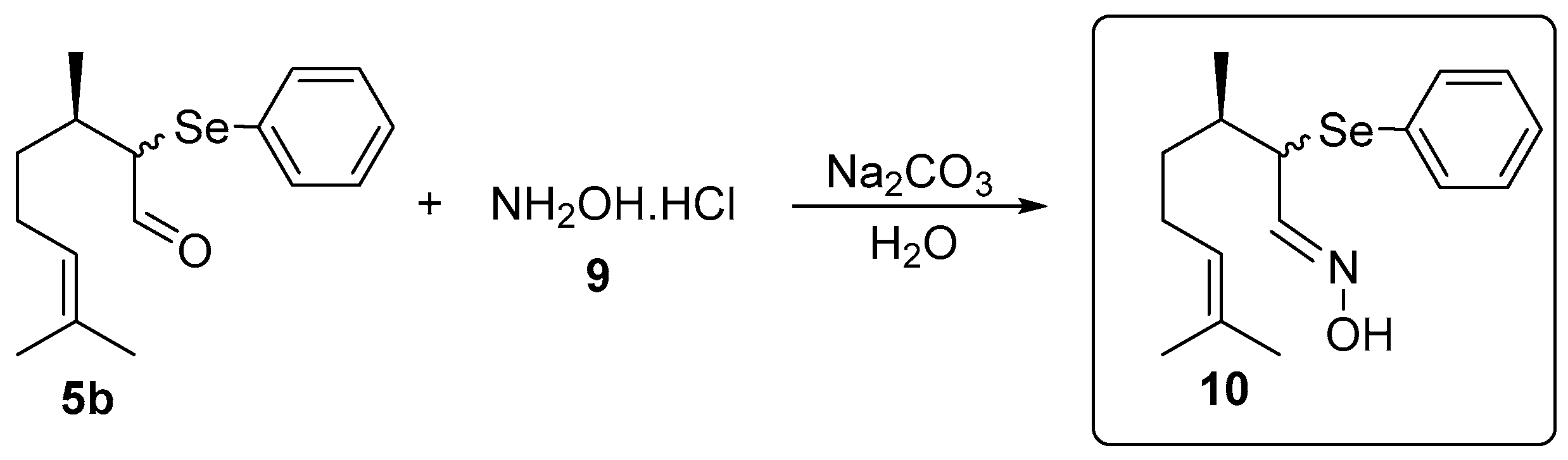Abstract
Background: The main constituents of Cymbopogonnardus (L) Rendle and C. citratus (DC) Stapfessential oils are (R)-citronellal and citral, respectively. Organochalcogen compounds can boost the biological activities of natural products. Methods: Several chalcogen-containing nitrones derived from (R)-citronellal and citral were prepared and evaluated for their antimicrobial and antioxidant activities. The antimicrobial activity was evaluated by the disc diffusion test and the antioxidant properties were evaluated in vitro by DPPH (1,1-diphenyl-2-picryl-hydrazyl), ABTS (2,2′-azino-bis(3-ethylbenzthiazoline-6-sulfonic acid), and FRAP (ferric ion reducing antioxidant power) assays. Results: In the antimicrobial assay, (E)-N,3,7-trimethyl-3-(phenylthio)oct-6-en-1-imine oxide 5c exhibited halos between 21.5 mm (Escherichia coli O157:H7) and 26.0 mm (Listeria monocytogenes), while (E)-N,3,7-trimethyloct-6-en-1-imine oxide 5d presented halos between 22.5 mm (E. coli O157:H7) and 31.0 mm (L. monocytogenes). (E)-N,3,7-Trimethyl-2-(phenylthio)oct-6-en-1-imine oxide 5a showed the lowest minimal inhibitory concentration (MIC) value against Bacillus cereus (0.48 mM), and 5c was the most potent bactericide, with a minimal bactericidal concentration (MBC) of 0.52 mM for E. coli O157:H7. In the antioxidant assays, 5c, 5d, and 10 ((E)-3,7-dimethyl-2-(phenylselanyl)oct-6-enal oxime) were the most actives in the DPPH, ABTS, and FRAP assays, respectively. Conclusions: The presence of a phenylthio group in the nitrone increases its antimicrobial activity against Gram-positive and Gram-negative foodborne pathogens in the disk diffusion test and the antioxidant activity in vitro.
1. Introduction
Oxidative and microbial changes are among the main causes of loss of quality and value of food products and infections and intoxication caused by pathogenic microorganisms bring risk to the consumer health [1]. To minimize or avoid these problems, antioxidants and preservatives are added for microbiological control. The search for a better understanding of the genetic mechanisms of microbial resistance and studies on new antimicrobials and antioxidants—synthetic, semi-synthetic, or natural ones—are of great interest [2].
