Recent Advances on Quinazoline Derivatives: A Potential Bioactive Scaffold in Medicinal Chemistry
Abstract
1. Introduction
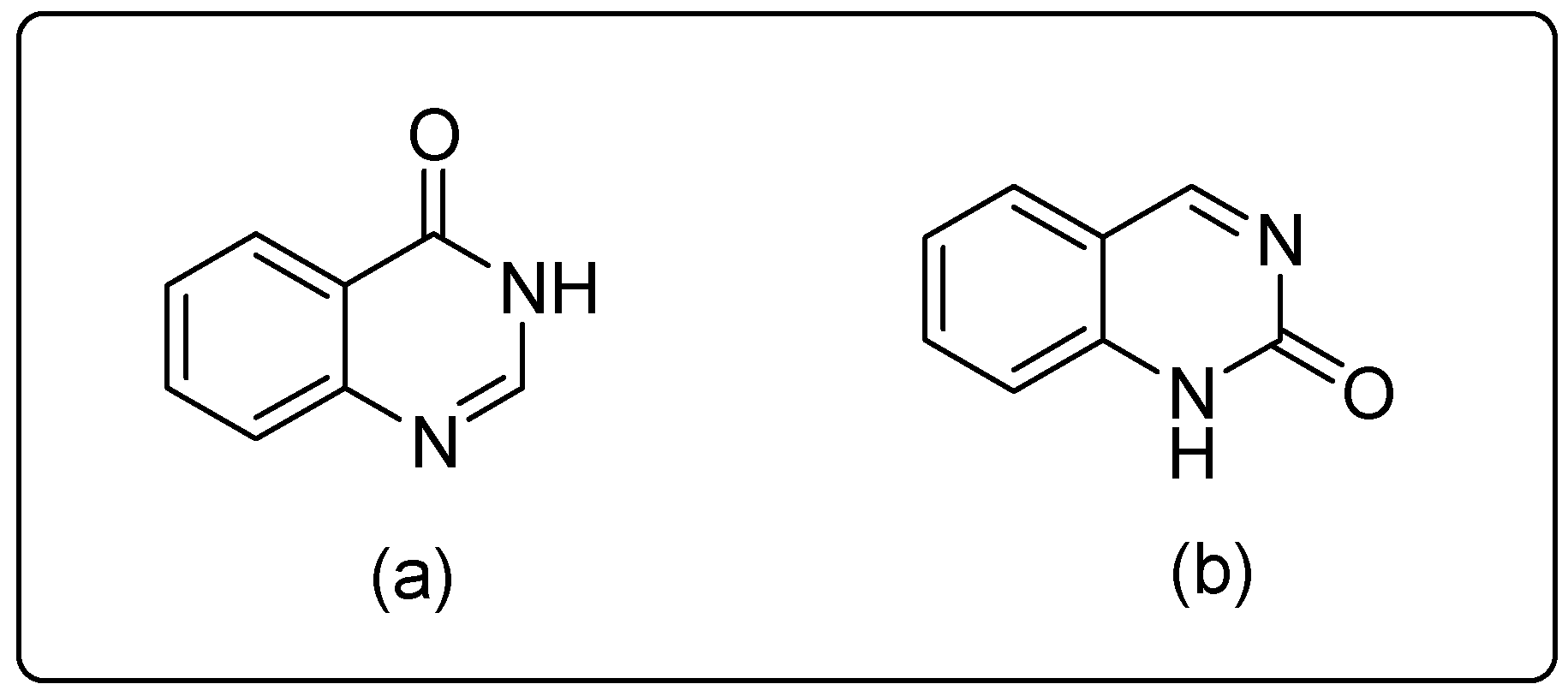
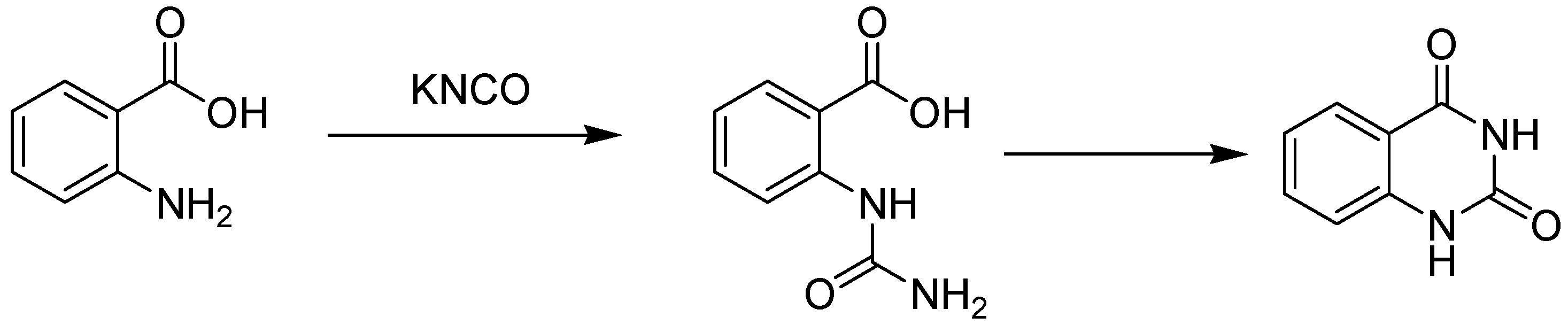
2. Synthesis Routes of Quinazoline
- (i)
- (ii)
- (iii)
- (iv)
- (v)
- (vi)
3. Pharmacological Significance of Quinazoline Derivatives
4. Quinazoline as Anti-Tumor Agents
5. Quinazoline as Anti-Viral
6. Quinazoline as Anti-Bacterial
7. Quinazoline as Anti-Tubercular Activity
8. Quinazoline as Anti-Oxidant Activity
9. Quinazoline as Anti-Convulsant
10. Quinazoline as Anti-Inflammatory Agents
11. Quinazoline as Sirtuin Modulating Agents
12. Quinazoline as Antidiabetic Agents
13. Quinazoline as Antifungal Agents
14. Quinazoline as Antiparasite Agents
15. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, D.; Gao, F. Quinazoline derivatives: Synthesis and bioactivities. Chem. Cent. J. 2013, 7, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Connolly, D.J.; Cusack, D.; O’Sullivan, T.P.; Guiry, P.J. Synthesis of quinazolinones and quinazolines. Tetrahedron 2005, 61, 10153–10202. [Google Scholar] [CrossRef]
- Meyer, J.F.; Wagner, E.C. The Niementowski reaction. The use of methyl anthranilate or isatoic anhydride with substituted amides or amidines in the formation of 3-substituted-4-keto-3,4-dihydroquinazolines. The course of the reaction. J. Org. Chem. 1943, 8, 239–252. [Google Scholar] [CrossRef]
- Alam, M.J.; Alam, O.; Naim, M.J.; Alam, P. A review: Recent investigations on quinazoline scaffold. Int. J. Adv. Res. 2015, 3, 1656–1664. [Google Scholar]
- Asif, M. Chemical Characteristics, Synthetic Methods, and Biological Potential of Quinazoline and Quinazolinone Derivatives. Int. J. Med. Chem. 2014, 2014, 395637. [Google Scholar] [CrossRef]
- Hameed, A.; Al-Rashida, M.; Uroos, M.; Ali, S.A.; Arshia; Ishtiaq, M.; Khan, K.M. Quinazoline and quinazolinone as important medicinal scaffolds: A comparative patent review (2011–2016). Expert Opin. Ther. Pat. 2018, 28, 281–297. [Google Scholar] [CrossRef]
- Sarker, S.D. Biological activity of magnolol: A review. Fitoterapia 1997, 68, 3–8. [Google Scholar]
- Auti, P.S.; George, G.; Paul, A.T. Recent advances in the pharmacological diversification of quinazoline/quinazolinone hybrids. RSC Adv. 2020, 10, 41353–41392. [Google Scholar] [CrossRef]
- Gupta, T.; Rohilla, A.; Pathak, A.; Akhtar, M.J.; Haider, M.R.; Yar, M.S. Current perspectives on quinazolines with potent biological activities: A review. Synth. Commun. 2018, 48, 1099–1127. [Google Scholar] [CrossRef]
- Bisht, A.S.; Negi, J.S.; Sharma, D.K. Chemistry and activity of quinazoline moiety: A systematic review study. Int. J. Pharm. Chem. Anal. 2020, 7, 61–65. [Google Scholar] [CrossRef]
- Sharif, M. Quinazolin-4(3H)-ones: A tangible synthesis protocol via an oxidative olefin bond cleavage using metal-catalyst free conditions. Appl. Sci. 2020, 10, 2815. [Google Scholar] [CrossRef]
- Pao, W.; Miller, V.; Zakowski, M.; Doherty, J.; Politi, K.; Sarkaria, I.; Singh, B.; Heelan, R.; Rusch, V.; Fulton, L.; et al. EGF receptor gene mutations are common in lung cancers from “never smokers” and are associated with sensitivity of tumors to gefitinib and erlotinib. Proc. Natl. Acad. Sci. USA 2004, 101, 13306–13311. [Google Scholar]
