Evaluation of the Effect of Particle Size and Biomass-to-Water Ratio on the Hydrothermal Carbonization of Sugarcane Bagasse
Abstract
1. Introduction
2. Materials and Methods
2.1. Sampling of Residual Biomass
2.2. Sugarcane Bagasse Primary Pretreatment
2.3. Biomass Characterization Techniques
2.3.1. Proximal Analysis
2.3.2. Ultimate Analysis
2.3.3. Structural Analysis
2.4. Hydrothermal Treatment of Biomass
2.4.1. Aqueous Phase
Identification and Quantification of the Formation of Platform Chemicals
2.4.2. Solid Phase (Hydrochar)
3. Results
3.1. Biomass Characterization
3.1.1. Proximal Analysis
3.1.2. Ultimate Analysis
3.1.3. Structural Analysis
3.2. Hydrothermal Treatment of Biomass
3.2.1. Aqueous Phase
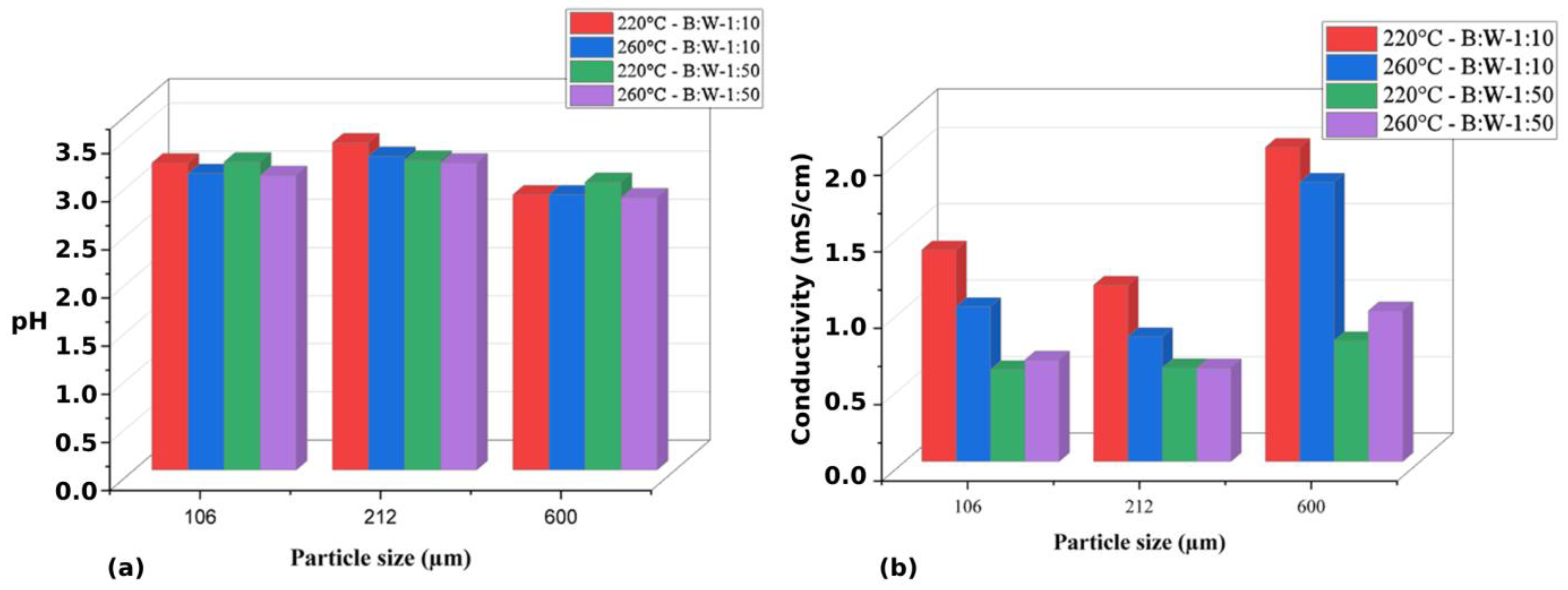
Conductivity Monitoring, pH Measurement, and Particle Size Variation
Experiments with B:W Ratio Variation, pH, and Conductivity Monitoring
Quantification of Platform Chemicals
3.2.2. Solid Phase (Hydrochar)
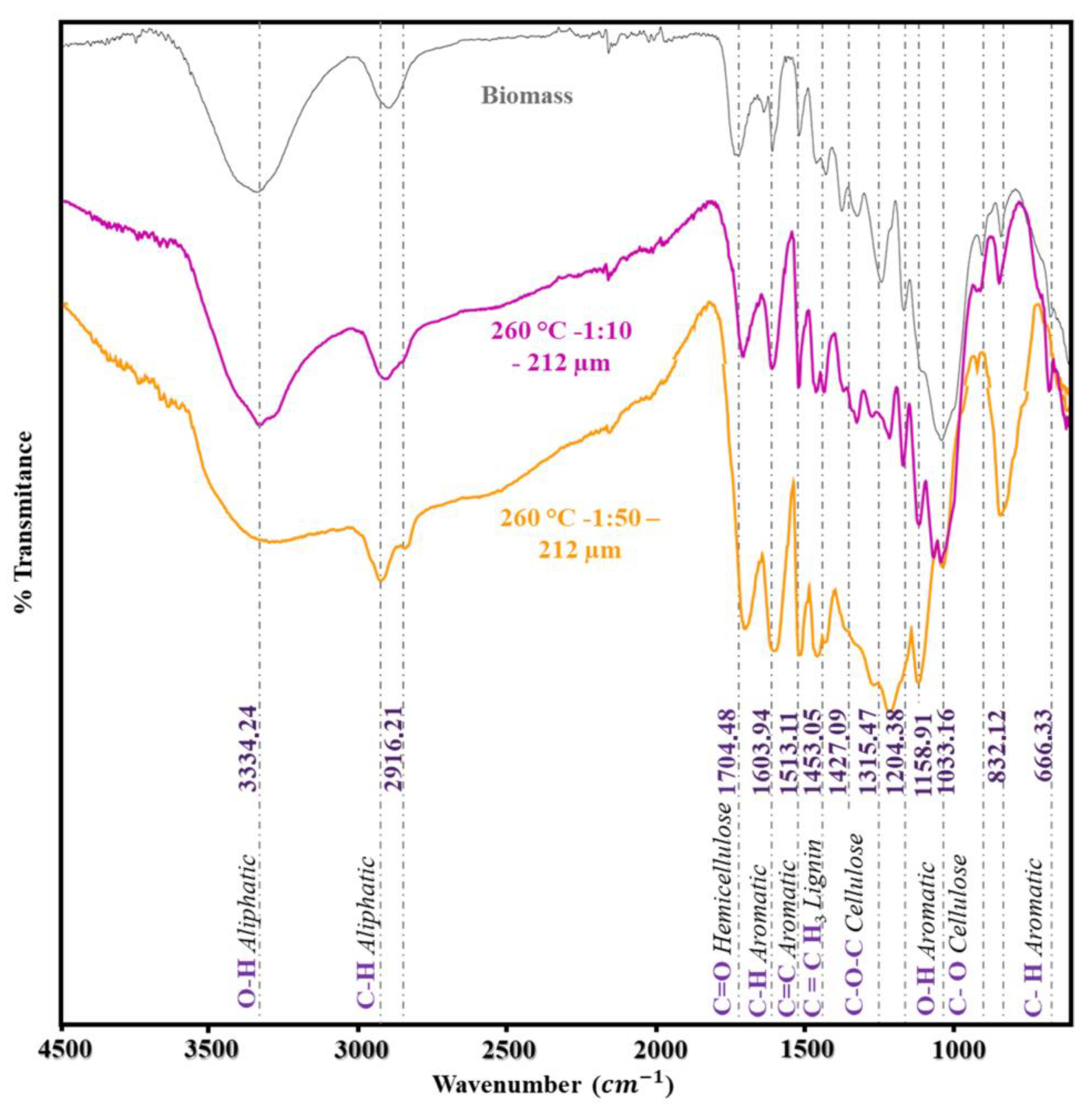
Infrared Spectroscopy
The Morphology of the Samples Microscopically
Elemental Analysis
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- FINAGRO. Crecimiento del Sector Agropecuario y AgroExpo 2023, un Reto Hacia el Desarrollo del Campo|Finagro. Available online: https://www.finagro.com.co/noticias/articulos/crecimiento-del-sector-agropecuario-agroexpo-2023-reto-desarrollo-del-campo-0 (accessed on 7 December 2023).