In this context, many studies looking for the prospection and identification of naturally-occurring bioactive compounds—such as essential oils (EOs) and their constituents—were recently described. A very versatile natural compound is citronellal, which is a monoterpene predominantly formed by the secondary metabolism of plants, usually found as a non-racemic mixture of R and S enantiomers. Citronellal is present in EOs of various plant species, being the main component of the Cymbopogon nardus EO, where (+)-(R)-citronellal is the only enantiomer found [3]. Several biological properties are associated with citronellal, including significant antioxidant and antimicrobial activities. Nevertheless, there are a number of limitations in the use of EOs or their constituents as food additives, which range from the low stability, interaction with ingredients, and other additives to the impact on the sensory characteristics of the product [4].
The development of new preservatives is directly linked to the economic importance of the food’s spoilage. The chemical modification of natural products has attracted the attention of many research groups, which aims to improve their original biological activities [5,6,7,8]. Recent studies have shown that semi-synthetic compounds functionalized with organochalcogen groups (sulfur and selenium) and nitrones could be an alternative to the synthetic additives currently used in food and pharmaceutical industries [8].
Organoselenium and organosulfur substrates have shown to be ideal to increase several biological and pharmaceutical activities, such as antibacterial, antifungal [9], and antioxidant [10]. This enhancing can be attributed, at least in part, to the ability of selenium and sulfur to stabilize free radicals [11].
Nitrones are molecules that contain nitrogen and oxygen heteroatoms and their 1,3-dipolar structure favors their participation in the synthesis of various biologically active nitrogen-containing substances [12,13]. Nitrone derivatives are industrially used in numerous applications, including as binders, antifungals, antimicrobials, and as starting materials in the synthesis of important organic compounds, such as oxaziridines, isoxazolidines, hydroxylamines, among others [14,15,16,17,18].
Therefore, based on the previous considerations and in continuation to our studies on the synthesis of semi-synthetic organochalcogen compounds, the objective of this work was to combine the bioactive properties of nitrone and the organochalcogen group with those of citronellal, a natural terpenoid, to obtain new multifunctional compounds. The new semi-synthetic compounds designed by molecular hybridization were evaluated for their antimicrobial and antioxidant activities in vitro.
2. Materials and Methods
2.1. Oil Material
Essential oil of citronella (C. nardus (L.) Rendle) was commercial product, produced in southern Brazil (Pólo Oleoquímico de Três Passos-RS). (R)-Citronellal was isolated from the essential oil by column chromatography using silicagel as a stationary phase and a solution of hexane/ethyl acetate (99:1) as the eluent. Citral (a 1:1 mixture of neral and geranial) and the other reagents were purchased from Aldrich (St. Louis, MO, USA).
