Exploring Biophysical Linkages between Coastal Forestry Management Practices and Aquatic Bivalve Contaminant Exposure
Abstract
:1. Introduction
1.1. Forest Management in Oregon’s Coastal Zone
1.2. Chemical Applications in Forestry Practices
1.3. Management Practices and Ecotoxicology
1.4. Monitoring Considerations
1.5. Project Goals
2. Materials and Methods
2.1. Site Selection
2.2. Field Sampling Methods
2.3. Biomonitoring of Bivalves
Laboratory Analytical Methods
2.4. Passive Water Sampling
2.5. Spatial Analysis of Oregon Coast Watersheds
2.6. Statistical Analyses
- CL= lipid-normalized concentration;
- Ci = initial concentration of the chemical in the bivalve tissue (ng/g);
- FL = fraction of the tissue that is lipid.
2.7. Quality Assurance/Quality Control
3. Results
3.1. Biomonitoring of Bivalves
3.1.1. Bivalve Lipid Content
3.1.2. Tissue Pesticide Analysis
3.2. Analysis of Passive Water Samples
3.2.1. POCIS Deployment
3.2.2. POCIS Detections
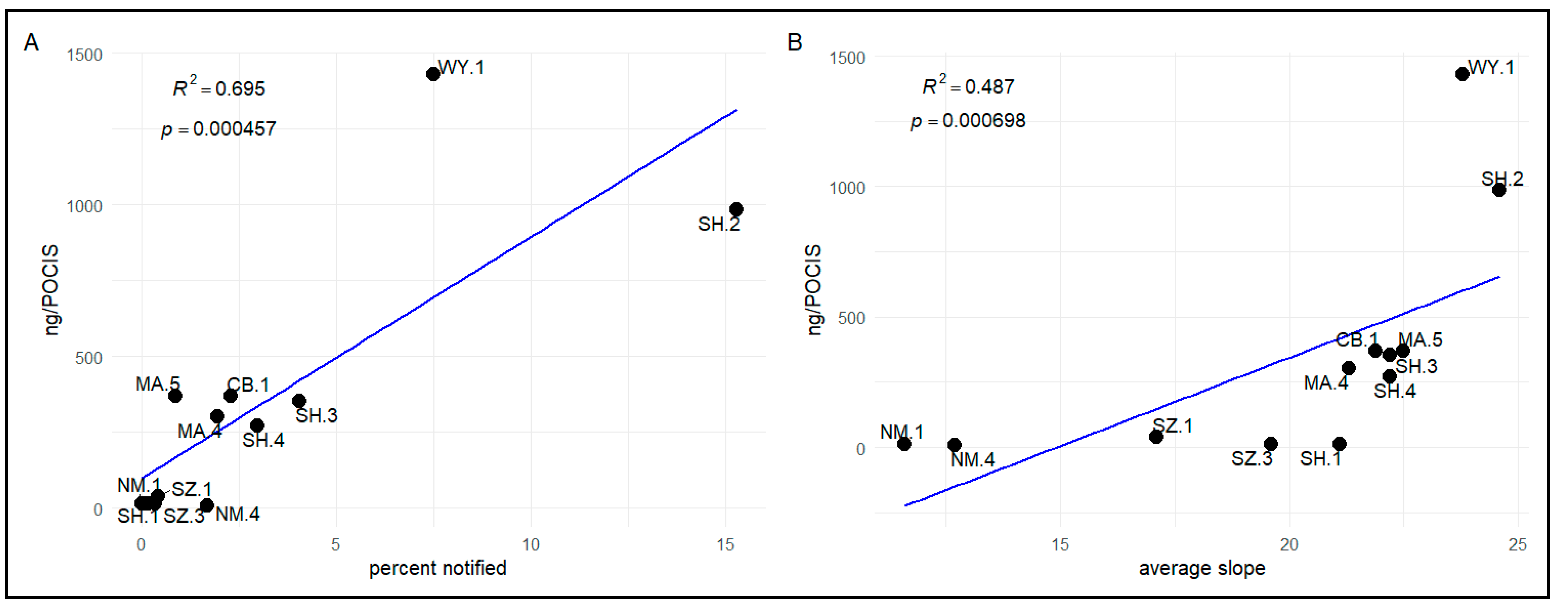
3.2.3. Relationships between Compound Detections and Forestland Management
3.3. Combined Chemical Results and Considerations
4. Discussion
4.1. Interpreting Project Goals and Analyses
4.1.1. Seasonal and Species Differences in Contaminant/Exposure Levels
4.1.2. Contrast in Compounds Detected in Waters and Bivalve Tissues
4.1.3. Forestland Management Regimes and Exposure of Bivalves to Pesticides
4.2. Additional Factors Affecting Pesticide Exposure and Transport in Coastal Watersheds
4.2.1. Spatial Scale and Complexity of Watershed Drainages
4.2.2. Ecotoxicity of Pesticide Mixtures and Pulsed Exposures
4.2.3. Management Practices
4.3. Caveats and Lessons Learned
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Granek, E.F.; Polasky, S.; Kappel, C.V.; Reed, D.J.; Stoms, D.M.; Koch, E.W.; Kennedy, C.J.; Cramer, L.A.; Hacker, S.D.; Barbier, E.B.; et al. Ecosystem Services as a Common Language for Coastal Ecosystem-Based Management. Conserv. Biol. 2010, 24, 207–216. [Google Scholar] [CrossRef]
- Lester, S.E.; McLeod, K.L.; Tallis, H.; Ruckelshaus, M.; Halpern, B.S.; Levin, P.S.; Chavez, F.P.; Pomeroy, C.; McCay, B.J.; Costello, C.; et al. Science in Support of Ecosystem-Based Management for the US West Coast and Beyond. Biol. Conserv. 2010, 143, 576–587. [Google Scholar] [CrossRef]
- Álvarez-Romero, J.G.; Pressey, R.L.; Ban, N.C.; Vance-Borland, K.; Willer, C.; Klein, C.J.; Gaines, S.D. Integrated Land-Sea Conservation Planning: The Missing Links. Annu. Rev. Ecol. Evol. Syst. 2011, 42, 381–409. [Google Scholar] [CrossRef] [Green Version]
- Stoms, D.M.; Davis, F.W.; Andelman, S.J.; Carr, M.H.; Gaines, S.D.; Halpern, B.S.; Hoenicke, R.; Leibowitz, S.G.; Leydecker, A.; Madin, E.M.; et al. Integrated Coastal Reserve Planning: Making the Land–Sea Connection. Front. Ecol. Environ. 2005, 3, 429–436. [Google Scholar] [CrossRef] [Green Version]
- Munns, W.R. Assessing Risks to Wildlife Populations from Multiple Stressors: Overview of the Problem and Research Needs. Ecol. Soc. 2006, 11, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Department of Land Conservation and Development: Oregon’s Coastal Zone: Oregon Coastal Management Program: State of Oregon. Available online: https://www.oregon.gov/lcd/OCMP/Pages/Coastal-Zone.aspx (accessed on 24 August 2020).
