; Chung, K.F.; Kuo, H.; Lee, K.; Chuang, K.; et al. The impact of air pollution on respiratory diseases in an era of climate change: A review of the current evidence. Sci. Total Environ. 2023, 898, 166340. [Google Scholar] [CrossRef] [PubMed]
- Eguiluz-Gracia, I.; Mathioudakis, A.; Bartel, S.; Vijverberg, S.; Fuertes, E.; Comberiati, P.; Cai, Y.; Tomazic, P.; Diamant, Z.; Vestbo, J.; et al. The need for clean air: The way air pollution and climate change affect allergic rhinitis and asthma. Allergy 2020, 75, 2170–2184. [Google Scholar] [CrossRef] [PubMed]
- Maio, S.; Sarno, G.; Tagliaferro, S.; Pirona, F.; Stanisci, I.; Baldacci, S.; Viegi, G. Outdoor air pollution and respiratory health. Int. J. Tuberc. Lung Dise. 2023, 27, 7–12. [Google Scholar] [CrossRef] [PubMed]
- Blaiss, M.S.; Hammerby, E.; Robinson, S.; Kennedy-Martin, T.; Buchs, S. The burden of allergic rhinitis and allergic rhinoconjunctivitis on adolescents: A literature review. Ann. Allergy Asthma Immunol. 2018, 121, 43–52.e3. [Google Scholar] [CrossRef] [PubMed]
- Meltzer, E.O. Allergic Rhinitis: Burden of Illness, Quality of Life, Comorbidities, and Control. Immunol. Allergy Clin. 2016, 36, 235–248. [Google Scholar]
- Zhang, Y.; Zhang, L. Increasing Prevalence of Allergic Rhinitis in China. Allergy Asthma Immunol. Res. 2019, 11, 156–169. [Google Scholar] [CrossRef]
- Cheng, L.; Chen, J.; Fu, Q.; He, S.; Li, H.; Liu, Z.; Tan, G.; Tao, Z.; Wang, D.; Wen, W.; et al. Chinese Society of Allergy Guidelines for Diagnosis and Treatment of Allergic Rhinitis. Allergy Asthma Immunol. Res. 2018, 10, 300–353. [Google Scholar] [CrossRef]
- Nur Husna, S.M.; Tan, H.T.; Md Shukri, N.; Mohd Ashari, N.S.; Wong, K.K. Allergic Rhinitis: A Clinical and Pathophysiological Overview. Front. Med.-Lausanne 2022, 9, 874114. [Google Scholar] [CrossRef]
- Lin, L.; Li, T.; Sun, M.; Liang, Q.; Ma, Y.; Wang, F.; Duan, J.; Sun, Z. Effect of particulate matter exposure on the prevalence of allergic rhinitis in children: A systematic review and meta-analysis. Chemosphere 2021, 268, 128841. [Google Scholar] [CrossRef]
- Piao, C.H.; Fan, Y.; Nguyen, T.V.; Shin, H.S.; Kim, H.T.; Song, C.H.; Chai, O.H. PM(2.5) Exacerbates Oxidative Stress and Inflammatory Response through the Nrf2/NF-κB Signaling Pathway in OVA-Induced Allergic Rhinitis Mouse Model. Int. J. Mol. Sci. 2021, 22, 8173. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Wu, W.; Wang, G.; Zhang, X.; Guo, Q.; Wang, B.; Cao, S.; Yan, M.; Pan, X.; Xue, T.; et al. Association between exposure to air pollution and risk of allergic rhinitis: A systematic review and meta-analysis. Environ. Res. 2022, 205, 112472. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Guo, Z.; Zhang, R.; Han, Z.; Huang, Y.; Deng, C.; Dong, W.; Zhuang, G. Effects of N-acetylcysteine on oxidative stress and inflammation reactions in a rat model of allergic rhinitis after PM2.5 exposure. Biochem. Biophys. Res. Commun. 2020, 533, 275–281. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Huang, Y.; Zhang, R.; Han, Z.; Zhou, L.; Sun, N.; Dong, W.; Zhuang, G. miR-338–3p inhibits autophagy in a rat model of allergic rhinitis after PM2.5 exposure through AKT/mTOR signaling by targeting UBE2Q1. Biochem. Biophys. Res. Commun. 2021, 554, 1–6. [Google Scholar] [CrossRef]