2.2. Synthesis of α-phenylselanyl citronellal 3a, α-phenylthio citronellal 3b, and β-phenylthio citronellal 8
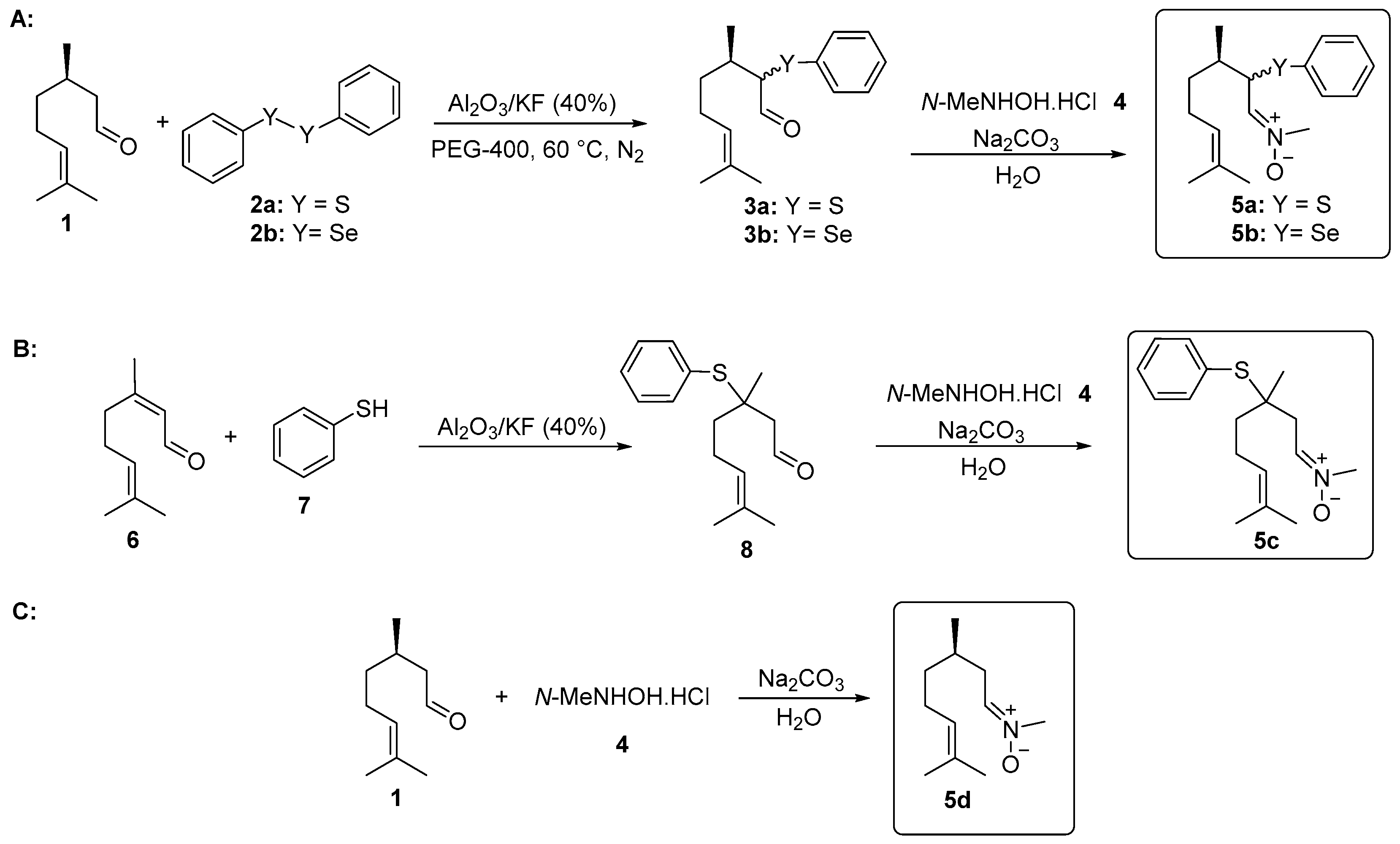
The synthesis of α-phenylchalcogencitronellal 3a–b was performed according to the methodology developed by Nazari and Movassagh [19], with modifications. In a 25 mL vial was added (R)-citronellal (1, 0.308 g, 2 mmol), diphenyl disulfide (2a, 1.5 mmol), or diphenyl diselenide (2b, 2 mmol) and PEG-400 (4.0 mL) under N2 atmosphere. Then, Al2O3/KF (40%) (0.324 g, 1.5 mmol) was added and the temperature was slowly increased to 60 °C. The progress of the reaction was monitored using thin layer chromatography (TLC) and after 22 h, compounds 3a–b were isolated and characterized (Figure 1A).
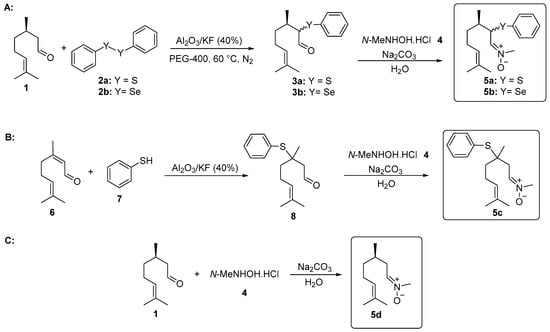
Figure 1.
Synthesis of nitrones derived from citronellal. (A) α-Phenylselanyl citronellal 3a, α-phenylthio citronellal 3b and nitrones 5a and 5b; (B) β-Phenylthio citronellal 8 and nitrone 5c; (C) Nitrone 5d.
The synthesis of β-phenylchalcogencitronellal 8 was performed according to previously described by our group [20]. In a test tube was added citral (6, 0.304 g, 2 mmol), benzenethiol (7, 0.352 g, 2.4 mmol) and Al2O3/KF (40%) (0.140 g, 0.65 mmol) under magnetic stirring at room temperature. The progress of the reaction was monitored using thin layer chromatography (TLC) and after 24 h, compound 8 was isolated and characterized (Figure 1B).
2.3. General Procedure for the Synthesis of Nitrones 5a–d Derived from Citronellal
The functionalized nitrones 5 were prepared using a synthetic route adapted from Isager et al. [21]: In a 25 mL vial, aldehyde 1, 3a–b, or 8 (0.5 mmol); N-methyl-hydroxylamine hydrochloride (4, 0.084 g, 1 mmol); and water (2 mL) as the solvent were combined. The mixture was stirred at room temperature for 30 min. Then, a 1M solution of sodium carbonate (Na2CO3) (1.0 mL) was added and the stirring was continued for additional 24 h. Compound 5a was purified by preparative chromatographic plate (silica gel) and compounds 5b–d were isolated by column chromatography using neutral alumina as a stationary phase and a solution of hexanes/ethyl acetate (90:10) as the eluent (Figure 1A–C).
2.4. Synthesis of the Selenium-Containing Oxime 10
The oxime 10 was prepared using a synthetic route adapted from Isager et al. [21]: In a 25 mL vial α-phenylselenocitronellal (5b, 0.156 g, 0.5 mmol), hydroxylamine hydrochloride (9, 0.069 g, 1 mmol) and water (2 mL) as the solvent were combined. After stirring for 30 min at room temperature, 0.5 mL of an aqueous solution of Na2CO3 (0.027 g, 0.26 mmol) was added and the stirring was continued for additional 22 h. After this time, the oxime 10 was isolated by column chromatography using silica gel as a stationary phase and a solution of hexanes/ethyl acetate (95:5) as the eluent (Figure 2).
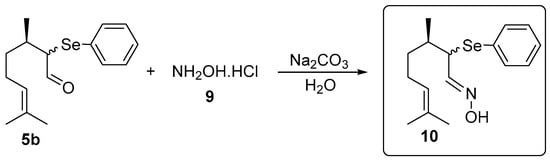
Figure 2.