- Stanaszek, W.F.; Kellerman, D.; Brogden, R.N.; Romankiewicz, J.A. Prazosin: A Review of its Pharmacological Properties and Heart Failure. Drugs 1983, 384, 339–384. [Google Scholar] [CrossRef]
- Dowell, J.; Minna, J.D.; Kirkpatrick, P. Erlotinib hydrochloride. Nat. Rev. Drug Discov. 2005, 4, 13–14. [Google Scholar] [CrossRef]
- Cherrier, L.; Nasar, A.; Goodlet, K.J.; Nailor, M.D.; Tokman, S.; Chou, S. Emergence of letermovir resistance in a lung transplant recipient with ganciclovir-resistant cytomegalovirus infection. Am. J. Transplant. 2018, 18, 3060–3064. [Google Scholar] [CrossRef]
- Commander, H.; Whiteside, G.; Perry, C. Vandetanib: First global approval. Drugs 2011, 71, 1355–1365. [Google Scholar] [CrossRef]
- Reckamp, K.L.; Giaccone, G.; Camidge, D.R.; Gadgeel, S.M.; Khuri, F.R.; Engelman, J.A.; Koczywas, M.; Rajan, A.; Campbell, A.K.; Gernhardt, D.; et al. A phase 2 trial of dacomitinib (PF-00299804), an oral, irreversible pan-HER (human epidermal growth factor receptor) inhibitor, in patients with advanced non-small cell lung cancer after failure of prior chemotherapy and erlotinib. Cancer 2014, 120, 1145–1154. [Google Scholar] [CrossRef]
- Dungo, R.T.; Keating, G.M. Afatinib: First global approval. Drugs 2013, 73, 1503–1515. [Google Scholar] [CrossRef] [PubMed]
- McKeage, K.; Plosker, G.L. Alfuzosin: A review of the therapeutic use of the prolonged-release formulation given once daily in the management of benign prostatic hyperplasia. Drugs 2002, 62, 633–653. [Google Scholar] [CrossRef] [PubMed]
- Senkovich, O.; Schormann, N.; Chattopadhyay, D. Structures of dihydrofolate reductase-thymidylate synthase of trypanosoma cruzi in the folate-free state and in complex with two antifolate drugs, trimetrexate and methotrexate. Acta Crystallogr. Sect. D Biol. Crystallogr. 2009, 65, 704–716. [Google Scholar] [CrossRef] [PubMed]
- Voigtlaender, M.; Schneider-Merck, T.; Trepel, M. Lapatinib. In Small Molecules in Oncology; Springer Nature: Basingstoke, UK, 2018; pp. 19–44. [Google Scholar] [CrossRef]
- Marsh, C.C.; Schuna, A.A.; Sundstrom, W.R. A Review of Selected Investigational Nonsteroidal Antiinflammatory Drugs of the 1980s. Pharmacother. J. Hum. Pharmacol. Drug Ther. 1986, 6, 10–25. [Google Scholar] [CrossRef] [PubMed]
- Guillon, R.; Pagniez, F.; Picot, C.; Hédou, D.; Tonnerre, A.; Chosson, E.; Duflos, M.; Besson, T.; Logé, C.; Le Pape, P. Discovery of a novel broad-spectrum antifungal agent derived from albaconazole. ACS Med. Chem. Lett. 2013, 4, 288–292. [Google Scholar] [CrossRef] [PubMed]
- Hammer, H.; Bader, B.M.; Ehnert, C.; Bundgaard, C.; Bunch, L.; Hoestgaard-Jensen, K.; Schroeder, O.H.U.; Bastlund, J.F.; Gramowski-Voß, A.; Jensen, A.A. A multifaceted GABAA receptor modulator: Functional properties and mechanism of action of the sedative-hypnotic and recreational drug methaqualone (Quaalude). Mol. Pharmacol. 2015, 88, 401–420. [Google Scholar] [CrossRef]
- Liu, Y.; Wu, W.; Hong, W.; Sun, X.; Wu, J.; Huang, Q. Raltitrexed-based chemotherapy for advanced colorectal cancer. Clin. Res. Hepatol. Gastroenterol. 2014, 38, 219–225. [Google Scholar] [CrossRef] [PubMed]
- Ewes, W.A.; Elmorsy, M.A.; El-Messery, S.M.; Nasr, M.N.A. Synthesis, biological evaluation and molecular modeling study of [1,2,4]-Triazolo[4,3-c]quinazolines: New class of EGFR-TK inhibitors. Bioorg. Med. Chem. 2020, 28, 115373. [Google Scholar] [CrossRef] [PubMed]
