Green Synthesis of Homogeneous Gold Nanoparticles Using Sargassum spp. Extracts and Their Enhanced Catalytic Activity for Organic Dyes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Extract Preparation
2.3. Synthesis of AuNPs
2.4. Characterization of AuNPs
2.5. Evaluation of Photocatalytic Properties
Kinetics Model for Photocatalytic Evaluation
3. Results
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Singh, R.K.; Behera, S.S.; Singh, K.R.; Mishra, S.; Panigrahi, B.; Sahoo, T.R.; Parhi, P.K.; Mandal, D. Biosynthesized gold nanoparticles as photocatalysts for selective degradation of cationic dye and their antimicrobial activity. J. Photochem. Photobiol. A 2020, 400, 112704. [Google Scholar] [CrossRef]
- Alle, M.; Lee, S.-H.; Kim, J.-C. Ultrafast synthesis of gold nanoparticles on cellulose nanocrystals via microwave irradiation and their dyes-degradation catalytic activity. J. Mater. Sci. Technol. 2020, 41, 168–177. [Google Scholar] [CrossRef]
- Uddin, M.J.; Ampiaw, R.E.; Lee, W. Adsorptive removal of dyes from wastewater using a metal-organic framework: A review. Chemosphere 2021, 284, 131314. [Google Scholar] [CrossRef]
- Parthipan, P.; Al-Dosary, M.A.; Al-Ghamdi, A.A.; Subramania, A. Eco-friendly synthesis of reduced graphene oxide as sustainable photocatalyst for removal of hazardous organic dyes. J. King Saud Univ. Sci. 2021, 33, 101438. [Google Scholar] [CrossRef]
- Singh, N.; Susan, A. Polymer Nanocomposites for Water Treatments. In Polymer-Based Nanocomposites for Energy and Environmental Applications; Elsevier: Amsterdam, The Netherlands, 2018; pp. 569–595. [Google Scholar]
- Susan Punnoose, M.; Bijimol, D.; Mathew, B. Microwave assisted green synthesis of gold nanoparticles for catalytic degradation of environmental pollutants. Environ Nanotechnol. Monit. Manag. 2021, 16, 100525. [Google Scholar] [CrossRef]
- Katwal, R.; Kothari, R.; Pathania, D. Chapter 10-An overview on degradation kinetics of organic dyes by photocatalysis using nanostructured electrocatalyst. In Delivering Low-Carbon Biofuels with Bioproduct Recovery; Singh, L., Mahapatra, D.M., Eds.; Elsevier: Amsterdam, The Netherlands, 2021; pp. 195–213. [Google Scholar]
- Kumari, P.; Meena, A. Green synthesis of gold nanoparticles from Lawsoniainermis and its catalytic activities following the Langmuir-Hinshelwood mechanism. Colloids Surf. A 2020, 606, 125447. [Google Scholar] [CrossRef]