Synthesis of (3R,E)-3,7-dimethyl-2-(phenylselanyl)oct-6-enal oxime 10.
2.5. Bacterial Strains
To evaluate the antimicrobial potential of the synthesized compounds, the following reference strains of bacteria, that are economically important in foods, were employed: Gram-positive Listeria monocytogenes ATCC 7644, Staphylococcus aureus ATCC 25923, and Bacillus cereus ATCC 11778; Gram-negative Salmonella Typhimurium ATCC 14028, Escherichia coli O157: H7 NCTC 12900 and the clinically important Pseudomonas aeruginosa ATCC 15442.
The antimicrobial activity assay using the disk diffusion test was realized using the methodology that is recommended by the Clinical Laboratory Standards Institute (CLSI) [22]. The determination of the minimum inhibitory concentration (MIC) and minimum bactericidal concentration (MBC) was performed using the macro dilution tube method, in accordance with Rota et al. [23], with modifications. Details of these assays are provided in the Supplemental Material.
2.6. Antioxidant Activity Assays
The antioxidant properties of the synthesized compounds (5a–d and 10) were evaluated by three different methods in vitro: DPPH (1,1-diphenyl-2-picryl-hydrazyl) and ABTS+ (2,2′-azino-bis(3-ethylbenzthiazoline-6-sulfonic acid) radical scavenging ability and ferric ion reducing antioxidant power (FRAP). All drugs were dissolved in dimethyl sulfoxide (DMSO) at concentrations of 1–500 µM. These assays were performed according to the literature and details of these experiments are described elsewhere (see the ‘Results and Discussion’ section for the references).
2.7. In Vitro Toxicity
The assay used to measure the activity of δ-ALA-D quantifies the products of the enzymatic action in two molecules, δ-aminolevulinic acid (ALA), and porphobilinogen (PBG), which after reaction with the Ehrlich’s solution, generates a red-pinkish color [24,25]. The activity of δ-ALA-D in the presence of compounds 5a–d and 10 at different concentrations (10–500 μM) was determined according to the method described by Sassa [24]. Detailed information is in the Supplemental Material.
3. Results and Discussion
3.1. Chemistry
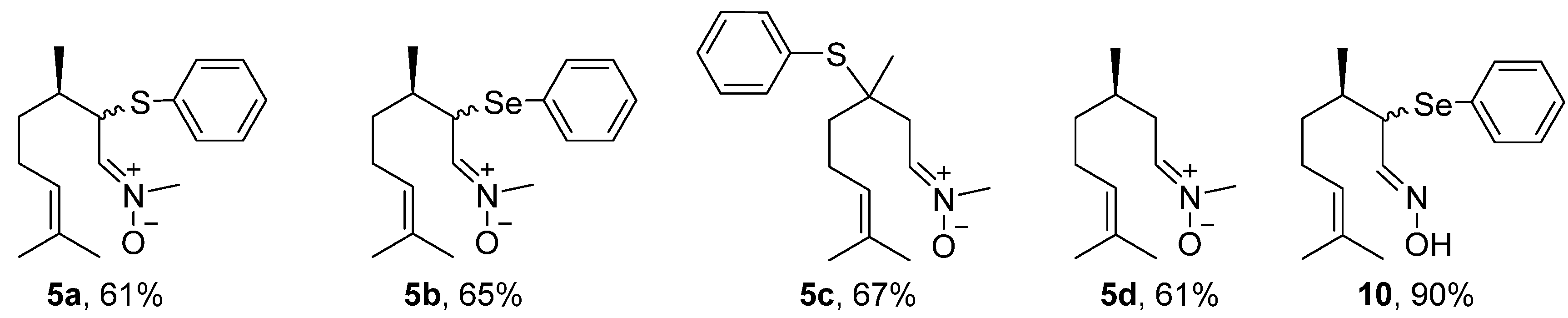
The first step in the synthesis of the chalcogen-containing nitrones 5a–c and the α-phenylselenoxime 10, derived from citronellal 1 involves the preparation of the key intermediates α- and β-phenylchalcogen citronellal 3a–b and 8. For 3a–b, a synthetic route described by Nazari and Movassagh [19] was used, while the β-phenylthio citronellal 8 was prepared using our previously reported procedure [20]. With the starting materials 3a–b and 8 in hands, they were subjected to the reaction with N-methyl-hydroxylamine hydrochloride 4 to prepare 5a–c; α-phenylselanyl citronellal 3b was also reacted with hydroxylamine hydrochloride 9 to prepare the oxime 10. Products were purified by preparative chromatographic plate (5a), column chromatography on neutral alumina (5b and 5c), or using silicagel (10). The nitrone derivative from citronellal 5d was prepared directly from the freshly purified aldehyde 1 and purified by column chromatography using neutral alumina. The structures and yields of the five prepared compounds are presented in Figure 3. In general, products were obtained in good yields (61–90%) and they showed a good stability after purification, being characterized by 1H and 13C-NMR and mass spectrometry. Figures of NMR spectra (1H and 13C) of compounds 5a–d are presented in the Supplementary Material. The NMR spectra of nitrones 5a (Figures S1 and S2), 5b (Figures S3 and S4), 5c (Figures S5 and S6), 5d (Figures S7 and S8) and oxime 10 (Figures S9 and S10) are in accordance with those expected for the compounds. Compounds 5a–b and 10 were obtained as a 1:1 mixture of diasteroisomers, once a second stereocenter is generated in the precursor β-phenylchalcogen citronellal 3a and 3b. Because it was not separable by the chromatographic methods employed by us, the mixture of isomers was used in the bioassays.
