Bisphenol A Exposure Induces Small Intestine Damage Through Oxidative Stress, Inflammation, and Microbiota Alteration in Rats
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents and Antibodies
2.2. Animals and Sampling
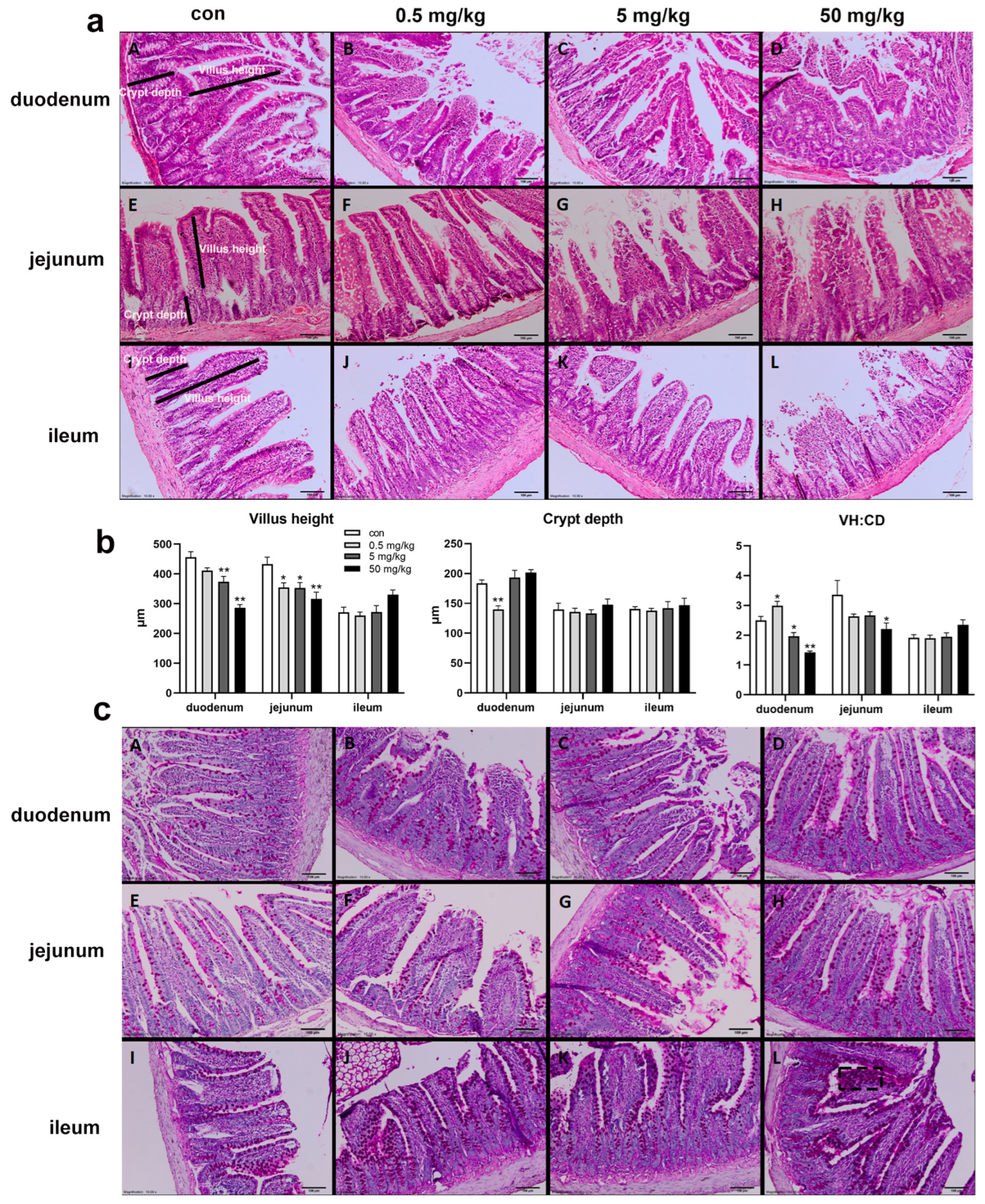
2.3. Morphological Analysis
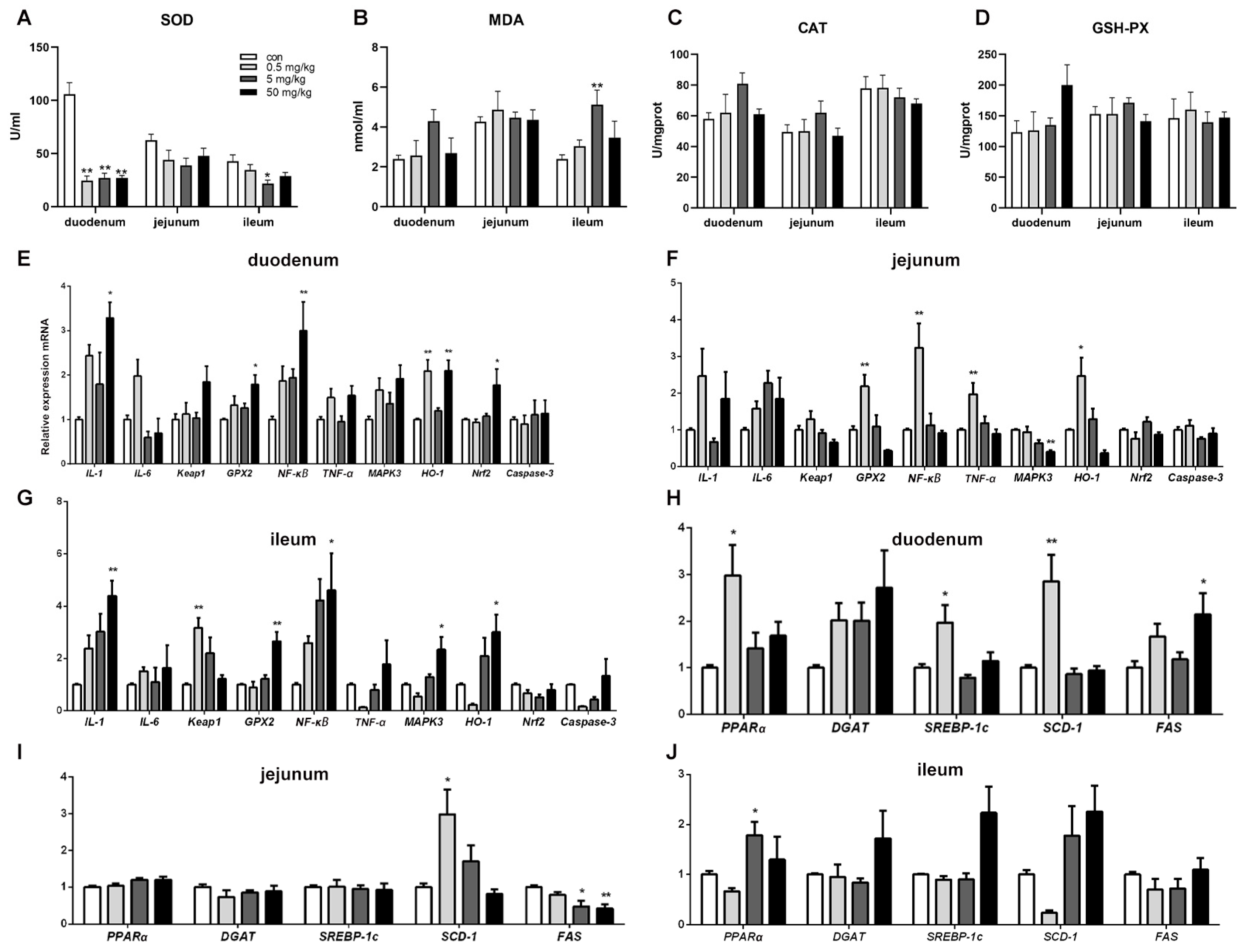
2.4. Oxidative Stress Determination
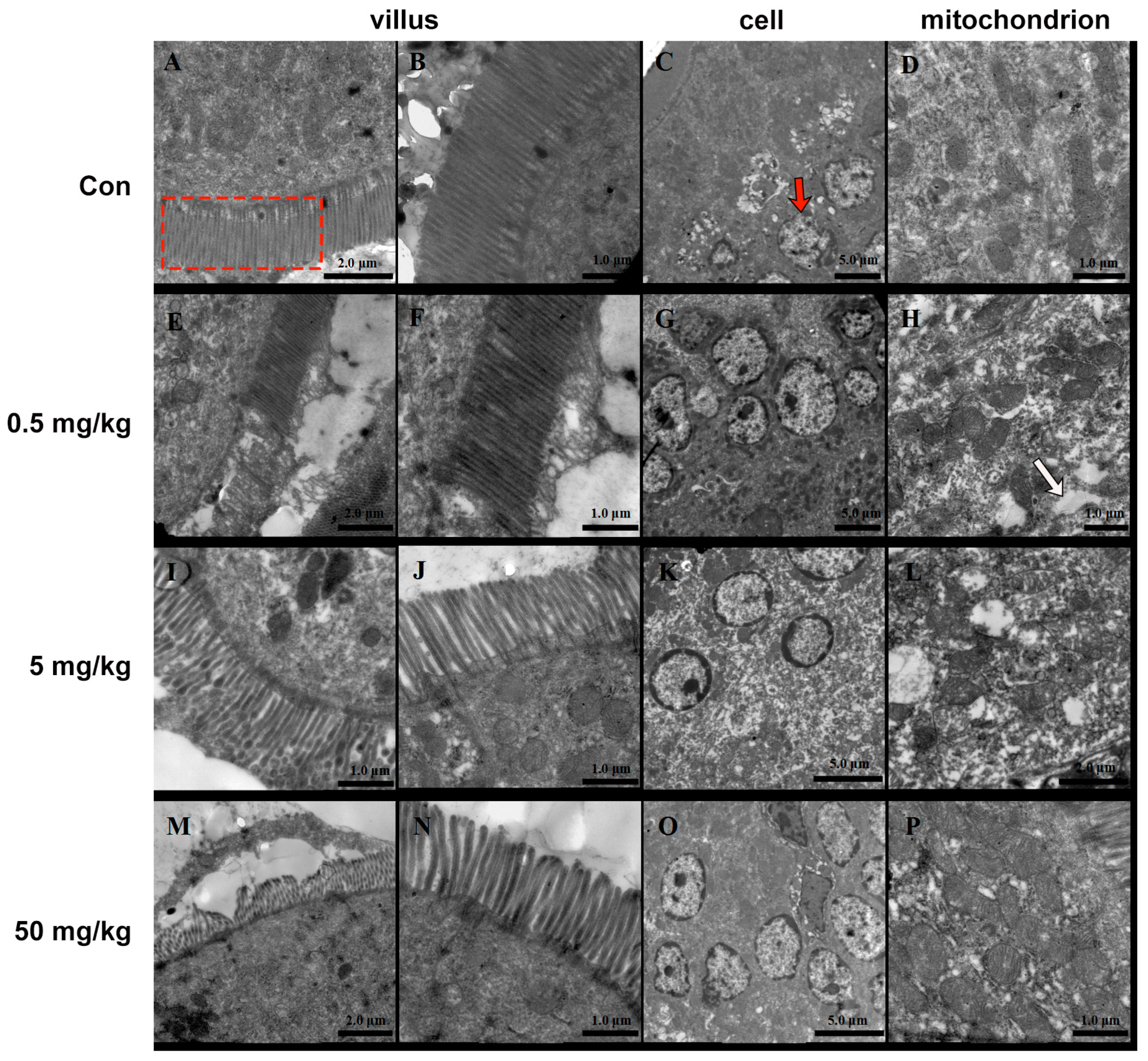
2.5. Ultrastructure Observation
2.6. Quantitative Real-Time PCR Analysis
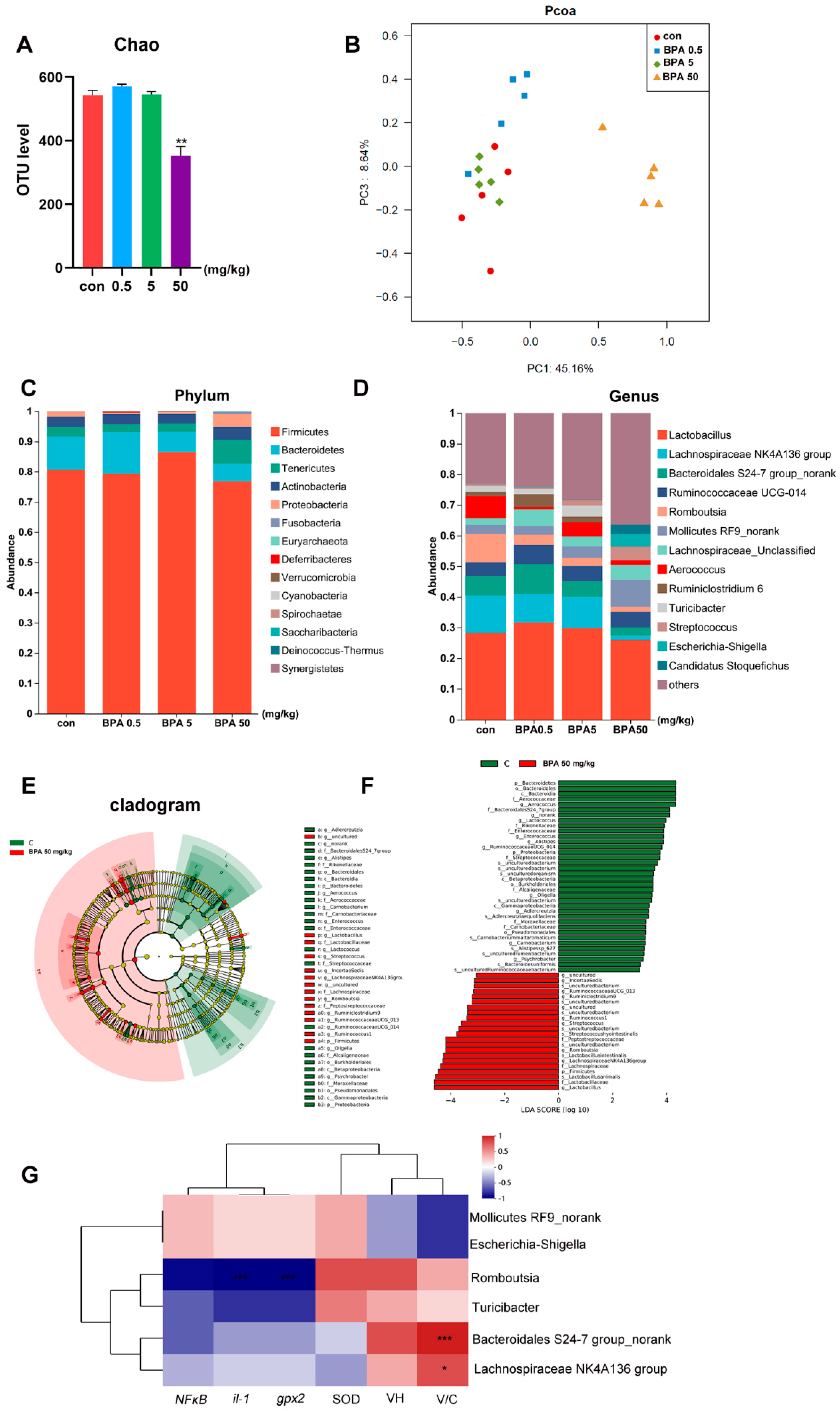
2.7. Fecal Microbial Community Analysis
2.8. Statistical Analysis
3. Results
3.1. Bisphenol A on the Structural Integrity of Rat Small Intestine
3.2. Bisphenol A on the Ultrastructure of the Rat Jejunum
3.3. Bisphenol A on Oxidative Stress in Rat Small Intestinal Tissue
3.4. Bisphenol A on the Expression of Oxidative Stress, Inflammation, and Apoptosis-Related Genes in Rat Small Intestinal Tissue
3.5. Bisphenol A on the Expression of Lipid-Related Genes in Rat Small Intestinal Tissue
3.6. Bisphenol A on Fecal Microbiota Composition in Rats
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kahn, L.G.; Philippat, C.; Nakayama, S.F.; Slama, R.; Trasande, L. Endocrine-disrupting chemicals: Implications for human health. Lancet Diabetes Endocrinol. 2020, 8, 703–718. [Google Scholar] [CrossRef] [PubMed]
- Halden, R.U. Plastics and health risks. Annu. Rev. Public Health 2010, 31, 179–194. [Google Scholar] [CrossRef] [PubMed]
- Barouki, R.; Samson, M.; Blanc, E.B.; Colombo, M.; Zucman-Rossi, J.; Lazaridis, K.N.; Miller, G.W.; Coumoul, X. The exposome and liver disease—How environmental factors affect liver health. J. Hepatol. 2023, 79, 492–505. [Google Scholar] [CrossRef]