- DANE. Boletín Encuesta Nacional Agropecuaria 2019. DANE. Available online: https://www.dane.gov.co/index.php/estadisticas-por-tema/agropecuario/encuesta-nacional-agropecuaria-ena (accessed on 7 December 2023).
- DANE. DANE—PIB Información Técnica. DANE. Available online: https://www.dane.gov.co/index.php/estadisticas-por-tema/cuentas-nacionales/cuentas-nacionales-trimestrales/pib-informacion-tecnica (accessed on 7 November 2021).
- DANE. Boletín mensual Insumos y Factores Asociados a la Producción Agropecuaria. 2020. Available online: http://www.agronet.gov.co/ (accessed on 6 November 2021).
- DANE. Un Camino para la Inclusión, la Equidad y el Reconocimiento. Available online: https://www.mineducacion.gov.co/1759/articles-362822_recurso.pdf (accessed on 8 December 2023).
- Felipe, J.; Bustamante, L. La Caña de Azucar (Saccharum officinarum) para la Producción de Panela. Caso: Nordeste del Departamento de Antioquia; National Open and Distance University UNAD: Bogota, Colombia, 2015. [Google Scholar]
- Minagricultura. Cadena Agroindustrial de la Panela. Colombia, 2019. Available online: https://sioc.minagricultura.gov.co/Panela/Documentos/2019-12-30CifrasSectoriales.pdf (accessed on 2 January 2022).
- Kambo, H.S.; Dutta, A. A comparative review of biochar and hydrochar in terms of production, physico-chemical properties and applications. Renew. Sustain. Energy Rev. 2015, 45, 359–378. [Google Scholar] [CrossRef]
- Peterson, A.A.; Vogel, F.; Lachance, R.P.; Fröling, M.; Antal, M.J., Jr.; Tester, J.W. Thermochemical biofuel production in hydrothermal media: A review of sub- and supercritical water technologies. Energy Environ. Sci. 2008, 1, 32. [Google Scholar] [CrossRef]
- Shen, Y. A review on hydrothermal carbonization of biomass and plastic wastes to energy products. Biomass Bioenergy 2020, 134, 105479. [Google Scholar] [CrossRef]
- Zhuang, X.; Zhan, H.; Song, Y.; He, C.; Huang, Y.; Yin, X.; Wu, C. Insights into the evolution of chemical structures in lignocellulose and non-lignocellulose biowastes during hydrothermal carbonization (HTC). Fuel 2019, 236, 960–974. [Google Scholar] [CrossRef]
- Coronella, C.J.; Lynam, J.G.; Reza, M.T.; Uddin, M.H. Hydrothermal Carbonization of Lignocellulosic Biomass. In Application of Hydrothermal Reactions to Biomass Conversion; Springer: Berlin/Heidelberg, Germany, 2014; pp. 275–311. [Google Scholar] [CrossRef]
- Heidari, M.; Dutta, A.; Acharya, B.; Mahmud, S. A review of the current knowledge and challenges of hydrothermal carbonization for biomass conversion. J. Energy Inst. 2019, 92, 1779–1799. [Google Scholar] [CrossRef]
- Nguyen, T.A.H.; Bui, T.H.; Guo, W.S.; Ngo, H.H. Valorization of the aqueous phase from hydrothermal carbonization of different feedstocks: Challenges and perspectives. Chem. Eng. J. 2023, 472, 144802. [Google Scholar] [CrossRef]
