Assessment and Mitigation of Groundwater Contamination from Phosphate Mining in Tunisia: Geochemical and Radiological Analysis
Abstract
:1. Introduction
2. Study Area
3. Socioeconomic of the Study Area
4. Lithological Proprieties
- 1-
- Alluvial sands and gravel (riverbed) are characterized by homogeneous fine-to-medium sands intercalated by laminates of silts, with a predominance of silt and clay (approximately between 60 and 66%), and a thickness of 0.5 to 2.5 m;
- 2-
- Limestones are characterized by carbonate and dolomite (fractured and karstified rocks) with a thickness of 2 to 50 m.
- 1-
- Aeolian deposits are mainly composed of fine–medium-grained sands, with a predominance of silts (55–65%) and clays (15%), and have a low level of organic matter. They have a variable thickness from one region to another, ranging from 0.5 to 2 m. Over the last ten years, this thickness has increased year by year, essentially due to the phenomenon of continuous desertification driven by sandstorms from south to north;
- 2-
- Alluvial sands (riverbeds) are characterized by homogeneous fine-to-medium sands intercalated by laminates of silts, with a predominance of silts (62–64%) and clays (approximately 16%), with a thickness ranging from 2 to 3 m.
5. Hydrology and Hydrogeology Setting
- 1-
- Alluvial aquifer, Pliocene, and Pleistocene–Holocene sands (shallow aquifer);
- 2-
- Mio-Plio-Quaternary aquifer (semi-confined aquifer);
- 3-
- Upper Cretaceous limestone/dolomite (semi-confined aquifer);
- 4-
- Lower Cretaceous sand (confined aquifer), contains fossil water (40–45 million years) and geothermal water (35–92 °C).
6. Material and Methods
7. Radiochemical Determination Methods
7.1. Determination of Po and Th
7.2. Determination of U
7.3. Radioactivity Measurements of Ra
8. Results and Discussions
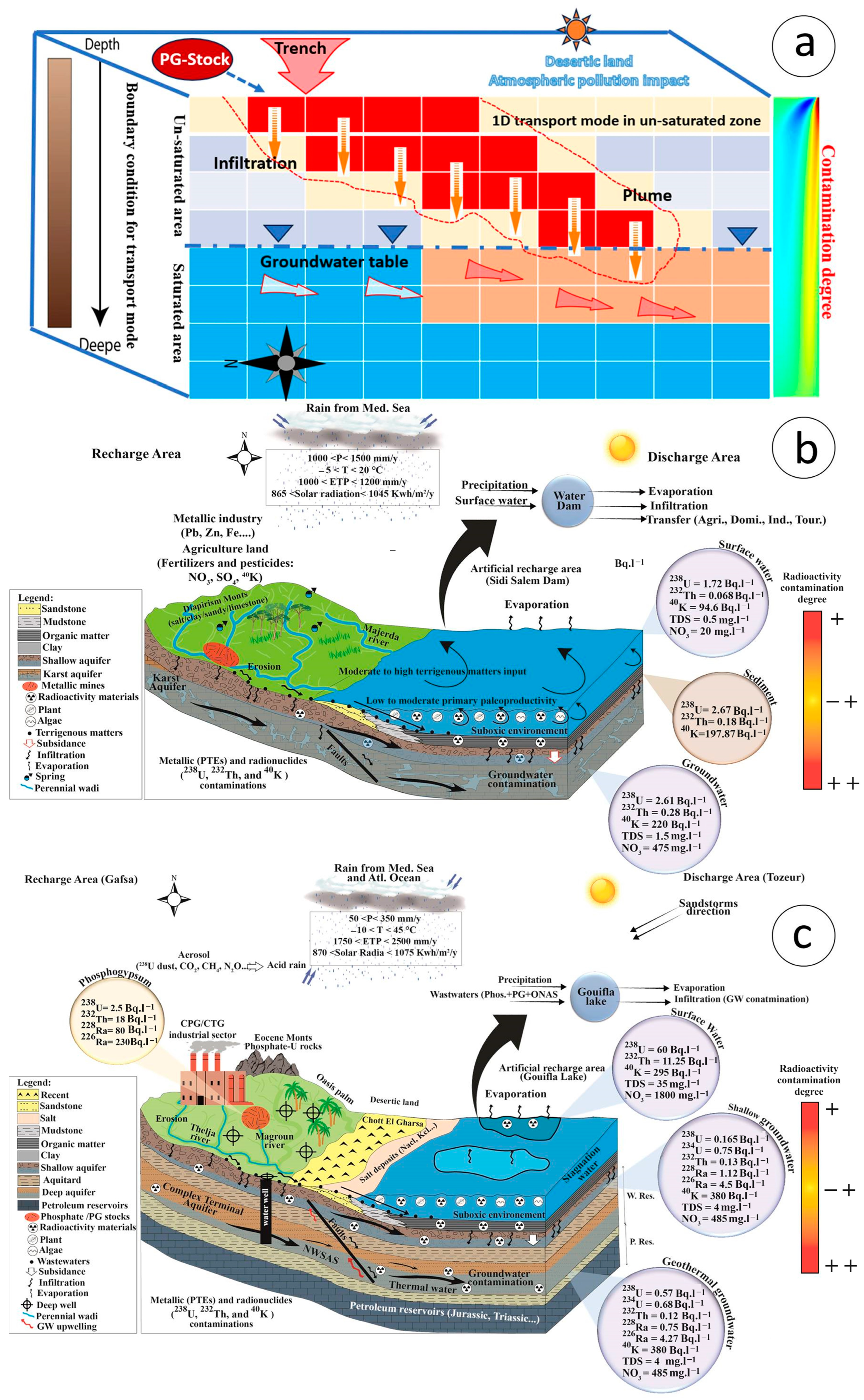
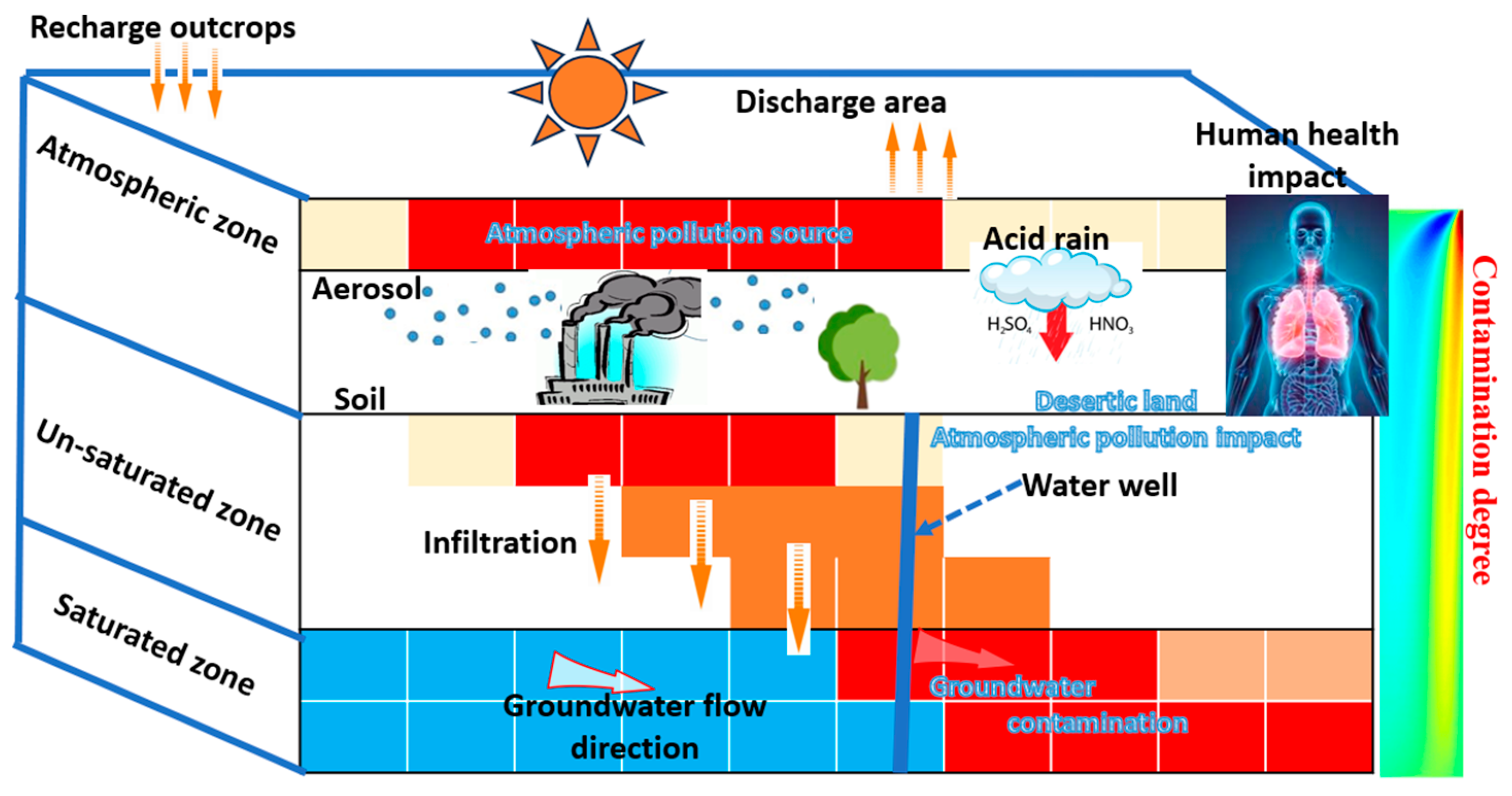
8.1. Radionuclides Transfer Mode and Environmental Impact
8.1.1. Environmental Contamination Measurements
8.1.2. Gamma Spectrometry Data
8.1.3. Mathematical Modeling of Radionuclide Transport
8.1.4. Modeling Approach and Findings
8.1.5. Radionuclide Distribution Patterns
8.1.6. Health and Ecological Risk Assessment
8.2. Groundwater Contamination
8.2.1. Northern Transboundary Part (Tuniso-Algerian Basin)
8.2.2. Southern Transboundary Part (Tuniso-Algerian Basin)
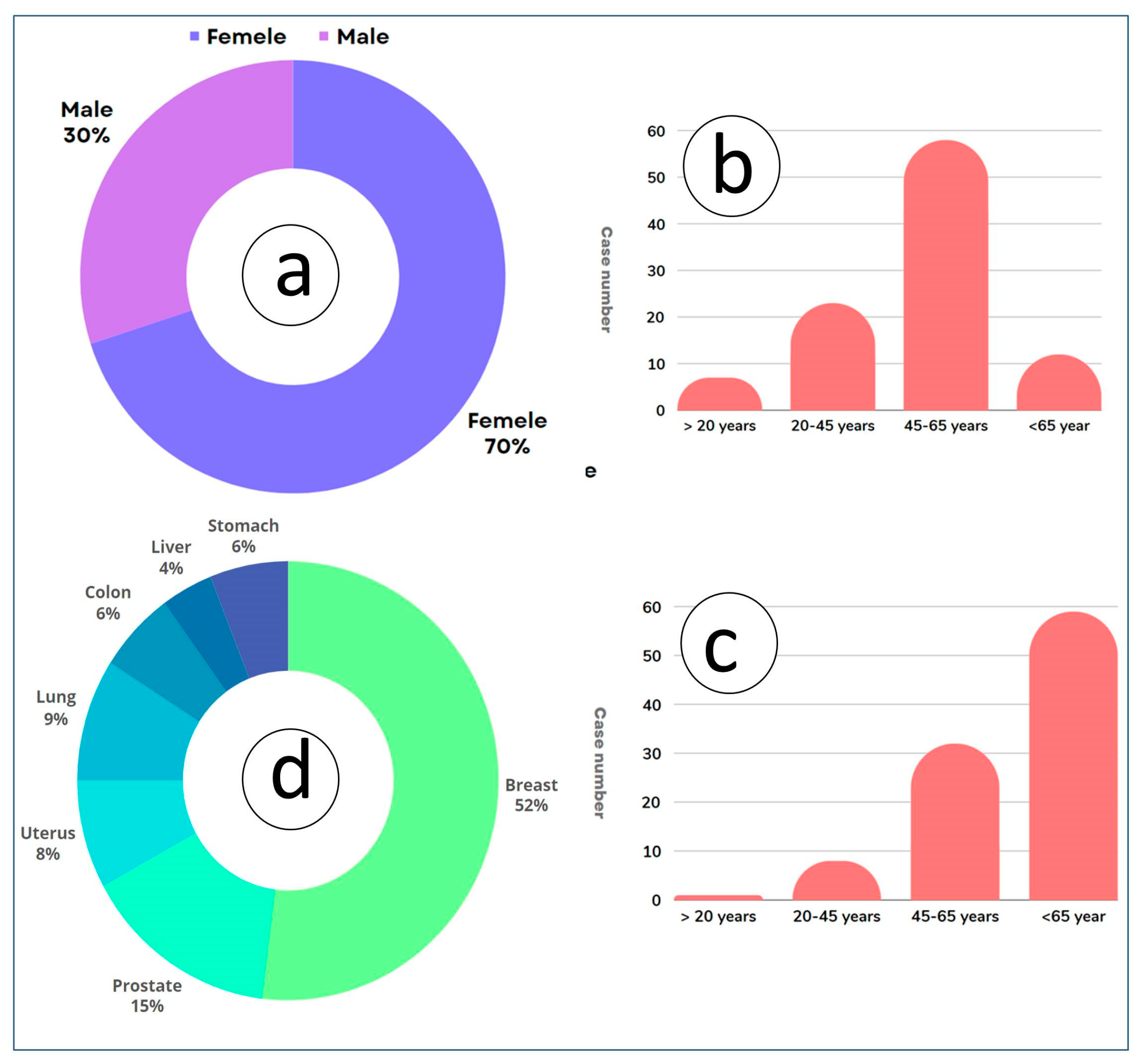
8.3. Human Health Impacts