- Akilandaeaswari, B.; Muthu, K. Green method for synthesis and characterization of gold nanoparticles using Lawsonia inermis seed extract and their photocatalytic activity. Mater. Lett. 2020, 277, 128344. [Google Scholar] [CrossRef]
- Anwar, Y.; Ullah, I.; Ul-Islam, M.; Alghamdi, K.M.; Khalil, A.; Kamal, T. Adopting a green method for the synthesis of gold nanoparticles on cotton cloth for antimicrobial and environmental applications. Arab. J. Chem. 2021, 14, 103327. [Google Scholar] [CrossRef]
- Al-Radadi, N.S. Facile one-step green synthesis of gold nanoparticles (AuNp) using licorice root extract: Antimicrobial and anticancer study against HepG2 cell line. Arab. J. Chem. 2021, 14, 102956. [Google Scholar] [CrossRef]
- Chen, X.; Xue, Z.; Ji, J.; Wang, D.; Shi, G.; Zhao, L.; Feng, S. Hedysarum polysaccharides mediated green synthesis of gold nanoparticles and study of its characteristic, analytical merit, catalytic activity. Mater. Res. Bull. 2021, 133, 111070. [Google Scholar] [CrossRef]
- Asnag, G.M.; Oraby, A.H.; Abdelghany, A.M. Green synthesis of gold nanoparticles and its effect on the optical, thermal and electrical properties of carboxymethyl cellulose. Compos. B Eng. 2019, 172, 436–446. [Google Scholar] [CrossRef]
- Hammami, I.; Alabdallah, N.M.; Jomaa, A.A.; Kamoun, M. Gold nanoparticles: Synthesis properties and applications. J. King Saud Univ. -Sci. 2021, 33, 101560. [Google Scholar] [CrossRef]
- Al-khattaf, F.S. Gold and silver nanoparticles: Green synthesis, microbes, mechanism, factors, plant disease management and environmental risks. Saudi J. Biol. Sci. 2021, 28, 3624–3631. [Google Scholar] [CrossRef] [PubMed]
- Yeh, Y.-C.; Creran, B.; Rotello, V.M. Gold nanoparticles: Preparation, properties, and applications in bionanotechnology. Nanoscale 2012, 4, 1871–1880. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Al-Misned, F.A.; El-Serehy, H.A.; Yang, L. Green synthesis of gold nanoparticles using aqueous extract of Mentha Longifolia leaf and investigation of its anti-human breast carcinoma properties in the in vitro condition. Arab. J. Chem. 2021, 14, 102931. [Google Scholar] [CrossRef]
- Thangamani, N.; Bhuvaneshwari, N. Green synthesis of gold nanoparticles using Simarouba glauca leaf extract and their biological activity of micro-organism. Chem. Phys. Lett. 2019, 732, 136587. [Google Scholar] [CrossRef]
- Satpathy, S.; Patra, A.; Ahirwar, B.; Hussain, M.D. Process optimization for green synthesis of gold nanoparticles mediated by extract of Hygrophila spinosa T. Anders and their biological applications. Phys. E Low Dimens. Syst. Nanostruct. 2020, 121, 113830. [Google Scholar] [CrossRef]