- Allam, H.A.; Aly, E.E.; Farouk, A.K.B.A.W.; El Kerdawy, A.M.; Rashwan, E.; Abbass, S.E.S. Design and Synthesis of some new 2,4,6-trisubstituted quinazoline EGFR inhibitors as targeted anticancer agents. Bioorg. Chem. 2020, 98, 103726. [Google Scholar] [CrossRef] [PubMed]
- Das, D.; Xie, L.; Wang, J.; Shi, J.; Hong, J. In vivo efficacy studies of novel quinazoline derivatives as irreversible dual EGFR/HER2 inhibitors, in lung cancer xenografts (NCI-H1975) mice models. Bioorg. Chem. 2020, 99, 103790. [Google Scholar] [CrossRef]
- Lin, S.Y.; Chang, C.F.; Coumar, M.S.; Chen, P.Y.; Kuo, F.M.; Chen, C.H.; Li, M.C.; Lin, W.H.; Kuo, P.C.; Wang, S.Y.; et al. Drug-like property optimization: Discovery of orally bioavailable quinazoline-based multi-targeted kinase inhibitors. Bioorg. Chem. 2020, 98, 103689. [Google Scholar] [CrossRef]
- El-Azab, A.S.; Abdel-Aziz, A.A.M.; Bua, S.; Nocentini, A.; AlSaif, N.A.; Alanazi, M.M.; El-Gendy, M.A.; Ahmed, H.E.; Supuran, C.T. S-substituted 2-mercaptoquinazolin-4(3H)-one and 4-ethylbenzensulfonamides act as potent and selective human carbonic anhydrase IX and XII inhibitors. J. Enzyme Inhib. Med. Chem. 2020, 35, 733–743. [Google Scholar] [CrossRef]
- Rahmannejadi, N.; Yavari, I.; Khabnadideh, S. Synthesis and antitumor activities of novel bis-quinazolin-4(3H)-ones. J. Heterocycl. Chem. 2020, 57, 978–982. [Google Scholar] [CrossRef]
- Khodair, A.I.; Alsafi, M.A.; Nafie, M.S. Synthesis, molecular modeling and anti-cancer evaluation of a series of quinazoline derivatives. Carbohydr. Res. 2019, 486, 107832. [Google Scholar] [CrossRef]
- Le, Y.; Gan, Y.; Fu, Y.; Liu, J.; Li, W.; Zou, X.; Zhou, Z.; Wang, Z.; Ouyang, G.; Yan, L. Design, synthesis and in vitro biological evaluation of quinazolinone derivatives as EGFR inhibitors for antitumor treatment. J. Enzym. Inhib. Med. Chem. 2020, 35, 555–564. [Google Scholar] [CrossRef]
- Li, W.; Chen, S.Y.; Hu, W.N.; Zhu, M.; Liu, J.M.; Fu, Y.H.; Wang, Z.C.; OuYang, G.P. Design, synthesis, and biological evaluation of quinazoline derivatives containing piperazine moieties as antitumor agents. J. Chem. Res. 2020, 44, 536–542. [Google Scholar] [CrossRef]
- Alkahtani, H.M.; Abdalla, A.N.; Obaidullah, A.J.; Alanazi, M.M.; Almehizia, A.A.; Alanazi, M.G.; Ahmed, A.Y.; Alwassil, O.I.; Darwish, H.W.; Alaa, A.M.; et al. Synthesis, cytotoxic evaluation, and molecular docking studies of novel quinazoline derivatives with benzenesulfonamide and anilide tails: Dual inhibitors of EGFR/HER2. Bioorg. Chem. 2020, 95, 103461. [Google Scholar] [CrossRef] [PubMed]
- Abuelizz, H.A.; Marzouk, M.; Ghabbour, H.; Al-Salahi, R. Synthesis and anticancer activity of new quinazoline derivatives. Saudi Pharm. J. 2017, 25, 1047–1054. [Google Scholar] [CrossRef] [PubMed]
- Vu, T.K. New Quinazolinone Derivatives: Sythesis and in vitro Cytotoxic Activity. Vietnam J. Sci. Technol. 2020, 58, 12. [Google Scholar] [CrossRef][Green Version]
- Zhang, Y.; Zhang, Y.; Liu, J.; Chen, L.; Zhao, L.; Li, B.; Wang, W. Synthesis and in vitro biological evaluation of novel quinazoline derivatives. Bioorg. Med. Chem. Lett. 2017, 27, 1584–1587. [Google Scholar] [CrossRef]
- Srinivas, S.; Aparna, V. Design, synthesis, biological evaluation and molecular docking studies of novel quinazoline derivatives as GSK-3β inhibitors. World J. Pharm. Pharm. Sci. 2013, 2, 5842–5851. [Google Scholar]
