A Comprehensive Review of the Harmful Compounds in Electronic Cigarettes
Abstract
1. Introduction
2. Materials and Methods
3. Inspection and Regulations
4. Bibliometric Analysis
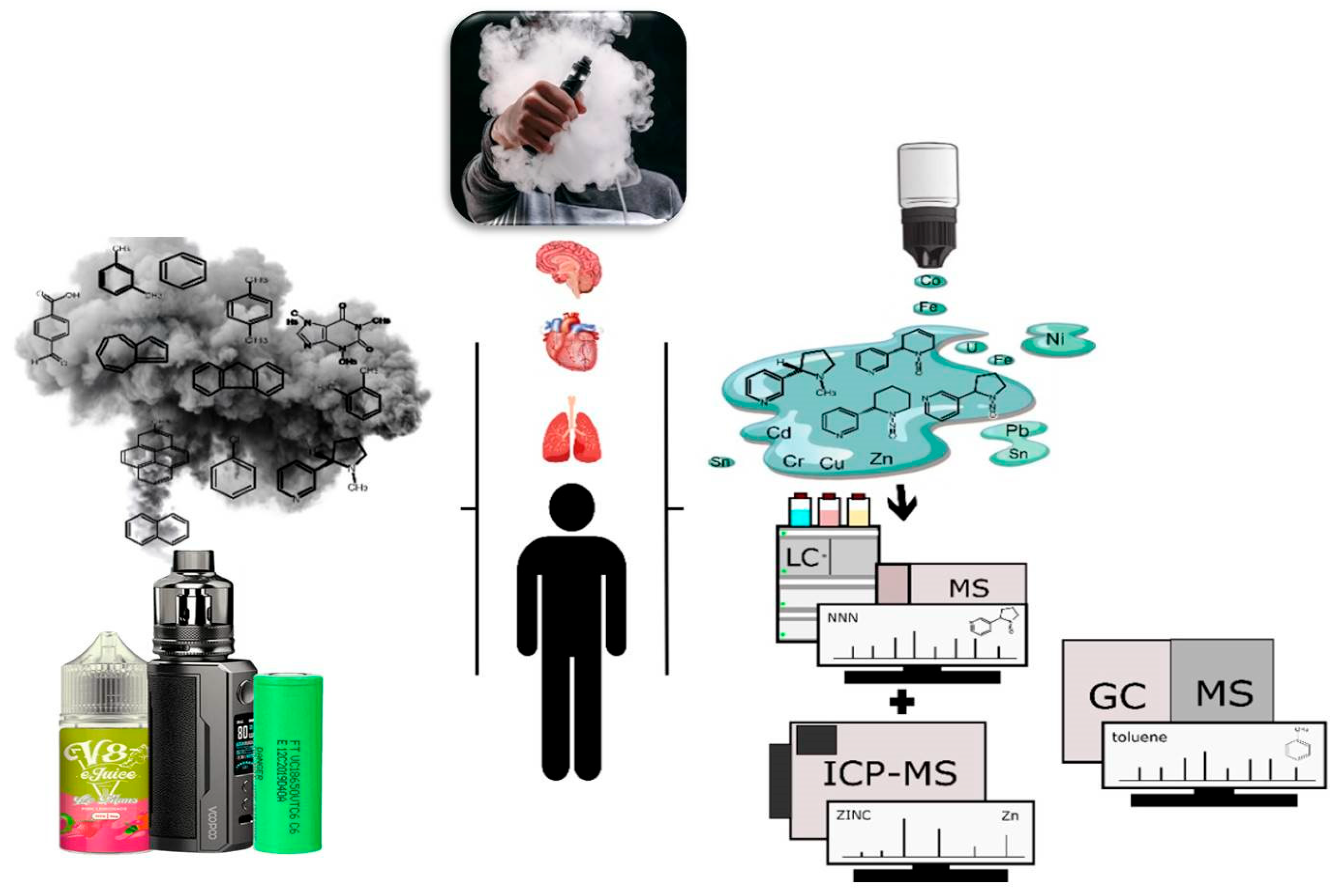
5. Impacts on Health and the Environment
6. Sample Preparation and Analysis
6.1. Gas Analysis
6.2. Liquid Analysis
7. Identified or Quantified Toxic Compounds in E-Liquids and Aerosols of Electronic Cigarettes
7.1. Nicotine
7.2. Polycyclic Aromatic Hydrocarbons (PAHs)
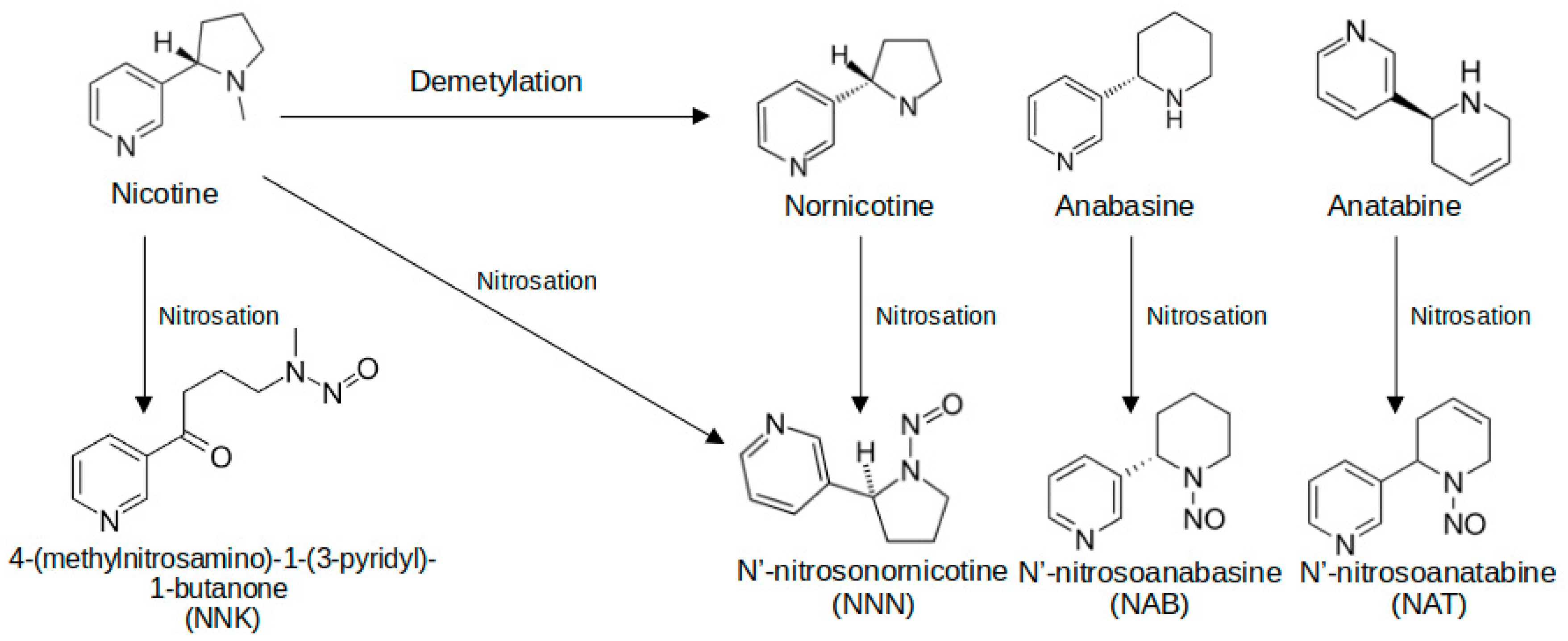
7.3. Nitrosamines (TSNAs)
7.4. Potentially Toxic Metals (PTMs)
8. Conclusions and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| PAHs | Polycyclic aromatic hydrocarbons |
| TSNAs | Tobacco-specific nitrosamines |
| THC | Tetrahydrocannabinol |
| CAGR | Compound annual growth rate |
| GC-MS | Gas chromatography–mass spectrometry |
| HPLC | High-performance liquid chromatography |
| ICP | Inductively coupled plasma |
| EU | European Union |
| TPD | Tobacco Products Directive |
| MHRA | Medicines and Healthcare Products Regulatory Agency |
| GABA | Gamma-AminoButyric Acid |
| TRPV1 | Transient Receptor Potential Vanilloid 1 |
| TRPA1 | Transient Receptor Potential Ankyrin 1 |
| GRAS | Generally Recognized as Safe |
| EVALI | e-cigarette or vaping product use-associated lung injury |
| DCP | Disease Control and Prevention |
| CBD | Cannabidiol |
| BAL | Bronchoalveolar lavage |
| PG | Propylene glycol |
| GLY | Glycerin |
| DnS | Dilute-and-shoot |
| SPE | Solid-phase extraction |
| LLE | Liquid–liquid extraction |
| SPME | Solid-phase microextraction |
| GCxIMS | Chromatography coupled with ion mobility spectrometry |
| LC–MS/MS | Liquid chromatography coupled with tandem mass spectrometry |
| LC-HRAM-MS | Liquid chromatography coupled with high-resolution accurate mass spectrometry |
| LC-UV | Liquid chromatography coupled with ultraviolet detection |
| UPLC-QTOF-HRMS | Quadrupole time-of-flight high-resolution mass spectrometry |
| EPA | Environmental Protection Agency |
| GC-FID | Gas chromatography with flame ionization detection |
| GC-TCD | Gas chromatography with thermal conductivity detection |
| ICP-MS | Inductively coupled plasma mass spectrometry |
| TXRF | Total reflection X-ray fluorescence |
| ICP-OES | Inductively coupled plasma optical emission spectrometry |
| RM | Reference material |
| LOD | Limit of detection |
| SEM | Scanning electron microscopy |
| WHO | World Health Organization |
| ICH | International Council for Harmonization of Technical Requirements for Pharmaceuticals for Human Use |
| ATSDR | Agency for Toxic Substances Disease Registry |
| NIOSH | National Institute for Occupational Safety and Health |
| LIBS | Laser-Induced Breakdown Spectroscopy |
| EIS | Electrochemical Impedance Spectroscopy |
| DPASV | Differential Pulse Anodic Stripping Voltammetry |
| SWASV | Square Wave Anodic Stripping Voltammetry |
| CV | Cyclic Voltammetry |
| 2D-IR | Two-Dimensional Infrared Spectroscopy |
References
- Li, Y.; Pang, T.; Zhang, Y.; Shi, J.; Song, Z.; Xu, Z. Study on the Chemical Composition of the Mainstream Cherry-red Tobacco Smoke. Curr. Anal. Chem. 2024, 20, 115–124. [Google Scholar] [CrossRef]
- Sajid, M.; Srivastava, S.; Joshi, L.; Bharadwaj, M. Impact of smokeless tobacco-associated bacteriome in oral carcinogenesis. Anaerobe 2021, 70, 102400. [Google Scholar] [CrossRef] [PubMed]
- Adesina, O.A.; Nwogu, A.S.; Sonibare, J.A. Indoor levels of polycyclic aromatic hydrocarbons (PAHs) from environment tobacco smoke of public bars. Ecotoxicol. Environ. Saf. 2021, 208, 111604. [Google Scholar] [CrossRef] [PubMed]
- Su, X.; Narayanan, M.; Shi, X.; Chen, X.; Li, Z.; Ma, Y. Mitigating heavy metal accumulation in tobacco: Strategies, mechanisms, and global initiatives. Sci. Total Environ. 2024, 926, 172128. [Google Scholar] [CrossRef]
- Swasticharan, L.; Srivastava, A.; Sharma, P.; Sharma, R.; Narula, A.K. A combined metagenome and disease enrichment analysis of nitrosamines present in smokeless tobacco, wiz using molecular docking and functional annotation approach. Gene Rep. 2024, 36, 101929. [Google Scholar] [CrossRef]
- Hiremath, P.; Patange, R.P.; Salunkhe, J.A.; Mohite, V.R.; Pawar, A.; Mulani, A. Maternal tobacco use and risk for congenital anomalies. Int. J. Community Med. Public Health 2019, 6, 1472–1475. [Google Scholar] [CrossRef]
- Li, X.; Ye, Z.; Wang, J.; Lin, P.; Zhang, X.; Xie, S.; Chen, C. Intake of tobacco nitrosamines of smokers in various provinces of China and their cancer risk: A meta-analysis. J. Environ. Sci. 2024, 141, 249–260. [Google Scholar] [CrossRef]
- Vargees, C.; Stroup, A.M.; Niznik, T.; Dunn, D.; Wyatt, R.; Hoetger, C.; Fetterman, J.L. Patterns of use, perceptions, and cardiopulmonary health risks of cigar products: A systematic review. BMC Public Health 2023, 23, 2357. [Google Scholar] [CrossRef]
- Upadhyay, S.; Rahman, M.; Johanson, G.; Palmberg, L.; Ganguly, K. Heated Tobacco Products: Insights into Composition and Toxicity. Toxics 2023, 11, 667. [Google Scholar] [CrossRef]
- National Academies of Sciences, Engineering, and Medicine; Health and Medicine; Board on Population Health and Public Health; Committee on the Review of the Health Effects of Electronic Nicotine Delivery Systems. Public Health Consequences of E-Cigarettes; Eaton, D.L., Kwan, L.Y., Stratton, K., Eds.; National Academies Press (US): Washington, DC, USA, 2018. [Google Scholar]