- Spies, T.A.; Johnson, K.N.; Burnett, K.M.; Ohmann, J.L.; McComb, B.C.; Reeves, G.H.; Bettinger, P.; Kline, J.D.; Garber-Yonts, B. Cumulative Ecological and Socioeconomic Effects of Forest Policies in Coastal Oregon. Ecol. Appl. 2007, 17, 5–17. [Google Scholar] [CrossRef]
- Johnson, S.L.; Jones, J.A. Stream Temperature Responses to Forest Harvest and Debris Flows in Western Cascades, Oregon. Can. J. Fish. Aquat. Sci. 2011. [Google Scholar] [CrossRef]
- Perry, T.D.; Jones, J.A. Summer Streamflow Deficits from Regenerating Douglas-Fir Forest in the Pacific Northwest, USA. Ecohydrology 2017, 10, e1790. [Google Scholar] [CrossRef]
- Clark, L.; Roloff, G.; Tatum, V.; Irwin, L.L. Forest Herbicide Effects on Pacific Northwest Ecosystems: A Literature Review. NCASI Tech. Bull. 2009, 1, 1–184. [Google Scholar]
- Norris, L.A.; Lorz, H.W.; Gregory, S.V. Forest chemicals. In Influences of Forest and Rangeland Management on Salmonid Fishes and Their Habitat; American Fisheries Society: Bethesda, MD, USA, 1991; pp. 207–296. [Google Scholar]
- Spies, T.A.; Stine, P.A.; Gravenmier, R.; Long, J.W.; Reilly, M.J.; Mazza, R. Synthesis of Science to Inform Land Management within the Northwest Forest Plan Area: Executive Summary; Gen. Tech. Rep. PNW-GTR-970; US Department of Agriculture, Forest Service, Pacific Northwest Research Station: Portland, OR, USA, 2018; Volume 970.
- Thomas, J.W.; Franklin, J.F.; Gordon, J.; Johnson, K.N. The Northwest Forest Plan: Origins, Components, Implementation Experience, and Suggestions for Change. Conserv. Biol. 2006, 20, 277–287. [Google Scholar] [CrossRef]
- Kaplan, D.M.; White, C.G. Integrating Landscape Ecology into Natural Resource Management; Cambridge University Press: Cambridge, UK, 2002; ISBN 978-0-521-78433-7. [Google Scholar]
- Forest Ecosystem Management Assessment Team. US Forest Ecosystem Management: An Ecological, Economic, and Social Assessment: Report of the Forest Ecosystem Management Assessment Team; The Service; Forest Ecosystem Management Assessment Team: Portland, OR, USA, 1993. [Google Scholar]
- Boisjolie, B.A.; Santelmann, M.V.; Flitcroft, R.L.; Duncan, S.L. Legal Ecotones: A Comparative Analysis of Riparian Policy Protection in the Oregon Coast Range, USA. J. Environ. Manag. 2017, 197, 206–220. [Google Scholar] [CrossRef] [Green Version]
- Adams, P.W. Policy and Management for Headwater Streams in the Pacific Northwest: Synthesis and Reflection. For. Sci. 2007, 53, 104–118. [Google Scholar] [CrossRef]
- Oregon Secretary of State Administrative Rules. Available online: https://secure.sos.state.or.us/oard/viewSingleRule.action?ruleVrsnRsn=162555 (accessed on 24 August 2020).
- Senate Bill 1602, 80th Oregon Legislative Assembly, 2020 Special Session; State of Oregon, Department of Environmental Quality: Portland, OR, USA, 2020.
- US Bureau of Land Management. Vegetation Treatments Using Herbicides on BLM Lands in Oregon (ROD); Bureau of Land Management: El Centro, CA, USA, 2010.
- Peachy, E. Pacific Northwest Weed Management Handbook [Online]; Oregon State University: Corvallis, OR, USA, 2020. [Google Scholar]
- Pesticide Information Center Online Database (PICOL). Available online: https://picol.cahnrs.wsu.edu/ (accessed on 24 August 2020).
- Dent, L.; Robben, J. Oregon Department of Forestry: Aerial Pesticide Application Monitoring Final Report; Technical Report 7; Oregon Department of Forestry, Forest Practices Monitoring Program: Salem, OR, USA, 2000; Volume 35.