9. Conclusions and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Beitz, H.; Schmidt, H.; Herzel, F. Occurrence, Toxicological and Ecotoxicological Significance of Pesticides in Groundwater and Surface Water. In Pesticides in Ground and Surface Water; Springer: Berlin/Heidelberg, Germany, 1994; pp. 1–56. [Google Scholar] [CrossRef]
- Balbus, J.M.; Boxall, A.B.A.; Fenske, R.A.; McKone, T.E.; Zeise, L. Implications of global climate change for the assessment and management of human health risks of chemicals in the natural environment. Environ. Toxicol. Chem. 2013, 32, 62–78. [Google Scholar] [CrossRef] [PubMed]
- El Gayar, A.; Hamed, Y. Climate change and water resources management in Arab countries. Springer international publishing AG-Euro-Mediterranean and surrounding regions. In Advances in Science, Technology & Innovation; Springer International Publishing AG: Cham, Switzerland, 2017. [Google Scholar]
- Khleifia, N.; Hannachi, A.; Abbes, N. Studies of uranium recovery from Tunisian wet process phosphoric acid. Int. J. Innov. Appl. Stud. 2013, 3, 1066–1071. [Google Scholar]
- Ayadi, Y.; Mokadem, N.; Besser, H.; Khelifi, F.; Harabi, S.; Hamad, A.; Boyce, A.; Laouar, R.; Hamed, Y. Hydrochemistry and stable isotopes (δ18O and δ2H) tools applied to the study of karst aquifers in Southern Mediterranean basin (Teboursouk area, NW Tunisia). J. Afr. Earth Sci. 2017, 137, 208–217. [Google Scholar] [CrossRef]
- Latifa, A.Y.; Lhoussaine, B.; Etienne, J.; Moussa, M.; Yassine, A.B.; Ahmed, E.M.; Jana, S.; Barbara, R. Impact of rock-water interactions and recharge on water resources quality of the Agadir-Essaouira basin, southwestern Morocco. Arab. J. Geosci. 2017, 10, 169. [Google Scholar] [CrossRef]
- Ayadi, Y.; Mokadem, N.; Besser, N.; Redhaounia, B.; Khelifi, F.; Harabi, S.; Nasri, T.; Hamed, Y. Statistical and geochemical assessment of groundwater quality in Teboursouk area (Northwestern Tunisian Atlas). Environ. Earth Sci. 2018, 77, 349. [Google Scholar] [CrossRef]
- Khelifi, F.; Besser, H.; Ayadi, Y.; Liu, G.; Yousaf, B.; Harabi, S.; Bedoui, S.; Zeghmi, K.; Hamed, Y. Evaluation of potentially toxic elements’ (PTEs) vertical distribution in sediments of Gafsa–Metlaoui mining basin (Southwestern Tunisia) using geochemical and multivariate. Environ. Earth Sci. 2019, 78, 53. [Google Scholar] [CrossRef]