- Shields, P.G.; Berman, M.; Brasky, T.M.; Freudenheim, J.L.; Mathe, E.; McElroy, J.P.; Wewers, M.D. A Review of Pulmonary Toxicity of Electronic Cigarettes in the Context of Smoking: A Focus on Inflammation. Cancer Epidemiol. Biomark. Prev. 2017, 26, 1175–1191. [Google Scholar] [CrossRef]
- O’Neal, R.A.; Carpenter, M.J.; Wahlquist, A.E.; Leavens, E.L.S.; Smith, T.T.; Fahey, M.C. The prospective relationship between a-priori intentions for and patterns of e-cigarette use among adults who smoke cigarettes. Addict. Behav. 2024, 156, 108067. [Google Scholar] [CrossRef] [PubMed]
- Evans-Polce, R.J.; Chen, B.; McCabe, S.E.; West, B.T. Longitudinal associations of e-cigarette use with cigarette, marijuana, and other drug use initiation among US adolescents and young adults: Findings from the Population Assessment of Tobacco and Health study (Waves 1–6). Drug Alcohol Depend. 2024, 263, 111402. [Google Scholar] [CrossRef] [PubMed]
- Pisinger, C.; Katsaounou, P.; Ravara, S.; Vestbo, J. E-cigarettes, heated tobacco and other novel nicotine-containing products: A help to smokers or a public health threat? In ERS Monograph; European Respiratory Society: Sheffield, UK, 2021; pp. 33–55. [Google Scholar] [CrossRef]
- Al-Delaimy, A.K.; Al-Ani, W.A.T. Prevalence of hookah smoking and associated factors among male high school students in Iraq. BMC Public Health 2021, 21, 1317. [Google Scholar] [CrossRef]
- Algabbani, A.; Althumiri, N.; Almarshad, A.; BinDhim, N. National prevalence, perceptions, and determinants of tobacco consumption in Saudi Arabia. Int. J. Drug Regul. Aff. 2019, 2, 1. [Google Scholar] [CrossRef]
- Romberg, A.R.; Miller Lo, E.J.; Cuccia, A.F.; Willett, J.G.; Xiao, H.; Hair, E.C.; King, B.A. Patterns of nicotine concentrations in electronic cigarettes sold in the United States, 2013–2018. Drug Alcohol Depend. 2019, 203, 1–7. [Google Scholar] [CrossRef]
- Majmundar, A.; Xue, Z.; Asare, S.; Bandi, P.; Patel, M.; Nargis, N. Concept flavor e-cigarette unit sales in the U.S.: 2019–2022. Prev. Med. Rep. 2023, 36, 102506. [Google Scholar] [CrossRef]
- Furlow, B. US FDA approves menthol e-cigarette products. Lancet Respir. Med. 2024, 12, 667. [Google Scholar] [CrossRef]
- Research, B. Global E-Cigarette Market. [ONLINE]. 2024. Available online: https://www.bccresearch.com/market-research/food-and-beverage/e-cigarette-market.html?srsltid=AfmBOoprSv7izVdHdK-swEOv9dN5sXd0hEp0E6H0R44Ktag4vMyp1n1I (accessed on 1 March 2025).
- Chen-Sankey, J.; La Capria, K.; Glasser, A.; Padon, A.A.; Moran, M.B.; Wagoner, K.G.; Berg, C.J. Associations between e-cigarette marketing exposure and vaping nicotine and cannabis among U.S. adults, 2021. Addict. Behav. 2024, 157, 108090. [Google Scholar] [CrossRef]
- Beutel, M.W.; Harmon, T.C.; Novotny, T.E.; Mock, J.; Gilmore, M.E.; Hart, S.C.; Holden, P.A. A Review of Environmental Pollution from the Use and Disposal of Cigarettes and Electronic Cigarettes: Contaminants, Sources, and Impacts. Sustainability 2021, 13, 12994. [Google Scholar] [CrossRef]
- Papaefstathiou, E.; Stylianou, M.; Agapiou, A. Main and side stream effects of electronic cigarettes. J. Environ. Manag. 2019, 238, 10–17. [Google Scholar] [CrossRef]
- Heide, M.; Engelhard, C. Chemical analysis of electronic cigarette liquids (e-liquids) and direct nicotine quantitation using surface-assisted flowing atmospheric-pressure afterglow desorption/ionization mass spectrometry (SA-FAPA-MS). RSC Adv. 2023, 13, 24150–24161. [Google Scholar] [CrossRef] [PubMed]
- Marco, E.; Grimalt, J.O. A rapid method for the chromatographic analysis of volatile organic compounds in exhaled breath of tobacco cigarette and electronic cigarette smokers. J. Chromatogr. A 2015, 1410, 51–59. [Google Scholar] [CrossRef] [PubMed]
- Strongin, R.M. E-Cigarette Chemistry and Analytical Detection. Annu. Rev. Anal. Chem. 2019, 12, 23–39. [Google Scholar] [CrossRef]
- FDA. How FDA Regulates Vapes. [ONLINE]. 2024. Available online: https://www.fda.gov/media/159412/download (accessed on 1 March 2025).
- Commission, E. Revision of the Tobacco Products Directive. [ONLINE]. 2024. Available online: https://health.ec.europa.eu/tobacco/product-regulation/implementing-tobacco-products-directive-directive-201440eu/revision-tobacco-products-directive_en (accessed on 1 March 2025).
- Medicines and Healthcare Products Regulatory Agency. E-Cigarettes: Regulations for Consumer Products. [ONLINE]. 2024. Available online: https://www.gov.uk/guidance/e-cigarettes-regulations-for-consumer-products (accessed on 1 March 2025).
- TGA. Vapes: Compliance and Enforcement. [ONLINE]. 2024. Available online: https://www.tga.gov.au/products/unapproved-therapeutic-goods/vaping-hub/vapes-compliance-and-enforcement (accessed on 1 March 2025).
- Canada. Tobacco and Vaping Products Act. [ONLINE]. 2024. Available online: https://laws-lois.justice.gc.ca/PDF/T-11.5.pdf (accessed on 1 March 2025).
- Cao, Y.; Yi, H.; Zhou, J.; Cheng, Y.; Mao, Y. Regulations on e-cigarettes: China is taking action. Pulmonology 2023, 29, 359–361. [Google Scholar] [CrossRef]
- ANVISA. Cigarro Eletrônico-Dispositivos Eletrônicos para Fumar (DEFs). [ONLINE]. 2024. Available online: https://www.gov.br/anvisa/pt-br/assuntos/tabaco/cigarro-eletronico (accessed on 1 March 2025).
- Federación. Decreto Presidencial. [ONLINE]. 2022. Available online: https://www.dof.gob.mx/nota_detalle.php?codigo=5653845&fecha=31/05/2022#gsc.tab=0 (accessed on 1 March 2025).