- Louch, J.; Tatum, V.; Allen, G.; Hale, V.C.; McDonnell, J.; Danehy, R.J.; Ice, G. Potential Risks to Freshwater Aquatic Organisms Following a Silvicultural Application of Herbicides in Oregon’s Coast Range. Integr. Environ. Assess. Manag. 2017, 13, 396–409. [Google Scholar] [CrossRef]
- Rashin, E.; Graber, C. Effectiveness of Best Management Practices for Aerial Application of Forest Pesticides; Washington State Department of Ecology, Environmental Investigations and Laboratory Services Program, Watershed Assessments Section: Olympia, WA, USA, 1993. [Google Scholar]
- Cox Caroline; Surgan Michael Unidentified Inert Ingredients in Pesticides: Implications for Human and Environmental Health. Environ. Health Perspect. 2006, 114, 1803–1806. [CrossRef] [Green Version]
- Laetz Cathy, A.; Baldwin David, H.; Collier Tracy, K.; Hebert, V.; Stark John, D.; Scholz Nathaniel, L. The Synergistic Toxicity of Pesticide Mixtures: Implications for Risk Assessment and the Conservation of Endangered Pacific Salmon. Environ. Health Perspect. 2009, 117, 348–353. [Google Scholar] [CrossRef] [Green Version]
- Greco, L.; Pellerin, J.; Capri, E.; Garnerot, F.; Louis, S.; Fournier, M.; Sacchi, A.; Fusi, M.; Lapointe, D.; Couture, P. Physiological Effects of Temperature and a Herbicide Mixture on the Soft-Shell Clam Mya Arenaria (Mollusca, Bivalvia). Environ. Toxicol. Chem. 2011, 30, 132–141. [Google Scholar] [CrossRef]
- Renault, T. Effects of Pesticides on Marine Bivalves: What Do We Know and What Do We Need to Know? In Pesticides in the Modern World—Risks and Benefits; Stoytcheva, M., Ed.; InTech: Rijeka, Croatia, 2011; ISBN 978-953-307-458-0. [Google Scholar]
- Gunderson, M.P.; Veldhoen, N.; Skirrow, R.C.; Macnab, M.K.; Ding, W.; van Aggelen, G.; Helbing, C.C. Effect of Low Dose Exposure to the Herbicide Atrazine and Its Metabolite on Cytochrome P450 Aromatase and Steroidogenic Factor-1 MRNA Levels in the Brain of Premetamorphic Bullfrog Tadpoles (Rana Catesbeiana). Aquat. Toxicol. Amst. Neth. 2011, 102, 31–38. [Google Scholar] [CrossRef] [Green Version]
- Tanguy, A.; Boutet, I.; Laroche, J.; Moraga, D. Molecular Identification and Expression Study of Differentially Regulated Genes in the Pacific Oyster Crassostrea Gigas in Response to Pesticide Exposure. FEBS J. 2005, 272, 390–403. [Google Scholar] [CrossRef]
- Kudsk, P.; Mathiassen, S.K. Joint Action of Amino Acid Biosynthesis-Inhibiting Herbicides. Weed Res. 2004, 44, 313–322. [Google Scholar] [CrossRef]
- Hayes, T.B.; Case, P.; Chui, S.; Chung, D.; Haeffele, C.; Haston, K.; Lee, M.; Mai, V.P.; Marjuoa, Y.; Parker, J.; et al. Pesticide Mixtures, Endocrine Disruption, and Amphibian Declines: Are We Underestimating the Impact? Environ. Health Perspect. 2006, 114, 40–50. [Google Scholar] [CrossRef] [PubMed]
- Michael, J.L. Best Management Practices for Silvicultural Chemicals and the Science behind Them. Water Air Soil Pollut. Focus 2004, 4, 95–117. [Google Scholar] [CrossRef]
- Kennish, M.J. Pollution Impacts on Marine Biotic Communities; CRC Press: Boca Raton, FL, USA, 1997; ISBN 978-0-8493-8428-8. [Google Scholar]
- Jacomini, A.E.; Avelar, W.E.P.; Martinêz, A.S.; Bonato, P.S. Bioaccumulation of Atrazine in Freshwater Bivalves Anodontites Trapesialis (Lamarck, 1819) and Corbicula Fluminea (Müller, 1774). Arch. Environ. Contam. Toxicol. 2006, 51, 387–391. [Google Scholar] [CrossRef]
- Metcalfe, C.D.; Helm, P.; Paterson, G.; Kaltenecker, G.; Murray, C.; Nowierski, M.; Sultana, T. Pesticides Related to Land Use in Watersheds of the Great Lakes Basin—ScienceDirect. Sci. Total Environ. 2019, 681–692. [Google Scholar] [CrossRef]
- National Research Council. Animals as Sentinels of Environmental Health Hazards; National Academies Press: Washington, DC, USA, 1991; ISBN 978-0-309-04046-4. [Google Scholar]
- Phillips, D.J.H.; Rainbow, P.S. Biomonitoring of Trace Aquatic Contaminants; Springer Science & Business Media: Berlin/Heidelberg, Germany, 1998; ISBN 978-94-011-2122-4. [Google Scholar]
- Siah, A.; Pellerin, J.; Benosman, A.; Gagné, J.-P.; Amiard, J.-C. Seasonal Gonad Progesterone Pattern in the Soft-Shell Clam Mya Arenaria. Comp. Biochem. Physiol. A. Mol. Integr. Physiol. 2002, 132, 499–511. [Google Scholar] [CrossRef]
- Haider, F.; Timm, S.; Bruhns, T.; Noor, M.N.; Sokolova, I.M. Effects of Prolonged Food Limitation on Energy Metabolism and Burrowing Activity of an Infaunal Marine Bivalve, Mya Arenaria. Comp. Biochem. Physiol. A. Mol. Integr. Physiol. 2020, 250, 110780. [Google Scholar] [CrossRef]
- Liu, W.; Li, Q.; Kong, L. Reproductive Cycle and Seasonal Variations in Lipid Content and Fatty Acid Composition in Gonad of the Cockle Fulvia Mutica in Relation to Temperature and Food. J. Ocean Univ. China 2013, 12, 427–433. [Google Scholar] [CrossRef]
- LeBlanc, G.A. Trophic-Level Differences in the Bioconcentration of Chemicals: Implications in Assessing Environmental Biomagnification. Environ. Sci. Technol. 1995, 29, 154–160. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.J. Impact of Herbicides on the Forest Ecosystem, Aquatic Ecosystems and Wildlife: The American Experience. Rev. For. Fr. Spec. 2002, 16, 593–608. [Google Scholar]
- Tzilivakis, J. Agricultural Substances Databases Background and Support Information. Available online: https://sitem.herts.ac.uk/aeru/ppdb/en/docs/5_1.pdf (accessed on 20 October 2020).