- Fang, J.; Zhan, L.; Ok, Y.S.; Gao, B. Minireview of potential applications of hydrochar derived from hydrothermal carbonization of biomass. J. Ind. Eng. Chem. 2018, 57, 15–21. [Google Scholar] [CrossRef]
- Rehman, A.; Nazir, G.; Heo, K.; Hussain, S.; Ikram, M.; Akhter, Z.; Algaradah, M.M.; Mahmood, Q.; Fouda, A.M. A focused review on lignocellulosic biomass-derived porous carbons for effective pharmaceuticals removal: Current trends, challenges and future prospects. Sep. Purif. Technol. 2024, 330, 125356. [Google Scholar] [CrossRef]
- Onokwai, A.O.; Ajisegiri, E.S.A.; Okokpujie, I.P.; Ibikunle, R.A.; Oki, M.; Dirisu, J.O. Characterization of lignocellulose biomass based on proximate, ultimate, structural composition, and thermal analysis. Mater. Today Proc. 2022, 65, 2156–2162. [Google Scholar] [CrossRef]
- Edreis, E.M.A.; Luo, G.; Yao, H. Investigations of the structure and thermal kinetic analysis of sugarcane bagasse char during non-isothermal CO2 gasification. J. Anal. Appl. Pyrolysis 2014, 107, 107–115. [Google Scholar] [CrossRef]
- Castro Vega, A.A. Estudio de la Naturaleza Química de Biocrudos Obtenidos Mediante Licuefacción Hidrotérmica de Biomasa Lignocelulósica. 2011. Available online: https://repositorio.unal.edu.co/handle/unal/8651 (accessed on 8 December 2023).
- Castro, A.A.V.; Varela, L.I.R.; Díaz Velásquez, J. Subcritical hydrothermal conversion of organic wastes and biomass. Reaction pathways. Ing. E Investig. 2007, 27, 41–50. [Google Scholar] [CrossRef]
- Qian, C.; Li, Q.; Zhang, Z.; Wang, X.; Hu, J.; Cao, W. Prediction of higher heating values of biochar from proximate and ultimate analysis. Fuel 2020, 265, 116925. [Google Scholar] [CrossRef]
- Preparation of Samples for Compositional Analysis; Technical Report NREL/TP-510-42620; National Renewable Energy Laboratory: Golden, CO, USA, 2008. Available online: https://www.nrel.gov/docs/gen/fy08/42620.pdf (accessed on 7 December 2023).
- Determination of Total Solids in Biomass and Total Dissolved Solids in Liquid Process Samples; Technical Report NREL/TP-510-42621; National Renewable Energy Laboratory: Golden, CO, USA, 2008. Available online: https://www.nrel.gov/docs/gen/fy08/42621.pdf (accessed on 7 December 2013).
- Determination of Ash in Biomass; National Renewable Energy Laboratory: Golden, CO, USA, (NREL/TP-510-42622); 2008. Available online: https://www.nrel.gov/docs/gen/fy08/42622.pdf (accessed on 7 December 2023).
- Standard Test Method for Volatile Matter in the Analysis of Particulate Wood Fuels (ASTM E872-82(2019)); ASTM International: West Conshohocken, PA, USA, 2019. Available online: https://www.astm.org/e0872-82r19.html (accessed on 7 December 2023).
- Standard Test Methods for Determination of Carbon, Hydrogen and Nitrogen in Analysis Samples of Coal and Carbon in Analysis Samples of Coal and Coke (ASTM D5373-21). 2021. Available online: https://www.astm.org/d5373-21.html (accessed on 7 December 2023).