- Braun, J.M. Early-life exposure to EDCs: Role in childhood obesity and neurodevelopment. Nat. Rev. Endocrinol. 2017, 13, 161–173. [Google Scholar] [CrossRef]
- Delbaere, K.; Roegiers, I.; Bron, A.; Durif, C.; Van de Wiele, T.; Blanquet-Diot, S.; Marinelli, L. The small intestine: Dining table of host-microbiota meetings. FEMS Microbiol. Rev. 2023, 47, fuad022. [Google Scholar] [CrossRef]
- Gustafsson, J.K.; Johansson, M. The role of goblet cells and mucus in intestinal homeostasis. Nat. Rev. Gastroenterol. Hepatol. 2022, 19, 785–803. [Google Scholar] [CrossRef]
- Diamante, G.; Cely, I.; Zamora, Z.; Ding, J.; Blencowe, M.; Lang, J.; Bline, A.; Singh, M.; Lusis, A.J.; Yang, X. Systems toxicogenomics of prenatal low-dose BPA exposure on liver metabolic pathways, gut microbiota, and metabolic health in mice. Environ. Int. 2021, 146, 106260. [Google Scholar] [CrossRef]
- Braniste, V.; Jouault, A.; Gaultier, E.; Polizzi, A.; Buisson-Brenac, C.; Leveque, M.; Martin, P.G.; Theodorou, V.; Fioramonti, J.; Houdeau, E. Impact of oral bisphenol A at reference doses on intestinal barrier function and sex differences after perinatal exposure in rats. Proc. Natl. Acad. Sci. USA 2010, 107, 448–453. [Google Scholar] [CrossRef]
- Sommer, F.; Anderson, J.M.; Bharti, R.; Raes, J.; Rosenstiel, P. The resilience of the intestinal microbiota influences health and disease. Nat. Rev. Microbiol. 2017, 15, 630–638. [Google Scholar] [CrossRef]
- Sies, H. Oxidative stress: A concept in redox biology and medicine. Redox Biol. 2015, 4, 180–183. [Google Scholar] [CrossRef]