- Nedeau, E.; Smith, A.K.; Stone, J.; Jepsen, S. Freshwater Mussels of the Pacific Northwest, 2nd ed.; Xerces Society for Invertebrate Conservation: Portland, OR, USA, 2009; p. 60. [Google Scholar]
- Blevins, E.; Jepsen, S.; Box, J.B.; Nez, D.; Howard, J.; Maine, A.; O’Brien, C. Extinction Risk of Western North American Freshwater Mussels: Anodonta Nuttalliana, the Anodonta Oregonensis/Kennerlyi Clade, Gonidea Angulata, and Margaritifera Falcata. Freshw. Mollusk Biol. Conserv. 2017, 20, 71–88. [Google Scholar] [CrossRef]
- Abraham, B.J.; Dillon, P.L. Species Profiles. Life Histories and Environmental Requirements of Coastal Fishes and Invertebrates (Mid-Atlantic). Softshell Clam; U.S. Department of the Interior, U.S. Fish and Wildlife Service: Burlington, MA, USA, 1986. [Google Scholar]
- Pauley, G.B.; Van Der Raay, D. Species Profiles: Life Histories and Environmental Requirements of Coastal Fishes and Invertebrates (Pacific Northwest): Pacific Oyster; Cooperative Fishery Research Unit, Washington University: Seattle, WA, USA, 1988. [Google Scholar]
- Haag, W.R. North American Freshwater Mussels: Natural History, Ecology, and Conservation; Cambridge University Press: Cambridge, UK, 2012; ISBN 978-1-139-56019-1. [Google Scholar]
- Hladik, M.L.; Vandever, M.; Smalling, K.L. Exposure of native bees foraging in an agricultural landscape to current-use pesticides. Sci. Total Environ. 2016, 542, 469–477. [Google Scholar] [CrossRef]
- Alvarez, D.A. Guidelines for the Use of the Semipermeable Membrane Device (SPMD) and the Polar Organic Chemical Integrative Sampler (POCIS) in Environmental Monitoring Studies. US Geol. Surv. Tech. Methods 2010, 1, 28. [Google Scholar]
- Oregon Health Authority. Public Health Assessment: Highway 36 Corridor Exposure Investigation; Oregon Health Authority: Salem, OR, USA, 2014; p. 138. [Google Scholar]
- Allard, D.J.; Whitesel, T.A.; Lohr, S.C.; Koski, M.L. Western Pearlshell Mussel Life History in Merrill Creek, Oregon: Reproductive Timing, Growth, and Movement. Northwest Sci. 2017, 91, 1–14. [Google Scholar] [CrossRef]
- Lindsay, S.; Chasse, J.; Butler, R.A.; Morrill, W.; Van Beneden, R.J. Impacts of Stage-Specific Acute Pesticide Exposure on Predicted Population Structure of the Soft-Shell Clam, Mya Arenaria. Aquat. Toxicol. 2010, 98, 265–274. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oregon Department of Forestry: Maps & Data: About ODF: State of Oregon. Available online: https://www.oregon.gov/ODF/AboutODF/Pages/MapsData.aspx (accessed on 25 August 2020).
- FERNS—Welcome. Available online: https://ferns.odf.oregon.gov/e-notification (accessed on 25 August 2020).