- Fiber (Acid Detergent) and Lignin (H2SO4) in Animal Feed. In AOAC (AOAC 973.18-1977); AOAC: Rockville, MD, USA, 2021; Available online: http://www.aoacofficialmethod.org/index.php?main_page=product_info&products_id=1165 (accessed on 7 December 2023).
- Van Soest, P.; Robertson, J.B.; Lewis, B.A. Methods for dietary fiber, neutral detergent fiber, and nonstarch polysaccharides in relation to animal nutrition. J. Dairy Sci. 1991, 74, 3583–3597. [Google Scholar] [CrossRef] [PubMed]
- Ayeni, A.O.; Adeeyo, O.A.; Oresegun, O.M.; Oladimeji, T.E. Compositional analysis of lignocellulosic materials: Evaluation of an economically viable method suitable for woody and non-woody biomass. Am. J. Eng. Res. 2015, 4, 14–19. Available online: https://www.ajer.org (accessed on 14 January 2024).
- Onokwai, A.O.; Okokpujie, I.P.; Ajisegiri, E.S.; Oki, M.; Adeoyeb, A.O.; Akinlabi, E.T. Characterization of Lignocellulosic Biomass Samples in Omu-Aran Metropolis, Kwara State, Nigeria, as Potential Fuel for Pyrolysis Yields. Int. J. Renew. Energy Dev. 2022, 11, 973–981. [Google Scholar] [CrossRef]
- Hincapié, G.; Soto, A.; López, D. Pre-tratamiento ácido y básico de bagazo de caña y de compuestos modelo para la producción de bio-aceite vía licuefacción hidrotérmica. Energética 2016, 47, 23–30. Available online: https://repositorio.unal.edu.co/handle/unal/64263 (accessed on 14 January 2024).
- Zhang, P.; Liao, W.; Kumar, A.; Zhang, Q.; Ma, H. Characterization of sugarcane bagasse ash as a potential supplementary cementitious material: Comparison with coal combustion fly ash. J. Clean. Prod. 2020, 277, 123834. [Google Scholar] [CrossRef]
- Khatami, R.; Stivers, C.; Joshi, K.; Levendis, Y.A.; Sarofim, A.F. Combustion behavior of single particles from three different coal ranks and from sugarcane bagasse in O2/N2 and O2/CO2 atmospheres. Combust. Flame 2012, 159, 1253–1271. [Google Scholar] [CrossRef]
- Yao, S.; Nie, S.; Yuan, Y.; Wang, S.; Qin, C. Efficient extraction of bagasse hemicelluloses and characterization of solid remainder. Bioresour. Technol. 2015, 185, 21–27. [Google Scholar] [CrossRef]
- Wüst, D.; Correa, C.R.; Jung, D.; Zimmermann, M.; Kruse, A.; Fiori, L. Understanding the influence of biomass particle size and reaction medium on the formation pathways of hydrochar. Biomass Convers. Biorefin. 2020, 10, 1357–1380. [Google Scholar] [CrossRef]
- Heidari, M.; Salaudeen, S.; Dutta, A.; Acharya, B. Effects of Process Water Recycling and Particle Sizes on Hydrothermal Carbonization of Biomass. Energy Fuels 2018, 32, 11576–11586. [Google Scholar] [CrossRef]
- Zhou, Y.; Remón, J.; Pang, X.; Jiang, Z.; Liu, H.; Ding, W. Hydrothermal conversion of biomass to fuels, chemicals and materials: A review holistically connecting product properties and marketable applications. Sci. Total Environ. 2023, 886, 163920. [Google Scholar] [CrossRef] [PubMed]