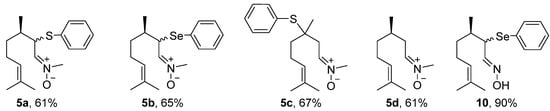
Figure 3.
Yields of nitrones derived from citronellal 5a–d and α-phenylselanyl oxime 10.
3.2. Antimicrobial Activity
In the first set of experiments, the antimicrobial activity of nitrones 5a–d and α-phenylselenoxime 10, derived from citronellal, was evaluated in vitro using the agar disk diffusion test. The strains L. monocytogenes, S. aureus, B. cereus, S. Typhimurium, E. coli O157:H7, and P. aeruginosa were qualitatively and quantitatively assessed for the presence or absence of inhibition zones (zone diameters) (Table 1), the minimum inhibitory concentration (MIC) and the minimum bactericidal concentration (MBC) (Table 2).

Table 1.
Antimicrobial activity of compounds 5a–d and 10 in the agar disk diffusion test.

Table 2.
Minimum inhibitory concentration (MIC) and minimum bactericidal concentration (MBC) of compounds 5a–d and 10.
As it can be seen on Table 1, nitrones 5c (containing a phenylthio group in the β-position) and 5d (without a chalcogen) and the seleno-containing oxime 10 generated inhibition zones for Gram-positive bacteria comparable to the control antibiotic streptomycin. Studies suggest that Gram-positive bacteria are more sensitive to EOs and their components than Gram-negative ones. This behavior appears to be related to the presence of the outer cell membrane in Gram-negative bacteria, which gives greater protection, making them more difficult to be inhibited [26]. Compounds 5c and 5d also showed a good antimicrobial potential for Gram-negative bacteria, with inhibition halos greater than those of streptomycin. These results are indicative that the chemical modification of the constituents of plants’ EOs can generate compounds with an improved antibacterial activity.
The high antimicrobial activity of compounds 5c and 5d were confirmed by their MIC and MBC values (Table 2), which were between 0.52 mM and 0.69 mM for compound 5c and between 1.13 mM and 1.26 mM for 5d in all the tested bacteria. Interestingly, compound 5a, having a phenylthio group in the α-position, inhibited B. cereus selectively, presenting MIC and MBC values of 0.48 mM and 0.59 mM, respectively. The antibacterial activity of 3-(p-chlorophenyl)thio citronellal, a close-related compound to 3a, was recently studied by us [27] and it showed inhibitory activity against Gram-positive L. monocytogenes and S. aureus (MIC of 2.1 mM) and Gram-negative bacteria S. Typhimurium, S. dysenteriae, E. coli, and P. fluorescens (MIC of 4.2 mM).
As we can see from the MIC and MBC values in Table 2, there is a clear improvement in the antimicrobial activity when an organosulfur and a nitrone group are present in the (R)-citronellal scaffold. The biological activities of these classes of compounds include antioxidant, anti-inflammatory, and antiviral ones; however, little information exists about the antimicrobial activity of compounds containing both organochalcogen and nitrone groups in the same molecule.
3.3. Antioxidant Activity
To verify the effect of the presence of the organochalcogen and nitrone moieties in the new molecules, the antioxidant activity of the nitrones derived from citronellal 5a–d (Figure 1) and α-phenylselenoxime citronellal 10 (Figure 2) was evaluated by different methods in vitro.
As can be seen in Table 3, only compounds 5a and 5c—which contain the organosulfur moiety—have demonstrated DPPH radical scavenging activity. The selenium-containing nitrone 5b, the nitrone 5d, without chalcogen, and the selenoxime 10 did not present effect in the DPPH test (results not presented).

Table 3.
DPPH radical scavenging of compounds 5a and 5c.
Table 4 shows the outcomes in the ABTS radical scavenging activity of compounds 5a–d and 10. In this assay, α-phenylchalcogen nitrones 5a and 5b and the α-phenylselenoxime 10 were the most active ones, with 10 presenting the best result (IC50 of 25 μM). The IC50 for α-phenylchalcogen nitrones 5a and 5b were of 297 and 315.3 μM respectively, while β-phenylthio nitrone 5c showed an IC50 value of 419.30 μM. This finding indicates that the proximity of the chalcogen atom to the nitrone or oxime groups affects the ABTS radical scavenging ability.

Table 4.
ABTS radical scavenging of compounds 5a–d and 10.