- Bruner, K.A.; Fisher, S.W.; Landrum, P.F. The Role of the Zebra Mussel, Dreissena Polymorpha, in Contaminant Cycling: I. The Effect of Body Size and Lipid Content on the Bioconcentration of PCBs and PAHs. J. Gt. Lakes Res. 1994, 20, 725–734. [Google Scholar] [CrossRef]
- Moore, D.G.; Loper, B.R. DDT Residues in Forest Floors and Soils of Western Oregon, September—November 1966. Pestic. Monit. J. 1980, 14, 77–85. [Google Scholar]
- Lewis, K.A.; Tzilivakis, J.; Warner, D.; Green, A. An International Database for Pesticide Risk Assessments and Management. Hum. Ecol. Risk Assess. Int. J. 2016, 22, 1050–1064. [Google Scholar] [CrossRef] [Green Version]
- Hapke, W.B.; Morace, J.L.; Nilsen, E.B.; Alvarez, D.A.; Masterson, K. Year-Round Monitoring of Contaminants in Neal and Rogers Creeks, Hood River Basin, Oregon, 2011-12, and Assessment of Risks to Salmonids. PLoS ONE 2016, 11, e0158175. [Google Scholar] [CrossRef]
- Capuzzo, J.M.; Farrington, J.W.; Rantamaki, P.; Clifford, C.H.; Lancaster, B.A.; Leavitt, D.F.; Jia, X. The Relationship between Lipid Composition and Seasonal Differences in the Distribution of PCBs in Mytilus Edulis L. Mar. Environ. Res. 1989, 28, 259–264. [Google Scholar] [CrossRef]
- Thompson, K.-L.; Picard, C.R.; Chan, H.M. Polycyclic Aromatic Hydrocarbons (PAHs) in Traditionally Harvested Bivalves in Northern British Columbia, Canada. Mar. Pollut. Bull. 2017, 121, 390–399. [Google Scholar] [CrossRef]
- Choi, J.Y.; Yang, D.B.; Hong, G.H.; Kim, K.; Shin, K.-H. Ecological and Human Health Risk from Polychlorinated Biphenyls and Organochlorine Pesticides in Bivalves of Cheonsu Bay, Korea. Environ. Eng. Res. 2016, 21, 373–383. [Google Scholar] [CrossRef]
- Kaapro, J.; Hall, J. Indaziflam—A New Herbicide for Pre-Emergent Control of Weeds in Turf, Forestry, Industrial Vegetation and Ornamentals. In Proceedings of the 23rd Asian-Pacific Weed Science Society Conference, Cairns City, Australia, 26–29 September 2012; Volume 4. [Google Scholar]
- National Center for Biotechnology Information Compound Summary for CID 44146693, Indaziflam. Available online: http://pubchem.ncbi.nlm.nih.gov/compound/44146693 (accessed on 23 January 2021).
- Caldwell, L.K.; Courter, L.A. Abiotic Factors Influence Surface Water Herbicide Concentrations Following Silvicultural Aerial Application in Oregon’s North Coast Range. Integr. Environ. Assess. Manag. 2020, 16, 114–127. [Google Scholar] [CrossRef] [Green Version]
- Boyle, J.R.; Warila, J.E.; Beschta, R.L.; Reiter, M.; Chambers, C.C.; Gibson, W.P.; Gregory, S.V.; Grizzel, J.; Hagar, J.C.; Li, J.L.; et al. Cumulative Effects of Forestry Practices: An Example Framework for Evaluation from Oregon, U.S.A. Biomass Bioenergy 1997, 13, 223–245. [Google Scholar] [CrossRef]
- Müller, K.; Trolove, M.; James, T.K.; Rahman, A. Herbicide Loss in Runoff: Effects of Herbicide Properties, Slope, and Rainfall Intensity. Soil Res. 2004, 42, 17–27. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, M. Modeling Effectiveness of Agricultural BMPs to Reduce Sediment Load and Organophosphate Pesticides in Surface Runoff. Sci. Total Environ. 2011, 409, 1949–1958. [Google Scholar] [CrossRef]
- Morselli, M.; Vitale, C.M.; Ippolito, A.; Villa, S.; Giacchini, R.; Vighi, M.; Di Guardo, A. Predicting Pesticide Fate in Small Cultivated Mountain Watersheds Using the DynAPlus Model: Toward Improved Assessment of Peak Exposure. Sci. Total Environ. 2018, 615, 307–318. [Google Scholar] [CrossRef]
- Schriever, C.A.; von der Ohe, P.C.; Liess, M. Estimating Pesticide Runoff in Small Streams. Chemosphere 2007, 68, 2161–2171. [Google Scholar] [CrossRef]
- Touart, L.W.; Maciorowski, A.F. Information Needs for Pesticide Registration in the United States. Ecol. Appl. 1997, 7, 1086–1093. [Google Scholar] [CrossRef]
- Lydy, M.; Belden, J.; Wheelock, C.; Hammock, B.; Denton, D. Challenges in Regulating Pesticide Mixtures. Ecol. Soc. 2004, 9. [Google Scholar] [CrossRef] [Green Version]
- Sobiech, S.A.; Henry, M.G. The Difficulty in Determining the Effects of Pesticides on Aquatic Communities. In Biological Response Signatures: Indicator Patterns Using Aquatic Communities; CRC Press: Boca Raton, FL, USA, 2002; ISBN 978-1-4200-4145-3. [Google Scholar]
- Gordon, A.K.; Mantel, S.K.; Muller, N.W.J. Review of Toxicological Effects Caused by Episodic Stressor Exposure. Environ. Toxicol. Chem. 2012, 31, 1169–1174. [Google Scholar] [CrossRef] [PubMed]
- Perry, K.; Lynn, J. Detecting Physiological and Pesticide-Induced Apoptosis in Early Developmental Stages of Invasive Bivalves. Hydrobiologia 2009, 628, 153–164. [Google Scholar] [CrossRef]
- Flynn, K.; Spellman, T. Environmental Levels of Atrazine Decrease Spatial Aggregation in the Freshwater Mussel, Elliptio Complanata. Ecotoxicol. Environ. Saf. 2009, 72, 1228–1233. [Google Scholar] [CrossRef] [PubMed]
- Cope, W.G.; Bringolf, R.B.; Buchwalter, D.B.; Newton, T.J.; Ingersoll, C.G.; Wang, N.; Augspurger, T.; Dwyer, F.J.; Barnhart, M.C.; Neves, R.J.; et al. Differential Exposure, Duration, and Sensitivity of Unionoidean Bivalve Life Stages to Environmental Contaminants. J. N. Am. Benthol. Soc. 2008, 27, 451–462. [Google Scholar] [CrossRef]
- Conners, D.E.; Black, M.C. Evaluation of Lethality and Genotoxicity in the Freshwater Mussel Utterbackiaimbecillis (Bivalvia: Unionidae) Exposed Singly and in Combination to ChemicalsUsed in Lawn Care. Arch. Environ. Contam. Toxicol. 2004, 46, 362–371. [Google Scholar] [CrossRef] [PubMed]
- Bringolf, R.B.; Cope, W.G.; Mosher, S.; Barnhart, M.C.; Shea, D. Acute and Chronic Toxicity of Glyphosate Compounds to Glochidia and Juveniles of Lampsilis Siliquoidea (Unionidae). Environ. Toxicol. Chem. 2007, 26, 2094–2100. [Google Scholar] [CrossRef] [PubMed]
- Kookana, R.; Holz, G.; Barnes, C.; Bubb, K.; Fremlin, R.; Boardman, B. Impact of Climatic and Soil Conditions on Environmental Fate of Atrazine Used under Plantation Forestry in Australia. J. Environ. Manag. 2010, 91, 2649–2656. [Google Scholar] [CrossRef]
- Mazza, R.; Olson, D. Heed the Head: Buffer Benefits along Headwater Streams; Science Findings 178; US Department of Agriculture, Forest Service, Pacific Northwest Research Station: Portland, OR, USA, 2015; Volume 178.