- Usman, M.; Chen, H.; Chen, K.; Ren, S.; Clark, J.H.; Fan, J.; Luo, G.; Zhang, S. Characterization and utilization of aqueous products from hydrothermal conversion of biomass for bio-oil and hydro-char production: A review. Green. Chem. 2019, 21, 1553–1572. [Google Scholar] [CrossRef]
- Zhou, Y.; Li, M.; Chen, Y.; Hu, C. Conversion of polysaccharides in Ulva prolifera to valuable chemicals in the presence of formic acid. J. Appl. Phycol. 2021, 33, 101–110. [Google Scholar] [CrossRef]
- Krysanova, K.; Krylova, A.; Kulikova, M.; Kulikov, A.; Rusakova, O. Biochar characteristics produced via hydrothermal carbonization and torrefaction of peat and sawdust. Fuel 2022, 328, 125220. [Google Scholar] [CrossRef]
- Zhu, R.; Yadama, V. Effects of hot water extraction pretreatment on physicochemical changes of Douglas fir. Biomass Bioenergy 2016, 90, 78–89. [Google Scholar] [CrossRef]
- Sabry, T.M.; El-Korashy, S.A.E.-H.; Jahin, H.E.S.; Khairy, G.M.; Aal, N.F.A. Hydrothermal carbonization of Calotropis procera leaves as a biomass: Preparation and characterization. J. Mol. Struct. 2024, 1302, 137397. [Google Scholar] [CrossRef]
- Sevilla, M.; Fuertes, A.B. The production of carbon materials by hydrothermal carbonization of cellulose. Carbon. N. Y. 2009, 47, 2281–2289. [Google Scholar] [CrossRef]
- Ju, Y.H.; Huynh, L.H.; Kasim, N.S.; Guo, T.J.; Wang, J.H.; Fazary, A.E. Analysis of soluble and insoluble fractions of alkali and subcritical water treated sugarcane bagasse. Carbohydr. Polym. 2011, 83, 591–599. [Google Scholar] [CrossRef]
- Iryani, D.A.; Kumagai, S.; Nonaka, M.; Sasaki, K.; Hirajima, T. Characterization and Production of Solid Biofuel from Sugarcane Bagasse by Hydrothermal Carbonization. Waste Biomass Valorization 2017, 8, 1941–1951. [Google Scholar] [CrossRef]
- Nicolae, S.A.; Au, H.; Modugno, P.; Luo, H.; Szego, A.E.; Qiao, M.; Li, L.; Yin, W.; Heeres, H.J.; Berge, N.D.; et al. Recent advances in hydrothermal carbonisation: From tailored carbon materials and biochemicals to applications and bioenergy. Green. Chem. 2020, 22, 4747–4800. [Google Scholar] [CrossRef]
- Kozarski, M.; Klaus, A.; Nikšić, M.; Van Griensven, L.J.L.D.; Vrvic, M.M.; Jakovljević, D. Polysaccharides of higher fungi: Biological role, structure, and antioxidative activity. Hemijska Industrija 2014, 68, 305–320. [Google Scholar] [CrossRef]
- Никoненкo, Н.А.; Buslov, D.K.; Sushko, N.I.; Zhbankov, R.G. Spectroscopic manifestation of stretching vibrations of glycosidic linkage in polysaccharides. J. Mol. Struct. 2005, 752, 20–24. [Google Scholar] [CrossRef]