- Abdoli, M.; Arkan, E.; Shekarbeygi, Z.; Khaledian, S. Green synthesis of gold nanoparticles using Centaurea behen leaf aqueous extract and investigating their antioxidant and cytotoxic effects on acute leukemia cancer cell line (THP-1). Inorg. Chem. Commun. 2021, 129, 108649. [Google Scholar] [CrossRef]
- Muniyappan, N.; Pandeeswaran, M.; Amalraj, A. Green synthesis of gold nanoparticles using Curcuma pseudomontana isolated curcumin: Its characterization, antimicrobial, antioxidant and anti- inflammatory activities. Environ. Chem. Ecotoxicol. 2021, 3, 117–124. [Google Scholar] [CrossRef]
- Varghese, B.A.; Nair, R.V.R.; Jude, S.; Varma, K.; Amalraj, A.; Kuttappan, S. Green synthesis of gold nanoparticles using Kaempferia parviflora rhizome extract and their characterization and application as an antimicrobial, antioxidant and catalytic degradation agent. J. Taiwan Inst. Chem. Eng. 2021, 126, 166–172. [Google Scholar] [CrossRef]
- Khan, M.; Ahmad, F.; Koivisto, J.T.; Kellomäki, M. Green synthesis of controlled size gold and silver nanoparticles using antioxidant as capping and reducing agent. Colloids Interface Sci. Commun. 2020, 39, 100322. [Google Scholar] [CrossRef]
- Naikoo, G.A.; Mustaqeem, M.; Hassan, I.U.; Awan, T.; Arshad, F.; Salim, H.; Qurashi, A. Bioinspired and green synthesis of nanoparticles from plant extracts with antiviral and antimicrobial properties: A critical review. J. Saudi Chem. Soc. 2021, 25, 101304. [Google Scholar] [CrossRef]
- Alabdallah, N.M.; Hasan, M.M. Plant-based green synthesis of silver nanoparticles and its effective role in abiotic stress tolerance in crop plants. Saudi J. Biol. Sci. 2021, 28, 5631–5639. [Google Scholar] [CrossRef] [PubMed]
- Vázquez-Delfín, E.; Freile-Pelegrín, Y.; Salazar-Garibay, A.; Serviere-Zaragoza, E.; Méndez-Rodríguez, L.C.; Robledo, D. Species composition and chemical characterization of Sargassum influx at six different locations along the Mexican Caribbean coast. Sci. Total Env. 2021, 795, 148852. [Google Scholar] [CrossRef]
- López-Miranda, J.L.; Esparza, R.; González-Reyna, M.A.; España-Sánchez, B.L.; Hernandez-Martinez, A.R.; Silva, R.; Estévez, M. Sargassum Influx on the Mexican Coast: A Source for Synthesizing Silver Nanoparticles with Catalytic and Antibacterial Properties. Appl. Sci. 2021, 11, 4638. [Google Scholar] [CrossRef]