- Liao, J.X.; Chen, Y.W.; Shih, M.K.; Tain, Y.L.; Yeh, Y.T.; Chiu, M.H.; Chang, S.; Hou, C.Y. Resveratrol Butyrate Esters Inhibit BPA-Induced Liver Damage in Male Offspring Rats by Modulating Antioxidant Capacity and Gut Microbiota. Int. J. Mol. Sci. 2021, 22, 5273. [Google Scholar] [CrossRef]
- Melebary, S.J.; AlGhamdi, M.S.; Elhalwagy, M.; Alsolmy, S.A.; Bin, D.A. Disturbance in Some Fertility Biomarkers Induced and Changes in Testis Architecture by Chronic Exposure to Various Dosages of Each of Nonylphenol or Bisphenol A and Their Mix. Life 2022, 12, 1555. [Google Scholar] [CrossRef]
- Linillos-Pradillo, B.; Rancan, L.; Paredes, S.D.; Schlumpf, M.; Lichtensteiger, W.; Vara, E.; Tresguerres, J. Low Dose of BPA Induces Liver Injury through Oxidative Stress, Inflammation and Apoptosis in Long-Evans Lactating Rats and Its Perinatal Effect on Female PND6 Offspring. Int. J. Mol. Sci. 2023, 24, 4585. [Google Scholar] [CrossRef]
- Helli, B.; Navabi, S.P.; Hosseini, S.A.; Sabahi, A.; Khorsandi, L.; Amirrajab, N.; Mahdavinia, M.; Rahmani, S.; Dehghani, M.A. The Protective Effects of Syringic Acid on Bisphenol A-Induced Neurotoxicity Possibly Through AMPK/PGC-1alpha/Fndc5 and CREB/BDNF Signaling Pathways. Mol. Neurobiol. 2024, 61, 7767–7784. [Google Scholar] [CrossRef]
- Ni, Y.; Hu, L.; Yang, S.; Ni, L.; Ma, L.; Zhao, Y.; Zheng, A.; Jin, Y.; Fu, Z. Bisphenol A impairs cognitive function and 5-HT metabolism in adult male mice by modulating the microbiota-gut-brain axis. Chemosphere 2021, 282, 130952. [Google Scholar] [CrossRef]