- Khelifi, F.; Caporale, A.G.; Hamed, Y.; Adamo, P. Bioaccessibility of potentially toxic metals in soil, sediments and tailings from a north Africa phosphate-mining area: Insight into human health risk assessment. J. Environ. Manag. 2021, 279, 111634. [Google Scholar] [CrossRef] [PubMed]
- Khelifi, F.; Mokadem, N.; Liu, G.; Yousaf, B.; Zhou, H.; Ncibi, K.; Hamed, Y. Occurrence, contamination evaluation and health risks of trace metals within soil, sediments and tailings in southern Tunisia. Int. J. Environ. Sci. Technol. 2021, 19, 6127–6140. [Google Scholar] [CrossRef]
- Hamed, Y.; Khelifi, F.; Houda, B.; Ben Saad, A.; Ncibi, K.; Hadji, R.; Melki, A. Phosphate mining pollution in southern Tunisia: Environmental, epidemiological, and socioeconomic investigation. Environ. Dev. Sustain. 2023, 25, 13619–13636. [Google Scholar] [CrossRef]
- Hamed, Y.; Ayadi, Y.; Hadji, R.; Ben Saad, A.; Gentilucci, M.; Elaloui, E. Environmental Radioactivity, Ecotoxicology (238U, 232Th and 40K) and Potentially Toxic Elements in Water and Sediments from North Africa Dams. Sustainability 2024, 16, 490. [Google Scholar] [CrossRef]
- Hassan, N.M.; Mansour, N.A.; Fayez-Hassan, M.; Sedqy, E. Assessment of natural radioactivity in fertilizers and phosphate ores in Egypt. J. Taibah Univ. Sci. 2016, 10, 296–306. [Google Scholar] [CrossRef]
- Boumala, D.; Mavon, C.; Belafrites, A.; Tedjani, A.; Groetz, J.E. Evaluation of radionuclide concentrations and external gamma radiation levels in phosphate ores and fertilizers commonly used in Algeria. J. Radioanal. Nucl. Chem. 2018, 317, 501–510. [Google Scholar] [CrossRef]
- Besser, H.; Hamed, Y. Environmental impacts of land management on the sustainability of natural resources in Oriental Erg Tunisia, North Africa. Environ. Dev. Sustain. 2021, 23, 11677–11705. [Google Scholar] [CrossRef]
- Zhang, F.; Wang, Y.; Liao, X. Recognition method for the health risks of potentially toxic elements in a headwater catchment. Sci. Total Environ. 2022, 839, 156287. [Google Scholar] [CrossRef] [PubMed]
- Arabi, A.M.; El Ahmed, N.K.; Din, K.S. Natural radionuclides and dose estimation in natural water resources from Elba protective area, Egypt. Radiat. Prot. Dosim. 2006, 121, 284–292. [Google Scholar] [CrossRef] [PubMed]
- Benmarce, K.; Hadji, R.; Zahri, F.; Khanchoul, K.; Chouabi, A.; Zighmi, K.; Hamed, Y. Hydrochemical and geothermometry characterization for a geothermal system in semiarid dry climate: The case study of Hamma spring (Northeast Algeria). J. Afr. Earth Sci. 2021, 182, 104285. [Google Scholar] [CrossRef]