- Hon, L. Flameless Atomizing Electronic Cigarette. Patent No. WO2004080216, 23 September 2004. [Google Scholar]
- Bullen, C.; McRobbie, H.; Thornley, S.; Glover, M.; Lin, R.; Laugesen, M. Effect of an electronic nicotine delivery device (e cigarette) on desire to smoke and withdrawal, user preferences and nicotine delivery: Randomised cross-over trial. Tob. Control 2010, 19, 98–103. [Google Scholar] [CrossRef]
- Cobb, N.K.; Abrams, D.B. E-cigarette or drug-delivery device? Regulating novel nicotine products. N. Engl. J. Med. 2011, 365, 193–195. [Google Scholar] [CrossRef]
- Cobb, N.K.; Byron, M.J.; Abrams, D.B.; Shields, P.G. Novel nicotine delivery systems and public health: The rise of the “e-cigarette”. Am. J. Public Health 2010, 100, 2340–2342. [Google Scholar] [CrossRef]
- Simpson, D. World: E-cigarettes are here. Tob. Control. 2009, 18, 80–81. [Google Scholar]
- Hadwiger, M.E.; Trehy, M.L.; Ye, W.; Moore, T.; Allgire, J.; Westenberger, B. Identification of amino-tadalafil and rimonabant in electronic cigarette products using high pressure liquid chromatography with diode array and tandem mass spectrometric detection. J. Chromatogr. A 2010, 1217, 7547–7555. [Google Scholar] [CrossRef]
- Yayan, J.; Franke, K.J.; Biancosino, C.; Rasche, K. Comparative systematic review on the safety of e-cigarettes and conventional cigarettes. Food Chem. Toxicol. 2024, 185, 114507. [Google Scholar] [CrossRef]
- Eniola, K. E-Cigarette Use Among Adolescents, a Gateway to Nicotine Addiction. J. Adolesc. Health 2023, 73, 602. [Google Scholar] [CrossRef] [PubMed]
- Mukerjee, R.; Hirschtick, J.L.; Arciniega, L.Z.; Xie, Y.; Barnes, G.D.; Arenberg, D.A.; Cook, S.F. ENDS, Cigarettes, and Respiratory Illness: Longitudinal Associations Among U.S. Youth. Am. J. Prev. Med. 2024, 66, 789–796. [Google Scholar] [CrossRef] [PubMed]
- Tobore, T.O. On the potential harmful effects of E-Cigarettes (EC) on the developing brain: The relationship between vaping-induced oxidative stress and adolescent/young adults social maladjustment. J. Adolesc. 2019, 76, 202–209. [Google Scholar] [CrossRef] [PubMed]
- Askwith, Z.; Grignon, J.; Ismail, M.; Martin, G.; McEachern, L.W.; Seabrook, J.A.; Gilliland, J.A. Environmental influences on E-cigarette use among young people: A systematic review. Health Place 2024, 87, 103212. [Google Scholar] [CrossRef]
- Gallegos-Carrillo, K.; Barrientos-Gutiérrez, I.; Arillo-Santillán, E.; Rodríguez-Bolaños, R.; Cruz-Jiménez, L.; Hardin, J.W.; Thrasher, J.F. Does e-cigarette use predict short-term smoking cessation behaviors among adults who smoke in Mexico? A longitudinal study. Addict. Behav. 2024, 157, 108077. [Google Scholar] [CrossRef]
- Smith, M.J.; Skivington, K.; Hilton, S.; Katikireddi, S.V. Exploring e-cigarette policy recommendations and the role of evidence in international public health guidelines: A citation network analysis. Lancet 2019, 394, S4. [Google Scholar] [CrossRef]
- Cashman, L.; Nutt, J. A comparison of levels of nicotine and cotinine in hair of tobacco smokers and users of e-cigarettes using GC-MS. Toxicol. Anal. Clin. 2019, 31 (Suppl. S2), S83. [Google Scholar] [CrossRef]
- Krüsemann, E.J.Z.; Pennings, J.L.A.; Cremers, J.W.J.M.; Bakker, F.; Boesveldt, S.; Talhout, R. GC–MS analysis of e-cigarette refill solutions: A comparison of flavoring composition between flavor categories. J. Pharm. Biomed. Anal. 2020, 188, 113364. [Google Scholar] [CrossRef]
- Winnicka, L.; Shenoy, M.A. EVALI and the Pulmonary Toxicity of Electronic Cigarettes: A Review. J. Gen. Intern. Med. 2020, 35, 2130–2135. [Google Scholar] [CrossRef]
- Temourian, A.A.; Song, A.V.; Halliday, D.M.; Gonzalez, M.; Epperson, A.E. Why do smokers use e-cigarettes? A study on reasons among dual users. Prev. Med. Rep. 2022, 29, 101924. [Google Scholar] [CrossRef]
- Wang, R.J.; Bhadriraju, S.; Glantz, S.A. E-Cigarette Use and Adult Cigarette Smoking Cessation: A Meta-Analysis. Am. J. Public Health 2021, 111, 230–246. [Google Scholar] [CrossRef] [PubMed]
- Pound, C.M.; Zhang, J.Z.; Kodua, A.T.; Sampson, M. Smoking cessation in individuals who use vaping as compared with traditional nicotine replacement therapies: A systematic review and meta-analysis. BMJ Open 2021, 11, e044222. [Google Scholar] [CrossRef] [PubMed]
- Lyzwinski, L.N.; Naslund, J.A.; Miller, C.J.; Eisenberg, M.J. Global youth vaping and respiratory health: Epidemiology, interventions, and policies. NPJ Prim. Care Respir. Med. 2022, 32, 14. [Google Scholar] [CrossRef] [PubMed]
- Rose, J.J.; Krishnan-Sarin, S.; Exil, V.J.; Hamburg, N.M.; Fetterman, J.L.; Ichinose, F.; Perez-Pinzon, M.A.; Rezk-Hanna, M.; Williamson, E.; Council on Epidemiology and Prevention; et al. Cardiopulmonary Impact of Electronic Cigarettes and Vaping Products: A Scientific Statement from the American Heart Association. Circulation 2023, 148, 703–728. [Google Scholar] [CrossRef]
- Papaefstathiou, E.; Bezantakos, S.; Stylianou, M.; Biskos, G.; Agapiou, A. Comparison of particle size distributions and volatile organic compounds exhaled by e-cigarette and cigarette users. J. Aerosol Sci. 2020, 141, 105487. [Google Scholar] [CrossRef]
- Sala, C.; Medana, C.; Pellegrino, R.; Aigotti, R.; Bello, F.D.; Bianchi, G.; Davoli, E. Dynamic measurement of newly formed carbonyl compounds in vapors from electronic cigarettes. Eur. J. Mass Spectrom. 2017, 23, 64–69. [Google Scholar] [CrossRef]
- Benowitz, N.L.; Fraiman, J.B. Cardiovascular effects of electronic cigarettes. Nat. Rev. Cardiol. 2017, 14, 447–456. [Google Scholar] [CrossRef]
- Sansone, L.; Milani, F.; Fabrizi, R.; Belli, M.; Cristina, M.; Zagà, V.; Russo, P. Nicotine: From Discovery to Biological Effects. Int. J. Mol. Sci. 2023, 24, 14570. [Google Scholar] [CrossRef]
- Eltorai, A.E.; Choi, A.R.; Eltorai, A.S. Impact of Electronic Cigarettes on Various Organ Systems. Respir. Care 2019, 64, 328–336. [Google Scholar] [CrossRef]
- Jaegers, N.R.; Hu, W.; Weber, T.J.; Hu, J.Z. Low-temperature (< 200 °C) degradation of electronic nicotine delivery system liquids generates toxic aldehydes. Sci. Rep. 2021, 11, 7800. [Google Scholar] [CrossRef]
- Erythropel, H.C.; Jabba, S.V.; DeWinter, T.M.; Mendizabal, M.; Anastas, P.T.; Jordt, S.E.; Zimmerman, J.B. Formation of flavorant-propylene Glycol Adducts with Novel Toxicological Properties in Chemically Unstable E-Cigarette Liquids. Nicotine Tob. Res. 2019, 21, 1248–1258. [Google Scholar] [CrossRef] [PubMed]
- Krüsemann, E.J.Z.; Boesveldt, S.; de Graaf, K.; Talhout, R. An E-Liquid Flavor Wheel: A Shared Vocabulary Based on Systematically Reviewing E-Liquid Flavor Classifications in Literature. Nicotine Tob. Res. 2019, 21, 1310–1319. [Google Scholar] [CrossRef] [PubMed]
- Muthumalage, T.; Lamb, T.; Friedman, M.R.; Rahman, I. E-cigarette flavored pods induce inflammation, epithelial barrier dysfunction, and DNA damage in lung epithelial cells and monocytes. Sci. Rep. 2019, 9, 19035. [Google Scholar] [CrossRef]
- Kassem, N.O.F.; Strongin, R.M.; Stroup, A.M.; Brinkman, M.C.; El-Hellani, A.; Erythropel, H.C.; Etemadi, A.; Exil, V.; Goniewicz, M.L.; Kassem, N.O.; et al. A Review of the Toxicity of Ingredients in e-Cigarettes, Including Those Ingredients Having the FDA’s “Generally Recognized as Safe (GRAS)” Regulatory Status for Use in Food. Nicotine Tob. Res. 2024, 26, 1445–1454. [Google Scholar] [CrossRef]
- Henry, T.S.; Kanne, J.P.; Kligerman, S.J. Imaging of Vaping-Associated Lung Disease. N. Engl. J. Med. 2019, 381, 1486–1487. [Google Scholar] [CrossRef]
- Layden, J.E.; Ghinai, I.; Pray, I.; Kimball, A.; Layer, M.; Tenforde, M.W.; Meiman, J. Pulmonary Illness Related to E-Cigarette Use in Illinois and Wisconsin-Final Report. N. Engl. J. Med. 2020, 382, 903–916. [Google Scholar] [CrossRef]
- Maddock, S.D.; Cirulis, M.M.; Callahan, S.J.; Keenan, L.M.; Pirozzi, C.S.; Raman, S.M.; Aberegg, S.K. Pulmonary Lipid-Laden Macrophages and Vaping. N. Engl. J. Med. 2019, 381, 1488–1489. [Google Scholar] [CrossRef]
- Blount, B.C.; Karwowski, M.P.; Shields, P.G.; Morel-Espinosa, M.; Valentin-Blasini, L.; Gardner, M.; Pirkle, J.L. Vitamin E Acetate in Bronchoalveolar-Lavage Fluid Associated with EVALI. N. Engl. J. Med. 2020, 382, 697–705. [Google Scholar] [CrossRef]
- Callahan, S.J.; Beck, E.; Warren, K.J.; Blagev, D.; Lanspa, M.; Lee Harris, D.; Paine, R. Vitamin E acetate continues to drive cases of evali. Chest 2023, 164, A6375–A6376. [Google Scholar] [CrossRef]
- Park, J.A.; Crotty Alexander, L.E.; Christiani, D.C. Vaping and Lung Inflammation and Injury. Annu. Rev. Physiol. 2022, 84, 611–629. [Google Scholar] [CrossRef]
- O’Callaghan, M.; Boyle, N.; Fabre, A.; Keane, M.P.; McCarthy, C. Vaping-Associated Lung Injury: A Review. Medicina 2022, 58, 412. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, H.; Kitzmiller, J.; Nguyen, K.; Chuong, N.; Bui, T. Oral Carcinoma Associated with Chronic Use of Electronic Cigarettes. Otolaryngology 2017, 7, 304. [Google Scholar] [CrossRef]
- Klawinski, D.; Hanna, I.; Breslin, N.K.; Katzenstein, H.M.; Indelicato, D.J. Vaping the Venom: Oral Cavity Cancer in a Young Adult With Extensive Electronic Cigarette Use. Pediatrics 2021, 147, e2020022301. [Google Scholar] [CrossRef] [PubMed]
- Shields, C.L.; Kim, M.; Lally, S.E.; Chévez-Barrios, P.; Shields, J.A. Eye cancer in a young male with a vaping history. Indian J. Ophthalmol. 2020, 68, 1699–1701. [Google Scholar] [CrossRef]
- Ballenberger, M.; Vojnic, M.; Indaram, M.; Machnicki, S.; Harshan, M.; Novoselac, A.V.; Singh, A.; Mina, B. A 33-Year-Old Man with Chest Pain. Chest 2022, 161, e43–e49. [Google Scholar] [CrossRef]
- Patel, D.; Taudte, R.V.; Nizio, K.; Herok, G.; Cranfield, C.; Shimmon, R. Headspace analysis of E-cigarette fluids using comprehensive two dimensional GC×GC-TOF-MS reveals the presence of volatile and toxic compounds. J. Pharm. Biomed. Anal. 2021, 196, 113930. [Google Scholar] [CrossRef]
- Cromwell, B.; Mota, L.; Levine, M. Detection of Potentially Toxic Additives in Electronic Cigarettes and Cigarette Flavourings. Anal. Lett. 2019, 53, 1407–1415. [Google Scholar] [CrossRef]
- Geng, X.; Wang, Y.; Li, H.; Chen, D.D.Y. Characterization of cigarette smokeomics by in situ solid-phase microextraction and confined-space direct analysis in real time mass spectrometry. Talant 2024, 280, 126680. [Google Scholar] [CrossRef]
- Kubica, P. Ultrasound-Assisted Solvent Extraction of a Porous Membrane Packed Sample for the Determination of Tobacco-Specific Nitrosamines in the Replacement Liquids for E-Cigarettes. Molecules 2019, 24, 4618. [Google Scholar] [CrossRef]
- Deng, H.; Tang, S.; Yang, F.; Chen, D.; Bian, Z.; Wang, Y.; Lee, H.K. Recent advances in the analysis of electronic cigarette liquids and aerosols: Sample preparation and chromatographic characterization. J. Chromatogr. A 2023, 1712, 464495. [Google Scholar] [CrossRef]