- Wallace, E.; Lyssikatos, J.; Topalav, G.; Buckmelter, A.; Zhao, Q. Qinazoline analogs as receptors tyrosine kinase inhibitors. U.S. Patent 9,676,791, 13 June 2017. [Google Scholar]
- Vishwakarma, R.A.; Bharate, S.B.; Bhushan, S.; Yadav, R.R.; Guru, S.K.; Joshi, P. 6-Aryl-4-phenylamino-quinazoline Analogs as Phosphoinositide-3-kinase Inhibitors. U.S. Patent 10,202,374, 12 February 2019. [Google Scholar]
- Golden, D.; Gb, M.; Hogan, P.J.; Gb, M.; Michael, D.; Martin, G. Chemical process for the Synthesis of 4-(4-bromo-2-fluoroanilino)-6-methoxy-7-(1-methylpiperidin-4-ylmethoxy)quinazoline. United States Patent. Vol. 2. U.S. Patent 10,344,015, 9 July 2019. [Google Scholar]
- Lindmark, B.; Ooi, L. Combination Therapy Comprising Varlitinib and an Anticancer Agent. Vol. 2. Singapore (SG). U.S. Patent 10,357,494, 23 July 2019. [Google Scholar]
- Ci, U.S.; Sui, I.; Cai, X.; Cn, J.; Jiang, Y.; Cn, J. 1-(Arylmethyl)quinazoline-2,4(1H,3H)-diones as PARP Inhibitors and the Use Thereof. Vol. 2. U.S. Patent 10,316,027, 11 June 2019. [Google Scholar]
- Wallace, E.; Tapalov, G.; Lyssikatose, J.; Buckmelter, A.; Qian, Z. Quinazoline Analogs as Receptor Tyrosine Kinase Inhibitors. Vol. 2. U.S. Patent 9,676,791, 13 June 2017. [Google Scholar]
- Ghorab, M.M.; Abdel-Kader, M.S.; Alqahtani, A.S.; Soliman, A.M. Synthesis of some quinazolinones inspired from the natural alkaloid L-norephedrine as EGFR inhibitors and radiosensitizers. J. Enzym. Inhib. Med. Chem. 2021, 36, 218–237. [Google Scholar] [CrossRef]
- Wang, M.; Zhang, G.; Wang, Y.; Wang, J.; Zhu, M.; Cen, S.; Wang, Y. Design, synthesis and anti-influenza A virus activity of novel 2,4-disubstituted quinazoline derivatives. Bioorg. Med. Chem. Lett. 2020, 30, 127143. [Google Scholar] [CrossRef]
- Rothan, H.; Faraj, F.L.; Teoh, T.C.; Yusof, R. Novel Quinazoline derivatives inhibited HCV Serine protease and viral replication in Huh-7 cells. Nov Quinazoline Deriv Inhib HCV Serine protease viral replication Huh-7 cells. bioRxiv 2019, 671313. [Google Scholar] [CrossRef]
- Fan, Z.; Shi, J.; Bao, X. Synthesis and antimicrobial evaluation of novel 1,2,4-triazole thioether derivatives bearing a quinazoline moiety. Mol. Divers. 2018, 22, 657–667. [Google Scholar] [CrossRef]
- Fröhlich, T.; Reiter, C.; Ibrahim, M.M.; Beutel, J.; Hutterer, C.; Zeitträger, I.; Bahsi, H.; Leidenberger, M.; Friedrich, O.; Kappes, B.; et al. Synthesis of Novel Hybrids of Quinazoline and Artemisinin with High Activities against Plasmodium falciparum, Human Cytomegalovirus, and Leukemia Cells. ACS Omega 2017, 2, 2422–2431. [Google Scholar] [CrossRef]
- Xie, D.; Shi, J.; Zhang, A.; Lei, Z.; Zu, G.; Fu, Y.; Gan, X.; Yin, L.; Song, B.; Hu, D. Syntheses, antiviral activities and induced resistance mechanisms of novel quinazoline derivatives containing a dithioacetal moiety. Bioorg. Chem. 2018, 80, 433–443. [Google Scholar] [CrossRef]
- Zhang, G.P.; Pan, J.K.; Zhang, J.; Wu, Z.X.; Liu, D.Y.; Zhao, L. Design, Synthesis, Antiviral Activities of Novel Phosphonate Derivatives Containing Quinazoline Based on Chalone Motif. J. Heterocycl. Chem. 2017, 54, 2548–2555. [Google Scholar] [CrossRef]
- Heil, M.L.; Cosford, N.D.; Ardecky, R.; Zou, J. Quinazolinone analogs and use of Quinazolinone analogs for treating or preventing certain Viral infection. U.S. Patent 10,611,733, 21 March 2017. [Google Scholar]