- Dragović, S.D.; Janković-Mandić, L.J.; Dragović, R.M.; Đorđević, M.M.; Đokić, M.M. Spatial distribution of the 226Ra activity concentrations in well and spring waters in Serbia and their relation to geological formations. J. Geochem. Explor. 2012, 112, 206–211. [Google Scholar] [CrossRef]
- Scanlon, B.R.; Faunt, C.C.; Longuevergne, L.; Reedy, R.C.; Alley, W.M.; McGuire, V.L.; McMahon, P.B. Groundwater depletion and sustainability of irrigation in the US High Plains and Central Valley. Proc. Natl. Acad. Sci. USA 2012, 109, 9320–9325. [Google Scholar] [CrossRef]
- Feng, W.; Zhong, M.; Lemoine, J.M.; Biancale, R.; Hsu, H.T.; Xia, J. Evaluation of groundwater depletion in North China using the Gravity Recovery and Climate Experiment (GRACE) data and ground-based measurements. Water Resour. Res. 2013, 49, 2110–2118. [Google Scholar] [CrossRef]
- Famiglietti, J.S. The global groundwater crisis. Nat. Clim. Chang. 2014, 4, 945–948. [Google Scholar] [CrossRef]
- Hamed, Y.; Ahmadi, R.; Demdoum, A.; Bouri, S.; Gargouri, I.; Ben Dhia, H.; Al-Gamal, S.; Laouar, R.; Choura, A. Use of geochemical, isotopic, and age tracer data to develop models of groundwater flow: A case study of Gafsa mining basin-Southern Tunisia. J. Afr. Earth Sci. 2014, 100, 418–436. [Google Scholar] [CrossRef]
- Hamed, Y.; Hadji, R.; Redhaounia, B.; Zighmi, K.; Bâali, F.; El Gayar, A. Climate impact on surface and groundwater in North Africa: A global synthesis of findings and recommendations. Euro-Mediterr. J. Environ. Integr. 2018, 3, 25. [Google Scholar] [CrossRef]
- Post, V.E.A.; Vassolo, S.I.; Tiberghien, C.; Baranyikwa, D.; Miburo, D. Weathering and evaporation controls on dissolved uranium concentrations in groundwater—A case study from northern Burundi. Sci. Total Environ. 2017, 607–608, 281–293. [Google Scholar] [CrossRef] [PubMed]
- Hadji, R.; Raïs, K.; Gadri, L.; Chouabi, A.; Hamed, Y. Slope failures characteristics and slope movement susceptibility assessment using GIS in a medium scale: A case study from OuledDriss and Machroha municipalities, Northeastern of Algeria. Arab. J. Sci. Eng. 2017, 42, 281–300. [Google Scholar] [CrossRef]
- Arif, K.; Khalid, P. Hydrostratigraphy and hydrogeophysical studies to delineate fresh and saline aquifer boundaries in Lesser Cholistan of Pakistan. PLoS ONE 2023, 18, e0292035. [Google Scholar] [CrossRef]
