Determination of Environmental Flow Using a Holistic Methodology in Three River Paths in the Tempisque River Basin, Costa Rica
Abstract
1. Introduction
2. Materials and Methods
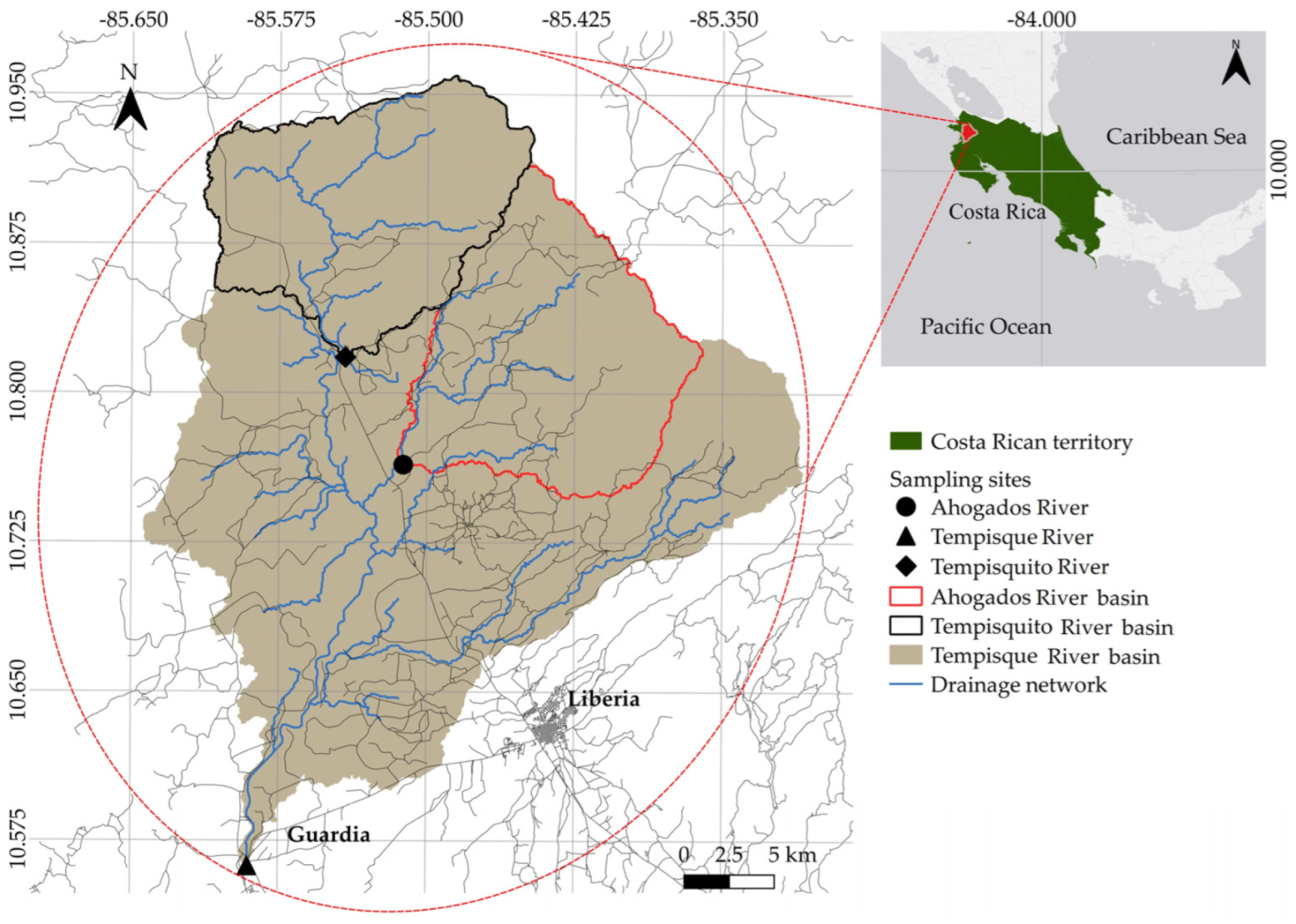
2.1. Hydrological Analysis
2.2. Selection and Analysis of Biological Indicators
2.3. Assessment of Regional Socioeconomic Factors
2.4. Establishment of the Environmental Flow Regime
3. Results
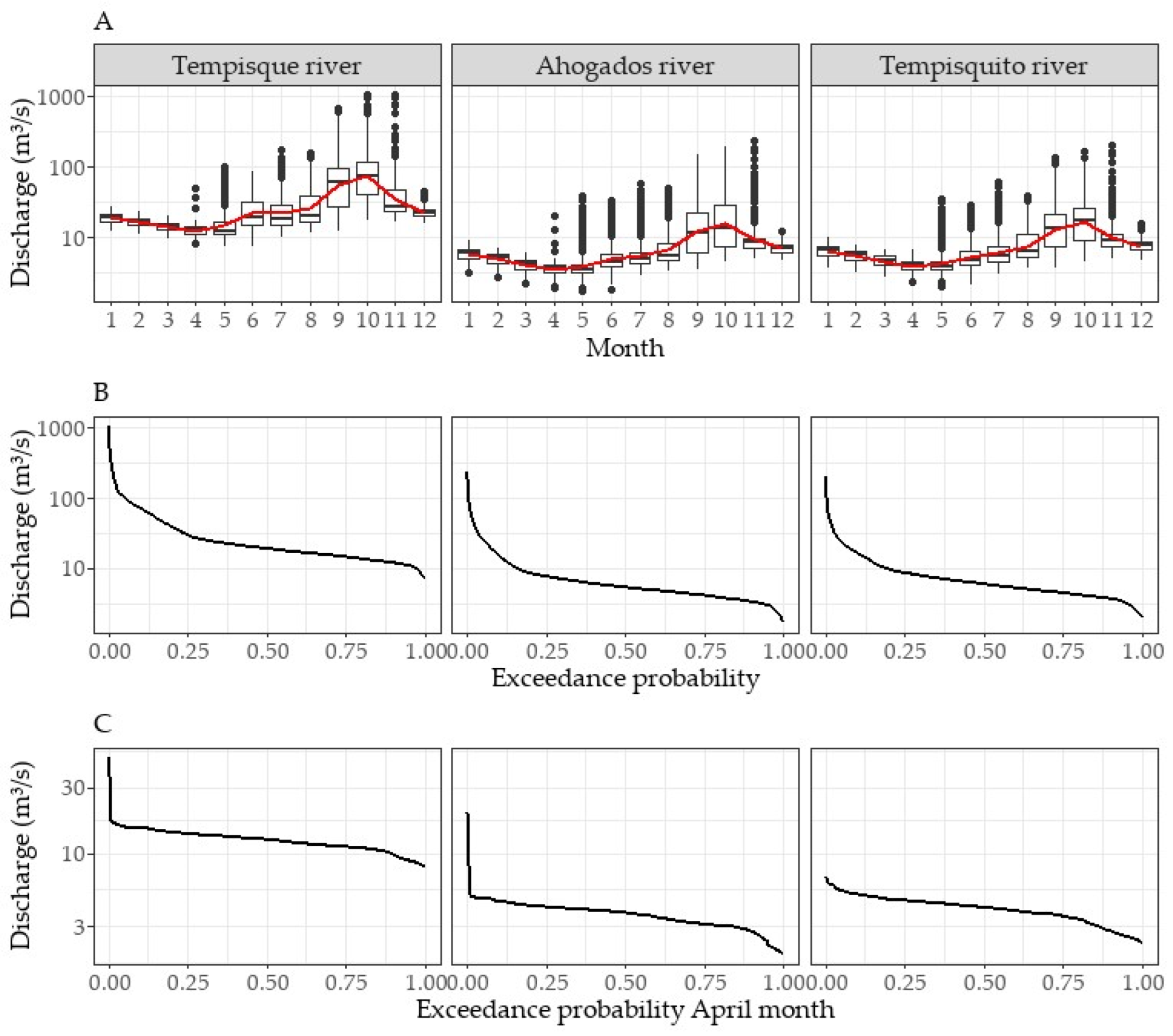
3.1. Hydrological Regime
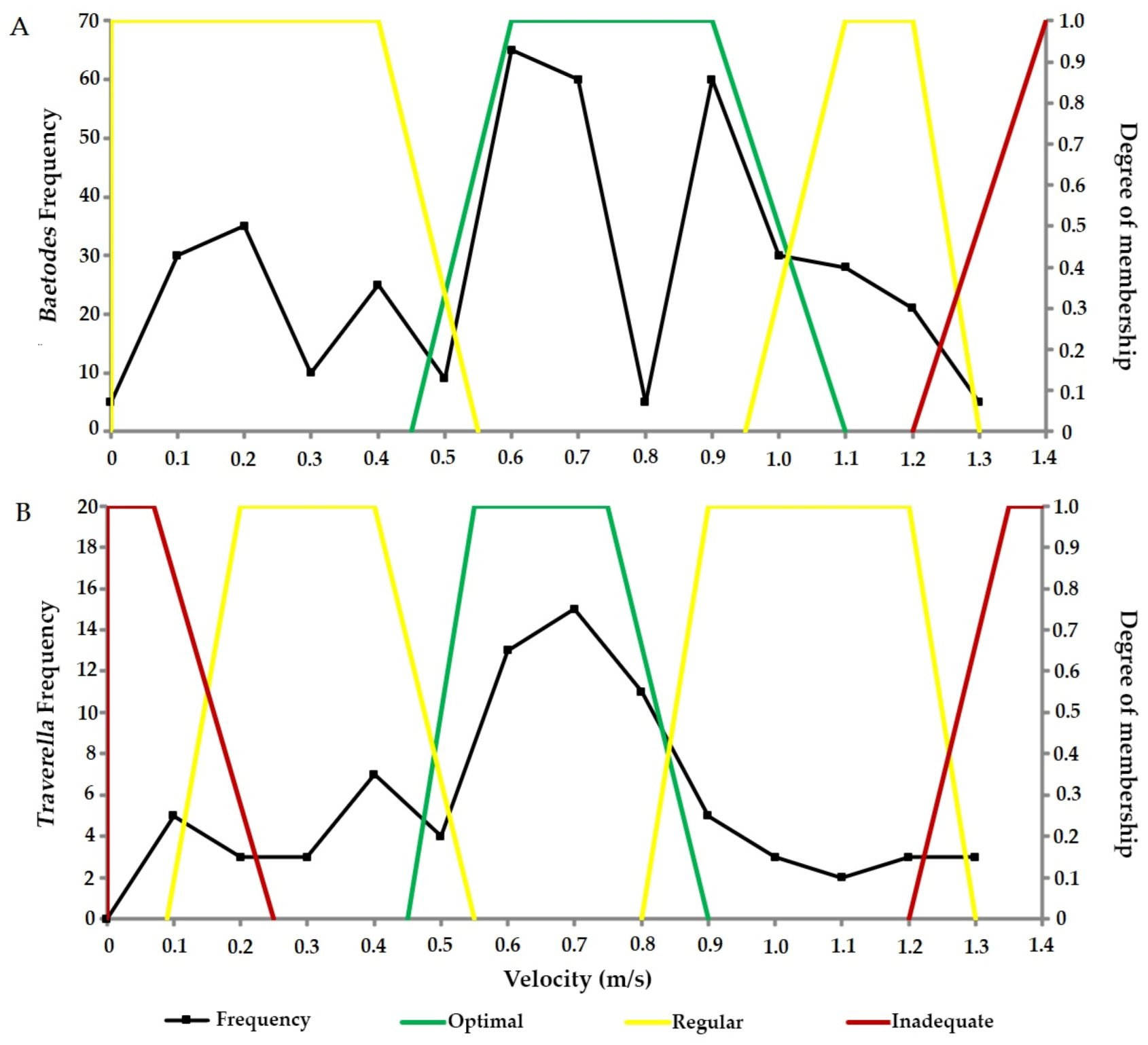
3.2. Biological Indicators for Environmental Flow Requirements
3.3. Socioeconomic Factors for Environmental Flow Requirements
3.4. Environmental Flow
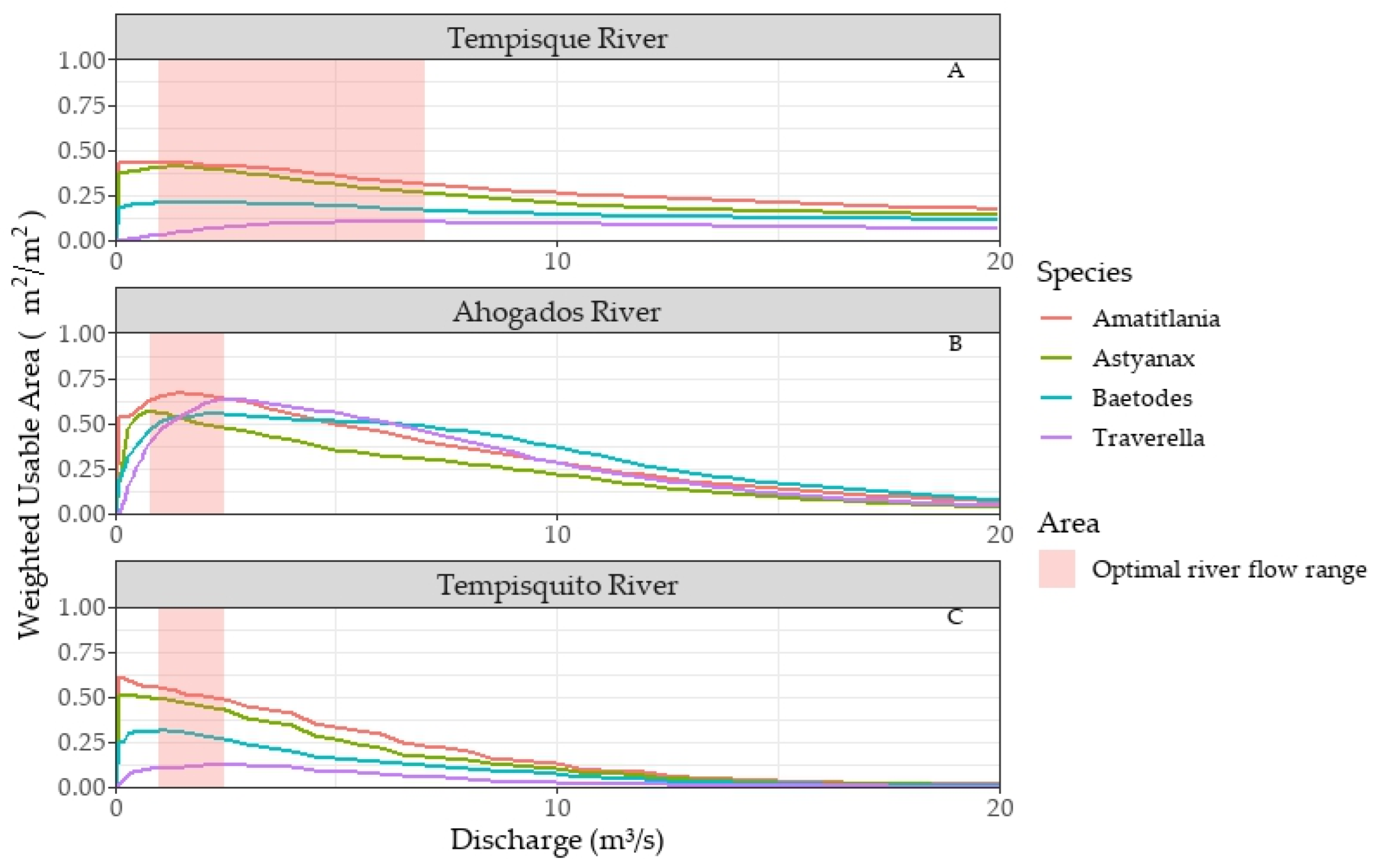
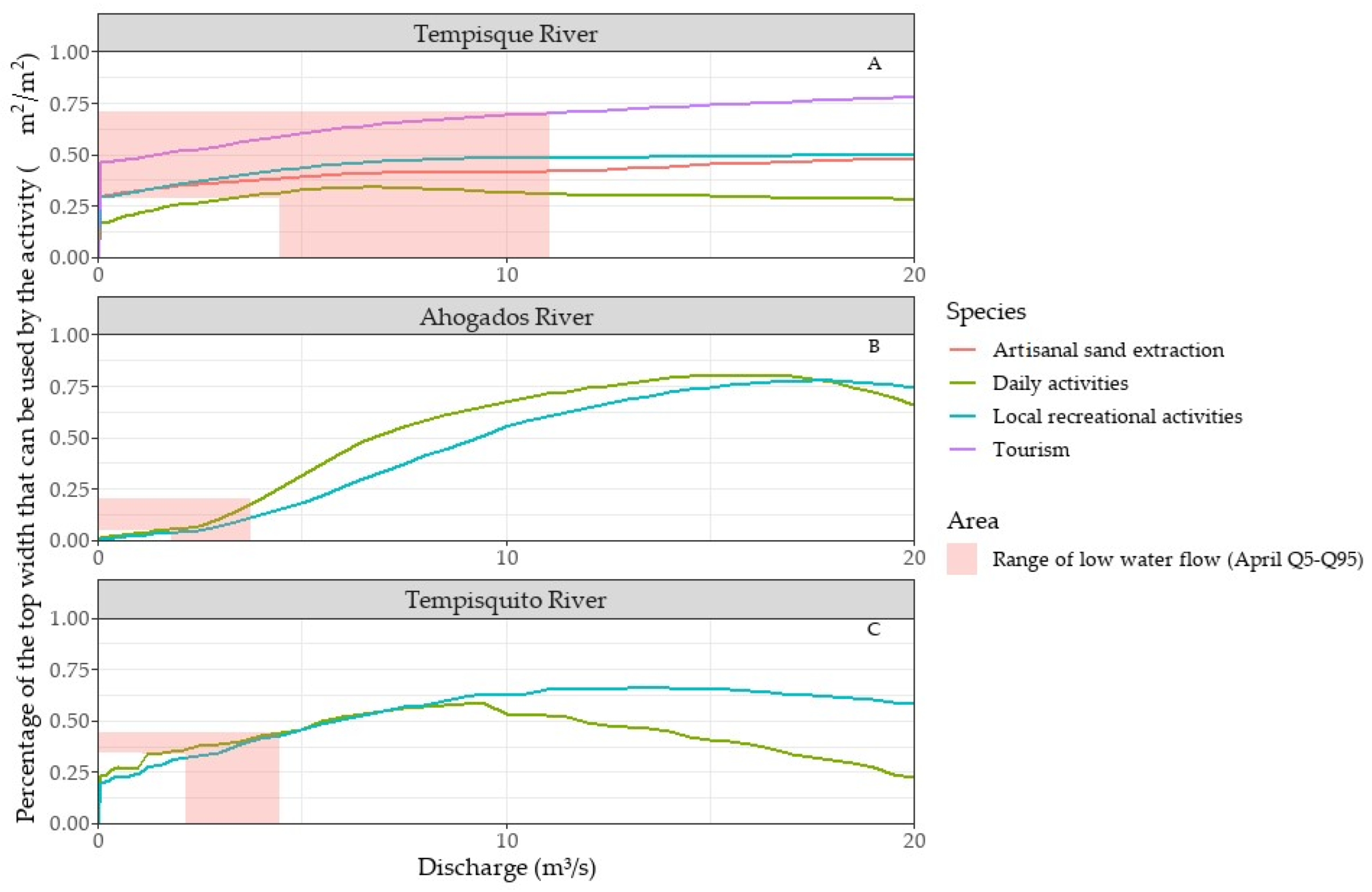
3.4.1. Weighted Usable Areas (WUAs)
3.4.2. Potentially Usable Areas (PUAs)
3.4.3. Environmental Flow Regime
4. Discussion
4.1. Biological Indicators
4.1.1. Aquatic Macroinvertebrates
4.1.2. Ichthyofauna
4.2. Socioeconomic Indicators
4.3. Environmental Flow
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sinnatamby, R.N.; Mayer, B.; Kruk, M.K.; Rood, S.B.; Farineau, A.; Post, J.R. Considering Multiple Anthropogenic Threats in the Context of Natural Variability: Ecological Processes in a Regulated Riverine Ecosystem. Ecohydrology 2020, 13, e2217. [Google Scholar] [CrossRef]