- Misra, A.; Dwivedi, J.; Shukla, S.; Kishore, D.; Sharma, S. Bacterial cell leakage potential of newly synthesized quinazoline derivatives of 1,5-benzodiazepines analogue. J. Heterocycl. Chem. 2020, 57, 1545–1558. [Google Scholar] [CrossRef]
- Sunil Kumar, A.; Kudva, J.; Lahtinen, M.; Peuronen, A.; Sadashiva, R.; Naral, D. Synthesis, characterization, crystal structures and biological screening of 4-amino quinazoline sulfonamide derivatives. J. Mol. Struct. 2019, 1190, 29–36. [Google Scholar] [CrossRef]
- Peter, J.; Mark, S.; Flanigan, D.L.; Helen, W.; George, H.; John, S. Disubstituted Quinazoline-2,4 Diamines and Uses Thereof. Vol. 1. U.S. Patent 10,323,007, 18 June 2019. [Google Scholar]
- Chang, M.; Mobashery, S.; Bouley, R. Quinazolinone Antibiotics. Vol. 2. U.S. Patent 10,329,262, 25 June 2019. [Google Scholar]
- Jadhavar, P.S.; Patel, K.I.; Dhameliya, T.M.; Saha, N.; Vaja, M.D.; Krishna, V.S.; Sriram, D.; Chakraborti, A.K. Benzimidazoquinazolines as new potent anti-TB chemotypes: Design, synthesis, and biological evaluation. Bioorg. Chem. 2020, 99, 103774. [Google Scholar] [CrossRef] [PubMed]
- Gawad, J.; Bonde, C. Design, synthesis and biological evaluation of novel 6-(trifluoromethyl)-N-(4-oxothiazolidin-3-yl)quinazoline-2-carboxamide derivatives as a potential DprE1 inhibitors. J. Mol. Struct. 2020, 1217, 128394. [Google Scholar] [CrossRef]
- Lupien, A.; Foo, C.S.Y.; Savina, S.; Vocat, A.; Piton, J.; Monakhova, N.; Benjak, A.; Lamprecht, D.A.; Steyn, A.J.; Pethe, K.; et al. New 2-Ethylthio-4-methylaminoquinazoline derivatives inhibiting two subunits of cytochrome bc1 in Mycobacterium tuberculosis. PLoS Pathog. 2020, 16, e1008270. [Google Scholar] [CrossRef] [PubMed]
- Al-Salahi, R.; Taie, H.A.A.; Bakheit, A.H.; Marzouk, M.; Almehizia, A.A.; Herqash, R.; Abuelizz, H.A. Antioxidant activities and molecular docking of 2-thioxobenzo[g]quinazoline derivatives. Pharmacol. Rep. 2019, 71, 695–700. [Google Scholar] [CrossRef]
- Almehizia, A.A.; Abuelizz, H.A.; Taie, H.A.A.; ElHassane, A.; Marzouk, M.; Al-Salahi, R. Investigation the antioxidant activity of benzo[g]triazoloquinazolines correlated with a DFT study. Saudi Pharm. J. 2019, 27, 133–137. [Google Scholar] [CrossRef]
- Dixit, A.; Pathak, D.; Sharma, G.K. Synthesis, antibacterial and antioxidant activity of novel 12-(N-arylmethaniminyl)indolo[1,2-c]quinazolines. Marmara Pharm. J. 2019, 23, 584–595. [Google Scholar] [CrossRef]
- Hricoviniova, J.; Hricoviniova, Z.; Kozica, K. Antioxidant, Cytotoxic, Genotoxic, and DNA Protective Potential of 2,3-Substituted Quinazolinones: Structure—Activity Relationship Study. Int. J. Mol. Sci. 2021, 22, 610. [Google Scholar] [CrossRef]
- Jain, R.K.; Kashaw, V. Design, synthesis and evaluation of novel 2,3-disubstituted-4-(3H) quinazolinone derivatives. Asian J. Pharm. Pharmacol. 2018, 4, 644–656. [Google Scholar] [CrossRef]
- Stavytskyi, V.; Voskoboinik, O.; Kazunin, I.; Nosulenko, I.; Shishkina, S.; Kovalenko, S. Substituted pyrrolo[1,2-a][1,2,4]triazolo-([1,2,4]triazino-)[c]quinazoline-4A(5a)-propanoic acids: Synthesis, spectral characteristics and anti-inflammatory activity. Vopr. Khimii I Khimicheskoi Tekhnologii. 2020, 2020, 61–70. [Google Scholar]
- Bansal, R. Design and Synthesis of Some Quinazoline Derivatives as Anti-Inflammatory Agents. Int. J. Engg. Tech. Sci. and Res. 2017, 4, 61–62. [Google Scholar]