- Mokadem, N.; Hamed, Y.; Ben Saad, A.; Gargouri, I. Atmospheric pollution in North Africa (ecosystems–atmosphere interactions): A case study in the mining basin of El Guettar–M’Dilla (southwestern Tunisia). Arab. J. Geosci. 2014, 7, 2071–2079. [Google Scholar] [CrossRef]
- Marzougui, S.; Sdiri, A.; Rekhiss, F. Heavy metals’ mobility from phosphate washing effluents discharged in the Gafsa area (southwestern Tunisia). Arab. J. Geosci. 2016, 9, 599. [Google Scholar] [CrossRef]
- Colman, A.S.; Holland, H.D. The Global Diagenetic Flux of Phosphorus from Marine Sediments to the Oceans: Redox Sensitivity and the Control of Atmospheric Oxygen Levels; SEPM Society for Sedimentary Geology: Claremore, OK, USA, 2000. [Google Scholar] [CrossRef]
- Selvakumar, R.; Ramadoss, G.; Menon, M.P.; Rajendran, K.; Thavamani, P.; Naidu, R.; Megharaj, M. Challenges and complexities in remediation of uranium contaminated soils: A review. J. Environ. Radioact. 2018, 192, 592–603. [Google Scholar] [CrossRef] [PubMed]
- Veríssimo, C.U.V.; Santos, R.V.; Parente, C.V.; de Oliveira, C.G.; Cavalcanti, J.A.D.; Neto, J.D.A.N. The Itataia phosphate-uranium deposit (Ceará, Brazil) new petrographic, geochemistry and isotope studies. J. S. Am. Earth Sci. 2016, 70, 115–144. [Google Scholar] [CrossRef]
- Gao, S.; Tang, G.; Hua, D.; Xiong, R.; Han, J.; Jiang, S.; Zhang, Q.; Huang, C. Stimuli-responsive bio-based polymeric systems and their applications. J. Mater. Chem. B 2019, 7, 709–729. [Google Scholar] [CrossRef]
- Hamed, Y.; Awad, S.; Ben Sâad, A. Nitrate contamination in groundwater in the Sidi Aïch-Gafsa oases region, Southern Tunisia. Environ. Earth Sci. 2013, 70, 2335–2348. [Google Scholar] [CrossRef]
- Khaing, H.; Thakur, P. Rapid sequential separation method for 210Po and actinides in air flter samples. J. Radioanal. Nucl. Chem. 2017, 314, 1383–1392. [Google Scholar] [CrossRef]
- Nelson, A.W.; Eitrheim, E.S.; Knight, A.W.; May, D.; Mehrhoff, M.A.; Shannon, R.; Litman, R.; Burnett, W.C.; Forbes, T.Z.; Schultz, M.K. Understanding the radioactive ingrowth and decay of naturally occurring radioactive materials in the environment: An analysis of produced fluids from the Marcellus Shale. Environ. Health Perspect. 2015, 123, 689–696. [Google Scholar] [CrossRef] [PubMed]
- Bugai, D.; Dewière, L. Global Model for the 90Sr Transport in the Aquifer at Chernobyl Pilot Site: Model Calibration and Sensitivity Analyses; IRSN: Fontenay-aux-Roses, France, 2004.