- Ryu, D.Y.; Pang, W.K.; Adegoke, E.O.; Rahman, M.S.; Park, Y.J.; Pang, M.G. Bisphenol-A disturbs hormonal levels and testis mitochondrial activity, reducing male fertility. Hum. Reprod. Open. 2023, 2023, hoad044. [Google Scholar] [CrossRef]
- Syage, J.A.; Maki, M.; Leffler, D.A.; Silvester, J.A.; Sealey-Voyksner, J.A.; Wu, T.T.; Murray, J.A. A Composite Morphometric Duodenal Biopsy Mucosal Scale for Celiac Disease Encompassing Both Morphology and Inflammation. Clin. Gastroenterol. Hepatol. 2024, 22, 1238–1244. [Google Scholar] [CrossRef]
- Malaise, Y.; Menard, S.; Cartier, C.; Lencina, C.; Sommer, C.; Gaultier, E.; Houdeau, E.; Guzylack-Piriou, L. Consequences of bisphenol a perinatal exposure on immune responses and gut barrier function in mice. Arch. Toxicol. 2018, 92, 347–358. [Google Scholar] [CrossRef]
- Birchenough, G.M.; Johansson, M.E.; Gustafsson, J.K.; Bergstrom, J.H.; Hansson, G.C. New developments in goblet cell mucus secretion and function. Mucosal Immunol. 2015, 8, 712–719. [Google Scholar] [CrossRef]
- Sun, W.; Xu, T.; Lin, H.; Yin, Y.; Xu, S. BPA and low-Se exacerbate apoptosis and autophagy in the chicken bursa of Fabricius by regulating the ROS/AKT/FOXO1 pathway. Sci. Total Environ. 2024, 908, 168424. [Google Scholar] [CrossRef]
- Liang, B.; Zhong, Y.; Huang, Y.; Lin, X.; Liu, J.; Lin, L.; Hu, M.; Jiang, J.; Dai, M.; Wang, B.; et al. Underestimated health risks: Polystyrene micro- and nanoplastics jointly induce intestinal barrier dysfunction by ROS-mediated epithelial cell apoptosis. Part. Fibre Toxicol. 2021, 18, 20. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, T.; Kumar, N.; Chawla, M.; Philpott, D.J.; Basak, S. The NF-kappaB signaling system in the immunopathogenesis of inflammatory bowel disease. Sci. Signal. 2024, 17, eadh1641. [Google Scholar] [CrossRef]