- Michael, J.L.; Neary, D.G. Herbicide Dissipation Studies in Southern Forest Ecosystems. Environ. Toxicol. Chem. 1993, 12, 405–410. [Google Scholar] [CrossRef]
- Tatum, V.L.; Jackson, C.R.; McBroom, M.W.; Baillie, B.R.; Schilling, E.B.; Wigley, T.B. Effectiveness of Forestry Best Management Practices (BMPs) for Reducing the Risk of Forest Herbicide Use to Aquatic Organisms in Streams. For. Ecol. Manag. 2017, 404, 258–268. [Google Scholar] [CrossRef]
- Milner-Gulland, E.J.; Shea, K. Embracing Uncertainty in Applied Ecology. J. Appl. Ecol. 2017, 54, 2063–2068. [Google Scholar] [CrossRef]
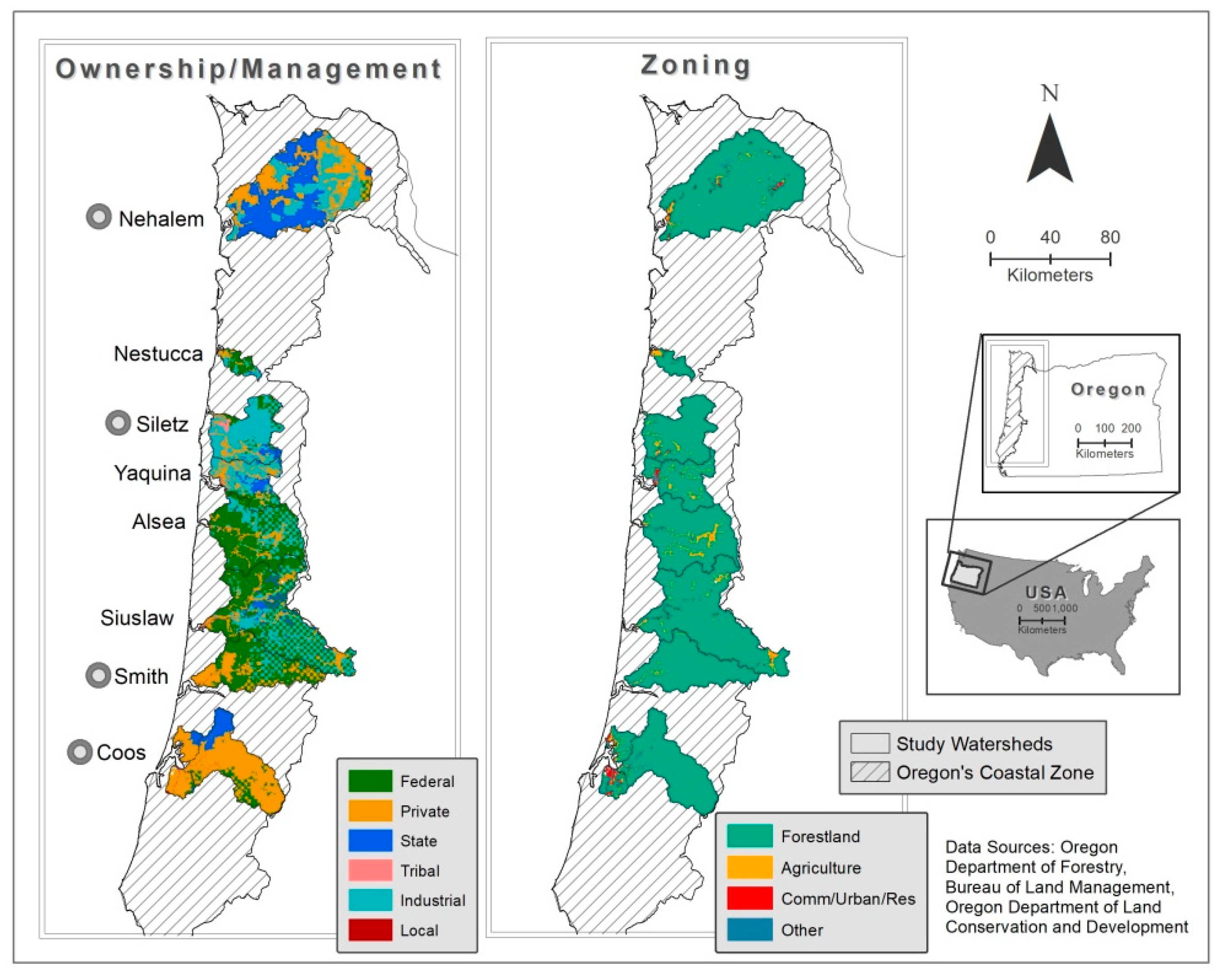





| Watershed | Watershed Area (sq. Kilometers) | Mean Annual Precip (Centimeters) | Mean Slope (Degrees) | Zoning (%) | Ownership/Management (%) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Forestland | Agriculture | Res/Comm/Indust | Other | Federal | State | Industrial/Private | Tribal | Local/Water | ||||
| Alsea | 1168.1 | 218.7 | 18.9 | 93.1 | 6.3 | 0.4 | 0.2 | 65.2 | 0.2 | 34.3 | 0.1 | 0.2 |
| Coos | 1358.7 | 178.1 | 17 | 92.5 | 2.5 | 2.7 | 2 | 10.9 | 13.4 | 74.9 | 0 | 0.8 |
| Nehalem | 2150.7 | 313.2 | 14.2 | 96.6 | 1.5 | 1.3 | 0.4 | 0.8 | 40.4 | 58.6 | 0 | 0.1 |
| Nestucca | 152.8 | 256.5 | 13.4 | 89.9 | 7.6 | 2.2 | 0.4 | 51.6 | 3.1 | 45.3 | 0 | 0.0 |
| Siletz | 787.4 | 266.7 | 17.2 | 95.3 | 3.4 | 0.7 | 0.5 | 11.2 | 3.8 | 82.2 | 2.4 | 0.4 |
| Siuslaw | 1779.3 | 176.3 | 19.6 | 96.2 | 2.8 | 0.9 | 0.1 | 51.7 | 5.3 | 42.6 | 0 | 0.4 |
| Smith | 955.7 | 185.9 | 22.2 | 98.1 | 1.4 | 0.1 | 0.5 | 57.7 | 0 | 41.9 | 0 | 0.3 |
| Yaquina | 569.8 | 193.8 | 17.4 | 90 | 6.3 | 2.2 | 1.5 | 15.2 | 13.2 | 70.8 | 0 | 0.8 |
| Species Attributes | Margaritifera falcata | Mya arenaria | Crassostrea gigas |
|---|---|---|---|
| Native Biogeographic Range | Western USA and Canada | East coast of USA, naturalized along west coast | Pacific coast of Asia |
| Habitat Type | Gravel and cobble substrates | Muddy substrate | Hard or rocky substrate |
| Water Salinity Preference (psu range) | Freshwater (0) | Upper estuarine; mesohaline, polyhaline (5–30) | Mid estuarine; polyhaline (20–25) |
| Management and conservation status | Designated as Near Threatened—(IUCN Red List) | Managed as a recreational fishery in Oregon | Commercial mariculture |
| Life-history Characteristics | Complex life-cycle with demersal glochidia larvae that attach to fish | Complex life-cycle with planktonic veliger larvae | Artificial propagation in hatcheries |
| Feeding Type | Suspension and deposit feeders | Suspension and deposit feeders | Suspension feeders |
| Life Span | >100 years | Up to 19 years, generally 10–12 years | Up to 40 years in northern latitudes |