- Dewière, L.; Bugai, D.; Grenier, C.; Kashparov, V.; Ahamdach, N. 90Sr migration to the geosphere from a waste burial in the Chernobyl exclusion zone. J. Env. Radioact. 2004, 74, 139–150. [Google Scholar] [CrossRef] [PubMed]
- Roux, C. Etude des Mécanismes de Transfert des Radionucléides en Aval de la Fosse T22 du Site Expérimental de Tchernobyl. Doctoral Dissertation, Aix-Marseille University, Marseille, France, 2013. [Google Scholar]
- Jenifer, M.A.; Jha, M.K. Comprehensive risk assessment of groundwater contamination in a weathered hard-rock aquifer system of India. J. Clean. Prod. 2018, 201, 853–868. [Google Scholar] [CrossRef]
- Yuan, Y.; Xiang, M.; Liu, C.; Theng, B.K.G. Chronic impact of an accidental wastewater spill from a smelter, China: A study of health risk of heavy metal(loid)s via vegetable intake. Ecotoxicol. Environ. Saf. 2019, 182, 109401. [Google Scholar] [CrossRef] [PubMed]
- Njuguna, S.M.; Makokha, V.A.; Yan, X.; Gituru, R.W.; Wang, Q.; Wang, J. Health risk assessment by consumption of vegetables irrigated with reclaimed wastewater: A case study in Thika (Kenya). J. Environ. Manag. 2019, 231, 576–581. [Google Scholar] [CrossRef] [PubMed]
- Langmuir, D. Aqueous Environmental Geochemistry; Prentice Hall: Upper Saddle River, NJ, USA, 1997; p. 600. [Google Scholar]
- De Souza, A.L.; Cotrim, M.E.B.; Pires, M.A.F. An overview of spectrometric techniques and sample preparation for the determination of impurities in uranium nuclear fuel grade. Microchem. J. 2013, 106, 194–201. [Google Scholar] [CrossRef]
- Kertes, A.S.; Guillaumont, R. Solubility of UO2. A comparative review. Nucl. Chem. Waste Manag. 1985, 5, 215–219. [Google Scholar]
- Bi, Y.; Hyun, S.P.; Kukkadapu, R.K.; Hayes, K.F. Oxidative dissolution of UO2 in a simulated groundwater containing synthetic nanocrystalline mackinawite. Geochim. Cosmochim. Acta 2013, 102, 175–190. [Google Scholar] [CrossRef]
- Durakoviæ, A. Medical effects of internal contamination with uranium. Croat. Med. J. 1999, 40, 49–66. [Google Scholar]
- Alam, M.S.; Cheng, T. Uranium release from sediment to groundwater: Influence of water chemistry and insights into release mechanisms. J. Contam. Hydrol. 2014, 164, 72–87. [Google Scholar] [CrossRef]
- Ulrich, S.; Gillow, J.; Roberts, S.; Byer, G.; Sueker, J.; Farris, K. Hydrogeochemical and mineralogical factors influencing uranium in background area groundwater wells: Grants, New Mexico. J. Hydrol. Reg. Stud. 2019, 26, 100636. [Google Scholar] [CrossRef]
- Van, K. Cadmium and Other Minor Elements in World Resources of Phosphate Rock; The Fertiliser Society Proceedings: York, UK, 1997; No. 400; pp. 15–22, 30–31. [Google Scholar]
- Van, K. World Phosphate Rock Reserves and Resources; IFDC Publication: Muscle Shoals, AL, USA, 2010; p. 33. [Google Scholar]
- U.S. Geological Survey. Mineral Year Book. In Phosphate Rock; U.S. Geological Survey: Reston, VA, USA, 2011. [Google Scholar]
- NEA; IAEA. Uranium 2020: Resources, Production and Demand; Public Online Report; NEA No. 7551; OECD: Paris, France, 2020. [Google Scholar]
- Gandhi, T.P.; Sampath, P.V.; Maliyekkal, S.M. A critical review of uranium contamination in groundwater: Treatment and sludge disposal. Sci. Total Environ. 2022, 825, 153947. [Google Scholar] [CrossRef]