- Augustini, A.L.R.M.; Sielemann, S.; Telgheder, U. Strategy for the identification of flavor compounds in e-liquids by correlating the analysis of GCxIMS and GC-MS. Talanta 2021, 230, 122318. [Google Scholar] [CrossRef] [PubMed]
- LeBouf, R.F.; Burns, D.A.; Ranpara, A.; Attfield, K.; Zwack, L.; Stefaniak, A.B. Headspace analysis for screening of volatile organic compound profiles of electronic juice bulk material. Anal. Bioanal. Chem. 2018, 410, 5951–5960. [Google Scholar] [CrossRef] [PubMed]
- Augustini, A.; Sielemann, S.; Telgheder, U. Quantitation of Flavor Compounds in Refill Solutions for Electronic Cigarettes Using HS-GCxIMS and Internal Standards. Molecules 2022, 27, 8067. [Google Scholar] [CrossRef]
- Barhdadi, S.; Mertens, B.; Van Bossuyt, M.; Van De Maele, J.; Anthonissen, R.; Canfyn, M.; Vanhaecke, T. Identification of flavouring substances of genotoxic concern present in e-cigarette refills. Food Chem. Toxicol. 2021, 147, 111864. [Google Scholar] [CrossRef]
- Budzyńska, E.; Sielemann, S.; Puton, J.; Surminski, A.L.R.M. Analysis of e-liquids for electronic cigarettes using GC-IMS/MS with headspace sampling. Talanta 2020, 209, 120594. [Google Scholar] [CrossRef]
- Omaiye, E.E.; Luo, W.; McWhirter, K.J.; Pankow, J.F.; Talbot, P. Flavour chemicals, synthetic coolants and pulegone in popular mint-flavoured and menthol-flavoured e-cigarettes. Tob. Control 2022, 31, e3–e9. [Google Scholar] [CrossRef]
- Barhdadi, S.; Canfyn, M.; Courselle, P.; Rogiers, V.; Vanhaecke, T.; Deconinck, E. Development and validation of a HS/GC–MS method for the simultaneous analysis of diacetyl and acetylpropionyl in electronic cigarette refills. J. Pharm. Biomed. Anal. 2017, 142, 218–224. [Google Scholar] [CrossRef]
- Poklis, J.L.; Wolf, C.E., 2nd; Peace, M.R. Ethanol concentration in 56 refillable electronic cigarettes liquid formulations determined by headspace gas chromatography with flame ionization detector (HS-GC-FID). Drug Test. Anal. 2017, 9, 1637–1640. [Google Scholar] [CrossRef]
- Augustini, A.L.R.M.; Borg, C.; Sielemann, S.; Telgheder, U. Making Every Single Puff Count—Simple and Sensitive E-Cigarette Aerosol Sampling for GCxIMS and GC-MS Analysis. Molecules 2023, 28, 6574. [Google Scholar] [CrossRef]
- Berenguer, C.; Pereira, J.A.M.; Câmara, J.S. Fingerprinting the volatile profile of traditional tobacco and e-cigarettes: A comparative study. Microchem. J. 2021, 166, 106196. [Google Scholar] [CrossRef]
- Papaefstathiou, E.; Stylianou, M.; Andreou, C.; Agapiou, A. Breath analysis of smokers, non-smokers, and e-cigarette users. J. Chromatogr. B 2020, 1160, 122349. [Google Scholar] [CrossRef] [PubMed]
- Krakowiak, R.I.; Poklis, J.L.; Peace, M.R. The Analysis of Aerosolized Methamphetamine from E-cigarettes Using High Resolution Mass Spectrometry and Gas Chromatography Mass Spectrometry. J. Anal. Toxicol. 2019, 43, 592–599. [Google Scholar] [CrossRef] [PubMed]
- Guo, W.; Vrdoljak, G.; Liao, V.C.; Moezzi, B. Major Constituents of Cannabis Vape Oil Liquid, Vapor and Aerosol in California Vape Oil Cartridge Samples. Front. Chem. 2021, 9, 694905. [Google Scholar] [CrossRef] [PubMed]
- Lim, H.H.; Shin, H.S. Measurement of Aldehydes in Replacement Liquids of Electronic Cigarettes by Headspace Gas Chromatography-mass Spectrometry. Bull. Korean Chem. Soc. 2013, 34, 2691–2696. [Google Scholar] [CrossRef]
- Lu, L.; Xiang, M.; Lu, H.; Tian, Z.; Gao, Y. Progress in quantification of nicotine content and form distribution in electronic cigarette liquids and aerosols. Anal. Meth. 2022, 14, 359–377. [Google Scholar] [CrossRef]
- Peace, M.R.; Krakowiak, R.I.; Wolf, C.E.; Poklis, A.; Poklis, J.L. Identification of MDMB-FUBINACA in commercially available e-liquid formulations sold for use in electronic cigarettes. Forensic Sci. Int. 2017, 271, 92–97. [Google Scholar] [CrossRef]
- Barhdadi, S.; Courselle, P.; Deconinck, E.; Vanhee, C. The analysis of cannabinoids in e-cigarette liquids using LC-HRAM-MS and LC-UV. J. Pharm. Biomed. Anal. 2023, 230, 115394. [Google Scholar] [CrossRef]
- Wang, C.; Li, W.; Zeng, Y.; Wu, Y.; Chen, D.; Meng, H.; Liu, K. Determination of four tobacco-specific nitrosamines in electronic cigarette liquids and aerosols by UPLC-QTOF-HRMS. Chin. J. Anal. Chem. 2024, 52, 100430. [Google Scholar] [CrossRef]
- Aszyk, J.; Kubica, P.; Kot-Wasik, A.; Namieśnik, J.; Wasik, A. Comprehensive determination of flavouring additives and nicotine in e-cigarette refill solutions. Part I: Liquid chromatography-tandem mass spectrometry analysis. J. Chromatogr. A. 2017, 1519, 45–54. [Google Scholar] [CrossRef]
- Almazrouei, E.S.; Bintamim, A.A.; Khalil, S.E.A.; Alremeithi, R.; Gewily, S. The identification of drugs of abuse in E-cigarette samples seized in Dubai between 2016 and 2020. Forensic Sci. Int. 2022, 333, 111233. [Google Scholar] [CrossRef]
- Kim, M.; An, S.; Kim, J.; Jung, H. Determination of cannabinoids in illegal e-cigarette fluids in Korea by LC-MS/MS. Toxicol. Anal. Clin. 2022, 34 (Suppl. S3), S129. [Google Scholar] [CrossRef]
- Outhous, A.E.; Holt, A.K.; Poklis, J.L.; Peace, M.R. Evaluation of cannabis product mislabeling: The development of a unified cannabinoid LC-MS/MS method to analyze e-liquids and edible products. Talanta Open 2024, 10, 100349. [Google Scholar] [CrossRef]
- Benowitz, N.L.; St Helen, G.; Liakoni, E. Clinical Pharmacology of Electronic Nicotine Delivery Systems (ENDS): Implications for Benefits and Risks in the Promotion of the Combusted Tobacco Endgame. J. Clin. Pharmacol. 2021, 61 (Suppl. S2), S18–S36. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Patra, J.K.; Shin, H.S. Analytical methods for determination of carbonyl compounds and nicotine in electronic No-Smoking aid refill solutions. Anal. Biochem. 2020, 588, 113470. [Google Scholar] [CrossRef]
- Kubica, P. Determination of Glycerol, Propylene Glycol, and Nicotine as the Main Components in Refill Liquids for Electronic Cigarettes. Molecules 2023, 28, 4425. [Google Scholar] [CrossRef]
- Dai, J.; Kim, K.H.; Szulejko, J.E.; Jo, S.H.; Kwon, K.; Choi, D.W. Quantification of nicotine and major solvents in retail electronic cigarette fluids and vaped aerosols. Microchem. J. 2018, 140, 262–268. [Google Scholar] [CrossRef]
- Alhusban, A.A.; Ata, S.A. Simple HPLC method for rapid quantification of nicotine content in e-cigarettes liquids. Acta Chromatogr. 2021, 33, 302–307. [Google Scholar] [CrossRef]
- Barhdadi, S.; Desmedt, B.; Courselle, P.; Rogiers, V.; Vanhaecke, T.; Deconinck, E. A simple dilute-and-shoot method for screening and simultaneous quantification of nicotine and alkaloid impurities in electronic cigarette refills (e-liquids) by UHPLC-DAD. J. Pharm. Biomed. Anal. 2019, 169, 225–234. [Google Scholar] [CrossRef]
- Beyer, J.; Jonsson, G.; Porte, C.; Krahn, M.M.; Ariese, F. Analytical methods for determining metabolites of polycyclic aromatic hydrocarbon (PAH) pollutants in fish bile: A review. Environ. Toxicol. Pharmacol. 2010, 30, 224–244. [Google Scholar] [CrossRef]
- ATSDR. Toxicological Profile for Polycyclic Aromatic Hydrocarbons; Agency for Toxic Substances and Disease Registry (US): Atlanta, GA, USA, 1995. Available online: https://wwwn.cdc.gov/TSP/MRLS/mrlsListing.aspx (accessed on 1 March 2024).
- McGrath, T.E.; Wooten, J.B.; Geoffrey Chan, W.; Hajaligol, M.R. Formation of polycyclic aromatic hydrocarbons from tobacco: The link between low temperature residual solid (char) and PAH formation. Food Chem. Toxicol. 2007, 45, 1039–1050. [Google Scholar] [CrossRef]
- Sánchez, N.E.; Callejas, A.; Millera, Á.; Bilbao, R.; Alzueta, M.U. Polycyclic Aromatic Hydrocarbon (PAH) and Soot Formation in the Pyrolysis of Acetylene and Ethylene: Effect of the Reaction Temperature. Energy Fuels 2012, 26, 4823–4829. [Google Scholar] [CrossRef]
- McGrath, T.E.; Chan, W.G.; Hajaligol, M.R. Low temperature mechanism for the formation of polycyclic aromatic hydrocarbons from the pyrolysis of cellulose. J. Anal. Appl. Pyrolysis 2003, 66, 51–70. [Google Scholar] [CrossRef]
- Parker, D.S.N.; Zhang, F.; Kim, Y.S.; Kaiser, R.I.; Landera, A.; Kislov, V.V.; Tielens, A.G.G.M. Low temperature formation of naphthalene and its role in the synthesis of PAHs (Polycyclic Aromatic Hydrocarbons) in the interstellar medium. Proc. Natl. Acad. Sci. USA 2012, 109, 53–58. [Google Scholar] [CrossRef] [PubMed]
- Beauval, N.; Antherieu, S.; Soyez, M.; Gengler, N.; Grova, N.; Howsam, M.; Garat, A. Chemical Evaluation of Electronic Cigarettes: Multicomponent Analysis of Liquid Refills and their Corresponding Aerosols. J. Anal. Toxicol. 2017, 41, 670–678. [Google Scholar] [CrossRef]
- Alarabi, A.B.; Lozano, P.A.; Khasawneh, F.T.; Alshbool, F.Z. The effect of emerging tobacco related products and their toxic constituents on thrombosis. Life Sci. 2022, 290, 120255. [Google Scholar] [CrossRef]
- Hecht, S.S.; Gupta, P.C.; Sturla, S.J.; Wang, Y. 50 Years of Research on Tobacco-Specific Nitrosamines: A Virtual Collection of Emerging Knowledge of Chemical Toxicology of Tobacco and Nicotine Delivery Systems and Call for Contributions to a Landmark Special Issue. Chem. Res. Toxicol. 2022, 35, 899–900. [Google Scholar] [CrossRef]
- Omare, M.O.; Kibet, J.K.; Cherutoi, J.K.; Kengara, F.O. A review of tobacco abuse and its epidemiological consequences. Z. Gesundh. Wiss. 2022, 30, 1485–1500. [Google Scholar] [CrossRef]
- Gushgari, A.J.; Halden, R.U. Critical review of major sources of human exposure to N-nitrosamines. Chemosphere 2018, 210, 1124–1136. [Google Scholar] [CrossRef]
- Wang, J.; Shi, H.; Zhou, J.; Bai, R.; Zhang, M.; Jin, T. Nitrate and Nitrite Promote Formation of Tobacco-Specific Nitrosamines via Nitrogen Oxides Intermediates during Postcured Storage under Warm Temperature. J. Chem. 2017, 2017, 6135215. [Google Scholar] [CrossRef]
- Stanfill, S.B.; Hecht, S.S.; Joerger, A.C.; González, P.J.; Maia, L.B.; Rivas, M.G.; Mehrotra, R. From cultivation to cancer: Formation of N-nitrosamines and other carcinogens in smokeless tobacco and their mutagenic implications. Crit. Rev. Toxicol. 2023, 53, 658–701. [Google Scholar] [CrossRef]
- Jin, X.; Wagner, K.; Melvin, M.; Smith, D.; Pithawalla, Y.; Gardner, W.; Karles, G. Influence of Nitrite on Formation of Tobacco-Specific Nitrosamines in Electronic Cigarette Liquids and Aerosols. Chem. Res. Toxicol. 2022, 35, 782–791. [Google Scholar] [CrossRef] [PubMed]
- IARC. Tobacco Habits Other than Smoking; Betel-Quid and Areca-Nut Chewing; and Some Related Nitrosamines. [ONLINE]. 1985. Available online: https://publications.iarc.fr/Book-And-Report-Series/Iarc-Monographs-On-The-Identification-Of-Carcinogenic-Hazards-To-Humans/Tobacco-Habits-Other-Than-Smoking-Betel-Quid-And-Areca-Nut-Chewing-And-Some-Related-Nitrosamines-1985 (accessed on 1 March 2025).