- Gebru, Y.A.; Pang, M.G. Modulatory effects of bisphenol A on the hepatic immune response. Environ. Pollut. 2023, 336, 122430. [Google Scholar] [CrossRef]
- He, W.; Gao, Z.; Liu, S.; Tan, L.; Wu, Y.; Liu, J.; Zheng, Z.; Fan, W.; Luo, Y.; Chen, Z.; et al. G protein-coupled estrogen receptor activation by bisphenol-A disrupts lipid metabolism and induces ferroptosis in the liver. Environ. Pollut. 2023, 334, 122211. [Google Scholar] [CrossRef]
- de Vos, W.M.; Tilg, H.; Van Hul, M.; Cani, P.D. Gut microbiome and health: Mechanistic insights. Gut 2022, 71, 1020–1032. [Google Scholar] [CrossRef]
- Hong, T.; Zou, J.; He, Y.; Zhang, H.; Liu, H.; Mai, H.; Yang, J.; Cao, Z.; Chen, X.; Yao, J.; et al. Bisphenol A induced hepatic steatosis by disturbing bile acid metabolism and FXR/TGR5 signaling pathways via remodeling the gut microbiota in CD-1 mice. Sci. Total Environ. 2023, 889, 164307. [Google Scholar] [CrossRef]
- Mao, W.; Mao, L.; Zhou, F.; Shen, J.; Zhao, N.; Jin, H.; Hu, J.; Hu, Z. Influence of Gut Microbiota on Metabolism of Bisphenol A, a Major Component of Polycarbonate Plastics. Toxics 2023, 11, 340. [Google Scholar] [CrossRef]
- Rizzatti, G.; Lopetuso, L.R.; Gibiino, G.; Binda, C.; Gasbarrini, A. Proteobacteria: A Common Factor in Human Diseases. Biomed. Res. Int. 2017, 2017, 9351507. [Google Scholar] [CrossRef]
- Jandhyala, S.M.; Talukdar, R.; Subramanyam, C.; Vuyyuru, H.; Sasikala, M.; Nageshwar, R.D. Role of the normal gut microbiota. World J. Gastroenterol. 2015, 21, 8787–8803. [Google Scholar] [CrossRef]