DPPH and ABTS are the most common spectrophotometric methods to determine the antioxidant activity of organic compounds, because they can directly react with the antioxidant species [28]. The principle of the DPPH assay is a single electron transfer (SET) reaction and a hydrogen-atom removal [29], while the ABTS assay is based only on a SET [30].
Several reports in the literature have demonstrated that the antioxidant activity might be correlated to the reducing power of a compound [31,32,33]. Thus, based on this evidence, the FRAP assay was used to determine the reducing power of compounds 5a–d and 10 to clarify the relationship between the antioxidant effect and the reducing power (Table 5).

Table 5.
Ferric ion reducing antioxidant power (FRAP) of compounds 5a–d and 10.
Among the tested compounds, 5d was the most effective in the FRAP test, with a concentration-dependent reducing power. In addition, our results revealed that all the (R)-citronellal derivatives presented a high ferric reducing ability.
The antioxidant and antidepressant-like activities of the semi-synthetic α-phenylselenocitronellal 3b and the natural terpenoid (R)-citronellal 1 were previously evaluated by Victória et al. [9] and both were inactive in the DPPH and ABTS assays. In the FRAP assay, 3b presented significant reducing power at concentrations equal and higher than 500 mM, while 1 did not present any effect at the same concentrations [9]. Taken together, these findings and the results of Table 3 and Table 4 indicate that the presence of the nitrone and oxime groups are crucial for the radical scavenging ability of the citronellal derivatives, while the aldehyde itself is not active, even when a chalcogen group is present.
3.4. In Vitro Toxicity
Based on the antimicrobial and antioxidant activities displayed by the semi-synthetic citronellal-based compounds 5a–d and 10 and searching for future technological applications in food preservation, their acute toxicity was investigated in mice’s tissues of liver, kidney, and brain (Table 6). The enzymatic activity of δ-Ala-D was evaluated at different concentrations (10, 50, 100, and 500 μM) of compounds 5a–d and 10 and no decrease was observed for 5a, 5c, 5d, and 10 in all the tested concentrations. This suggests the absence of acute toxicity in vitro. However, nitrone 5b, containing a phenylselanyl group at the α-position of the nitrone, inhibit the δ-Ala-D activity at all concentrations in the liver and at 100 and 500 μM in kidney. This suggests an acute toxicity of 5b in vitro.

Table 6.
Assay of δ-ALA-D in liver, kidney, and brain of rats after treatment with compounds 5a–d and 10.
4. Conclusions
A new class of chalcogen-containing nitrones derivatives from (R)-citronellal, obtained from a renewable source, C. nardus (L.) Rendle essential oil, was synthesized and evaluated for their antimicrobial and antioxidant activities in vitro. From this study, it can be concluded that the combination of an organochalcogen group with nitrone and oxime in the same molecule increases the antibacterial and antioxidant capabilities of the precursor (R)-citronellal. The synthesized compounds were effective in controlling foodborne pathogenic bacteria, including Gram-positive L. monocytogenes, S. aureus, and B. cereus; and Gram-negative S. Typhimurium and E. coli O157:H7, as well as the clinically important bacteria P. aeruginosa. Promising results on the antimicrobial activity were obtained in the disk diffusion test. Nitrone 5a, with a phenylthio group at the α-position, presented the lowest MIC, for B. cereus (0.48 mM), while the lowest MBC was observed using compound 5c against E. coli O157:H7 (0.52 mM). Regarding the antioxidant activities, all of the synthesized compounds were active. Compounds 5c (sulfur-containing), oxime 10 (selenium-containing) and nitrone 5d (without chalcogen) presented the best results in DPPH, ABTS, and FRAP assays, respectively. Among the five tested compounds, only α-phenylselanyl nitrone 5b showed acute toxicity, reducing the δ-ALA-D activity in liver and kidney of mice. The in vitro data presented here indicate that thio- and selenium-containing nitrones and oxime derivatives from (R)-citronellal have potential to be explored in the food industry to enhance the safety of food products, protecting from oxidation and foodborne bacteria. Additional studies on the long-term toxicity need to be performed before a possible commercial utilization.
Supplementary Materials
The following are available online at www.mdpi.com/2305-6320/4/2/39/s1, Figure S1: 1H NMR of compound 5a in CDCl3 (300 MHz), Figure S2: 13C NMR of compound 5a in CDCl3 (75 MHz), Figure S3: 1H NMR of compound 5b in CDCl3 (300 MHz), Figure S4: 13C NMR of compound 5b in CDCl3 (75 MHz), Figure S5: 1H NMR of compound 5c in CDCl3 (300 MHz), Figure S6: 13C NMR of compound 5c in CDCl3 (75 MHz), Figure S7: 1H NMR of compound 5d in CDCl3 (300 MHz), Figure S8: 13C NMR of compound 5d in CDCl3 (75 MHz), Figure S9: 1H NMR of compound 10 in CDCl3 (400 MHz), Figure S10: 13C NMR of compound 10 in CDCl3 (100 MHz).
Acknowledgments
The authors are grateful to CNPq, CAPES, and FAPERGS for financial support. CNPq is also acknowledged for the fellowship for Eder J. Lenardão, Raquel G. Jacob, Wladimir P. da Silva and Lucielli Savegnago.