- IARC. Smokeless Tobacco and Some Tobacco-specific N-Nitrosamines. [ONLINE]. 2007. Available online: https://publications.iarc.fr/Book-And-Report-Series/Iarc-Monographs-On-The-Identification-Of-Carcinogenic-Hazards-To-Humans/Smokeless-Tobacco-And-Some-Tobacco-specific-Em-N-Em--Nitrosamines-2007 (accessed on 1 December 2024).
- Nestor, T.B.; Gentry, J.S.; Peele, D.M.; Riddick, M.G.; Conner, B.T.; Edwards, M.E. Role of Oxides of Nitrogen in Tobacco-Specific Nitrosamine Formation in Flue-Cured Tobacco. Beitr. Tabakforsch. Int. 2014, 20, 467–475. [Google Scholar] [CrossRef]
- FDA. Harmful and Potentially Harmful Constituents in Tobacco Products and Tobacco Smoke; Established List. [ONLINE]. 2012. Available online: https://www.federalregister.gov/documents/2012/04/03/2012-7727/harmful-and-potentially-harmful-constituents-in-tobacco-products-and-tobacco-smoke-established-list (accessed on 1 March 2025).
- Pérez-Ortuño, R.; Martínez-Sánchez, J.M.; Fu, M.; Ballbè, M.; Quirós, N.; Fernández, E.; Pascual, J.A. Assessment of tobacco specific nitrosamines (TSNAs) in oral fluid as biomarkers of cancer risk: A population-based study. Environ. Res. 2016, 151, 635–641. [Google Scholar] [CrossRef]
- Pérez-Ortuño, R.; Martínez-Sánchez, J.M.; Fu, M.; Fernández, E.; Pascual, J.A. Evaluation of tobacco specific nitrosamines exposure by quantification of 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone (NNK) in human hair of non-smokers. Sci. Rep. 2016, 6, 25043. [Google Scholar] [CrossRef]
- Martínez-Sánchez, J.M.; Ballbè, M.; Pérez-Ortuño, R.; Fu, M.; Sureda, X.; Pascual, J.A.; Fernández, E. Secondhand exposure to aerosol from electronic cigarettes: Pilot study of assessment of tobacco-specific nitrosamine (NNAL) in urine. Gac. Sanit. 2019, 33, 575–578. [Google Scholar] [CrossRef]
- Lee, J.W.; Kim, S. Comparison of a Tobacco-Specific Carcinogen in Tobacco Cigarette, Electronic Cigarette, and Dual Users. J. Korean Med. Sci. 2023, 38, e140. [Google Scholar] [CrossRef]
- Lee, Y.S.; Kim, K.H.; Lee, S.S.; Brown, R.J.C.; Jo, S.H. Analytical Method for Measurement of Tobacco-Specific Nitrosamines in E-Cigarette Liquid and Aerosol. Appl. Sci. 2018, 8, 2699. [Google Scholar] [CrossRef]
- World Health Organization. Standard Operating Procedure for Determination of Tobacco-Specific Nitrosamines in Mainstream Cigarette Smoke Under ISO and Intense Smoking Conditions: WHO TobLabNet Official Method SOP 03. [ONLINE]. World Health Organization, 2014; Available online: https://iris.who.int/handle/10665/136000 (accessed on 1 March 2024).
- CORESTA. Determination of Tobacco-Specific Nitrosamines in Mainstream Smoke by LC-MS/MS. [ONLINE]. 2022. Available online: https://www.coresta.org/determination-tobacco-specific-nitrosamines-mainstream-smoke-lc-msms-29198.html (accessed on 1 December 2024).
- Farsalinos, K.E.; Gillman, G.; Poulas, K.; Voudris, V. Tobacco-Specific Nitrosamines in Electronic Cigarettes: Comparison between Liquid and Aerosol Levels. Int. J. Environ. Res. Public Health 2015, 12, 9046–9053. [Google Scholar] [CrossRef]
- Smith, D.M.; Shahab, L.; Blount, B.C.; Gawron, M.; Kosminder, L.; Sobczak, A.; Goniewicz, M.L. Differences in Exposure to Nicotine, Tobacco-Specific Nitrosamines, and Volatile Organic Compounds among Electronic Cigarette Users, Tobacco Smokers, and Dual Users from Three Countries. Toxics 2020, 8, 88. [Google Scholar] [CrossRef]
- Goniewicz, M.L.; Gawron, M.; Smith, D.M.; Peng, M.; Jacob, P., 3rd; Benowitz, N.L. Exposure to Nicotine and Selected Toxicants in Cigarette Smokers Who Switched to Electronic Cigarettes: A Longitudinal Within-Subjects Observational Study. Nicotine Tob. Res. 2017, 19, 160–167. [Google Scholar] [CrossRef]
- Round, E.K.; Chen, P.; Taylor, A.K.; Schmidt, E. Biomarkers of Tobacco Exposure Decrease After Smokers Switch to an E-Cigarette or Nicotine Gum. Nicotine Tob. Res. 2019, 21, 1239–1247. [Google Scholar] [CrossRef] [PubMed]
- Behar, R.Z.; Hua, M.; Talbot, P. Puffing topography and nicotine intake of electronic cigarette users. PLoS ONE 2015, 10, e0117222. [Google Scholar] [CrossRef]
- Robinson, R.J.; Hensel, E.C.; Morabito, P.N.; Roundtree, K.A. Electronic Cigarette Topography in the Natural Environment. PLoS ONE 2015, 10, e0129296. [Google Scholar] [CrossRef]
- Cho, Y.J.; Mehta, T.; Hinton, A.; Sloan, R.; Nshimiyimana, J.; Tackett, A.P.; Wagener, T.L. E-Cigarette Nicotine Delivery Among Young Adults by Nicotine Form, Concentration, and Flavor: A Crossover Randomized Clinical Trial. JAMA Netw. Open 2024, 7, e2426702. [Google Scholar] [CrossRef]
- Gholap, V.V.; Kosmider, L.; Golshahi, L.; Halquist, M.S. Nicotine forms: Why and how do they matter in nicotine delivery from electronic cigarettes? Expert Opin. Drug Deliv. 2020, 17, 1727–1736. [Google Scholar] [CrossRef]
- Abdelghani, J.; Alkhwarah, M.; El-Sheikh, A. Monitoring Heavy Metals in e-Liquids upon Vaping using Nano-Magnetic Graphene Oxide Extractor with ICP-OES Detection. Res. Sq. 2024. [Google Scholar] [CrossRef]
- Gray, N.; Halstead, M.; Gonzalez-Jimenez, N.; Valentin-Blasini, L.; Watson, C.; Pappas, R.S. Analysis of Toxic Metals in Liquid from Electronic Cigarettes. Int. J. Environ. Res. Public Health 2019, 16, 4450. [Google Scholar] [CrossRef]
- Gray, N.; Halstead, M.; Valentin-Blasini, L.; Watson, C.; Pappas, R.S. Toxic Metals in Liquid and Aerosol from Pod-Type Electronic Cigarettes. J. Anal. Toxicol. 2022, 46, 69–75. [Google Scholar] [CrossRef]
- Halstead, M.; Gray, N.; Gonzalez-Jimenez, N.; Fresquez, M.; Valentin-Blasini, L.; Watson, C.; Pappas, R.S. Analysis of Toxic Metals in Electronic Cigarette Aerosols Using a Novel Trap Design. J. Anal. Toxicol. 2020, 44, 149–155. [Google Scholar] [CrossRef]
- Kamilari, E.; Farsalinos, K.; Poulas, K.; Kontoyannis, C.G.; Orkoula, M.G. Detection and quantitative determination of heavy metals in electronic cigarette refill liquids using Total Reflection X-ray Fluorescence Spectrometry. Food Chem. Toxicol. 2018, 116, 233–237. [Google Scholar] [CrossRef]
- Liu, Q.; Huang, C.; Chris Le, X. Arsenic species in electronic cigarettes: Determination and potential health risk. J. Environ. Sci. Stud. 2020, 91, 168–176. [Google Scholar] [CrossRef] [PubMed]
- Ting, C.Y.; Ahmad Sabri, N.A.; Tiong, L.L.; Zailani, H.; Wong, L.P.; Agha Mohammadi, N.; Anchah, L. Heavy metals (Cr, Pb, Cd, Ni) in aerosols emitted from electronic cigarettes sold in Malaysia. J. Environ. Sci. Health A Toxic Hazard Subst. Environ. Eng. 2020, 55, 55–62. [Google Scholar] [CrossRef] [PubMed]
- Zervas, E.; Matsouki, N.; Kyriakopoulos, G.; Poulopoulos, S.; Ioannides, T.; Katsaounou, P. Transfer of metals in the liquids of electronic cigarettes. Inhal. Toxicol. 2020, 32, 240–248. [Google Scholar] [CrossRef] [PubMed]
- Zhao, D.; Navas-Acien, A.; Ilievski, V.; Slavkovich, V.; Olmedo, P.; Adria-Mora, B.; Hilpert, M. Metal concentrations in electronic cigarette aerosol: Effect of open-system and closed-system devices and power settings. Environ. Res. 2019, 174, 125–134. [Google Scholar] [CrossRef]
- Zhao, D.; Ilievski, V.; Slavkovich, V.; Olmedo, P.; Domingo-Relloso, A.; Rule, A.M.; Hilpert, M. Effects of e-liquid flavor, nicotine content, and puff duration on metal emissions from electronic cigarettes. Environ. Res. 2022, 204, 112270. [Google Scholar] [CrossRef]
- Eshraghian, E.A.; Al-Delaimy, W.K. A review of constituents identified in e-cigarette liquids and aerosols. Tob. Prev. Cessat. 2021, 7, 10. [Google Scholar] [CrossRef]
- Kim, J.J.; Sabatelli, N.; Tutak, W.; Giuseppetti, A.; Frukhtbeyn, S.; Shaffer, I.; Ondov, J.M. Universal electronic-cigarette test: Physiochemical characterization of reference e-liquid. Tob. Induc. Dis. 2017, 15, 14. [Google Scholar] [CrossRef]
- Mallampati, S.R.; McDaniel, C.; Wise, A.R. Strategies for Nonpolar Aerosol Collection and Heavy Metals Analysis of Inhaled Cannabis Products. ACS Omega 2021, 6, 17126. [Google Scholar] [CrossRef]
- Omaiye, E.E.; Williams, M.; Bozhilov, K.N.; Talbot, P. Design features and elemental/metal analysis of the atomizers in pod-style electronic cigarettes. PLoS ONE 2021, 16, e0248127. [Google Scholar] [CrossRef]
- Hess, C.A.; Olmedo, P.; Navas-Acien, A.; Goessler, W.; Cohen, J.E.; Rule, A.M. E-cigarettes as a source of toxic and potentially carcinogenic metals. Environ. Res. 2017, 152, 221–225. [Google Scholar] [CrossRef]
- Mulder, H.A.; Stewart, J.B.; Blue, I.P.; Krakowiak, R.I.; Patterson, J.L.; Karin, K.N.; Peace, M.R. Characterization of E-cigarette coil temperature and toxic metal analysis by infrared temperature sensing and scanning electron microscopy-energy-dispersive X-ray. Inhal. Toxicol. 2020, 32, 447–455. [Google Scholar] [CrossRef] [PubMed]
- Williams, M.; Bozhilov, K.; Ghai, S.; Talbot, P. Elements including metals in the atomizer and aerosol of disposable electronic cigarettes and electronic hookahs. PLoS ONE 2017, 12, e0175430. [Google Scholar] [CrossRef]
- Williams, M.; Bozhilov, K.N.; Talbot, P. Analysis of the elements and metals in multiple generations of electronic cigarette atomizers. Environ. Res. 2019, 175, 156–166. [Google Scholar] [CrossRef] [PubMed]
- Na, C.J.; Jo, S.H.; Kim, K.H.; Sohn, J.R.; Son, Y.S. The transfer characteristics of heavy metals in electronic cigarette liquid. Environ. Res. 2019, 174, 152–159. [Google Scholar] [CrossRef]
- Zhao, J.; Nelson, J.; Dada, O.; Pyrgiotakis, G.; Kavouras, I.; Demokritou, P. Assessing electronic cigarette emissions: Linking physico-chemical properties to product brand, e-liquid flavoring additives, operational voltage and user puffing patterns. Inhal. Toxicol. 2018, 30, 78–88. [Google Scholar] [CrossRef]
- Kapiamba, K.F.; Hao, W.; Owusu, S.Y.; Liu, W.; Huang, Y.W.; Wang, Y. Examining Metal Contents in Primary and Secondhand Aerosols Released by Electronic Cigarettes. Chem. Res. Toxicol. 2022, 35, 954–962. [Google Scholar] [CrossRef]
- WHO. WHO Study Group on Tobacco Product Regulation. [ONLINE]. 2015. Available online: https://iris.who.int/bitstream/handle/10665/161512/9789241209892.pdf?sequence=1 (accessed on 1 March 2025).