- Oalmann, C.; Perni, R.B.; Us, M.A.; White, B. Quinazolinone, Quinolone Related Analogs as Sirtuin Modulators. Vol. 2. U.S. Patent 10,329,262, 3 May 2016. [Google Scholar]
- Santos-Ballardo, L.; García-Páez, F.; Picos-Corrales, L.A.; Ochoa-Terán, A.; Bastidas, P.; Calderón-Zamora, L.; Rendón-Maldonado, G.; Osuna-Martínez, U.; Sarmiento-Sánchez, J.I. Synthesis, biological evaluation and molecular docking of 3-substituted quinazoline-2,4(1H, 3H)-diones. J. Chem. Sci. 2020, 132, 100. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, J.; Ma, Y.; Ren, D.; Cheng, P.; Zhao, J.; Zhang, F.; Yao, Y. One-pot synthesis and antifungal activity against plant pathogens of quinazolinone derivatives containing an amide moiety. Bioorg. Med. Chem. Lett. 2016, 26, 2273–2277. [Google Scholar] [CrossRef]
- Nangare, A.; Randive, D.; Barkade, G. Synthesis and Biological evaluation of Novel Quinazoline derivatives as Anticancer, Antibacterial and Antifungal agents. Bull. Env. Pharmacol. Life Sci. 2021, 10, 179–190. [Google Scholar]
- Agrawal, G.; Khan, N.; Dwivedi, S.; Patel, R.; Singh, G. Synthesis, Characterization and Anti-Microbial Evaluation of a Series of Quinazoline analogs. J. Adv. Sci. Res. 2021, 12, 123–130. [Google Scholar]
- Mishra, M.; Mishra, V.K.; Kashaw, V.; Agrawal, R.K.; Kashaw, S.K. Novel Quinazoline Chalcone Hybrids as Antiplasmodium Agents: Synthesis, Biological Evaluation and Molecular Docking. J. Adv. Sci. Res. 2020, 11, 85–99. [Google Scholar]
- Tahghighi, A.; Mehrizi, A.; Zakeri, S. In vitro anti-plasmodial activity of new synthetic derivatives of 1-(heteroaryl)-2-((5-nitroheteroaryl)methylene) hydrazine. Asian Pac. J. Trop. Med. 2021, 14, 128–138. [Google Scholar]
- Mendoza-Martínez, C.; Galindo-Sevilla, N.; Correa-Basurto, J.; Ugalde-Saldivar, V.M.; Rodriguez-Delgado, R.G.; Hernández-Pineda, J.; Padierna-Mota, C.; Flores-Alamo, M.; Hernández-Luis, F. Antileishmanial activity of quinazoline derivatives: Synthesis, docking screens, molecular dynamic simulations and electrochemical studies. Eur. J. Med. Chem. 2015, 92, 314–331. [Google Scholar] [CrossRef] [PubMed]
- Amrane, D.; Gellis, A.; Hutter, S.; Prieri, M.; Verhaeghe, P.; Azas, N.; Vanelle, P.; Primas, N. Synthesis and Antiplasmodial Evaluation of 4-Carboxamido- and 4-Alkoxy-2-Trichloromethyl Quinazolines. Molecules 2020, 25, 3929. [Google Scholar] [CrossRef]





| S. no. | Commercial Name | Structure | Usage | Ref. |
|---|---|---|---|---|
| 1. | Gifitinib | 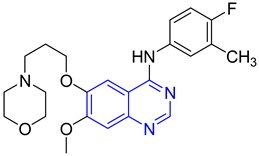 | For treatment of non-small cell lung cancer | [12] |
| 2. | Prazocin |  | For high blood pressure | [13] |
| 3. | Erlotinib | 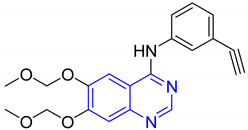 | For non-small cell lung cancer, pancreatic cancer and several other types of cancer | [14] |
| 4. | Letermovir | 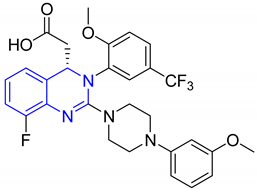 | Antiviral drug | [15] |
| 5. | Vandetanib | 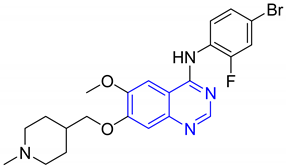 | Antagonist of the vascular endothelial growth factor receptor | [16] |
| 6. | Dacomitinib |  | Non small cell lung carcinoma | [17] |
| 7. | Afatinib | 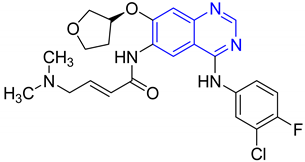 | For treatment of cancers resistant to gefinitib and erlotinib | [18] |
| 8. | Alfuzosin |  | Prostatic hyperplasia | [19] |
| 9. | Trimetrexate | 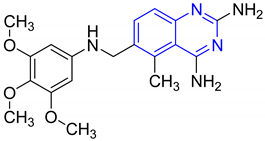 | Antineoplastic agent, and as an antiparasitic agent against pneumocystis | [20] |