- Chávez, V.; Uribe-Martínez, A.; Cuevas, E.; Rodríguez-Martínez, R.E.; van Tussenbroek, B.I.; Francisco, V.; Estévez, M.; Celis, L.B.; Monroy-Velázquez, L.V.; Leal-Bautista, R. Massive Influx of Pelagic Sargassum spp. on the Coasts of the Mexican Caribbean 2014–2020: Challenges and Opportunities. Water 2020, 12, 2908. [Google Scholar] [CrossRef]
- Robledo, D.; Vázquez-Delfín, E.; Freile-Pelegrín, Y.; Vásquez-Elizondo, R.M.; Qui-Minet, Z.N.; Salazar-Garibay, A. Challenges and Opportunities in Relation to Sargassum Events Along the Caribbean Sea. Front. Mar. Sci. 2021, 8. [Google Scholar] [CrossRef]
- van Tussenbroek, B.I.; Arana, H.A.H.; Rodríguez-Martínez, R.E.; Espinoza-Avalos, J.; Canizales-Flores, H.M.; González-Godoy, C.E.; Barba-Santos, M.G.; Vega-Zepeda, A.; Collado-Vides, L. Severe impacts of brown tides caused by Sargassum spp. on near-shore Caribbean seagrass communities. Mar. Pollut. Bull. 2017, 122, 272–281. [Google Scholar] [CrossRef]
- Thompson, T.M.; Ramin, P.; Udugama, I.; Young, B.R.; Gernaey, K.V.; Baroutian, S. Techno-economic and environmental impact assessment of biogas production and fertiliser recovery from pelagic Sargassum: A biorefinery concept for Barbados. Energy Convers. Manag. 2021, 245, 114605. [Google Scholar] [CrossRef]
- López-Miranda, J.L.; Silva, R.; Molina, G.A.; Esparza, R.; Hernandez-Martinez, A.; Hernández-Carteño, J.; Estévez, M. Evaluation of a Dynamic Bioremediation System for the Removal of Metal Ions and Toxic Dyes Using Sargassum Spp. J. Mar. Sci. Eng. 2020, 8, 899. [Google Scholar] [CrossRef]
- Desrochers, A.; Cox, S.; Oxenford, H.; Van Tussenbroek, B. Sargassum Uses Guide: A Resource for Caribbean Researchers, Entrepreneurs and Policy Makers. In Entrepreneurs and Policy Makers; CERMES Technical Report: Indies, Barbados, 2020; p. 125. [Google Scholar]
- Hosny, M.; Fawzy, M.; Abdelfatah, A.M.; Fawzy, E.E.; Eltaweil, A.S. Comparative study on the potentialities of two halophytic species in the green synthesis of gold nanoparticles and their anticancer, antioxidant and catalytic efficiencies. Adv. Powder Technol. 2021, 32, 3220–3233. [Google Scholar] [CrossRef]
- Antonisamy, J.M.; Eahamban, K. UV VIS Spectroscopic and HPLC Studies on Dictyota bartayresiana Lamour. Asian Pac. J. Trop. Biomed. 2012, 2, S514–S518. [Google Scholar] [CrossRef]
- Davis, D.; Simister, R.; Campbell, S.; Marston, M.; Bose, S.; McQueen-Mason, S.J.; Gomez, L.D.; Gallimore, W.A.; Tonon, T. Biomass composition of the golden tide pelagic seaweeds Sargassum fluitans and S. natans (morphotypes I and VIII) to inform valorisation pathways. Sci. Total Environ. 2021, 762, 143134. [Google Scholar] [CrossRef] [PubMed]