- Li, Y.; Zhou, J.; Rui, X.; Zhou, L.; Mo, X. PM2.5 exposure exacerbates allergic rhinitis in mice by increasing DNA methylation in the IFN-γ gene promoter in CD4+T cells via the ERK-DNMT pathway. Toxicol. Lett. 2019, 301, 98–107. [Google Scholar] [CrossRef]
- Kim, S.H.; Lee, J.; Oh, I.; Oh, Y.; Sim, C.; Bang, J.; Park, J.; Kim, Y. Allergic rhinitis is associated with atmospheric SO2: Follow-up study of children from elementary schools in Ulsan, Korea. PLoS ONE 2021, 16, e0248624. [Google Scholar] [CrossRef]
- Dąbrowiecki, P.; Chciałowski, A.; Dąbrowiecka, A.; Piórkowska, A.; Badyda, A. Exposure to ambient air pollutants and short-term risk for exacerbations of allergic rhinitis: A time-stratified, case-crossover study in the three largest urban agglomerations in Poland. Respir. Physiol. Neurobiol. 2023, 315, 104095. [Google Scholar] [CrossRef]
- Ye, M.; Liu, H.; Li, H.; Liu, Q.; Zhou, Z.; Wang, T.; Tan, G. Long-Term Exposure to Sulfur Dioxide Before Sensitization Decreased the Production of Specific IgE in HDM-Sensitized Allergic Rhinitis Mice. J. Inflamm. Res. 2022, 15, 2477–2490. [Google Scholar] [CrossRef]
- Wang, J.; Lu, M.; An, Z.; Jiang, J.; Li, J.; Wang, Y.; Du, S.; Zhang, X.; Zhou, H.; Cui, J.; et al. Associations between air pollution and outpatient visits for allergic rhinitis in Xinxiang, China. Environ. Sci. Pollut. Res. Int. 2020, 27, 23565–23574. [Google Scholar] [CrossRef]
- Wang, M.; Wang, S.; Wang, X.; Tian, Y.; Wu, Y.; Cao, Y.; Song, J.; Wu, T.; Hu, Y. The association between PM(2.5) exposure and daily outpatient visits for allergic rhinitis: Evidence from a seriously air-polluted environment. Int. J. Biometeorol. 2020, 64, 139–144. [Google Scholar] [CrossRef]
- Wu, R.; Guo, Q.; Fan, J.; Guo, C.; Wang, G.; Wu, W.; Xu, J. Association between air pollution and outpatient visits for allergic rhinitis: Effect modification by ambient temperature and relative humidity. Sci. Total Environ. 2022, 821, 152960. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Zeng, Y.; Lau, A.K.; Guo, C.; Wei, X.; Lin, C.; Huang, B.; Lao, X.Q. Chronic exposure to ambient PM2.5/NO2 and respiratory health in school children: A prospective cohort study in Hong Kong. Ecotoxicol. Environ. Saf. 2023, 252, 114558. [Google Scholar] [CrossRef] [PubMed]
- Lu, C.; Wang, F.; Liu, Z.; Li, B.; Yang, W.; Liao, H. Intrauterine and early postnatal exposure to air pollution associated with childhood allergic rhinitis. Chemosphere 2023, 336, 139296. [Google Scholar] [CrossRef] [PubMed]
- Rosario, C.S.; Urrutia-Pereira, M.; Murrieta-Aguttes, M.; D’Amato, G.; Chong-Silva, D.C.; Godoi, R.H.M.; Rosario Filho, N.A. Air pollution and rhinitis. Front. Allergy 2024, 5, 1387525. [Google Scholar] [CrossRef]