- Zhou, J.; Li, M.; Chen, Q.; Li, X.; Chen, L.; Dong, Z.; Zhu, W.; Yang, Y.; Liu, Z.; Chen, Q. Programmable probiotics modulate inflammation and gut microbiota for inflammatory bowel disease treatment after effective oral delivery. Nat. Commun. 2022, 13, 3432. [Google Scholar] [CrossRef]
- Shao, H.; Zhang, C.; Wang, C.; Tan, Z. Intestinal mucosal bacterial diversity of antibiotic-associated diarrhea (AAD) mice treated with Debaryomyces hansenii and Qiweibaizhu powder. 3 Biotech 2020, 10, 392. [Google Scholar] [CrossRef] [PubMed]
- Song, R.; Xu, Y.; Jia, Z.; Liu, X.; Zhang, X. Integration of intestinal microbiota and metabonomics to elucidate different alleviation impacts of non-saponification and saponification astaxanthin pre-treatment on paracetamol-induced oxidative stress in rats. Food Funct. 2022, 13, 1860–1880. [Google Scholar] [CrossRef]
- Gao, X.; Wang, Z.; Liu, B.; Cheng, Y. Causal association of gut microbiota and esophageal cancer: A Mendelian randomization study. Front. Microbiol. 2023, 14, 1286598. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, Y.; Li, W.; Zhang, B.; Yin, J.; Liuqi, S.; Wang, J.; Peng, B.; Wang, S. Fucoidan Ameliorated Dextran Sulfate Sodium-Induced Ulcerative Colitis by Modulating Gut Microbiota and Bile Acid Metabolism. J. Agric. Food Chem. 2022, 70, 14864–14876. [Google Scholar] [CrossRef]
- Lynch, J.B.; Gonzalez, E.L.; Choy, K.; Faull, K.F.; Jewell, T.; Arellano, A.; Liang, J.; Yu, K.B.; Paramo, J.; Hsiao, E.Y. Gut microbiota Turicibacter strains differentially modify bile acids and host lipids. Nat. Commun. 2023, 14, 3669. [Google Scholar] [CrossRef]