Author Contributions
R.G.J., L.S., W.P.S., and E.J.L. conceived and designed the experiments; M.C.F. and R.A.M. performed the synthesis and purifications; D.H.O. performed the antioxidant assays; D.S.V.M. performed the antimicrobial experiments; R.G.J., L.S., W.P.S., E.J.L., and D.H.O. analyzed the data and wrote the paper.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Damodaran, S.; Parkin, K.; Fennema, O.R. Fennema’s Food Chemistry, 4th ed.; Taylor & Francis: Boca Raton, FL, USA, 2008. [Google Scholar]
- Hoeden, B.P.; Davies, J.K.; Johnson, P.D.R.; Stinear, T.P.; Grayson, M.L. Reduced vancomycin susceptibility in Staphylococcus aureus, including vancomycin intermediate and heterogeneous vancomycin-intermediate strains: Resistance mechanisms, laboratory detection, and clinical implications. Clin. Microbiol. 2010, 23, 99–139. [Google Scholar] [CrossRef] [PubMed]
- Pybus, D.; Sell, C. The Chemistry of Fragrances: From Perfumer to Consumer; The Royal Society of Chemistry: Cambridge, UK, 1999. [Google Scholar]
- Burt, S. Essential oils: Their antibacterial properties and potential applications in foods—a review. Int. J. Food Microbiol. 2004, 94, 223–253. [Google Scholar] [CrossRef] [PubMed]
- Martinez, D.M.; Barcellos, A.; Casaril, A.M.; Perin, G.; Schiesser, C.H.; Callaghan, K.L.; Lenardão, E.J.; Savegnago, L. Twice acting antioxidants: Synthesis and antioxidant properties of selenium and sulfur-containing zingerone derivatives. Tetrahedron Lett. 2015, 56, 2243–2246. [Google Scholar] [CrossRef]
- Neukirch, H.; D’Ambrosio, M.; Sosa, S.; Altinier, G.; Loggia, R.D.; Guerriero, A. Improved anti-inflammatory activity of three new terpenoids derived, by systematic chemical modifications, from the abundant triterpenes of the flower plant Calendula officinalis. Chem. Biodivers. 2005, 2, 657–671. [Google Scholar] [CrossRef] [PubMed]
- Hong, Y.; Junkai, L.; Shengtao, X.; Zheying, Z.; Jinyi, X. The structural modification of natural products for novel drug discovery. Exp. Opin. Drug Disc. 2017, 12, 121–140. [Google Scholar] [CrossRef]
- Lenardão, E.J.; Silva, W.P.; Jacob, R.G.; Volcan, D.S.M.; Goldbeck, J.C.S.; Fonseca, S.F. Semi-Synthetic Compounds as Antimicrobial Agents in Food Preservation. In The Battle against Microbial Pathogens: Basic Science, Technological Advances and Educational Programs; Méndez-Vilas, A., Ed.; Formatex Research Center: Badajoz, Spain, 2015; Volume 1, pp. 576–583. [Google Scholar]
- Victória, F.N.; Radatz, C.S.; Sachini, M.; Jacob, R.G.; Alves, D.; Savegnago, L.; Perin, G.; Motta, A.S.; Silva, W.P.; Lenardão, E.J. Further analysis of the antimicrobial activity of α-phenylseleno citronellal and α-phenylseleno citronellol. Food Control 2012, 23, 95–99. [Google Scholar] [CrossRef]
- Savegnago, L.; Borges, V.C.; Alves, D.; Jesse, C.R.; Rocha, J.B.T.; Nogueira, C.W. Evaluation of antioxidant activity and potential toxicity of 1-buthyltelurenyl-2-methylthioheptene. Life Sci. 2006, 79, 1546–1552. [Google Scholar] [CrossRef] [PubMed]
- Victória, F.N.; Martinez, D.M.; Castro, M.; Casaril, A.M.; Alves, D.; Lenardão, E.J.; Salles, H.D.; Schneider, P.H.; Savegnago, L. Antioxidant properties of (R)-Se-aryl thiazolidine-4-carboselenoate. Chem. Biol. Interact. 2013, 205, 100–107. [Google Scholar] [CrossRef] [PubMed]
- Pellissier, H. Asymmetric 1,3-dipolar cycloadditions. Tetrahedron 2007, 63, 3235–3285. [Google Scholar] [CrossRef]
- Hamer, J.; Macaluso, A. Nitrones. Chem. Rev. 1964, 64, 473–795. [Google Scholar] [CrossRef]
- McQueen, D.M. U.S. Patent 2,426,894. Available online: https://www.google.com/patents/US2426894#forward-citations (accessed on 23 May 2017).