- McAdam, K.; Waters, G.; Moldoveanu, S.; Margham, J.; Cunningham, A.; Vas, C.; Porter, A.; Digard, H. Diacetyl and Other Ketones in e-Cigarette Aerosols: Some Important Sources and Contributing Factors. Front. Chem. 2021, 9, 742538. [Google Scholar] [CrossRef]
- ICH. ICH Guideline Q3D (R1) on Elemental Impurities. [ONLINE]. 2019. Available online: https://www.ema.europa.eu/en/documents/scientific-guideline/international-conference-harmonisation-technical-requirements-registration-pharmaceuticals-human-use-ich-q3d-elemental-impurities-step-5-revision-1_en.pdf (accessed on 1 March 2025).
- ATSDR. Minimal Risk Levels (MRLs) for Hazardous Substances. [ONLINE]. 2024. Available online: https://wwwn.cdc.gov/TSP/ToxProfiles/ToxProfiles.aspx?id=122&tid=25 (accessed on 1 March 2025).
- NIOSH. NIOSH Pocket Guide to Chemical Hazards. [ONLINE]. 2020. Available online: https://www.cdc.gov/niosh/npg/default.html (accessed on 1 March 2025).
- Fang, W.; Yang, Y.; Xu, Z. PM10 and PM2.5 and health risk assessment for heavy metals in a typical factory for cathode ray tube television recycling. Environ. Sci. Technol. 2013, 47, 12469–12476. [Google Scholar] [CrossRef]
- Guo, W.; Zhang, X.; Yue, J.; Gao, Y.; Tillotson, M.R.; Zhao, X. Nasal filter reveal exposure risks of inhalable particulates and heavy metals in urban women. Environ. Int. 2024, 188, 108743. [Google Scholar] [CrossRef]
- Heidari, M.; Darijani, T.; Alipour, V. Heavy metal pollution of road dust in a city and its highly polluted suburb; quantitative source apportionment and source-specific ecological and health risk assessment. Chemosphere 2021, 273, 129656. [Google Scholar] [CrossRef]
- IARC. Cobalt in hard metals and cobalt sulfate, gallium arsenide, indium phosphide and vanadium pentoxide. IARC Monogr. Eval. Carcinog. Risks Hum. 2006, 86, 1–294. [Google Scholar]
- Chen, C.; Huo, C.; Mattey-Mora, P.P.; Bidulescu, A.; Parker, M.A. Assessing the association between e-cigarette use and cardiovascular disease: A meta-analysis of exclusive and dual use with combustible cigarettes. Addict. Behav. 2024, 157, 108086. [Google Scholar] [CrossRef]
- Kumar, K.; Anjali, S.; Sharma, S. Effect of lead exposure on histone modifications: A review. J. Biochem. Mol. Toxicol. 2024, 38, e23547. [Google Scholar] [CrossRef]
- Yau, W.H.; Chen, S.C.; Wu, D.W.; Chen, H.C.; Lin, H.H.; Wang, C.W.; Kuo, C.H. Blood lead (Pb) is associated with lung fibrotic changes in non-smokers living in the vicinity of petrochemical complex: A population-based study. Environ. Sci. Pollut. Res. Int. 2023, 30, 75225–75234. [Google Scholar] [CrossRef]
- Dill, M.; Barhdadi, S.; Vanhee, C.; Deconinck, E. Sample preparation methods for elemental analysis in electronic cigarette aerosols: A critical review. Anal. Methods 2025, 17, 1997–2014. [Google Scholar] [CrossRef]
- Ganapathy, V.; Jaganathan, R.; Chinnaiyan, M.; Chengizkhan, G.; Sadhasivam, B.; Manyanga, J.; Ramachandran, I.; Queimado, L. E-Cigarette effects on oral health: A molecular perspective. Food Chem. Toxicol. 2025, 196, 115216. [Google Scholar] [CrossRef]
- Chong-Silva, D.C.; Sant’Anna, M.d.F.B.P.; Riedi, C.A.; Sant’Anna, C.C.; Ribeiro, J.D.; Vieira, L.M.N.; Pinto, L.A.; Terse-Ramos, R.; Morgan, M.A.P.; Godinho, R.N.; et al. Electronic cigarettes: “wolves in sheep’s clothing”. J. Pediatr. 2025, 101, 122–132. [Google Scholar] [CrossRef]
- Aizezi, N.; Ye, Y.; Chen, Z.; Liu, Y. Impact of soldering temperatures on heavy metal and dust emissions: A LIBS-based environmental pollution analysis. Spectrochim. Acta Part B At. Spectrosc. 2025, 225, 107124. [Google Scholar] [CrossRef]
- Inobeme, A.; Mathew, J.T.; Jatto, E.; Inobeme, J.; Adetunji, C.O.; Muniratu, M.; Onyeachu, B.I.; Adekoya, M.A.; Ajai, A.I.; Mann, A.; et al. Recent advances in instrumental techniques for heavy metal quantification. Environ. Monit. Assess. 2023, 195, 452. [Google Scholar] [CrossRef]
- Clavijo Morales, J.A.; Zea Ramírez, H.R. Simultaneous quantification of Hg(II) and Pb(II) by square wave anodic stripping voltammetry using Bi/graphite electrode. Heliyon 2024, 10, e34656. [Google Scholar] [CrossRef]
- Laghlimi, C.; Moutcine, A.; Elamrani, M.; Chtaini, A.; Isaad, J.; Belkhanchi, H.; Ziat, Y. Investigation on square wave and cyclic voltammetry approaches of the Pb2+, Cd2+, Co2+ and Hg2+ in tap water of Beni Mellal City (Morocco). Desalination Water Treat. 2022, 280, 251–261. [Google Scholar] [CrossRef]
- Collin, M.S.; Venkatraman, S.K.; Vijayakumar, N.; Kanimozhi, V.; Arbaaz, S.M.; Stacey, R.G.S.; Anusha, J.; Choudhary, R.; Lvov, V.; Tovar, G.I.; et al. Bioaccumulation of lead (Pb) and its effects on human: A review. JHM Adv. 2022, 7, 100094. [Google Scholar] [CrossRef]
- Rasin, P.; V, A.A.; Basheer, S.M.; Haribabu, J.; Santibanez, J.F.; Garrote, C.A.; Arulraj, A.; Mangalaraja, R.V. Exposure to cadmium and its impacts on human health: A short review. JHM Adv. 2025, 17, 100608. [Google Scholar] [CrossRef]
- Genchi, G.; Carocci, A.; Lauria, G.; Sinicropi, M.S.; Catalano, A. Nickel: Human Health and Environmental Toxicology. Int. J. Environ. Res. Public Health 2020, 17, 679. [Google Scholar] [CrossRef]
- Hossini, H.; Shafie, B.; Niri, A.D.; Nazari, M.; Esfahlan, A.J.; Ahmadpour, M.; Nazmara, Z.; Ahmadimanesh, M.; Makhdoumi, P.; Mirzaei, N.; et al. A comprehensive review on human health effects of chromium: Insights on induced toxicity. Environ. Sci. Pollut. Res. Int. 2022, 29, 70686–70705. [Google Scholar] [CrossRef]

| Matrix Analyzed | Number of Compounds | Identified or Quantified Compounds (CAS) | Instrumental Technique | Reference |
|---|---|---|---|---|
| Blood orange-flavored e-liquid | 37 | Ethanol (64-17-5), Ethyl acetate (141-78-6), 1-Butanol (71-36-3), 1-Butanol (105-37-3), 3-Methyl-1-butanol (123-51-3), 1,2-Propanediol (57-55-6), Isobutyl acetate (110-19-0), Ethyl butanoate (105-54-4), Butyl acetate (123-86-4), Ethyl 2-methylbutanoate (7452-79-1), 3-Hexen-1-ol (Z) (928-96-1), 1-Hexanol (111-27-3), 3-Methylbutyl acetate (123-92-2), 2-Methylbutyl acetate (624-41-9), Ethyl 4-pentenoate (1968-40-7), Heptanal (111-71-7), (+)-α-Pinene (7785-26-4), 1-Heptanol (111-70-6), (−)-α-Pinene (7785-70-8), 6-Methyl-5-hepten-2-on (110-93-0), β-Myrcene (123-35-3), Ethyl hexanoate (123-66-0), Octanal (1234-13-0), Limonene (138-86-3), Eucalyptol (470-82-6), 3-Methylbutyl butanoate (106-27-4), 1-Octanol (111-87-5), Linalool (78-70-6), Nonanal (124-19-6), Citronellal (106-23-0), Menthon (126-81-8), Menthol (89-78-1), Decanal (112-31-2), Geraniol (106-24-1), Carvone (99-49-0), Undecanal (112-44-7), nicotine (54-11-5) | HS-GCxIMS # and HS-GC-MS | [82] |
| 146 e-liquids (flavor categories: brown, fruit, hybrid dairy, menthol, mint, none, tobacco, and others) | 51 | Ethanol (64-17-5), Acetaldehyde (75-07-0), D-Limonene (5989-27-), Isopropyl alcohol (67-63-0), Acetone (67-64-1), 2,3-Butanedione (431-03-8), α-Pinene (80-56-8), 2,3-Pentanedione (600-14-6), Benzene (71-43-2), m,p-Xylene (108-38-3, 106-42-3), Toluene (108-88-3), o-Xylene (95-47-6), 2,3-Hexanedione (3848-24-6), Methylene chloride (75-09-2), Ethylbenzene (100-41-4), Methyl methacrylate (80-62-6), n-Hexane (110-54-3), Styrene (100-42-5), Chloroform (67-66-3), Ethyl acetate (141-78-6), Ethyl butanoate (105-54-4), Ethyl propionate (105-37-3), Ethyl 2-methylbutanoate (7452-79-1), Isoamyl acetate (123-92-2), β-Pinene (127-91-3), 2-Methylbutyl acetate (624-41-9), Isopentyl isovalerate (659-70-1), Isobutyl acetate (110-19-0), p-Cymene (99-87-6), Ethyl 3-methylbutanoate (108-64-5), γ-Terpinene (99-85-4), 1,3-Dioxolane, 2,2,4-trimethyl (1193-11-9), Ethyl hexanoate (123-66-0), Benzaldehyde (100-52-7), Hexyl acetate (142-92-7), Isobutyl isovalerate (589-59-3), Pentyl acetate (628-63-7), Cyclohexanone, 5-methyl-2-(1-methylethyl)-, (2R-cis) (1196-31-2), Camphene (9-92-5), β-Ocimene (13877-91-3), α-Thujene (2867-05-2), 1,1-Diethoxyethane (105-57-7), Terpinolene (586-62-9), 4-Hexen-1-ol, acetate (7223736-6), (Z)-Ocimene (3338-55-4), 2-Heptanone (110-43-0), Butyl isovalerate (109-19-3), Methylcyclopentane (96-37-7), Phellandral (21391-98-0), 1,8-Cineole (470-82-6) | HS-GC-MS | [83] |