- Dudgeon, D. Multiple Threats Imperil Freshwater Biodiversity in the Anthropocene. Curr. Biol. 2019, 29, R960–R967. [Google Scholar] [CrossRef] [PubMed]
- Graham, P.M.; Pattinson, N.B.; Stassen, R.; Pike, T.; Hamidan, N.A.F. Using Environmental Flows to Inform Integrated Water Resource Management in Critically Water Scarce Regions. Ecohydrology 2024, e2705. [Google Scholar] [CrossRef]
- de Graaf, I.E.M.; Gleeson, T.; van Beek, L.P.H.; Sutanudjaja, E.H.; Bierkens, M.F.P. Environmental Flow Limits to Global Groundwater Pumping. Nature 2019, 574, 90–94. [Google Scholar] [CrossRef]
- Mott Lacroix, K.E.; Tapia, E.; Springer, A. Environmental Flows in the Desert Rivers of the United States and Mexico: Synthesis of Available Data and Gap Analysis. J. Arid. Environ. 2017, 140, 67–78. [Google Scholar] [CrossRef]
- Arthington, A.H.; Bhaduri, A.; Bunn, S.E.; Jackson, S.E.; Tharme, R.E.; Tickner, D.; Young, B.; Acreman, M.; Baker, N.; Capon, S.; et al. The Brisbane Declaration and Global Action Agenda on Environmental Flows (2018). Front. Environ. Sci. 2018, 6, 45. [Google Scholar] [CrossRef]