- Zhang, S.; Fu, Q.; Wang, S.; Jin, X.; Tan, J.; Ding, K.; Zhang, Q.; Li, X. Association between air pollution and the prevalence of allergic rhinitis in Chinese children: A systematic review and meta-analysis. Allergy Asthma Proc. 2022, 43, e47–e57. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.Y.; Kim, H.; Kim, H.J.; Na, G.; Jang, Y.; Kim, S.H.; Kim, N.H.; Kim, H.C.; Park, Y.; Kim, H.C.; et al. The impact of ambient air pollution on lung function and respiratory symptoms in elite athletes. Sci. Total Environ. 2023, 855, 158862. [Google Scholar] [CrossRef]
- Sharma, A.; Mandal, T.K.; Sharma, S.; Shukla, D.; Singh, S.N. Relationships of surface ozone with its precursors, particulate matter and meteorology over Delhi. J. Atmos. Chem. 2017, 74, 451–474. [Google Scholar] [CrossRef]
- Kelly, J.T.; Reff, A.; Gantt, B. A method to predict PM2.5 resulting from compliance with national ambient air quality standards. Atmos. Environ. 2017, 162, 1–10. [Google Scholar] [CrossRef]
- Su, T.; Li, J.; Tian, C.; Zong, Z.; Chen, D.; Zhang, G. Source and formation of fine particulate nitrate in South China: Constrained by isotopic modeling and online trace gas analysis. Atmos. Environ. 2020, 231, 117563. [Google Scholar] [CrossRef]
- Tang, W.; Sun, L.; Wang, J.; Li, K.; Liu, S.; Wang, M.; Cheng, Y.; Dai, L. Exploring Associations Between Short-Term Air Pollution and Daily Outpatient Visits for Allergic Rhinitis. Risk Manag. Healthc. Policy 2023, 16, 1455–1465. [Google Scholar] [CrossRef]
- Liu, W.; Cai, J.; Fu, Q.; Zou, Z.; Sun, C.; Zhang, J.; Huang, C. Associations of ambient air pollutants with airway and allergic symptoms in 13,335 preschoolers in Shanghai, China. Chemosphere 2020, 252, 126600. [Google Scholar] [CrossRef] [PubMed]
- Luo, P.; Ying, J.; Li, J.; Yang, Z.; Sun, X.; Ye, D.; Liu, C.; Wang, J.; Mao, Y. Air Pollution and Allergic Rhinitis: Findings from a Prospective Cohort Study. Environ. Sci. Technol. 2023, 57, 15835–15845. [Google Scholar] [CrossRef] [PubMed]
- Burte, E.; Leynaert, B.; Marcon, A.; Bousquet, J.; Benmerad, M.; Bono, R.; Carsin, A.; de Hoogh, K.; Forsberg, B.; Gormand, F.; et al. Long-term air pollution exposure is associated with increased severity of rhinitis in 2 European cohorts. J. Allergy Clin. Immunol. 2020, 145, 834–842.e6. [Google Scholar] [CrossRef] [PubMed]
- Zhou, P.; Qian, Z.; McMillin, S.; Vaughn, M.; Xie, Z.; Xu, Y.; Lin, L.; Hu, L.; Yang, B.; Zeng, X.; et al. Relationships between Long-Term Ozone Exposure and Allergic Rhinitis and Bronchitic Symptoms in Chinese Children. Toxics 2021, 9, 221. [Google Scholar] [CrossRef]
- Chai, G.; He, H.; Sha, Y.; Zhai, G.; Zong, S. Effect of PM(2.5) on daily outpatient visits for respiratory diseases in Lanzhou, China. Sci. Total Environ. 2019, 649, 1563–1572. [Google Scholar] [CrossRef] [PubMed]