- Wang, C.; Gu, Y.; Chu, Q.; Wang, X.; Ding, Y.; Qin, X.; Liu, T.; Wang, S.; Liu, X.; Wang, B.; et al. Gut microbiota and metabolites as predictors of biologics response in inflammatory bowel disease: A comprehensive systematic review. Microbiol. Res. 2024, 282, 127660. [Google Scholar] [CrossRef]
- Quaglio, A.; Grillo, T.G.; De Oliveira, E.; Di Stasi, L.C.; Sassaki, L.Y. Gut microbiota, inflammatory bowel disease and colorectal cancer. World J. Gastroenterol. 2022, 28, 4053–4060. [Google Scholar] [CrossRef]
- Liu, B.; Piao, X.; Niu, W.; Zhang, Q.; Ma, C.; Wu, T.; Gu, Q.; Cui, T.; Li, S. Kuijieyuan Decoction Improved Intestinal Barrier Injury of Ulcerative Colitis by Affecting TLR4-Dependent PI3K/AKT/NF-κB Oxidative and Inflammatory Signaling and Gut Microbiota. Front. Pharmacol. 2020, 11, 1036. [Google Scholar] [CrossRef]
- Feng, L.; Chen, S.; Zhang, L.; Qu, W.; Chen, Z. Bisphenol A increases intestinal permeability through disrupting intestinal barrier function in mice. Environ. Pollut. 2019, 254 Pt A, 112960. [Google Scholar] [CrossRef]
| Gene | Sequence (5′ to 3′) | Size (bp) |
|---|---|---|
| β-actin | F: CTAAGGCCAACCGTGAAAAGAT R: CACAGCCTGGATGGCTACGT | 83 |
| IL-1β | F: GCCAACAAGTGGTATTCTCCA R: TGCCGTCTTTCATCACACAG | 120 |
| IL-6 | F: AGTTGCCTTCTTGGGACTGA R: ACTGGTCTGTTGTGGGTGGT | 102 |
| MAPK3 | F: CTACACGCAGCTGCAGTACATC R: GTGCGCTGACAGTAGGTTTGA | 153 |
| NF-kB | F: CGACGTATTGCTGTGCCTTC R: TTGAGATCTGCCCAGGTGGTA | 198 |
| TNF-α | F: TTCCGTCCCTCTCATACACTG R: AGACACCGCCTGGAGTTCT | 149 |
| Keap1 | F: CATCGGCATCGCCAACTTC R: GCTGGCAGTGTGACAGGTTGA | 278 |