- Singh, T.; Jayaprakash, A.; Alsuwaidi, M.; Madhavan, A.A. Green synthesized gold nanoparticles with enhanced photocatalytic activity. Mater. Today Proc. 2021, 42, 1166–1169. [Google Scholar] [CrossRef]
- Qu, Y.; Pei, X.; Shen, W.; Zhang, X.; Wang, J.; Zhang, Z.; Li, S.; You, S.; Ma, F.; Zhou, J. Biosynthesis of gold nanoparticles by Aspergillum sp. WL-Au for degradation of aromatic pollutants. Phys. E Low-Dimens. Syst. Nanostruct. 2017, 88, 133–141. [Google Scholar] [CrossRef]
- Singh, A.K.; Tiwari, R.; Singh, V.K.; Singh, P.; Khadim, S.R.; Singh, U.; Laxmi; Srivastava, V.; Hasan, S.H.; Asthana, R.K. Green synthesis of gold nanoparticles from Dunaliella salina, its characterization and in vitro anticancer activity on breast cancer cell line. J. Drug Deliv. Sci. Technol. 2019, 51, 164–176. [Google Scholar] [CrossRef]
- Lim, S.H.; Ahn, E.Y.; Park, Y. Green Synthesis and Catalytic Activity of Gold Nanoparticles Synthesized by Artemisia capillaris Water Extract. Nanoscale Res Lett 2016, 11, 474. [Google Scholar] [CrossRef] [Green Version]
- Coates, J. Interpretation of Infrared Spectra, A Practical Approach. In Encyclopedia of Analytical Chemistry; John Wiley & Sons Ltd.: Chichester, UK, 2006. [Google Scholar]
- Dursun, S.; Yavuz, E.; Çetinkaya, Z. In situ reduction of chloroauric acid (HAuCl4) for generation of catalytic Au nanoparticle embedded triazine based covalent organic polymer networks. RSC Adv. 2019, 9, 38538–38546. [Google Scholar] [CrossRef] [Green Version]
- Islam, N.U.; Amin, R.; Shahid, M.; Amin, M. Gummy gold and silver nanoparticles of apricot (Prunus armeniaca) confer high stability and biological activity. Arab. J. Chem. 2019, 12, 3977–3992. [Google Scholar] [CrossRef] [Green Version]
- Botteon, C.E.A.; Silva, L.B.; Ccana-Ccapatinta, G.V.; Silva, T.S.; Ambrosio, S.R.; Veneziani, R.C.S.; Bastos, J.K.; Marcato, P.D. Biosynthesis and characterization of gold nanoparticles using Brazilian red propolis and evaluation of its antimicrobial and anticancer activities. Sci. Rep. 2021, 11, 1974. [Google Scholar] [CrossRef]
- Elbagory, A.M.; Meyer, M.; Cupido, C.N.; Hussein, A.A. Inhibition of Bacteria Associated with Wound Infection by Biocompatible Green Synthesized Gold Nanoparticles from South African Plant Extracts. Nanomaterials 2017, 7, 417. [Google Scholar] [CrossRef] [Green Version]