- Tharme, R.E. A Global Perspective on Environmental Flow Assessment: Emerging Trends in the Development and Application of Environmental Flow Methodologies for Rivers. River Res. Appl. 2003, 19, 397–441. [Google Scholar] [CrossRef]
- Atazadeh, E.; Barton, A.; Shirinpour, M.; Zarghami, M.; Rajabifard, A. River Management and Environmental Water Allocation in Regulated Ecosystems of Arid and Semi-Arid Regions—A Review. Fundam. Appl. Limnol. 2020, 193, 327–345. [Google Scholar] [CrossRef]
- Dourado, G.F.; Rallings, A.M.; Viers, J.H. Overcoming Persistent Challenges in Putting Environmental Flow Policy into Practice: A Systematic Review and Bibliometric Analysis. Environ. Res. Lett. 2023, 18, 043002. [Google Scholar] [CrossRef]
- Bhuiyan, C. Environmental Flows: Issues and Gaps—A Critical Analysis. Sustain. Sci. 2022, 17, 1109–1128. [Google Scholar] [CrossRef]
- Schmutz, S.; Sendzimir, J. Riverine Ecosystem Management, 1st ed.; Schmutz, S., Sendzimir, J., Eds.; Springer Cham: New York, NY, USA, 2018. [Google Scholar] [CrossRef]
- Ibáñez, C.; Caiola, N.; Belmar, O. Environmental Flows in the Lower Ebro River and Delta: Current Status and Guidelines for a Holistic Approach. Water 2020, 12, 2670. [Google Scholar] [CrossRef]