- Hidouri, N.; Missaoui, R.; Jraba, A.; Yousaf, B.; Sleimi, N.; Hamed, Y. Assessing the vertical distribution and health risks of trace metal elements in phosphogypsum-enriched agricultural soils of typical peri-urban areas in Southwestern Tunisia. Water Air Soil. Pollut. 2023, 234, 332. [Google Scholar] [CrossRef]
- Serio, F.; Miglietta, P.P.; Lamastra, L.; Ficocelli, S.; Intini, F.; De Leo, F.; De Donno, A. Groundwater nitrate contamination and agricultural land use: A grey water footprint perspective in Southern Apulia Region (Italy). Sci. Total Environ. 2018, 645, 1425–1431. [Google Scholar] [CrossRef] [PubMed]
- He, S.; Wu, J. Hydrogeochemical characteristics, groundwater quality and health risks from hexavalent chromium and nitrate in groundwater of Huanhe Formation in Wuqi County, northwest China. Expo Health 2019, 11, 125–137. [Google Scholar] [CrossRef]
- Li, P.; He, X.; Guo, W. Spatial groundwater quality and potential health risks due to nitrate ingestion through drinking water: A case study in Yan’an City on the Loess Plateau of northwest China. Hum. Ecol. Risk Assess. 2019, 25, 11–31. [Google Scholar] [CrossRef]
- Hamdi, A.; Ben Jamaa, N.; KallelKamoun, I. Potential use of phosphogypsum in paving blocks. Green Mater. 2021, 9, 97–107. [Google Scholar] [CrossRef]
- Garbaya, H.; Jraba, A.; Khadimallah, M.A.; Elaloui, E. The Development of a New Phosphogypsum-Based Construction Material: A Study of the Physicochemical, Mechanical and Thermal Characteristics. Materials 2021, 14, 7369. [Google Scholar] [CrossRef]
- Hashim, M.A.; Mukhopadhyay, S.; Sahu, J.N.; Sengupta, B. Remediation technologies for heavy metal contaminated groundwater. J. Environ. Manag. 2011, 92, 2355–2388. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Li, P. Surface water pollution in the middle Chinese Loess Plateau with special focus on hexavalent chromium (Cr6+): Occurrence, sources, and health risks. Expo Health 2020, 12, 385–401. [Google Scholar] [CrossRef]




| Lithology | Mineralogy | Porosity (%) | Density (kg/m3) | Silt (%) (<0.01 mm) | Thickness (m) |
|---|---|---|---|---|---|
| Alluvial sands and gravel | Quartz (97–99%) Silt and organic matter (1–3%) | 36–42 | 1.68 | 0.72–1.85 | 0.5–2.5 |
| Limestones | Carbonate (87–94%) Dolomite (6–13%) | 25–32 | 1.71 | 0.8–2.4 | 2–50 |
| Lithology | Mineralogy | Porosity (%) | Density (kg/m3) | Silt (%) (<0.01 mm) | Thickness (m) |
|---|---|---|---|---|---|
| Aeolian deposits | Quartz (97–99%) Silt and organic matter (1–3%) | 33–37 | 1.74 | 0.8–2.5 | 0.5–2 |
| Alluvial sands | Quartz (85–92%) Silt and organic matter (8–15%) | 30–38 | 1.71 | 1.4–10.4 | 2–3 |
| Region | Sample ID | Gamma Radioactivity mSv/y | 238U | 234U | 228Th | 228Ra | 226Ra |
|---|---|---|---|---|---|---|---|
| M Dilla1 | * GW1 | 0.18 | 0.19 | 0.83 | 0.24 | 2.05 | 7.95 |
| M Dilla2 | * GW2 | 0.16 | 0.2 | 0.75 | 0.22 | 2.00 | 7.98 |
| M Dilla3 | * GW3 | 0.22 | 0.22 | 1.22 | 0.1 | 0.84 | 4.48 |
| El Guettar1 | * GW4 | 0.24 | 0.22 | 1.23 | 0.11 | 0.88 | 4.56 |
| El Guettar2 | * GW5 | 0.19 | 0.20 | 1.09 | 0.1 | 0.78 | 4.49 |