- Sampaio, S.; Viana, J.C. Production of silver nanoparticles by green synthesis using artichoke (Cynara scolymus L.) aqueous extract and measurement of their electrical conductivity. Adv. Nat. Sci. 2018, 9, 045002. [Google Scholar] [CrossRef]
- Garg, N.; Bera, S.; Rastogi, L.; Ballal, A.; Balaramakrishna, M.V. Synthesis and characterization of L-asparagine stabilised gold nanoparticles: Catalyst for degradation of organic dyes. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2020, 232, 118126. [Google Scholar] [CrossRef] [PubMed]
- Mallick, K.; Witcomb, M.J.; Scurrell, M.S. Redox catalytic property of gold nanoclusters: Evidence of an electron-relay effect. Appl. Phys. A 2005, 80, 797–801. [Google Scholar] [CrossRef]
- Kgatle, M.; Sikhwivhilu, K.; Ndlovu, G.; Moloto, N. Degradation Kinetics of Methyl Orange Dye in Water Using Trimetallic Fe/Cu/Ag Nanoparticles. Catalysts 2021, 11, 428. [Google Scholar] [CrossRef]
- Meydan, E.; Demirci, S.; Aktas, N.; Sahiner, N.; Ozturk, O.F. Catalytic performance of boron-containing magnetic metal nanoparticles in methylene blue degradation reaction and mixture with other pollutants. Inorg. Chem. Commun. 2021, 126, 108474. [Google Scholar] [CrossRef]
- Cavuslar, O.; Nakay, E.; Kazakoglu, U.; Abkenar, S.K.; Ow-Yang, C.W.; Acar, H.Y. Synthesis of stable gold nanoparticles using linear polyethyleneimines and catalysis of both anionic and cationic azo dye degradation. Mater. Adv. 2020, 1, 2407–2417. [Google Scholar] [CrossRef]
- León, E.R.; Rodríguez, E.L.; Beas, C.R.; Plascencia-Villa, G.; Palomares, R.A.I. Study of Methylene Blue Degradation by Gold Nanoparticles Synthesized within Natural Zeolites. J. Nanomater. 2016, 2016, 9541683. [Google Scholar] [CrossRef]
- Rani, A.; Patel, A.S.; Chakraborti, A.; Singh, K.; Sharma, P. Enhanced photocatalytic activity of plasmonic Au nanoparticles incorporated MoS2 nanosheets for degradation of organic dyes. J. Mater. Sci. Mater. Electron. 2021, 32, 6168–6184. [Google Scholar] [CrossRef]
- Ghosh, S.; Roy, S.; Naskar, J.; Kole, R.K. Process optimization for biosynthesis of mono and bimetallic alloy nanoparticle catalysts for degradation of dyes in individual and ternary mixture. Sci. Rep. 2020, 10, 277. [Google Scholar] [CrossRef]
- Kumar, B.; Smita, K.; Debut, A.; Cumbal, L. Utilization of Persea americana (Avocado) oil for the synthesis of gold nanoparticles in sunlight and evaluation of antioxidant and photocatalytic activities. Environ. Nanotechnol. Monit. Manag. 2018, 10, 231–237. [Google Scholar] [CrossRef]