- Nikonenko, N.A.; Buslov, D.K.; Sushko, N.I.; Zhbankov, R.G. Investigation of stretching vibrations of glycosidic linkages in disaccharides and polysaccharides with use of IR spectra deconvolution. Biopolym. Orig. Res. Biomol. 2000, 57, 257–262. [Google Scholar] [CrossRef]
- Thite, V.S.; Nerurkar, A.S. Valorization of sugarcane bagasse by chemical pretreatment and enzyme mediated deconstruction. Sci. Rep. 2019, 9. [Google Scholar] [CrossRef] [PubMed]
- Corrales, R.C.N.R.; De Souza Nogueira Sardinha Mendes, F.; Perrone, C.C.; Sant’Anna, C.; De Souza, W.; Abud, Y.; Bon, E.P.S.; Ferreira-Leitão, V.S. Structural evaluation of sugar cane bagasse steam pretreated in the presence of CO2 and SO2. Biotechnol. for Biofuels 2012, 5. [Google Scholar] [CrossRef] [PubMed]
- Ibbett, R.; Gaddipati, S.; Davies, S.M.; Hill, S.E.; Tucker, G.A. The mechanisms of hydrothermal deconstruction of lignocellulose: New insights from thermal–analytical and complementary studies. Bioresour. Technol. 2011, 102, 9272–9278. [Google Scholar] [CrossRef]
- Santín, C.; Doerr, S.H.; Merino, A.; Bucheli, T.D.; Bryant, R.; Ascough, P.; Gao, X.; Masiello, C.A. Carbon sequestration potential and physicochemical properties differ between wildfire charcoals and slow-pyrolysis biochars. Sci. Rep. 2017, 7, 11233. [Google Scholar] [CrossRef]




| Experiment | Temperature (°C) | Biomass-to-Water Ratio B:W | Particle Size (µm) |
|---|---|---|---|
| 1 | 220 | 1:10 | 106 |
| 2 | 260 | 1:10 | 106 |
| 3 | 220 | 1:50 | 106 |
| 4 | 260 | 1:50 | 106 |
| 5 | 220 | 1:10 | 212 |
| 6 | 260 | 1:10 | 212 |
| 7 | 220 | 1:50 | 212 |
| 8 | 260 | 1:50 | 212 |
| 9 | 220 | 1:10 | 600 |
| 10 | 260 | 1:10 | 600 |
| 11 | 220 | 1:50 | 600 |
| 12 | 260 | 1:50 | 600 |
| Ref. | Biomass | % Moisture | % Ash | % Volatile Matter | % Fixed Carbon |
|---|---|---|---|---|---|
| Sample | Sugarcane bagasse 1 | 7.910 ± 0.07 | 1.556 ± 0.02 | 80.93 ± 0.09 | 9.601 |
| [22] | Sugarcane straw 2 | 0.90 | 9.60 | 77.25 | 13.31 |
| [23] | Sugarcane bagasse 2 | 7.32 | 4.76 | 83 | 12.9 |
| [24] | Pennisetum pasture 2 | 8.17 | 10.73 | 73.44 | 15.83 |
| Ref. | Biomass | % C | % H | % N | % O | % S |
|---|---|---|---|---|---|---|
| Sample | Sugarcane bagasse 1 | 45.22 | 5.94 | 0.292 | 48.56 | 0 |
| [22] | Sugarcane straw 2 | 44.80 | 5.94 | 0.10 | 48.89 | 0.27 |
| [29] | Sugarcane bagasse 2 | 46.2 | 5.9 | 0.21 | 47.5 | - |
| [31] | Rice shell 2 | 40.82 | 5.25 | 0.38 | 53.38 | 0.17 |
| Ref. | Biomass | % Cellulose | % Hemicellulose | % Lignin |
|---|---|---|---|---|
| Sample | Sugarcane bagasse 1 | 61.4 | 23.6 | 8.1 |
| [33] | Sugarcane straw 2 | 50.81 | 20.36 | 9.18 |
| [32] | Sugarcane bagasse 2 | 45.28 | 22.13 | 22.39 |
| [27] | Sugarcane bagasse 2 | 35.28 | 33.25 | 25.20 |
| Experiment Number | Concentration (g/L) | % | ||||
|---|---|---|---|---|---|---|