- Poff, N.L.; Tharme, R.E.; Arthington, A.H. Evolution of Environmental Flows Assessment Science, Principles, and Methodologies. In Water for the Environment; Elsevier Inc.: London, UK, 2017; pp. 203–236. [Google Scholar] [CrossRef]
- Mussehl, M.L.; Horne, A.C.; Webb, J.A.; Poff, N.L. Purposeful Stakeholder Engagement for Improved Environmental Flow Outcomes. Front. Environ. Sci. 2022, 9, 749864. [Google Scholar] [CrossRef]
- Laporte, S.A.D.Ĺ.; Pacheco, A.; Rodriguez, C.R. Estimation of Minimum Acceptable (Compensatory) Flow for the Rivers of Costa Rica. In Fifth FRIEND World Conference: Climate Variability and Change—Hydrological Impacts; IAHS Publications 308: Havana, Cuba, 2006; pp. 558–562. [Google Scholar]
- Chaves, A.; Krasovskaia, I.; Gottschalk, L. Environmental Demands for Sustainable Regulation Schemes in the Humid Tropics. In Fifth FRIEND World Conference: Climate Variability and Change—Hydrological Impacts; IAHS Publications 308: Havana, Cuba, 2006; pp. 569–572. [Google Scholar]
- González Leiva, J.A.; Segovia de González, J.V.; Cornejo Hernández, G.A.; Revelo Vidaurre, J.C.; Morán Villatoro, J.M. Idoneidad Del Hábitat de Amatitlania Nigrofasciata y Caudales Ecológicos Del Río Cara Sucia, El Salvador. Real. y Reflexión 2021, 54, 95–111. [Google Scholar] [CrossRef]