| Pesticide Class | Detected Compounds | C. gigas | M. arenaria | M. falcata | |||
|---|---|---|---|---|---|---|---|
| Frequency | Max Conc. (ng/g dry weight) | Frequency | Max Conc. (ng/g dry weight) | Frequency | Max Conc. (ng/g dry weight) | ||
| Summer 2017 | |||||||
| Fungicides | Fenbuconazole | 1/6 | 16.7 | 1/18 | 21.1 | 0/14 | ND |
| Fluopicolide | 1/6 | 114.8 | 4/18 | 532.5 | 3/14 | 191.7 | |
| Pyraclostrobin | 0/6 | ND | 1/18 | 13.1 | 0/14 | ND | |
| Insecticides | Permethrin | 0/6 | ND | 1/18 | 238.8 | 0/14 | ND |
| Bifenthrin | 0/6 | ND | 2/18 | 12.7 | 0/14 | ND | |
| * Clothianidin Desmethyl | 1/6 | 52.2 | 1/18 | 24.6 | 0/14 | ND | |
| p,p’-DDT | 0/6 | ND | 0/18 | ND | 1/14 | 10.5 | |
| * p,p’-DDD | 0/6 | ND | 0/18 | ND | 1/14 | 10.9 | |
| * p,p’-DDE | 2/6 | 8.7 | 0/18 | ND | 1/14 | 9.8 | |
| Herbicides | Metolachlor | 0/6 | ND | 0/18 | ND | 1/14 | 7.8 |
| Indaziflam | 0/6 | ND | 1/18 | 235.8 | 1/14 | 26.6 | |
| Spring 2018 | |||||||
| Fungicides | Fenbuconazole | 1/6 | 11.8 | 2/24 | 215.7 | 0/9 | ND |
| Fluopicolide | 1/6 | 264.6 | 9/24 | 2421.3 | 0/9 | ND | |
| Insecticides | Bifenthrin | 0/6 | ND | 0/24 | ND | 4/9 | 11.6 |
| Indoxacarb | 0/6 | ND | 2/24 | 374.6 | 0/9 | ND | |
| Herbicide | Indaziflam | 1/6 | 107.4 | 2/24 | 1298.2 | 0/9 | ND |
| Compound | Sampling Matrix | Detection Matrix and Frequency | Year Introduced | Active Registration (in OR Forestry) | Pesticide Class | Mode of Action | Solubility—In Water at 20 °C (mg L−1) | Log Kow at pH 7, 20 °C | Koc | Groundwater Ubiquity Score (Leaching Potential) | Bioconcentration Factor (Potential Concern) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Atrazine | Tissue, water | Water, 60.0% (n = 15) | 1957 | Yes (yes) | Herbicide | Inhibits photosynthesis (photosystem II) | 35 | 2.7 | 100 | 2.57 (Moderate) | 4.3 (Low) |
| Bifenthrin | Tissue | Tissue, 7.8% (n = 77) | 1984 | Yes (yes) | Insecticide | Sodium channel modulator | 0.001 | 6.6 | 236,610 | −2.66 (Low) | 1703 (Threshold for concern) |
| Clothianidin Desmethyl * | Tissue | Tissue, 2.6% (n = 77) | Yes (no) | Insecticide * | n/a | n/a | n/a | n/a | n/a | n/a | |
| DDTs | Tissue | Tissue, 3.9% (n = 77) | 1944 | No (no) | Insecticide | Sodium channel modulator | 0.006 | 6.91 | 151,000 | −3.89 (Low) | 3173 (Threshold for concern) |
| Fenbuconazole | Tissue | Tissue, 6.5% (n = 77) | 1992 | Yes (no) | Fungicide | Inhibits sterol biosynthesis in fungi | 2.47 | 3.79 | 0.63 (Low) | 160 (threshold for concern | |
| Fluopicolide | Tissue | Tissue, 23.4% (n = 77) | 2006 | Yes (no) | Fungicide | Delocalizes spectrin-like proteins (novel) | 2.8 | 2.9 | 3.2 | 121 (Threshold for concern) | |