| Carbohydrates | Formic Acid | Levulinic Acid | HMF | Furfural | Total Yield | |
| 1 | 0.685 | 4.854 | 9.306 | 2.014 | 2.547 | 18.253 |
| 2 | 0.334 | 6.326 | 9.413 | 0.007 | 0.000 | 15.654 |
| 3 | 0.115 | 1.274 | 0.597 | 0.559 | 0.661 | 15.880 |
| 4 | 0.148 | 2.724 | 0.780 | 0.000 | 0.000 | 17.729 |
| 5 | 0.316 | 8.348 | 3.635 | 1.277 | 0.941 | 13.926 |
| 6 | 0.347 | 7.894 | 3.838 | 0.000 | 0.000 | 12.082 |
| 7 | 0.232 | 2.991 | 0.758 | 0.970 | 0.884 | 31.072 |
| 8 | 0.157 | 4.18 | 0.983 | 0.025 | 0.000 | 13.463 |
| 9 | 0.820 | 6.049 | 3.531 | 3.051 | 2.600 | 14.028 |
| 10 | 0.301 | 7.920 | 3.961 | 0.000 | 0.705 | 12.247 |
| 11 | 0.222 | 1.657 | 0.718 | 0.812 | 0.714 | 19.857 |
| 12 | 0.069 | 2.309 | 0.791 | 0.000 | 0.000 | 15.608 |
| Experiment Number | % | O/C | ||||
|---|---|---|---|---|---|---|
| C | O | N | Other | Conversion by Weight of Solid Product | ||
| Biomass | 44.22 | 45.56 | 0.292 | 1.030 | ||
| 1 | 68.605 | 25.655 | 5.140 | Si = 0.39; F = 0.21 | 49.592 | 0.375 |
| 2 | 70.255 | 20.335 | 68.605 | Si = 4.76 | 59.132 | 0.297 |
| 3 | 61.965 | 33.165 | 4.420 | F = 0.45 | 52.921 | 0.536 |
| 4 | 77.565 | 15.975 | 6.465 | 72.187 | 0.206 | |
| 5 | 68.520 | 25.890 | 5.465 | Si = 0.12 | 56.712 | 0.380 |
| 6 | 74.920 | 10.730 | 3.650 | 58.490 | 0.142 | |
| 7 | 64.110 | 27.330 | 9.235 | 65.860 | 0.426 | |
| 8 | 74.310 | 19.540 | 6.150 | 85.851 | 0.263 | |
| 9 | 67.540 | 27.345 | 5.115 | 51.196 | 0.405 | |
| 10 | 74.180 | 19.570 | 6.250 | 62.759 | 0.264 | |
| 11 | 63.200 | 31.825 | 4.985 | 57.454 | 0.504 | |
| 12 | 75.865 | 17.910 | 6.225 | 74.213 | 0.236 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moreno-Chocontá, L.N.; Lozano-Pérez, A.S.; Guerrero-Fajardo, C.A. Evaluation of the Effect of Particle Size and Biomass-to-Water Ratio on the Hydrothermal Carbonization of Sugarcane Bagasse. ChemEngineering 2024, 8, 43. https://doi.org/10.3390/chemengineering8020043
Moreno-Chocontá LN, Lozano-Pérez AS, Guerrero-Fajardo CA. Evaluation of the Effect of Particle Size and Biomass-to-Water Ratio on the Hydrothermal Carbonization of Sugarcane Bagasse. ChemEngineering. 2024; 8(2):43. https://doi.org/10.3390/chemengineering8020043
Chicago/Turabian StyleMoreno-Chocontá, Leidy Natalia, Alejandra Sophia Lozano-Pérez, and Carlos Alberto Guerrero-Fajardo. 2024. "Evaluation of the Effect of Particle Size and Biomass-to-Water Ratio on the Hydrothermal Carbonization of Sugarcane Bagasse" ChemEngineering 8, no. 2: 43. https://doi.org/10.3390/chemengineering8020043
APA StyleMoreno-Chocontá, L. N., Lozano-Pérez, A. S., & Guerrero-Fajardo, C. A. (2024). Evaluation of the Effect of Particle Size and Biomass-to-Water Ratio on the Hydrothermal Carbonization of Sugarcane Bagasse. ChemEngineering, 8(2), 43. https://doi.org/10.3390/chemengineering8020043