- Dunbar, M.J.; Alfredsen, K.; Harby, A. Hydraulic-habitat Modelling for Setting Environmental River Flow Needs for Salmonids. Fish. Manag. Ecol. 2012, 19, 500–517. [Google Scholar] [CrossRef]
- Im, D.; Choi, S.-U.; Choi, B. Physical Habitat Simulation for a Fish Community Using the ANFIS Method. Ecol. Inform. 2018, 43, 73–83. [Google Scholar] [CrossRef]
- Gebreegziabher, G.A.; Degefa, S.; Furi, W.; Legesse, G. Evolution and Concept of Environmental Flows (e-Flows): Meta-Analysis. Water Supply 2023, 23, 2466–2490. [Google Scholar] [CrossRef]
- Mateo-Vega, J. Características Generales de La Cuenca Del Río Tempisque. In La Cuenca del río Tempisque: Perspectivas para un Manejo Integrado; Jiménez, R.J.A., González, J.E., Eds.; Organización para Estudios Tropicales: San José, Costa Rica, 2001; pp. 32–72. [Google Scholar]
- Comité Regional de Recursos Hidráulicos (CRRH). Pacífico Norte. In El Clima, su Variabilidad y Cambio Climático en Costa Rica; Instituto Meteorológico Nacional (IMN): San José, Costa Rica, 2008. [Google Scholar]
- Dirección de Agua. Plan Nacional de Monitoreo de La Calidad de Los Cuerpos de Agua Superficiales; 2013. Available online: https://da.go.cr/monitoreo-de-calidad-del-recurso-hidrico/ (accessed on 17 September 2024).