- Ji, J.; Chen, K.; Dong, J.; Yu, H.; Zhang, Y. Associations between air pollution and outpatient visits for allergic rhinitis in Lanzhou, China. Environ. Sci. Pollut. Res. Int. 2023, 30, 91453–91465. [Google Scholar] [CrossRef]
- Liu, C.; Liu, Y.; Zhou, Y.; Feng, A.; Wang, C.; Shi, T. Short-term effect of relatively low level air pollution on outpatient visit in Shennongjia, China. Environ. Pollut. 2019, 245, 419–426. [Google Scholar] [CrossRef]
| Variable | Mean ± SD or N (%) |
|---|---|
| Age (years) | 35.4 ± 12.1 |
| Gender | |
| Male | 60 (35.9%) |
| Female | 107 (64.1%) |
| High (cm) | |
| Male | 175 ± 5.2 |
| Female | 160.9 ± 5.6 |
| Weight (kg) | |
| Male | 73.4 ± 12.7 |
| Female | 55 ± 7.3 |
| BMI (kg/m2) | |
| Male | 23.9 ± 3.9 |
| Female | 21.1 ± 2.5 |
| IgE (IU/mL) | 376.5 ± 398 |
| Smoking status | |
| Smoker | 13 (7.8%) |
| Never smoker | 154 (92.2%) |
| Drinking status | |
| Drinker | 24 (14.4%) |
| Never drinker | 143 (85.6%) |
| Sneezing a | 1.4 ± 0.8 |
| Nasal blockage a | 2.3 ± 0.8 |
| Rhinorrhea a | 1.7 ± 0.7 |
| Itchy nose a | 1.5 + 0.8 |
| Itchy eyes a | 1.4 ± 0.9 |
| May | June | July | August | September | October | November | December | January | |
|---|---|---|---|---|---|---|---|---|---|
| PM2.5 (μg/m3) a | 27.6 ± 9.8 | 23.2 ± 9.4 | 14.4 ± 5.4 | 19.1 ± 7.7 | 22.1 ± 9.3 | 29.7 ± 15.3 | 34.5 ± 13.6 | 53.4 ± 37.1 | 66.8 ± 32 |
| PM10 (μg/m3) a | 49.6 ± 18.1 | 39.2 ± 15.4 | 25.8 ± 7.9 | 31.8 ± 10.9 | 34 ± 12.9 | 47.3 ± 19.7 | 63.1 ± 22.1 | 81.1 ± 50.8 | 93 ± 42.4 |
| SO2 (μg/m3) a | 6.2 ± 0.8 | 5.9 ± 0.8 | 5.6 ± 0.5 | 5.7 ± 0.4 | 5.9 ± 0.6 | 7.1 ± 0.6 | 7.3 ± 1 | 7.5 ± 1.2 | 7.6 ± 1.3 |
| NO2 (μg/m3) a | 26 ± 6.3 | 22.2 ± 4.4 | 16 ± 3.7 | 16.6 ± 3.3 | 19.5 ± 3.2 | 32.4 ± 11.2 | 41.3 ± 12.2 | 49.2 ± 21.2 | 47.3 ± 14.7 |
| CO (mg/m3) a | 0.6 ± 0.1 | 0.6 ± 0.1 | 0.6 ± 0.1 | 0.6 ± 0.1 | 0.7 ± 0.1 | 0.6 ± 0.1 | 0.7 ± 0.1 | 0.8 ± 0.2 | 0.9 ± 0.2 |
| O3 (μg/m3) b | 128.7 ± 37.5 | 131.9 ± 57.6 | 96.1 ± 33.5 | 130.5 ± 45.7 | 123.9 ± 43.8 | 126.5 ± 29.7 | 77.6 ± 27.9 | 51 ± 22.8 | 57.5 ± 21.4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, W.; Ding, C.; Jiang, Z.; Zheng, X.; Jiang, J.; Xu, H. The Impact of Ambient Air Pollution on Allergic Rhinitis Symptoms: A Prospective Follow-Up Study. Toxics 2024, 12, 663. https://doi.org/10.3390/toxics12090663
Sun W, Ding C, Jiang Z, Zheng X, Jiang J, Xu H. The Impact of Ambient Air Pollution on Allergic Rhinitis Symptoms: A Prospective Follow-Up Study. Toxics. 2024; 12(9):663. https://doi.org/10.3390/toxics12090663
Chicago/Turabian StyleSun, Wen, Chan Ding, Zhuoying Jiang, Xinliang Zheng, Jinlan Jiang, and Huadong Xu. 2024. "The Impact of Ambient Air Pollution on Allergic Rhinitis Symptoms: A Prospective Follow-Up Study" Toxics 12, no. 9: 663. https://doi.org/10.3390/toxics12090663
APA StyleSun, W., Ding, C., Jiang, Z., Zheng, X., Jiang, J., & Xu, H. (2024). The Impact of Ambient Air Pollution on Allergic Rhinitis Symptoms: A Prospective Follow-Up Study. Toxics, 12(9), 663. https://doi.org/10.3390/toxics12090663