- Ahmed, M.A.; Abou-Gamra, Z.M.; Alshakhanbeh, M.A.; Medien, H. Control synthesis of metallic gold nanoparticles homogeneously distributed on hexagonal ZnO nanoparticles for photocatalytic degradation of methylene blue dye. Environ. Nanotechnol. Monit. Manag. 2019, 12, 100217. [Google Scholar] [CrossRef]
- Baruah, D.; Goswami, M.; Yadav, R.N.S.; Yadav, A.; Das, A.M. Biogenic synthesis of gold nanoparticles and their application in photocatalytic degradation of toxic dyes. J. Photochem. Photobiol. B Biol. 2018, 186, 51–58. [Google Scholar] [CrossRef] [PubMed]
- Desai, M.P.; Sangaokar, G.M.; Pawar, K.D. Kokum fruit mediated biogenic gold nanoparticles with photoluminescent, photocatalytic and antioxidant activities. Process Biochem. 2018, 70, 188–197. [Google Scholar] [CrossRef]
- Salomatina, E.V.; Fukina, D.G.; Koryagin, A.V.; Titaev, D.N.; Suleimanov, E.V.; Smirnova, L.A. Preparation and photocatalytic properties of titanium dioxide modified with gold or silver nanoparticles. J. Environ. Chem. Eng. 2021, 9, 106078. [Google Scholar] [CrossRef]
- Khan, A.U.; Yuan, Q.; Wei, Y.; Khan, G.M.; Khan, Z.U.H.; Khan, S.; Ali, F.; Tahir, K.; Ahmad, A.; Khan, F.U. Photocatalytic and antibacterial response of biosynthesized gold nanoparticles. J. Photochem. Photobiol. B 2016, 162, 273–277. [Google Scholar] [CrossRef]
- Dabhane, H.; Chatur, S.; Jadhav, G.; Tambade, P.; Medhane, V. Phytogenic synthesis of gold nanoparticles and applications for removal of methylene blue dye: A review. Environ. Chem. Ecotoxicol. 2021, 3, 160–171. [Google Scholar] [CrossRef]
- Sha, Y.; Mathew, I.; Cui, Q.; Clay, M.; Gao, F.; Zhang, X.J.; Gu, Z. Rapid degradation of azo dye methyl orange using hollow cobalt nanoparticles. Chemosphere 2016, 144, 1530–1535. [Google Scholar] [CrossRef] [PubMed]
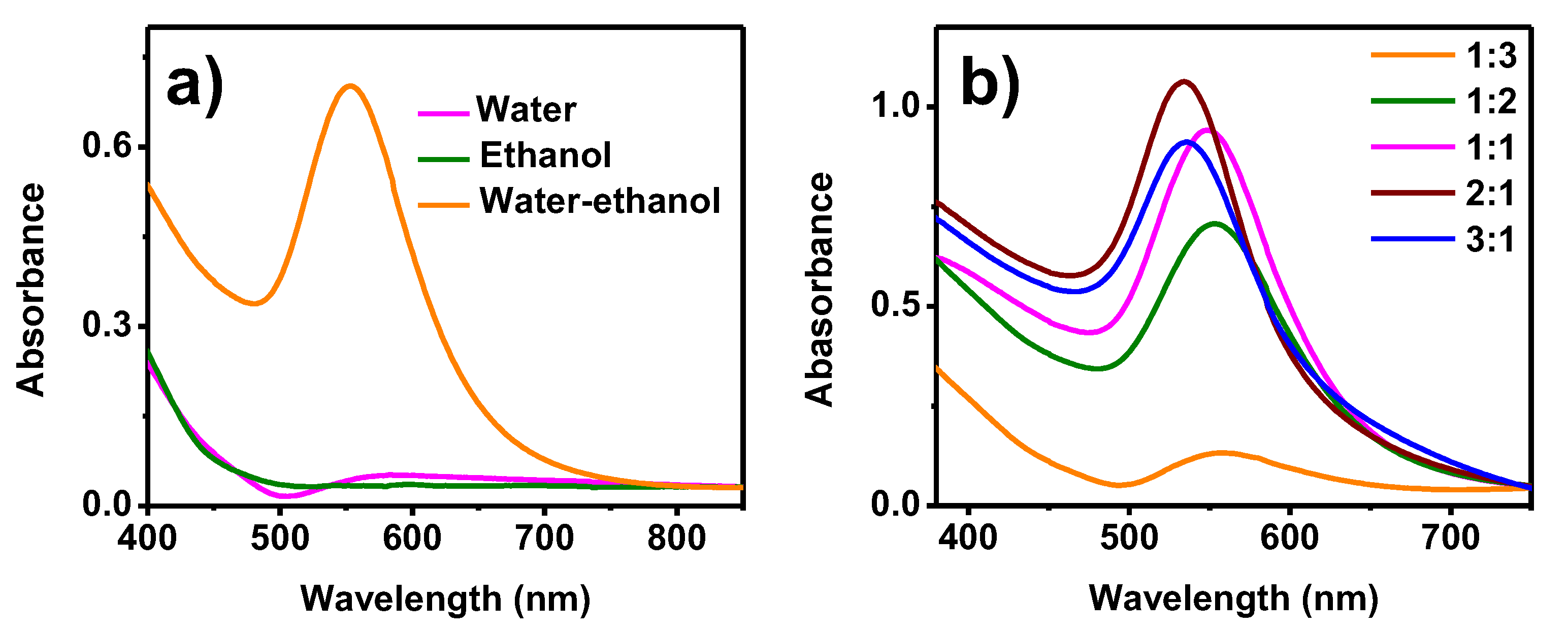
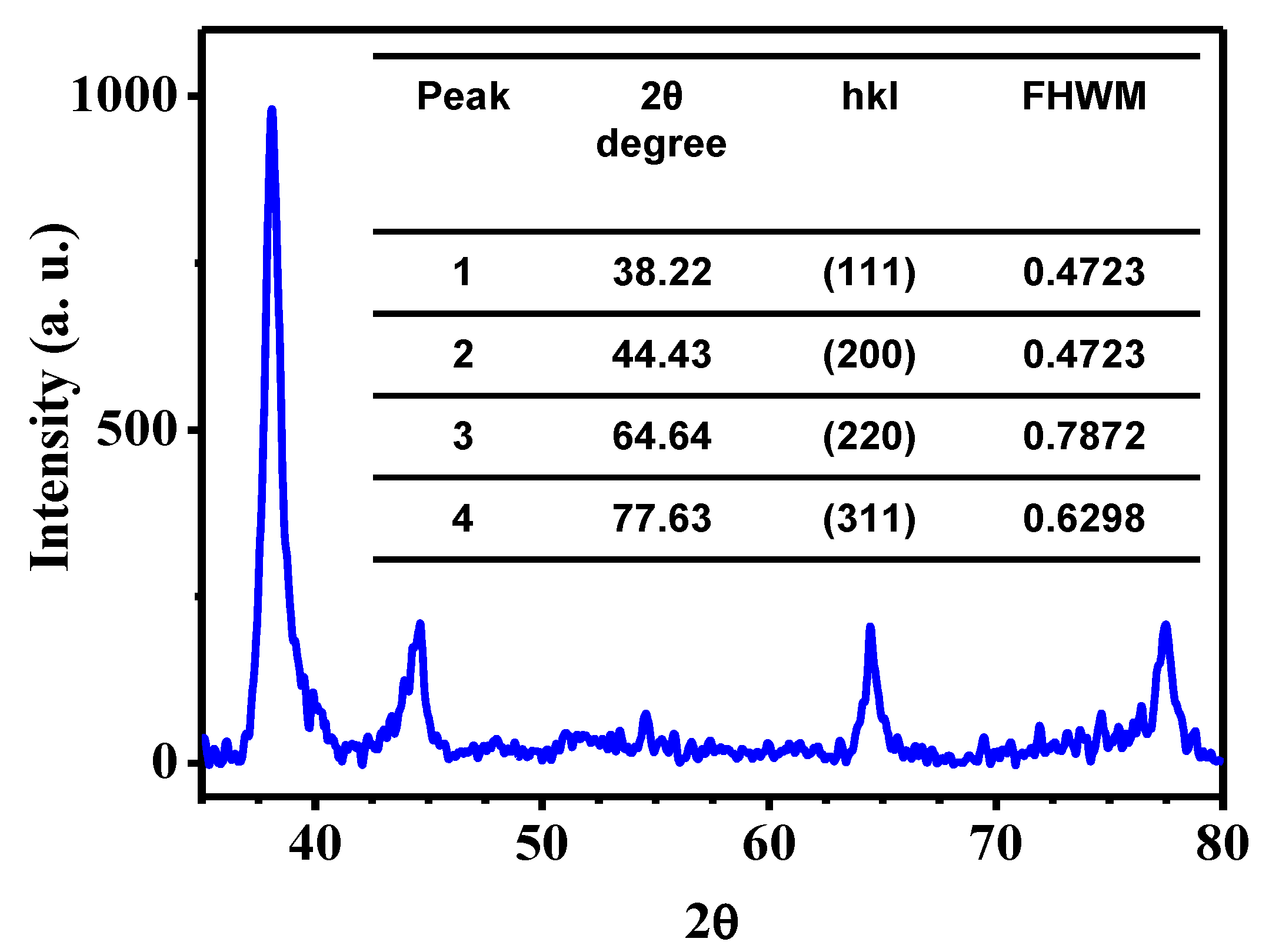


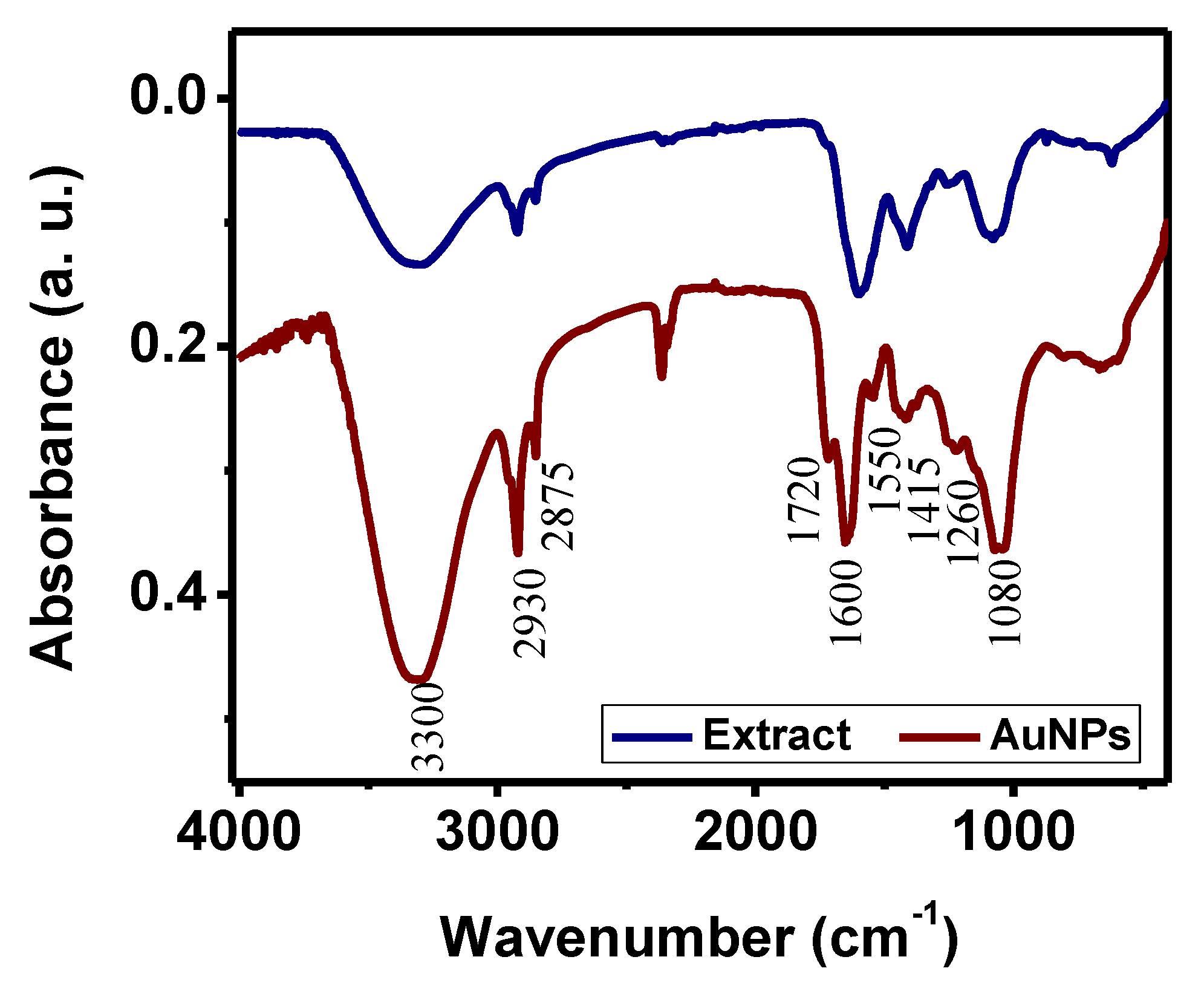

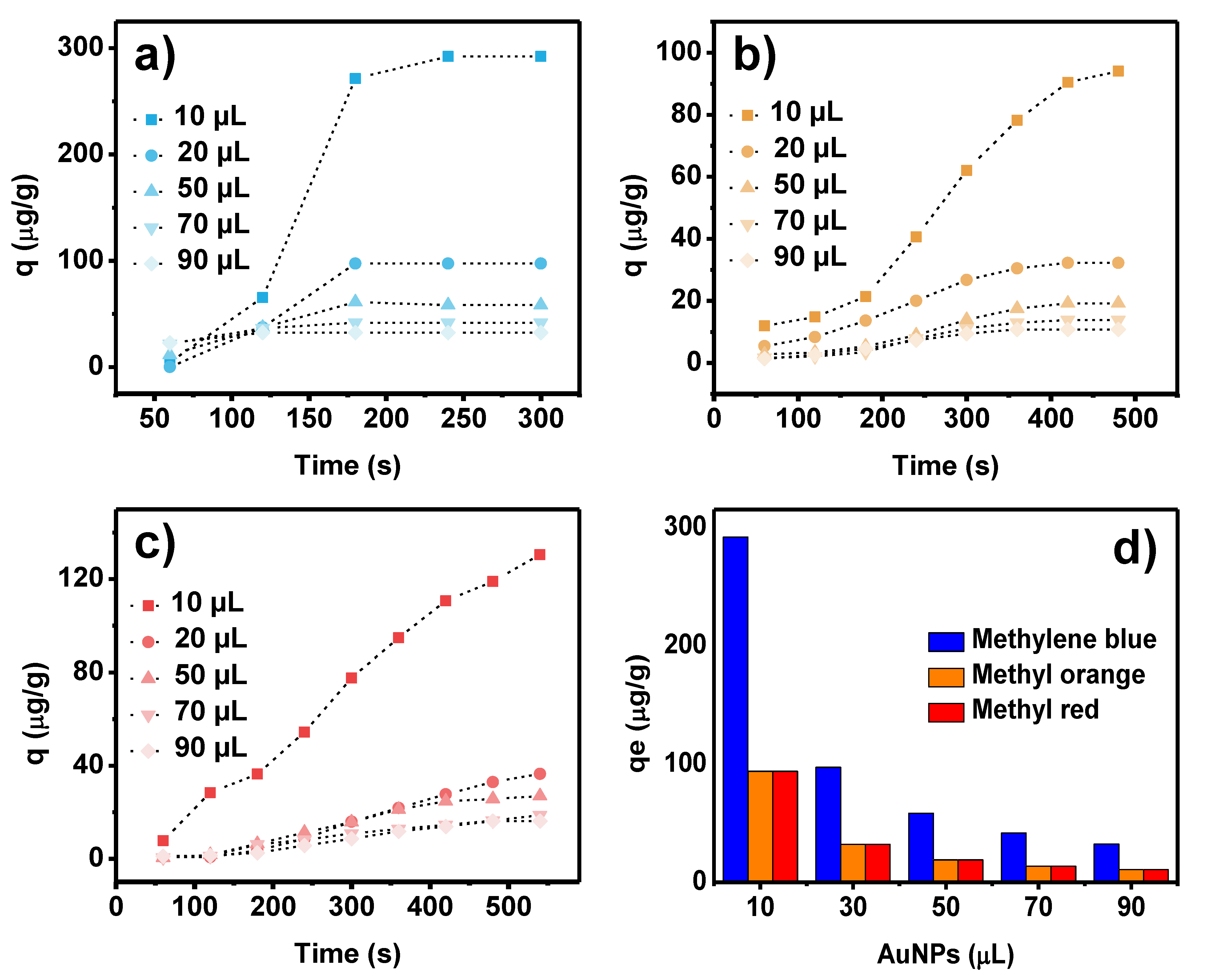
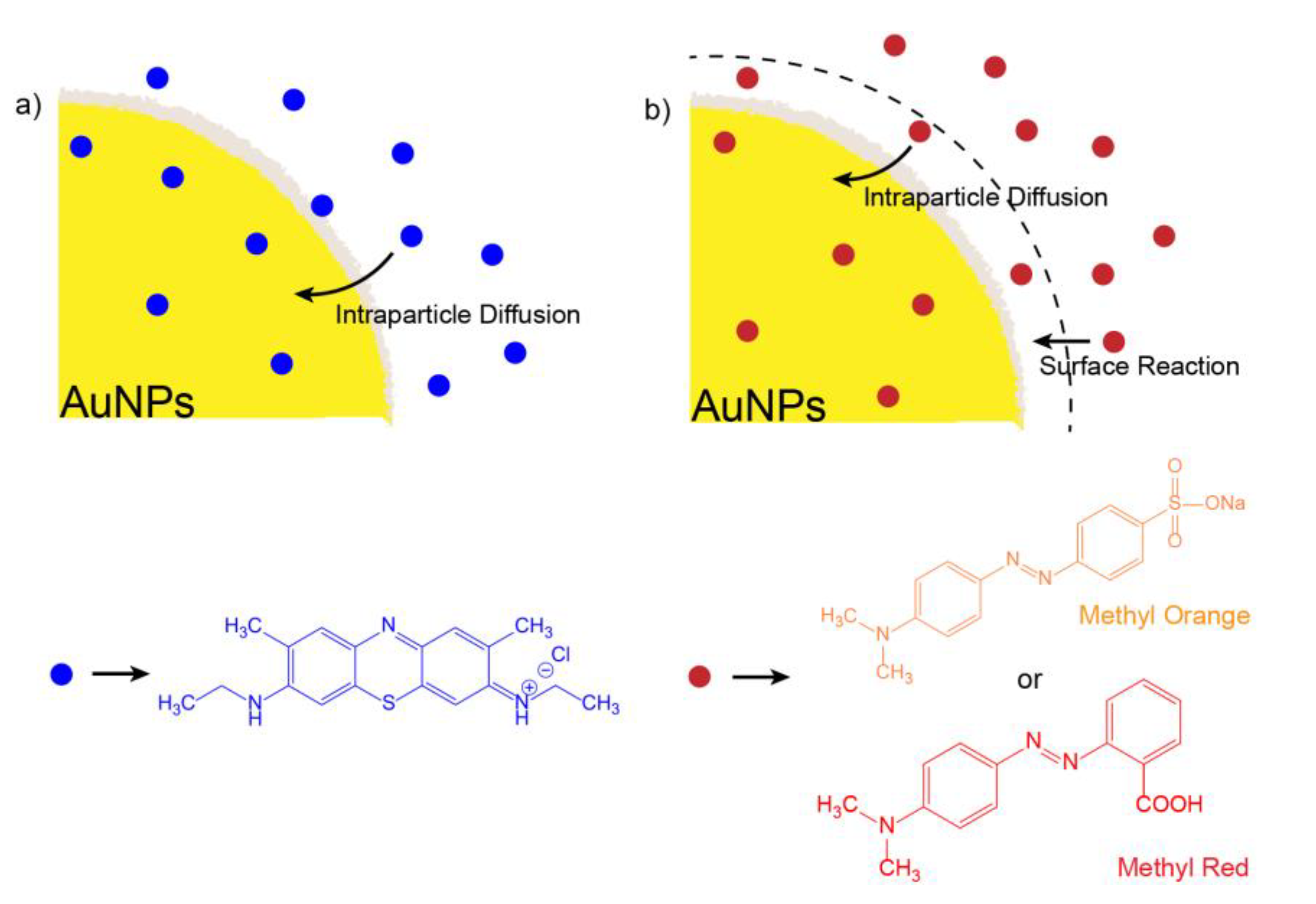
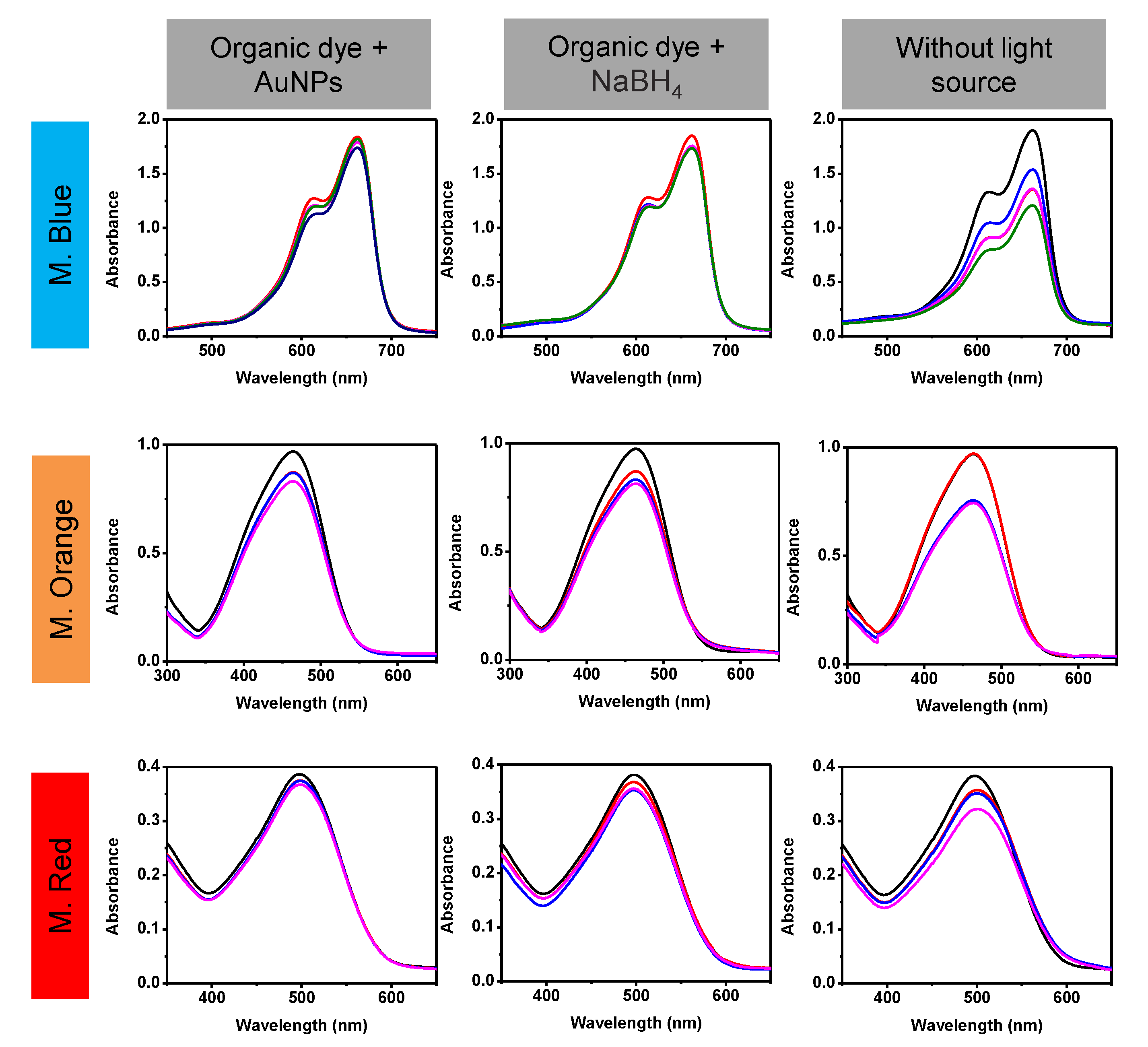


| Methylene Blue | Methyl Orange | Methyl Red | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Kinetic Model | Characteristic Parameter | R2 | Characteristic Parameter | R2 | Characteristic Parameter | R2 | |||
| PFO | k1 | 1.3150 | 0.6339 | k1 | 0.4776 | 0.7787 | k1 | 0.3344 | 0.9198 |
| qe | 1533.10 | qe | 226.05 | qe | 224.70 | ||||
| PSO | k2 | 0.0094 | 0.3937 | k2 | 0.0003 | 0.1862 | k2 | 0.0002 | 0.3453 |
| h | 5.5036 | h | 8.1037 | h | 9.9900 | ||||
| qe | −24.1546 | qe | −172.4138 | qe | −200 | ||||
| Elovich model | α | 194.1453 | 0.8460 | α | 37.7868 | 0.8148 | α | 49.4101 | 0.9098 |