- Renöfält, B.M.; Jansson, R.; Nilsson, C. Effects of Hydropower Generation and Opportunities for Environmental Flow Management in Swedish Riverine Ecosystems. Freshw. Biol. 2010, 55, 49–67. [Google Scholar] [CrossRef]
- Quesada-Alvarado, F.; Umaña-Villalobos, G.; Springer, M.; Picado-Barboza, J. Classification of Aquatic Macroinvertebrates in Flow Categories for the Adjustment of the LIFE Index to Costa Rican Rivers. Ecohydrol. Hydrobiol. 2021, 21, 368–376. [Google Scholar] [CrossRef]
- Bussing, W.A. Peces de Las Aguas Continentales de Costa Rica/Freshwater Fishes of Costa Rica, 2nd ed.; Editorial UCR: San José, Costa Rica, 2002. [Google Scholar]
- Noack, M.; Schneider, M.; Wieprecht, S. The Habitat Modelling System CASiMiR: A Multivariate Fuzzy Approach and Its Applications. In Ecohydraulics: An Integrated Approach; John Wiley & Sons, Ltd.: West Sussex, UK, 2013; pp. 75–91. [Google Scholar] [CrossRef]
- Serrano-Núñez, V.; Watson-Hernández, F.; Guzmán-Arias, I.; Chavarría-Pizarro, L.; Quesada-Alvarado, F. Correction of Empirical Equations Known as “Strickler-Type” for the Calculation of the Manning’s Roughness Coefficient for Costa Rica’s Northern Pacific Conditions. Hydrology 2022, 9, 71. [Google Scholar] [CrossRef]
- Bovee, K.D. A Guide to Stream Habitat Analysis Using the Instream Flow Incremental Methodology. IFIP No. 12; U.S. Fish and Wildlife Service: Washington, DC, USA, 1982. [Google Scholar]
- Sanz-Ramos, M.; Bladé, E.; Niñerola, D.; Palau-Ibars, A. Evaluación Numérico-Experimental Del Comportamiento Histérico Del Coeficiente de Rugosidad de Los Macrófitos. Ing. del Agua 2018, 22, 109–124. [Google Scholar] [CrossRef]
- Bustos-Vázquez, A.L.; Fernández Chévez, K.; Chacón Gutiérrez, K. Informe Caudal Ambiental, Metodología Holística En Cuenca Del Río Tempisque; Instituto Meteorológico Nacional: San José, Costa Rica, 2023. [Google Scholar]
- Jiménez, J.; Calvo, J.; Pizarro, F.; González, E. Conceptualización de Caudal Ambiental En Costa Rica: Determinación Inicial Para El Río Tempisque; UICN-Unión Mundial para la Naturaleza, Oficina Regional para Mesoamérica (UICN/ORMA): San José, Costa Rica, 2005; Volume 53. [Google Scholar]
- Brittain, J.E.; Saltveit, S.J. A Review of the Effect of River Regulation on Mayflies (Ephemeroptera). Regul. Rivers Res. Manag. 1989, 3, 191–204. [Google Scholar] [CrossRef]
- Da-Silva, E.R.; Nessimian, J.L.; Coelho, L.B.N. Leptophlebiidae Ocorrentes No Estado Do Rio de Janeiro, Brasil: Hábitats, Meso-Hábitats e Hábitos Das Ninfas (Insecta: Ephemeroptera). Biota Neotrop. 2010, 10, 87–93. [Google Scholar] [CrossRef]
- Sierra-Labastidas, T.K.; Tamaris-Turizo, C.E.; Reyes Picón, S.A.; Rueda-Delgado, G. Densidad, Biomasa y Hábitos Alimentarios de Anacroneuria Klapálek 1909 (Plecoptera: Perlidae) En Un Río Tropical. Actual. Biológicas 2018, 39, 66–74. [Google Scholar] [CrossRef][Green Version]
- Domínguez, E.; Encalada, A.; Fernández, H.R.; Giorgi, A.; Marchese, M.; Miserendino, M.L.; Munné, A.; Prat, N.; Ríos-Touma, B.; Rodrigues Capítulo, A. Biomonitoreo En Ríos de La Argentina: Un Camino Por Recorrer. Ecol. Austral 2021, 32, 229–244. [Google Scholar] [CrossRef]
- Silveira, M.P.; Buss, D.F.; Nessimian, J.L.; Baptista, D.F. Spatial and Temporal Distribution of Benthic Macroinvertebrates in a Southeastern Brazilian River. Braz. J. Biol. 2006, 66, 623–632. [Google Scholar] [CrossRef]
- Mackie, J.K.; Chester, E.T.; Matthews, T.G.; Robson, B.J. Macroinvertebrate Response to Environmental Flows in Headwater Streams in Western Victoria, Australia. Ecol. Eng. 2013, 53, 100–105. [Google Scholar] [CrossRef]
- Castillo Sánchez, K.; Aguirre, Y.; Ríos González, T.; BERNAL VEGA, J.A. Anacroneuria (Plecoptera: Perlidae) Del Río Caldera, Chiriquí, Panamá, Con Nuevos Registros de Distribución, y Comentarios Sobre Distribución Altitudinal y Variación Estacional. Rev. Biol. Trop. 2017, 66, 164–177. [Google Scholar] [CrossRef]