| 10. | Lapatinib | 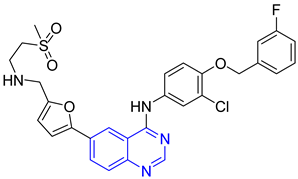 | For treatment of advanced-stage or metastatic breast cancer | [21] |
| 11. | Proquazone |  | Non-steroidal anti-inflammatory drug | [22] |
| 12. | Albaconazole | 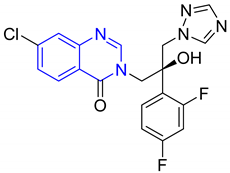 | Anti-fungal agent | [23] |
| 13. | Methaqualone |  | Sedative effects | [24] |
| 14. | Raltitrexed |  | Cancer of large intestine | [25] |
| Compound no. | Structure | Activity Tested against the Cells | Cytotoxicity | Ref. |
|---|---|---|---|---|
| 1 |  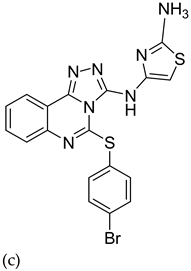 |
| IC50 (uM) (a)
| [26] |
| 2 |  |
| IC50 (uM)
| [27] |
| 3 |  |
| IC50 (nM) (a)
| [28] |
| 4 | 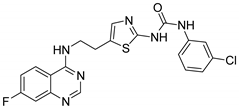 |
| (IC50 = 30.5 nM)
| [29] |
| 5 |   |
| KI (nM) (a)
| [30] |
| 6 | 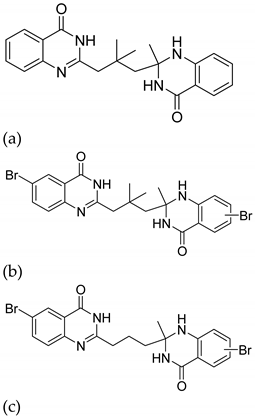 |
| IC50 (μM) (a)
| [31] |
| 7 |  |
| IC50 (μM)
| [32] |
| 8 |  |
| (a)
| [33] |
| 9 | 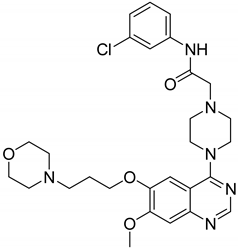 |
| IC50 (μM)
| [34] |
| 10 | 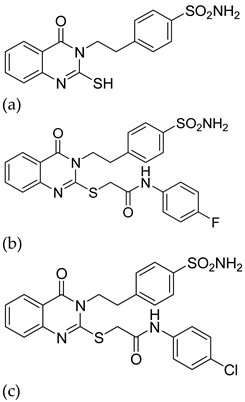 |
| IC50 (mM) (a)
| [35] |
| 11 | 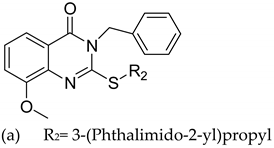 |
| IC50 (mM) (a)
| [36] |
| 12 |  |
| IC50
| [37] |
| 13 | 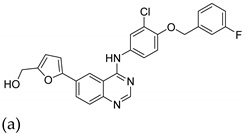 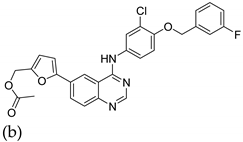 |
| IG50 (μM) (a)
| [38] |
| 14 |  | Glycogen synthase kinase (GSK-3) inhibitors | Docking score
| [39] |
| 15 | 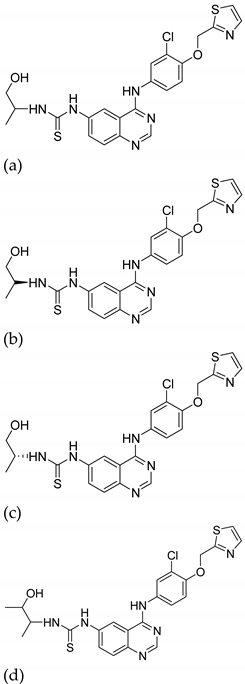 | Type-I receptor tyrosine kinase inhibitors | Effective for the treatment of hyperpro-liferative diseases, e.g., cancer | [45] |
| 16 |  |
| (a)
| [41] |
| 17 | 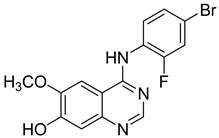 | vascular endothelial cell growth (VEGF) | -- | [42] |
| 18 |  | HER2 positive or HER2 amplified | .-- | [43] |
| 19 | 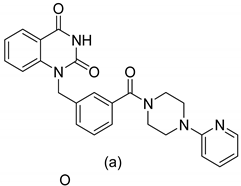  | ADP ribose polymerase (PARP) inhibitors | effective for breast cancer treatment | [44] |
| 20 |  | Type 1 receptor tyrosine kinase inhibitors. | Effective for the treatment of hyperproliferative diseases such as cancer | [45] |
| 21 |  |
| (a)
| [46] |
| Compound no. | Structure | Microbe Selected | Activity | Ref. |
|---|---|---|---|---|
| 1 | 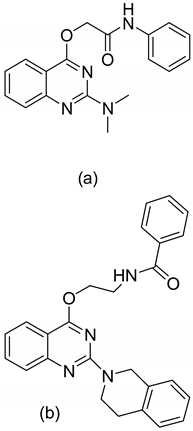 | Anti-IAV A/WSN/33 (H1N1) | IC50 (μM)
| [47] |
| 2 | 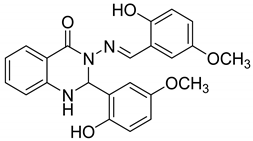 |
| IC50 (μM)
| [48] |
| 3 | 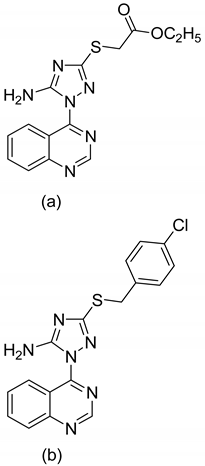  |
| EC50 (μg/mL)
| [49] |
| 4 | 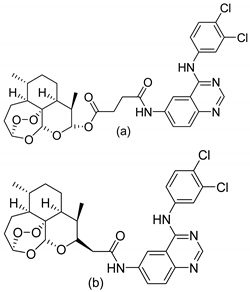 |
| EC50 (μg/mL) (a)
| [50] |
| 5 | 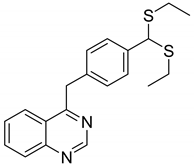 |
|
| [51] |
| 6 |  | CMV
| (a)
| [52] |
| 7 | 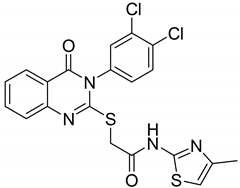 |
| -- | [53] |
| Compound no. | Structure | Microbe Selected | Activity | Ref. |
|---|---|---|---|---|
| 1 | 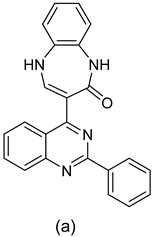 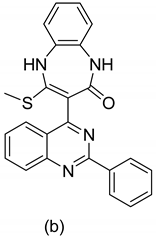 |
| IC50 (μg/mL)(a)
| [54] |
| 2 | 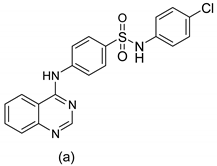 |
| MIC in mg/mL
| [55] |
| 3 | 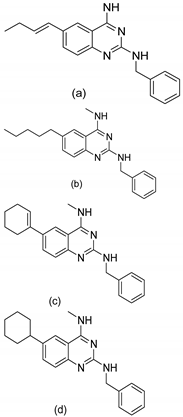 | A. baumannii bacteria | -- | [56] |
| 4 | 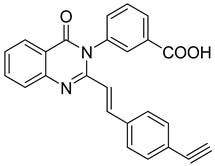 | Staphylococcus aureus M (RSA) (gram positive bacteria) | -- | [57] |
| Compound no. | Structure | Activity | Observed Values | Ref. |
|---|---|---|---|---|
| 1 | 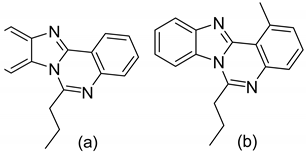 | As anti-TB agents M. tuberculosis | MIC values in the range of 12.5 and 0.78 μg/mL | [58] |
| 2 | 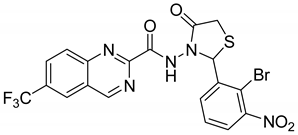 |
|
| [59] |
| 3 | 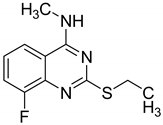 |