| GPx2 | F: CCGTGCTGATTGAGAATGTG R: AGGGAAGCCGAGAACCACTA | 113 |
| Caspase-3 | F: AAGCCGAAACTCTTCATC R: TGAGCATTGACACAATACAC | 349 |
| PPARα | F: CTCGTGCAGGTCATCAAGAA R: CAGCCCTCTTCATCTCCAAG | 158 |
| Nrf2 | F: CAGTGCTCCTATGCGTGAA R: GCGGCTTGAATGTTTGTC | 109 |
| HO-1 | F: ACAGATGGCGTCACTTCG R: TGAGGACCCACTGGAGGA | 128 |
| SREBP1c | F: GCCATGGATTGCACATTG R: TGTGTCTCCTGTCTCACCCC | 102 |
| SCD1 | F: CCTTAACCCTGAGATCCCGTAGA R: AGCCCATAAAAGATTTCTGCAA | 237 |
| FAS | F: GGACATGGTCACAGACGATGAC R: GGAGGCGTCGAACTTGGA | 194 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, K.; Tang, J.; Shen, D.; Li, Y.; Nagaoka, K.; Li, C. Bisphenol A Exposure Induces Small Intestine Damage Through Oxidative Stress, Inflammation, and Microbiota Alteration in Rats. Toxics 2025, 13, 331. https://doi.org/10.3390/toxics13050331
Wang K, Tang J, Shen D, Li Y, Nagaoka K, Li C. Bisphenol A Exposure Induces Small Intestine Damage Through Oxidative Stress, Inflammation, and Microbiota Alteration in Rats. Toxics. 2025; 13(5):331. https://doi.org/10.3390/toxics13050331
Chicago/Turabian StyleWang, Kai, Juan Tang, Dan Shen, Yansen Li, Kentaro Nagaoka, and Chunmei Li. 2025. "Bisphenol A Exposure Induces Small Intestine Damage Through Oxidative Stress, Inflammation, and Microbiota Alteration in Rats" Toxics 13, no. 5: 331. https://doi.org/10.3390/toxics13050331
APA StyleWang, K., Tang, J., Shen, D., Li, Y., Nagaoka, K., & Li, C. (2025). Bisphenol A Exposure Induces Small Intestine Damage Through Oxidative Stress, Inflammation, and Microbiota Alteration in Rats. Toxics, 13(5), 331. https://doi.org/10.3390/toxics13050331