- Dewson, Z.S.; James, A.B.W.; Death, R.G. A Review of the Consequences of Decreased Flow for Instream Habitat and Macroinvertebrates. J. N. Am. Benthol. Soc. 2007, 26, 401–415. [Google Scholar] [CrossRef]
- McIntosh, M.D.; Benbow, M.E.; Burky, A.J. Effects of Stream Diversion on Riffle Macroinvertebrate Communities in a Maui, Hawaii, Stream. River Res. Appl. 2002, 18, 569–581. [Google Scholar] [CrossRef]
- Kinzie, R.A.; Chong, C.; Devrell, J.; Lindstrom, D.; Wolff, R.H. Effects of Water Removal on a Hawaiian Stream Ecosystem. Pac. Sci. 2006, 60, 1–47. [Google Scholar] [CrossRef]
- Vásquez, D.; Flowers, R.W.; Springer, M. Life History of Five Small Minnow Mayflies (Ephemeroptera: Baetidae) in a Small Tropical Stream on the Caribbean Slope of Costa Rica. Aquat. Insects 2009, 31 (Suppl. S1), 319–332. [Google Scholar] [CrossRef]
- Parasiewicz, P.; Dunbar, M.J. Physical Habitat Modelling for Fish—A Developing Approach. River Syst. 2001, 12, 239–268. [Google Scholar] [CrossRef]
- Leal, C.G.; Junqueira, N.T.; Pompeu, P.S. Morphology and Habitat Use by Fishes of the Rio Das Velhas Basin in Southeastern Brazil. Environ. Biol. Fishes 2011, 90, 143–157. [Google Scholar] [CrossRef]
- Quesada-Alvarado, F.; Campos-Calderón, F. Morphometry and Burst Swimming in Six Continental Fish Species from Costa Rica. UNED Res. J. 2019, 11, 395–402. [Google Scholar] [CrossRef]
- Kim, S.K.; Choi, S.-U. Prediction of Suitable Feeding Habitat for Fishes in a Stream Using Physical Habitat Simulations. Ecol. Model. 2018, 385, 65–77. [Google Scholar] [CrossRef]
- Bussing, W.A. Peces de Las Aguas Continentales de Costa Rica, 1st ed.; Editorial UCR: San José, Costa Rica, 1998. [Google Scholar]
- Cortes, R.M.V.; Ferreira, M.T.; Oliveira, S.V.; Oliveira, D. Macroinvertebrate Community Structure in a Regulated River Segment with Different Flow Conditions. River Res. Appl. 2002, 18, 367–382. [Google Scholar] [CrossRef]
- Carvajal Vanegas, D. Dinámica Hídrica Bajo Condiciones Climáticas Cambiantes En La Subcuenca Del Río Tempisquito, Cuenca Del Tempisque, Guanacaste, Costa Rica; Centro Agronómico Tropical de Investigación y Enseñanza (CATIE): Turrialba, Costa Rica, 2017. [Google Scholar]
- Harris, A.; Porter, M.; McKay, S.K.; Mulchandani, A.; Stone, M. Hydraulic Analysis for Assessing Environmental Flow Selection and Ecological Model Formulation. Ecohydrology 2024, 17, e2681. [Google Scholar] [CrossRef]
- Karimi, S.; Salarijazi, M.; Ghorbani, K.; Heydari, M. Comparative Assessment of Environmental Flow Using Hydrological Methods of Low Flow Indexes, Smakhtin, Tennant and Flow Duration Curve. Acta Geophys. 2021, 69, 285–293. [Google Scholar] [CrossRef]
- Suwal, N.; Kuriqi, A.; Huang, X.; Delgado, J.; Młyński, D.; Walega, A. Environmental Flows Assessment in Nepal: The Case of Kaligandaki River. Sustainability 2020, 12, 8766. [Google Scholar] [CrossRef]
- Calvo Alvarado, J.; Jiménez, A.J.A.; González, E.; Pizarro, F.; Jiménez, A. Determinación Preliminar Del Caudal Ambiental En El Río Tempisque, Costa Rica: El Enfoque Hidrológico Con Limitación de Datos. Rev. For. Mesoam. Kurú 2008, 5, 1–18. [Google Scholar]
- Extence, C.A.; Balbi, D.M.; Chadd, R.P. River Flow Indexing Using British Benthic Macroinvertebrates: A Framework for Setting Hydroecological Objectives. Regul. Rivers Res. Manag. 1999, 15, 545–574. [Google Scholar] [CrossRef]
- Dunbar, M.J.; Pedersen, M.L.; Cadman, D.; Extence, C.; Waddingham, J.; Chadd, R.; Larsen, S.E. River Discharge and Local-scale Physical Habitat Influence Macroinvertebrate LIFE Scores. Freshw. Biol. 2010, 55, 226–242. [Google Scholar] [CrossRef]
- Worrall, T.P.; Dunbar, M.J.; Extence, C.A.; Laizé, C.L.R.; Monk, W.A.; Wood, P.J. The Identification of Hydrological Indices for the Characterization of Macroinvertebrate Community Response to Flow Regime Variability. Hydrol. Sci. J. 2014, 59, 645–658. [Google Scholar] [CrossRef]


| Habitat | Velocity (m/s) | Depth (m) | ||||||
|---|---|---|---|---|---|---|---|---|
| Baetodes | Traverella | Astyanax | Amatitlania | Baetodes | Traverella | Astyanax | Amatitlania | |