| MIC μg/mL
| [60] |
| Compound no. | Structure | Activity | Observed Values | Ref. |
|---|---|---|---|---|
| 1 |  | Anti-Oxidant activity DPPH and free radical scavenging activities were evaluated | Dock score kcal/mol
| [61] |
| 2 | 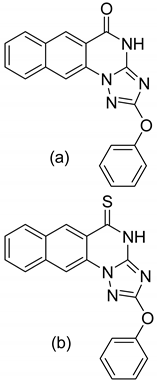 |
| (a)
| [62] |
| 3 | 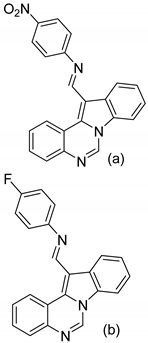 |
| IC50 μg/mL (a)
| [63] |
| 4 | 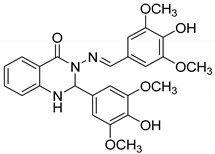 |
|
| [64] |
| Compound no. | Structure | Activity | Results | Ref. |
|---|---|---|---|---|
| 1 | 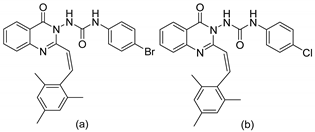 | Evaluated as anti-convulsant activity | Synthesized compounds were performed against maximal electroshock-induced seizures and PTZ-induced clonic seizures | [65] |
| 2 | 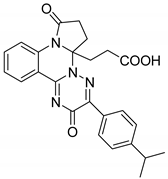 | Evaluated as anti-inflammatory agent | Synthesized compounds showed high anti-inflammatory activity. Fluorine atom has crucial role in the anti-inflammatory activity of the synthesized compounds | [66] |
| 3 |  | Evaluated as anti-inflammatory agent | Introduction of Fluorine atom on the phenyl ring leads to strengthening anti-inflammatory activity | [67] |
| 4 |  | Evaluated as Sirtuin Modulating agents | Increases the mitochondrial activity and lifespan of a cell; uses for various diseases and disorders. | [68] |
| 5 | 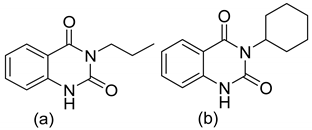 | Evaluated as Antidiabetic agents | Alpha-amylase and alpha-glucosidase inhibitors | [69] |
| 6 | 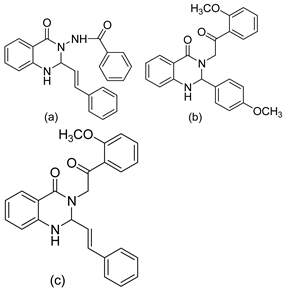 | Evaluated as antifungal | [70] | |
| 7 | 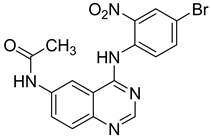 | Evaluated as antifungal | Antifungal activity against Fusarium moniliforme | [71] |
| 8 | 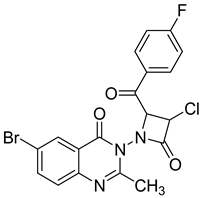 | Evaluated as antifungal | Antifungal activity against Candida albicans and Aspergillus flavus | [72] |
| 9 | 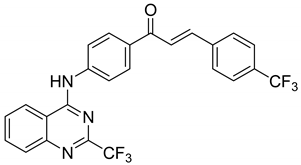 | Evaluated as Antiparasite agents | Β-hematin formation inhibitors | [73] |
| 10 | 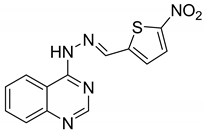 | Evaluated as Antiparasite agents | Most effective derivatives against P. falciparum 3D7 and K1 strains | [74] |
| 11 |  | Evaluated as Antiparasite agents | Showed activity on promastigotes and intracellular amastigotes | [75] |
| 12 | 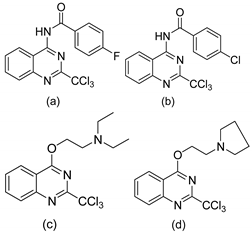 | Evaluated as Antiplasmodium agents | IC50 P. falci. K1 = 0.94 μM IC50 P. falci. K1 = 0.9 μM | [76] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Karan, R.; Agarwal, P.; Sinha, M.; Mahato, N. Recent Advances on Quinazoline Derivatives: A Potential Bioactive Scaffold in Medicinal Chemistry. ChemEngineering 2021, 5, 73. https://doi.org/10.3390/chemengineering5040073
Karan R, Agarwal P, Sinha M, Mahato N. Recent Advances on Quinazoline Derivatives: A Potential Bioactive Scaffold in Medicinal Chemistry. ChemEngineering. 2021; 5(4):73. https://doi.org/10.3390/chemengineering5040073
Chicago/Turabian StyleKaran, Ram, Pooja Agarwal, Mukty Sinha, and Neelima Mahato. 2021. "Recent Advances on Quinazoline Derivatives: A Potential Bioactive Scaffold in Medicinal Chemistry" ChemEngineering 5, no. 4: 73. https://doi.org/10.3390/chemengineering5040073
APA StyleKaran, R., Agarwal, P., Sinha, M., & Mahato, N. (2021). Recent Advances on Quinazoline Derivatives: A Potential Bioactive Scaffold in Medicinal Chemistry. ChemEngineering, 5(4), 73. https://doi.org/10.3390/chemengineering5040073