| El Guettar3 | * GW6 | 0.16 | 0.21 | 0.76 | 0.24 | 2.04 | 8.02 |
| Gafsa North1 | * GW7 | 0.09 | 0.12 | 0.67 | 0.12 | 1.24 | 7.87 |
| Gafsa North2 | * GW8 | 0.06 | 0.09 | 0.5 | 0.09 | 1.17 | 7.32 |
| Gafsa North3 | * GW9 | 0.07 | 0.08 | 0.48 | 0.085 | 1.15 | 7.28 |
| Gafsa North4 | * GW10 | 0.08 | 0.11 | 0.52 | 0.11 | 1.18 | 7.64 |
| Metaloui1 | * GW11 | 0.14 | 0.2 | 0.61 | 0.11 | 1.21 | 6.98 |
| Metlaoui2 | * GW12 | 0.15 | 0.19 | 0.58 | 0.12 | 1.24 | 7.09 |
| Metlaoui3 | * GW13 | 0.18 | 0.23 | 0.66 | 0.14 | 0.96 | 1.87 |
| Moulares1 | ** GW14 | 0.08 | 0.1 | 0.32 | 0.13 | 0.52 | 0.89 |
| Moulares2 | ** GW15 | 0.07 | 0.11 | 0.33 | 0.16 | 0.66 | 1.05 |
| Moulares3 | ** GW16 | 0.09 | 0.12 | 0.42 | 0.18 | 0.79 | 2.07 |
| S. Ahmed Zarroug | ** GW17 | 0.24 | 1.05 | 0.52 | 0.21 | 1.02 | 7.56 |
| Tozeur1 | ** GW18 | 0.23 | 0.82 | 0.47 | 0.19 | 0.81 | 5.21 |
| Tozeur2 | ** GW19 | 0.26 | 0.98 | 0.41 | 0.2 | 0.72 | 4.45 |
| Tozeur3 | ** GW20 | 0.24 | 0.87 | 0.52 | 0.09 | 0.55 | 7.02 |
| Sidi Boubaker1 | ** GW21 | 0.10 | 0.09 | 0.31 | 0.04 | 0.42 | 2.63 |
| Sidi Boubaker2 | ** GW22 | 0.09 | 0.1 | 0.34 | 0.05 | 0.33 | 2.91 |
| Oum Laksab1 | * GW23 | 0.07 | 0.08 | 0.31 | 0.02 | 0.29 | 2.78 |
| Oum Laksab2 | * GW24 | 0.07 | 0.05 | 0.38 | 0.02 | 0.29 | 2.78 |
| Oum Laksab3 | * GW25 | 0.08 | 0.04 | 0.35 | 0.01 | 0.31 | 3.21 |
| Gabès1 | * GW26 | 0.26 | 0.29 | 1.04 | 0.13 | 0.2 | 0.98 |
| Gabès2 | ** GW27 | 0.29 | 0.32 | 0.98 | 0.12 | 0.14 | 1.02 |
| Gabès3 | ** GW28 | 0.28 | 0.24 | 0.78 | 0.1 | 0.16 | 1.12 |
| Gabès4 | ** GW29 | 0.31 | 0.33 | 1.05 | 0.11 | 0.12 | ND * |
| Jerba1 | * GW30 | 0.06 | 0.04 | 0.28 | 0.02 | 0.24 | 3.01 |
| Jerba2 | ** GW31 | 0.24 | 0.23 | 0.66 | 0.11 | 0.14 | 1.14 |
| Jerba3 | ** GW32 | 0.22 | 0.31 | 0.74 | 0.14 | 0.42 | 2.02 |
| Jerba4 | ** GW33 | 0.3 | 0.33 | 0.81 | 0.16 | 0.36 | ND * |
| --- | Mean-shallow | 0.13 | 0.165 | 0.75 | 0.12 | 1.12 | 4.5 |
| --- | Mean-Deep | 0.202 | 0.57 | 0.68 | 0.12 | 0.75 | 4.27 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hamed, Y.; Gentilucci, M.; Mokadem, N.; Khalil, R.; Ayadi, Y.; Hadji, R.; Elaloui, E. Assessment and Mitigation of Groundwater Contamination from Phosphate Mining in Tunisia: Geochemical and Radiological Analysis. Hydrology 2024, 11, 84. https://doi.org/10.3390/hydrology11060084
Hamed Y, Gentilucci M, Mokadem N, Khalil R, Ayadi Y, Hadji R, Elaloui E. Assessment and Mitigation of Groundwater Contamination from Phosphate Mining in Tunisia: Geochemical and Radiological Analysis. Hydrology. 2024; 11(6):84. https://doi.org/10.3390/hydrology11060084
Chicago/Turabian StyleHamed, Younes, Matteo Gentilucci, Naziha Mokadem, Rayan Khalil, Yosra Ayadi, Riheb Hadji, and Elimame Elaloui. 2024. "Assessment and Mitigation of Groundwater Contamination from Phosphate Mining in Tunisia: Geochemical and Radiological Analysis" Hydrology 11, no. 6: 84. https://doi.org/10.3390/hydrology11060084
APA StyleHamed, Y., Gentilucci, M., Mokadem, N., Khalil, R., Ayadi, Y., Hadji, R., & Elaloui, E. (2024). Assessment and Mitigation of Groundwater Contamination from Phosphate Mining in Tunisia: Geochemical and Radiological Analysis. Hydrology, 11(6), 84. https://doi.org/10.3390/hydrology11060084