| Flavorless e-liquid and a banana-flavored liquid | 50 | Ethanol (64-17-5), 2-Methylpropanal (78-84-2), Diacetyl (431-03-8), 2-Butanone (78-93-3), Ethyl acetate (141-78-6), Isobutanol (78-83-1), 2-Methylbutanal (96-17-3), 3-Methylbutanal (590-86-3), 2-Pentanone (107-87-9), Ethyl propanoate (105-37-3), Isoamyl alcohol (123-51-3), Methyl isobutyl ketone (108-10-1), Propylene glycol (57-55-6), Isobutyl acetate (110-19-0), 2,3-Hexanedione(110-13-4), 2-Hexanone (591-78-6), Hexanal (66-25-1), Butyl acetate (123-86-4), Furfural (98-01-1), Ethyl 2-methylbutanoate (7452-79-1), trans-2-Hexenal (1335-39-3), trans-2-Hexenol (928-95-0), 3-Methylbutyl acetate (123-92-2), 2-Methylbutyl acetate (585-07-9), 2-Heptanone (110-43-0), Ethyl pentanoate (539-82-2), Methyl hexanoate (106-70-7), (+)-α-Pinene (80-56-8), Benzaldehyde (100-52-7), 1-Octen-3-one (4312-99-6), 2-Octanone (111-13-7), Octanal (124-13-0), trans-2,4-Heptadienal (4313-03-5), D(+)-Limonene ( 5989-27-5), Isoamyl butanoate (106-27-4), 1-Octanol (111-87-5), Allyl hexanoate (123-68-2), 2-Nonanone (821-55-6), Linalool (78-70-6), Isoamyl isovalerate (659-70-1), L-Menthone (14073-97-3), D-Menthone (89-80-5), D/L-Menthol (89-78-1), 2-Decanone (693-54-9), Decanal (112-31-2), Neral (106-26-3), L-Carvone (6485-40-1), Geranial (106-24-1), trans-Anethole (4180-23-8), and Eugenol (97-53-0). | HS-GCxIMS # and HS-GC-MS | [84] |
| 129 e-liquids | 807 | 807 individual substances were identified, including nicotine, nicotine impurities, VOC impurities, additives (diacetin), and flavoring substances (incl. synthetic substances and components from essential oils or other herbal extracts)—list of compounds not reported by the authors | HS-GC-MS | [85] |
| Commercially available e-liquids | 10 | Menthol (2216-51-5), Menthone (14073-97-3), Geraniol (106-24-1), Linalool (78-70-6), 2,3-Butanedione (431-03-8), 2,3-Pentanedione (600-14-6), 2,3-Hexanedione (3848-24-6), 2,3-Heptanedione (96-04-8), Methyl eugenole (93-15-2), Estragole (140-67-0) | HS-GC-IMS/MS | [86] |
| Mint-flavored and menthol-flavored e-cigarettes | 2 | Pulegone (89-82-7), menthol (2216-51-5) | HS-GC-MS | [87] |
| Flavored e-liquid | 2 | Diacetyl (431-03-8) and acetylpropionyl (600-14-6) | HS-GC-MS | [88] |
| Flavored e-liquid | 1 | Ethanol (64-17-5) | HS-GC-FID | [89] |
| Flavored e-liquid | 26 | 2-Methylpropanal (78-84-2), Diacetyl (431-03-8), Ethyl acetate (141-78-6), 2-Methylpropanol (78-83-1), 2-Methylbutanal (96-17-3), Isoamyl alcohol (123-51-3), Methyl isobutyl ketone (108-10-1), 2,3-Hexanedione (3848-24-6), Hexanal (66-25-1), Butyl acetate (123-86-4), Furfural (98-01-1), Ethyl 2-methylbutanoate ((E)-2-Hexenal) (6728-26-3), (E)-2-Hexenol (928-95-0), Ethyl pentanoate (539-82-2), Methyl hexanoate (106-70-7), (+)-α-Pinene (7785-70-8), Benzaldehyde (100-52-7), 1-Octen-3-one (4312-99-6), Octanal (124-13-0), (E,E)-2,4-Heptadienal (05.03.4313), D(+)-Limonene (5989-27-5), Octanol (111-87-5), (821-55-6), Linalool (78-70-6), L/D-Menthone (89-80-5), D/L-Menthol (1490-04-6), Neral/Geranial (5392-40-5) | HS-GCxIMS # and HS-GC-MS | [90] |
| Flavored e-liquid (flavor categories: vanilla, butterscotch, tobacco, cinnamon, apple, menthol, and others) | ≈1000 | 1000 individual substances were identified, including carboxylic acids (18), esters (204), aldehydes (73), ketones (131), alcohols (163), ethers (112), halogenateds (44), aromatics (287), hydrocarbons (alkanes, alkenes, alkines) (267), and miscellaneous (56) | HS-SPME-GC × GC-TOF-MS | [77] |
| Commercially available e-liquids (two brands) | 92 | 92 VOCs were identified, including 31 esters, 18 alcohols, 10 hydrocarbons, 8 carbonyl compounds, 7 acetals, 5 pyrazines, 4 terpenes, 3 miscellaneous, 2 carboxylic acids, 2 lactones, 1 amine, and 1 volatile phenol | HS-SPME-GC–MS | [91] |
| Breath analysis (e-liquid flavors) | 206 | Propylene glycol, hydrocarbons (58), chloride compounds (3), alcohols (23), aldehydes (16), ketones (22), acids (7), esters (33), ethers (1), furans and pyrans (13), nitrogen compounds (14), and aromatic hydrocarbons (16) | HS-SPME-GC–MS | [92] |
| Three lab-formulated e-liquids | 1 | Methamphetamine (537-46-2) | HS-SPME-GC–MS, SPME-DART-MS+ | [93] |
| Cannabis vape oil liquid, vapor and aerosol | 206 | 100 terpenes and natural extracts, 19 cannabinoids, and other potential toxic additives such as vitamin E acetate, polyethylene glycols, and medium-chain triglycerides | HS-SPME-GC–MS | [94] |
| Two different types of e-cigarettes and different puff durations were evaluated | 3 | Formaldehyde (50-00-0.), acetaldehyde (75-07-0), and acrolein (107-02-8) | HS-SPME-GC–MS | [57] |
| 225 replacement liquids were purchased from 17 e-cigarette shops | 3 | Formaldehyde (50-00-0.), acetaldehyde (75-07-0), and acrolein (107-02-8) | HS-SPME-GC–MS | [95] |
| Electronic cigarette liquids and aerosols | 1 | Nicotine (65-31-6) | HS-SPME-GC–MS | [96] |
| Three commercially available e-liquids | 1 | MDMB-fubinaca (Methyl (S)-2-(1-(4-fluorobenzyl)-1H-indazole-3-carboxamido)-3,3-dimethylbutanoate) (1971007-93-8) | HS-SPME-GC–MS | [97] |
| Flavored e-liquid | 72 | Ethanol (64-17-5) 1,3-Butadiene, 2-methyl (78-79-5) Propanal (123-38-6) Acetone (67-64-1) Dimethyl sulfide (75-18-3) 1,3-Cyclopentadiene (542-92-7) 2-Butene, 2,3-dimethyl- (563-79-1) 1-Propanol (71-23-8) Furan, 3-methyl (930-27-8) Ethyl acetate (141-78-6) Hexane, 2-methyl (591764) Pentane, 2,3-dimethyl (565-59-3) Hexane, 3-methyl (589-34-4) Benzene (71-43-2) Heptane (142-82-5) Acetic acid (64-19-7) Propane, 1-(methylthio)- (3877-15-4) Furan, 2,5-dimethyl (625-86-5) 2-Pentanone (107-87-9) Hexane, 2,4-dimethyl (589-43-5) 3-Hexanone (589-38-8) Heptane, 4-methyl (589-53-7) Acetoin (513-86-0) Propanoic acid (79-09-4) Toluene (108-88-3) Octane (111-65-9) Hexane, 2,3,5-trimethyl (1069-53-0) Heptane, 2,4-dimethyl (2213-23-2) Butanoic acid, ethyl ester (105-54-4) Hexanal (66-25-1) 2,4-Dimethyl-1-heptene (19549-87-2) Heptane, 2,3-dimethyl (3074-71-3) Octane, 4-methyl (2216-34-4) Benzene, 1-ethynyl-4-methyl (766-97-2) Heptane, 2,4,6-trimethyl (2613-61-8) Butanoic acid, 2-methyl-, ethyl ester (7452-79-1) Ethylbenzene (100-41-4) Nonane (111-84-2) p-Xylene (106-42-3) 3-Hexen-1-ol (928-96-1) Pyrazine, 2,6-dimethyl (108-50-9) Pyrazine, ethyl- (6924-68-1) Benzene, 1-methyl-4-(1-methylethenyl)- (1195-32-0) Benzonitrile. 4-methyl (104-85-8) Heptane, 3,3,5-trimethyl (7154-80-5) Nonanal (124-19-6) Hexanoic acid, ethyl ester (123-66-0) Benzaldehyde (100-52-7) 3-Hexen-1-ol, acetate, (E)- (3681-82-1) Pyrazine, 2-ethyl-6-methyl- (13925-03-6) Acetic acid, hexyl ester (142-92-7) Pyrazine, trimethyl (14667-55-1) Octanal (124-13-0) D-Limonene (5989-27-5) p-Cymene (99-87-6) Decane, 3,7-dimethyl (17312548) Decane, 3,6-dimethyl (17312-53-7) Acetylpyrazine (22047-25-2) Ethanone, 1-(2-pyridinyl)- (1122-62-9) Phenol (108-95-2) 1,2-Cyclopentanedione, 3-methyl (765-70-8) Pyrazine, tetramethyl (1124-11-4) o-Isopropenyltoluene (7399-49-7) Butanoic acid, 3-methyl, 3-methylbutyl ester (106-27-4)) Nonanal (124-19-6) Heptadecane, 8-methyl (13287-23-5) Pyrazine, 2-methyl-3-(methylthio)- (2882-20-4) Decanal (112-31-2) Dodecane, 4-methyl (6117-97-1) Octane, 5-ethyl-2-methyl (62016186) Hexadecane, 2,6,10,14-tetramethyl (638-36-8) | HS-SPME-GC–MS | [56] |