| Hexazinone | Tissue, Water | Water, 73.3% (n = 15) | 1975 | Yes (yes) | Herbicide | Inhibits photosynthesis (photosystem II) | 33,000 | 1.17 | 54 | 4.43 (High) | 7 (Low) |
| Indaziflam | Tissue | Tissue, 6.5% (n = 77) | 2010 | Yes (yes) | Herbicide | Inhibits cellulose biosynthesis (CB Inhibitor). | 2.8 | 2.8 | 1000 | 2.18 (Moderate) | Low risk (based on Kow) |
| Indoxacarb | Tissue | Tissue, 2.6% (n = 77) | 1996 | Yes (no) | Insecticide | Voltage-dependent sodium channel blocker. | 0.2 | 4.65 | 4483 | 0.27 (Low) | 77.3 (Low) |
| Metolachlor | Tissue | Tissue, 1.3% (n = 77) | 1976 | Yes (yes) | Herbicide | Inhibition of VLCFA (inhibition of cell division) | 530 | 3.4 | 120 | 2.36 (Moderate) | 68.8 (Low) |
| Metsulfuron- methyl | Water | Water, 6.7% (n = 15) | 1983 | Yes (yes) | Herbicide | Inhibits plant amino acid synthesis | 2790 | −1.87 | 3.28 (High) | 1 (Low) | |
| Permethrin | Tissue | Tissue, 1.3% (n = 77) | 1973 | Yes (yes) | Insecticide | Sodium channel modulator | 0.2 | 6.1 | 100,000 | −1.62 (Low) | 300 (Threshold for concern) |
| Pyraclostrobin | Tissue | Tissue, 1.3% (n = 77) | 2000 | Yes (yes) | Fungicide | Respiration inhibitor (QoL fungicide) | 1.9 | 3.99 | 9304 | 0.05 (Low) | 706 (threshold for concern) |
| Sulfometuron-methyl | Tissue, Water | Water, 40.0% (n = 15) | 1982 | Yes (yes) | Herbicide | Inhibits plant amino acid synthesis | 244 | −0.51 | 85 | 3.92 (High) | (Low) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Scully-Engelmeyer, K.; Granek, E.F.; Nielsen-Pincus, M.; Lanier, A.; Rumrill, S.S.; Moran, P.; Nilsen, E.; Hladik, M.L.; Pillsbury, L. Exploring Biophysical Linkages between Coastal Forestry Management Practices and Aquatic Bivalve Contaminant Exposure. Toxics 2021, 9, 46. https://doi.org/10.3390/toxics9030046
Scully-Engelmeyer K, Granek EF, Nielsen-Pincus M, Lanier A, Rumrill SS, Moran P, Nilsen E, Hladik ML, Pillsbury L. Exploring Biophysical Linkages between Coastal Forestry Management Practices and Aquatic Bivalve Contaminant Exposure. Toxics. 2021; 9(3):46. https://doi.org/10.3390/toxics9030046
Chicago/Turabian StyleScully-Engelmeyer, Kaegan, Elise F. Granek, Max Nielsen-Pincus, Andy Lanier, Steven S. Rumrill, Patrick Moran, Elena Nilsen, Michelle L. Hladik, and Lori Pillsbury. 2021. "Exploring Biophysical Linkages between Coastal Forestry Management Practices and Aquatic Bivalve Contaminant Exposure" Toxics 9, no. 3: 46. https://doi.org/10.3390/toxics9030046
APA StyleScully-Engelmeyer, K., Granek, E. F., Nielsen-Pincus, M., Lanier, A., Rumrill, S. S., Moran, P., Nilsen, E., Hladik, M. L., & Pillsbury, L. (2021). Exploring Biophysical Linkages between Coastal Forestry Management Practices and Aquatic Bivalve Contaminant Exposure. Toxics, 9(3), 46. https://doi.org/10.3390/toxics9030046