| β | 0.0049 | β | 0.0226 | β | 0.0171 | ||||
| IPD | ki | 1 | 1 | ki | 52.0833 | 0.9149 | ki | 64.6271 | 0.9768 |
| Ci | 0 | Ci | −54.4762 | Ci | −65.3418 | ||||
| Dye | AuNPs (µL) | TOF (h−1) |
|---|---|---|
| Methylene Blue | 10 | 3.60 |
| 90 | 1.07 | |
| Methyl Orange | 10 | 4.98 × 10−1 |
| 90 | 6.18 × 10−2 | |
| Methyl Red | 10 | 7.75 × 10−1 |
| 90 | 0.96 × 10−1 |
| Material | Synthesis Method | Organic Dye | % Degradation | Reference |
|---|---|---|---|---|
| AuNPs (This work) | Green synthesis | Methylene blue | 99.6 | |
| Methyl orange | 98.2 | |||
| Methyl red | 94.9 | |||
| AuNPs | Chemical reduction | Methylene blue | 99.4 | [51] |
| Methyl orange | 91 | |||
| AuNPs-zeolite | Chemical reduction | Methylene blue | 50 | [52] |
| AuNPs-MoS2 | Chemical reduction | Methylene blue | 94.3 | [53] |
| Methyl red | 96.7 | |||
| AuNPs | Green synthesis | Methylene blue | 96.26 | [37] |
| Au-Ag alloy NPs | Green synthesis | Methylene blue | 96.59 | [54] |
| Methyl orange | 80.06 | |||
| Methylene violet | 86.88 | |||
| AuNPs | Green synthesis | Methylene blue | 84 | [55] |
| Au-ZnO NPs | Sol-gel | Methylene blue | 82.1 | [56] |
| AuNPs | Green synthesis | Acid Orange II | 94.6 | [38] |
| Acid yellow II | 92.7 | |||
| Reactive red | 95.3 | |||
| AuNPs | Green synthesis | Methyl orange | 83.25 | [57] |
| Rhodamine B | 87.64 | |||
| AuNPs | Green synthesis | Methylene violet | 89.17 | [58] |
| TiO2-AuNPs | Chemical reduction/UV irradiation | Methylene blue | 90 | [59] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
López-Miranda, J.L.; Molina, G.A.; Esparza, R.; González-Reyna, M.A.; Silva, R.; Estévez, M. Green Synthesis of Homogeneous Gold Nanoparticles Using Sargassum spp. Extracts and Their Enhanced Catalytic Activity for Organic Dyes. Toxics 2021, 9, 280. https://doi.org/10.3390/toxics9110280
López-Miranda JL, Molina GA, Esparza R, González-Reyna MA, Silva R, Estévez M. Green Synthesis of Homogeneous Gold Nanoparticles Using Sargassum spp. Extracts and Their Enhanced Catalytic Activity for Organic Dyes. Toxics. 2021; 9(11):280. https://doi.org/10.3390/toxics9110280
Chicago/Turabian StyleLópez-Miranda, J. Luis, Gustavo A. Molina, Rodrigo Esparza, Marlen Alexis González-Reyna, Rodolfo Silva, and Miriam Estévez. 2021. "Green Synthesis of Homogeneous Gold Nanoparticles Using Sargassum spp. Extracts and Their Enhanced Catalytic Activity for Organic Dyes" Toxics 9, no. 11: 280. https://doi.org/10.3390/toxics9110280