| Matrix Analyzed | Number of Compounds | Identified or Quantified Compounds (CAS) | Instrumental Technique | Reference |
|---|---|---|---|---|
| E-liquids | 17 | CBD (13956-29-1), Δ9-tetrahydrocannabinol (Δ9-THC) (1972-08-3), cannabinol (CBN) (521-35-7), cannabidiolic acid (CBDA) (1244-58-2), Δ9-tetrahydrocannabinolic acid A (Δ9-THCA) (23978-85-0), Δ8-tetrahydrocannabinol (Δ8-THC) (5957-75-5), cannabinol (CBN) (521-35-7), cannabigerol (CBG) (25654-31-3), cannabichromene (CBC) (20675-51-8), Cannabicyclol (CBL) (21366-63-2), cannabidivarin (CBDV) (24274-48-4), tetrahydrocannabivarin (THCV) (31262-37-0), cannabicitran (CBT) (31508-71-1), tetrahydrocannabivarinic acid (THCVA) (39986-26-0), cannabinolic acid (CBNA) (2808-39-1), cannabigerolic acid (CBGA) (25555-57-1), and cannabidivarinic acid (CBDVA) (31932-13-5) | LC-HRAM-MS and LC-UV | [98] |
| E-liquids and aerosols | 8 | N-nitrosonornicotine (NNN) (80508-23-2), N′-nitrosoanatabine (NAT) (887407-16-1), 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone (NNK) (64091-91-4), N-nitrosoanabasine (NAB) (37620-20-5), 4-(methylnitrosamino)-4-(3-pyridyl)-1-butanol (iso-NNAL) (59578-66-4), 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanol (NNAL) (76014-81-8), 4-(methylnitrosamino)-4-3-pyridyl) butyric acid (iso-NNAC) (123743-84-0), and 4-(methylnitrosamino)-4-(3-pyridyl)-butanal (NNA) (64091-90-3) | UPLC-QTOF-HRMS | [99] |
| E-cigarette refill solutions | 42 | 2-acetylpyrazine (22047-25-2), 2-acetylpyridine (1122-62-9), 2-acetylpyrrole (1072-83-9), 2-isopropyl-4-methylthiazole (15679-13-7), 2-methylpyrazine (109-08-0), 2,5-dimethylpyrazine (123-32-0), 2,6-dimethylpyridine (108-48-5), 2,3,5-trimethylpyrazine (14667-55-1), 2,3,5,6-tetramethylpyrazine (1124-11-4), 3-ethylpyridine (108-99-6), 3-methyl-3-phenylglycidate (77-83-8), 4-methyl acetophenone (122-00-9), 5-methylfurfural (620-02-0), carvone (6485-40-1), cocoa (8002-31-1), diethyl malonate (105-53-3), diethyl succinate (123-25-1), ethyl acetoacetate (141-97-9), ethyl cinnamate (103-36-6), ethyl lactate (687-47-8), ethyl phenylacetate (101-97-3), ethyl vanillin (121-32-4), ethyl-2-methylbutyrate (7452-79-1), ethyl 3-(methylthio)propionate (13327-56-5), ethyl maltol (4940-11-8), furaneol (3658-77-3), geraniol (106-24-1), ionone α (127-41-3), ionone β (14901-07-6), linalool (78-70-6), linalool oxide (60047-17-8), maltol (118-71-8), menthol (2216-51-5), menthone (10458-14-7), methyl cinnamate (103-26-4), methyl cyclopentenolone (765-70-8), methyl heptanone (110-93-0), methyl salicylate (119-36-8), nerol (106-25-2), nicotine (65-31-6), pyridine (110-86-1), vanillin (121-33-5), β-damascone (35044-68-9), γ-valeroactone (108-29-2), and γ-hexalactone (695-06-7) | HPLC-ESI–MS/MS | [100] |
| E-liquid samples | 4 | Tetrahydrocannabinol (THC) (1972-08-3), Mephedrone (1189805-46-6), Cumy-PeGaClone (Synthetic Cannabinoid) (2160555-55-3), and Methamphetamine (537-46-2) | DART-Q-TOF-MS/MS | [101] |
| E-cigarette fluids | 4 | Δ8-THC (5957-75-5), Δ9-THC (1972-08-3), CBD (13956-29-1), and CBN (521-35-7) | LC-MS/MS | [102] |
| E-liquid samples | 21 | Δ8-THC (5957-75-5), Δ9-THC (1972-08-3), Δ10-THC (95543-62-7), Δ6a,10a-THC (95720-02-8), CBDA (1244-58-2), THCA-A (23978-85-0), HHC (36403-90-4), Δ9-THCB (60008-00-6), Δ9-THCP (6465-30-1), Δ8-THCP (5957-75-5), Δ9-THCH (81586-39-2), cannabicitran (CBT) (31508-71-1), CBG (25654-31-3), CBN (521-35-7), CBD (13956-29-1), CBC (20675-51-8), CBDV (24274-48-4), acetate ester of Δ9-THC (885123-57-9), acetate ester of CBD (23050-54-6), acetate ester of CBN (51895-51-3), and acetate ester of HHC (not reported). | LC-MS/MS | [103] |
| Matrix Analyzed | Analytes (Range) | Instrumental Technique | Reference |
|---|---|---|---|
| 3 brands of e-liquids (residual) | Cd (0.035 µg/g) Co (0.51–0.55 µg/g) Cr (3.22–5.33 µg/g) Cu (3.50–194.6 µg/g) Ni (10.90–12.42 µg/g) Pb (0.15–0.61 µg/g) | ICP OES | [143] |
| E-liquid refills within e-cigarettes | Cd (<LQ) Cr (0.033–0.396 µg/g) Cu (9.18–903 µg/g) Ni (0.040–4.04 µg/g) Pb (<LQ) Sn (0.119–1.35 µg/g) Zn (3.00–454 µg/g) | ICP-MS | [144] |
| E-liquids and aerosols from pod-based devices from three manufacturers | Cd (0.100–0.108 µg/g) Cr (0.025–1.64 µg/g) Cu (2.00–927 µg/g) Ni (0.025–61.3 µg/g) Pb (0.066–2.56 µg/g) Sn (0.099–58.2 µg/g) Zn (1.00–14.9 µg/g) | ICP-MS | [145] |
| Aerosols emitted from 12 brands of e-liquids | Cd (<LOD) Cr (0.231–1.85 ng/10 puffs) Cu (2.53–488 ng/10 puffs) Ni (0.472–9.63 ng/10 puffs) Pb (0.128–11.4 ng/10 puffs) Sn (0.341–1.71 ng/10 puffs) Zn (27.9–339 ng/10 puffs) | ICP-MS | [146] |
| 22 e-liquid refills and their constituents | Cd (0.001–0.141 µg/g) Cr (0.003–0.036 µg/g) Cu (0.004–0.055 µg/g) Ni (0.002–0.092 µg/g) Pb (0.001–0.011 µg/g) As (ND) | TXRF | [147] |
| Aerosols emitted from 8 brands of e-liquids (chemical speciation of arsenic) | iASIII (1.06–4.66 µg/kg) iASV (0.71–8.30 µg/kg) MMA (0.05–2.28 µg/kg) | HPLC-ICP-MS | [148] |
| Aerosols emitted from 50 brands of e-liquids | Cd (0.01–1.60 ng/m3) Cr (0.41–126.17 ng/m3) Ni (0.03–4.49 ng/m3) Pb (0.06–7.88 ng/m3) | ICP-MS | [149] |
| Aerosols emitted from 4 synthetic e-liquids | Cu (0.0001–8.3 mg/L) Fe (0.07–55.0 mg/L) Ni (0.08–24.0 mg/L) Pb (0.04–5.8 mg/L) Zn (0.02–22.0 mg/L) | TXRF | [150] |
| Aerosols emitted from 16 brands of e-liquids and 4 e-cigarettes | Al (5.39–34.7 µg/kg) As (0.11–7.76 µg/kg) Cd (0.06–1.98 µg/kg) Cr (0.06–39.4 µg/kg) Cu (6.3–1936 µg/kg) Fe (4.44–200 µg/kg) Mn (0.42–96.1 µg/kg) Ni (8.00–2491 µg/kg) Pb (6.50–1079 µg/kg) Sb (0.06–13.2 µg/kg) Sn (0.51–322 µg/kg) U (0.04–0.06 µg/kg) W (0.05–0.15 µg/kg) Zn (387–6952 µg/kg) | ICP-MS | [151] |
| Aerosols emitted from 16 brands of e-liquids and 4 e-cigarettes | Al (6.02–16.7 µg/kg) As (0.09–2.07 µg/kg) Cd (0.04–0.16 µg/kg) Cr (0.05–7.99 µg/kg) Cu (15.4–615 µg/kg) Fe (3.46–194 µg/kg) Mn (0.77–51.4 µg/kg) Ni (0.73–1181 µg/kg) Pb (2.38–377 µg/kg) Sb (0.33–4.29 µg/kg) Sn (0.37–208 µg/kg) U (0.04–0.06 µg/kg) W (0.04–0.25 µg/kg) Zn (704–3420 µg/kg) | ICP-MS | [152] |
| Metal | ICH (µg/day) | ATSDR (µg/m3) | NIOSH (mg/m3) | NIOSH Daily Value (mg) |
|---|---|---|---|---|
| Cd | 3 | 0.03 | 0.005 | 0.03 |
| Cr | 3 | NI | 0.5 | 3.3 |
| Cu | 30 | NI | 1.0 | 6.7 |
| Ni | 6 | 0.09 | 0.015 | 0.1 |
| Pb | 5 | NI | 0.03 | 0.2 |
| Zn | NI | NI | 5.0 | 33.3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Toledo, E.F.V.; Simões, I.F.; Farias, M.T.d.; Minho, L.A.C.; Conceição, J.d.L.; Santos, W.N.L.d.; Mesquita, P.R.R.d.; Júnior, A.d.F.S. A Comprehensive Review of the Harmful Compounds in Electronic Cigarettes. Toxics 2025, 13, 268. https://doi.org/10.3390/toxics13040268
Toledo EFV, Simões IF, Farias MTd, Minho LAC, Conceição JdL, Santos WNLd, Mesquita PRRd, Júnior AdFS. A Comprehensive Review of the Harmful Compounds in Electronic Cigarettes. Toxics. 2025; 13(4):268. https://doi.org/10.3390/toxics13040268
Chicago/Turabian StyleToledo, Eduard Ferney Valenzuela, Ivana Ferreira Simões, Marcel Tavares de Farias, Lucas Almir Cavalcante Minho, Jaquelide de Lima Conceição, Walter Nei Lopes dos Santos, Paulo Roberto Ribeiro de Mesquita, and Aníbal de Freitas Santos Júnior. 2025. "A Comprehensive Review of the Harmful Compounds in Electronic Cigarettes" Toxics 13, no. 4: 268. https://doi.org/10.3390/toxics13040268
APA StyleToledo, E. F. V., Simões, I. F., Farias, M. T. d., Minho, L. A. C., Conceição, J. d. L., Santos, W. N. L. d., Mesquita, P. R. R. d., & Júnior, A. d. F. S. (2025). A Comprehensive Review of the Harmful Compounds in Electronic Cigarettes. Toxics, 13(4), 268. https://doi.org/10.3390/toxics13040268