- Petkesa, H.I.; Gál, E.; Gãinã, L.; Sabou, M.; Majdik, C.; Silaghi-Dumitrescu, L. Synthesis and antibacterial properties of new phenothiazinyl- and phenyl-nitrones. C. R. Chim. 2014, 17, 1050–1056. [Google Scholar] [CrossRef]
- Shijum, C.; Kang, Z.; Gang, C. Synthesis and application of phenyl nitrone derivatives as acidic and microbial corrosion inhibitors. J. Chem. 2015, 2015, 1–7. [Google Scholar] [CrossRef]
- Franco, S.; Merchan, F.L.; Merino, P.; Tejero, T. An Improved Synthesis of Ketonitrones. Synth. Commun. 1995, 25, 2275–2284. [Google Scholar] [CrossRef]
- Flick, A.C.; Padwa, A. A conjugate addition-dipolar cycloaddition approach towards the synthesis of various alkaloids. ARKIVOC 2011, vi, 137–161. [Google Scholar]
- Nazari, M.; Movassagh, B. α-Phenylselenenylation of aldehydes and ketones with diphenyl diselenide mediated by KF/Al2O3. Tetrahedron Lett. 2009, 50, 1453–1455. [Google Scholar] [CrossRef]
- Lenardão, E.J.; Trecha, D.O.; Ferreira, P.C.; Jacob, R.G.; Perin, G. Green Michael addition of thiols to electron deficient alkenes using KF/alumina and recyclable solvent or solvent-free conditions. J. Braz. Chem. Soc. 2009, 20, 93–99. [Google Scholar] [CrossRef]
- Isager, P.; Thomsen, I.; Torssell, K.B.G. Reactions with α,β-unsaturated nitrile oxides. Synthetic studies in the terpene field. Synthesis of tagetones, ocimenones, deodarone and atlantone. Acta Chem. Scand. 1990, 44, 806–813. [Google Scholar] [CrossRef]
- Clinical Laboratory Standards Institute (CLSI). Performance Standards for Antimicrobial Susceptibility Testing; Twenty-Second Informational Supplement (M100-S25); CLSI: Wayne, PA, USA, 2015. [Google Scholar]
- Rota, C.; Carramiñana, J.J.; Burillo, J.; Herrera, A. In vitro antimicrobial activity of essential oils from aromatic plants against selected foodborne pathogens. J. Food Prot. 2004, 67, 1252–1256. [Google Scholar] [CrossRef]
- Sassa, S. Delta-aminolevulinic acid dehydratase assay. Enzyme 1982, 28, 133–145. [Google Scholar] [PubMed]
- Costa, J.R.M.A.; Mela, M.; Assis, H.C.S.; Pelletier, E.; Randi, M.A.F.; Ribeiro, C.A.O. Enzymatic inhibition and morphological changes in Hopliasmalabaricus from dietary exposure to lead(II) or methylmercury. Ecotoxicol. Environ. Saf. 2007, 67, 82–88. [Google Scholar] [CrossRef] [PubMed]
- Smith-Palmer, A.; Stewart, J.; Fyfe, L. Antimicrobial properties of plant essential oils and essences against five important food-borne pathogens. Lett. Appl. Microbiol. 1998, 26, 118–124. [Google Scholar] [CrossRef]
- Goldbeck, J.C.; Victória, F.N.; Motta, A.; Savegnago, L.; Jacob, R.G.; Perin, G.; Lenardão, E.J.; da Silva, W.P. Bioactivity and morphological changes of bacterial cells after exposure to 3-(p-chlorophenyl)thio citronellal. LWT Food Sci. Technol. 2014, 59, 813–819. [Google Scholar] [CrossRef]
- Wiedander, E.; Engman, L.; Suensjö, E.; Erlansson, M.; Johansson, U.; Linden, M.; Andersson, C.M.; Brattsand, R. Antioxidative properties of organotellurium compounds in cell systems. Biochem. Pharmacol. 1998, 55, 573–584. [Google Scholar] [CrossRef]
- Gunlçin, I. Antioxidant activity of L-adrenaline: A structure–activity insight. Chem. Biol. Interact. 2009, 179, 71–80. [Google Scholar] [CrossRef] [PubMed]
- Soong, Y.; Barlow, P.J. Antioxidant activity and phenolic content of selected fruit seeds. Food Chem. 2004, 88, 411–417. [Google Scholar] [CrossRef]
- Prior, R.L.; Wu, X.; Schaich, K. Standardized methods for the determination of antioxidant capacity and phenolics in foods and dietary supplements. J. Agric. Food Chem. 2005, 53, 4290–4303. [Google Scholar] [CrossRef] [PubMed]
- Martins, A.C.; Bukman, L.; Vargas, A.M.M.; Barizão, E.O.; Moraes, J.C.G.; Visentainer, J.V.; Almeida, V.C. The antioxidant activity of teas measured by the FRAP method adapted to the FIA system: Optimizing the conditions using the response surface methodology. Food Chem. 2013, 138, 574–580. [Google Scholar] [CrossRef] [PubMed]
- Berker, K.I.; Güçlü, K.; Tor, I.; Apak, R. Comparative evaluation of Fe(III) reducing power- based antioxidant capacity assays in the presence of phenanthroline, batho-phenanthroline, tripyridyltriazine (FRAP), and ferricyanide reagents. Talanta 2007, 72, 1157–1165. [Google Scholar] [CrossRef] [PubMed]
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).