| Inadequate | [0.00–0.04[ | [0.00–0.05[ | [0.00–0.04[ | |||||
| Regular | [0.00–0.44[ | [0.04–0.10[ | [0.05–0.10[ | [0.04–0.10[ | [0.00–0.10[ | [0.00–0.20[ | ||
| Optimal | [0.00–1.10[ | [0.10–0.40[ | [0.00–0.40[ | [0.00–0.55[ | [0.10–0.30[ | [0.10–0.40[ | [0.10–0.40[ | [0.20–0.50[ |
| Regular | [1.10–1.30[ | [0.40–0.60[ | [0.40–1.00[ | [0.55–1.30[ | [0.30–0.69[ | [0.40–0.60[ | [0.40–0.70[ | [0.50–0.70[ |
| Inadequate | ≥1.30 | ≥0.60 | ≥1.00 | ≥1.30 | ≥0.69 | ≥0.60 | ≥0.70 | ≥0.70 |
| Activities | Water Depth Requirement Relative Distribution (%) | |||
|---|---|---|---|---|
| 132 cm | 78 cm | 45 cm | ||
| High | Medium | Low | Total | |
| Tourism | 44.50 | 39.00 | 16.50 | 100.00 |
| Agriculture (irrigation for intensive crops) | 77.80 | 15.00 | 7.20 | 100.00 |
| Mechanized sand mining | 36.70 | 28.80 | 34.50 | 100.00 |
| Artisanal sand mining | 20.50 | 29.20 | 50.30 | 100.00 |
| Local recreation | 18.50 | 56.00 | 25.50 | 100.00 |
| Daily activities | 7.20 | 44.30 | 48.50 | 100.00 |
| Self-consumption fishing | 28.50 | 46.00 | 25.50 | 100.00 |
| Component | Flow Range (m3/s) | |||||
|---|---|---|---|---|---|---|
| Ahogados River | Tempisquito River | Tempisque River (Guardia) | ||||
| Lower Limit | Upper Limit | Lower Limit | Upper Limit | Lower Limit | Upper Limit | |
| Biological | 0.80 | 2.50 | 1.00 | 2.50 | 1.00 | 7.00 |
| Socio-economic | 1.82 | 3.75 | 2.17 | 4.47 | 4.46 | 11.08 |
| Intersection | 1.82 | 2.50 | 2.17 | 2.50 | 4.46 | 7.00 |
| Month | Flow Regime (m3/s) | |||||
|---|---|---|---|---|---|---|
| Ahogados River | Tempisquito River | Tempisque River (Guardia) | ||||
| Lower Limit | Upper Limit | Lower Limit | Upper Limit | Lower Limit | Upper Limit | |
| January | 2.82 | 3.79 | 3.39 | 3.81 | 8.24 | 14.18 |
| February | 2.40 | 3.27 | 2.91 | 3.28 | 5.64 | 10.98 |
| March | 2.13 | 2.84 | 2.54 | 2.83 | 4.63 | 7.96 |
| April | 1.82 | 2.50 | 2.17 | 2.50 | 4.46 | 7.00 |
| May | 1.57 | 2.29 | 1.90 | 2.51 | 4.51 | 7.95 |
| June | 2.20 | 3.03 | 2.75 | 3.26 | 7.11 | 14.07 |
| July | 2.87 | 3.09 | 3.29 | 3.47 | 9.63 | 15.57 |
| August | 3.23 | 3.75 | 3.33 | 3.93 | 10.68 | 17.17 |
| September | 3.81 | 5.00 | 4.13 | 5.21 | 10.38 | 24.36 |
| October | 5.29 | 7.55 | 5.55 | 7.95 | 12.88 | 31.55 |
| November | 4.78 | 5.07 | 4.64 | 5.39 | 11.98 | 25.26 |
| December | 4.34 | 4.52 | 4.19 | 4.72 | 11.38 | 18.27 |
| Discharge Exceedance Probability (%) | 94.90 | 88.42 | 94.74 | 89.12 | 95.00 | 63.86 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chavarría-Pizarro, L.; Watson-Hernández, F.; Quesada-Alvarado, F.; Serrano-Núñez, V.; Bustos-Vásquez, A.L.; Fernández-Chévez, K.; Chacón-Gutierrez, J.; Guzmán-Arias, I. Determination of Environmental Flow Using a Holistic Methodology in Three River Paths in the Tempisque River Basin, Costa Rica. Hydrology 2024, 11, 159. https://doi.org/10.3390/hydrology11100159
Chavarría-Pizarro L, Watson-Hernández F, Quesada-Alvarado F, Serrano-Núñez V, Bustos-Vásquez AL, Fernández-Chévez K, Chacón-Gutierrez J, Guzmán-Arias I. Determination of Environmental Flow Using a Holistic Methodology in Three River Paths in the Tempisque River Basin, Costa Rica. Hydrology. 2024; 11(10):159. https://doi.org/10.3390/hydrology11100159
Chicago/Turabian StyleChavarría-Pizarro, Laura, Fernando Watson-Hernández, Francisco Quesada-Alvarado, Valeria Serrano-Núñez, Ana Lucía Bustos-Vásquez, Karina Fernández-Chévez, Jendry Chacón-Gutierrez, and Isabel Guzmán-Arias. 2024. "Determination of Environmental Flow Using a Holistic Methodology in Three River Paths in the Tempisque River Basin, Costa Rica" Hydrology 11, no. 10: 159. https://doi.org/10.3390/hydrology11100159
APA StyleChavarría-Pizarro, L., Watson-Hernández, F., Quesada-Alvarado, F., Serrano-Núñez, V., Bustos-Vásquez, A. L., Fernández-Chévez, K., Chacón-Gutierrez, J., & Guzmán-Arias, I. (2024). Determination of Environmental Flow Using a Holistic Methodology in Three River Paths in the Tempisque River Basin, Costa Rica. Hydrology, 11(10), 159. https://doi.org/10.3390/hydrology11100159













