β-Glucosidase and Its Application in Bioconversion of Ginsenosides in Panax ginseng
Abstract
:1. Introduction
Ginseng and Ginsenosides
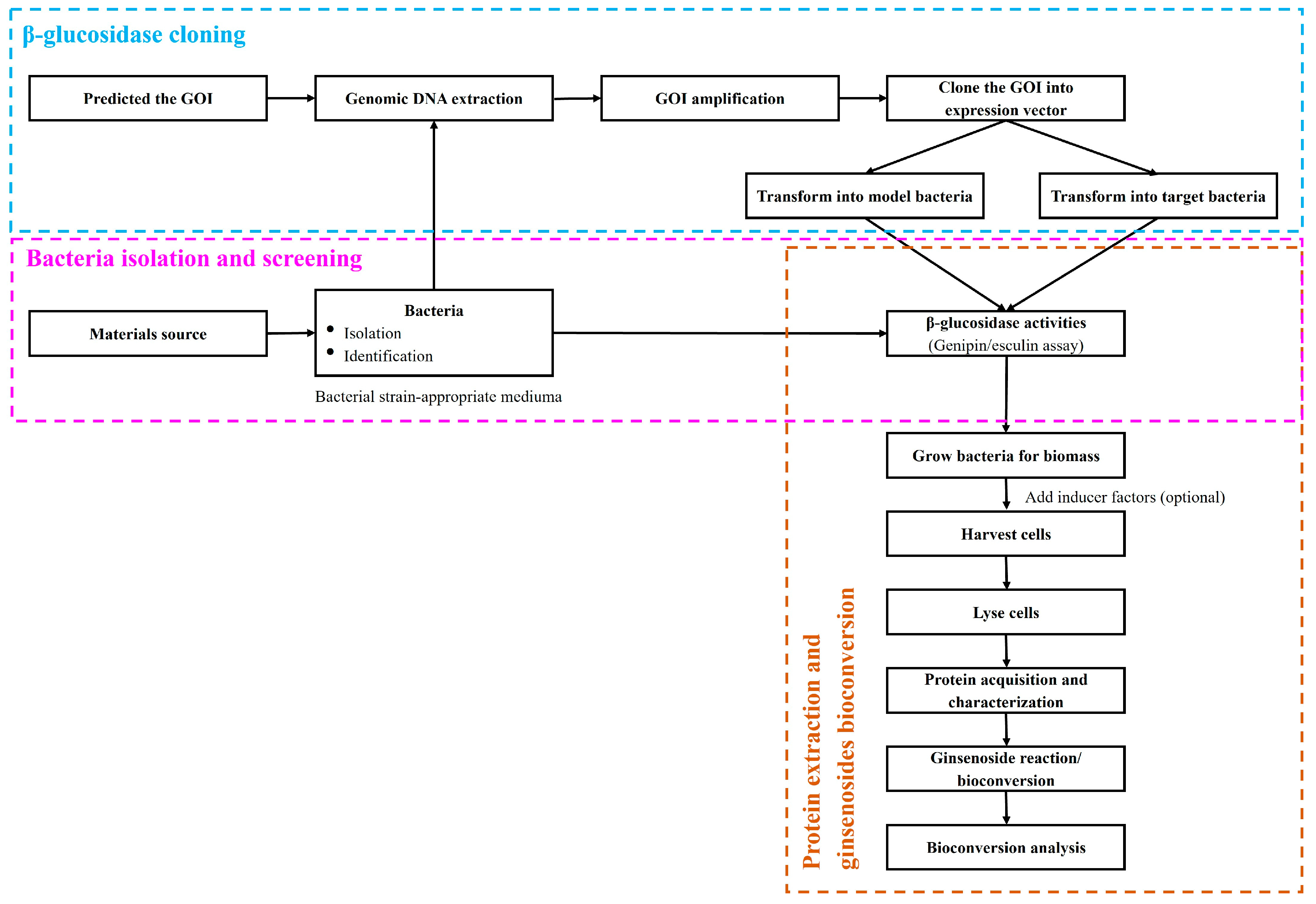
2. β-Glucosidases and Their Functions
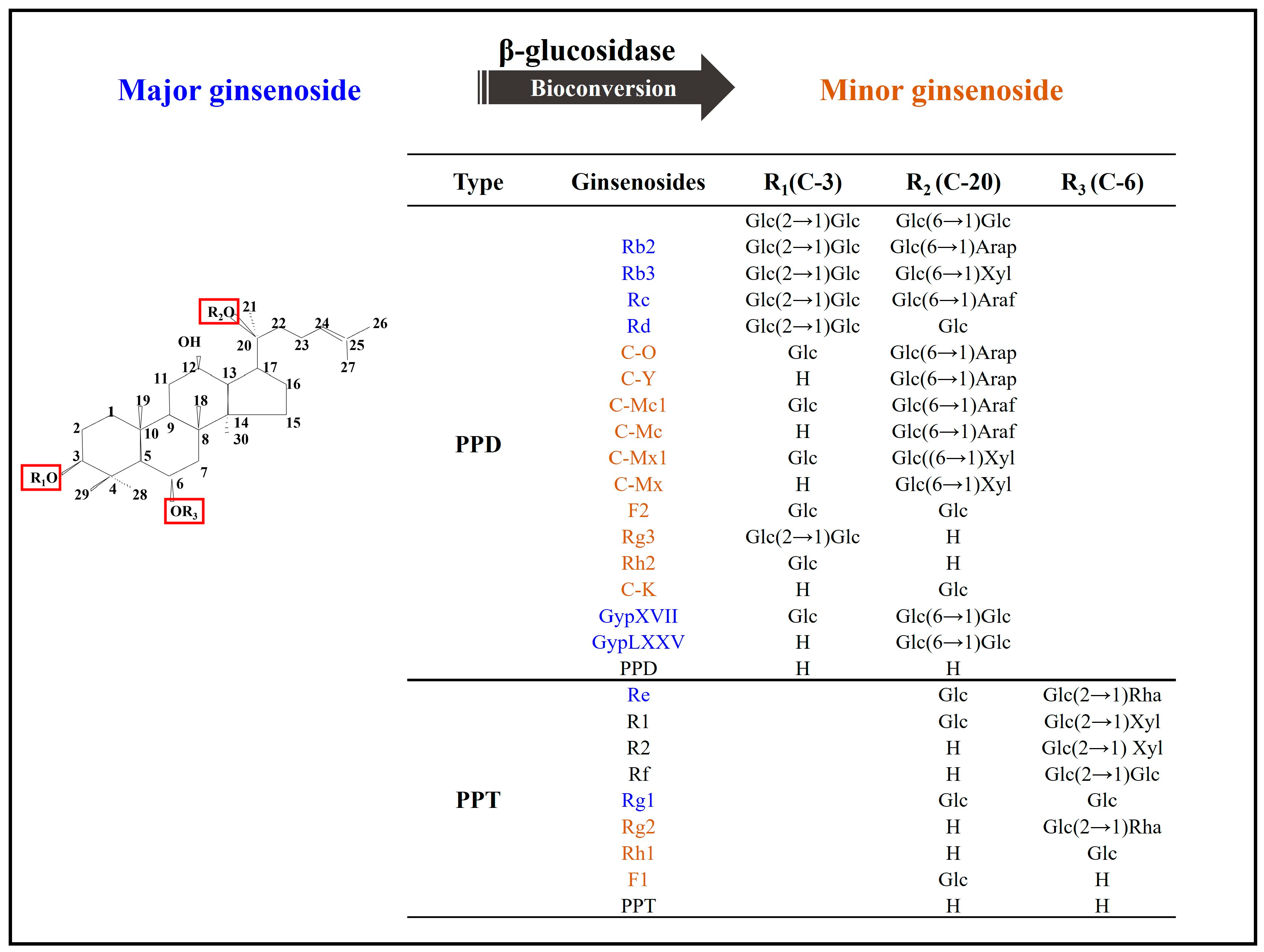
3. Bioconversion of Ginsenosides
4. β-Glucosidases Applications in Bioconversion of Ginsenosides
5. Bioconversion of Ginsenosides by β-Glucosidase Enzymes Obtained from Microorganism
| No. | Microorganism | Strain | Source | Pathway | Ref. |
|---|---|---|---|---|---|
| 1 | Aspergillus niger | KCCM 11239 | Rb1→Rd→F2→CK Rb1→Rd→Rg3 Rb2→Rd CO→F2 CY→CK Rc→Rd Mc1→F2 Mc→CK | [28,69,70] | |
| 2 | Aspergillus niger | XD101 | Soil | Rb1→CK | [71] |
| 3 | Armillaria mellea | KACC 50013 | Mushroom mycelia | G-Rc→C-Mc1→C-Mc G-Rc→G-Rd→G-F2→CK | [15] |
| 4 | Chryseobacterium panacisoli sp. | Gsoil 183T | Soil | Rb1→F2 | [80] |
| 5 | Chryseobacterium ginsengiterrae sp. | DCY68T | Rb1→F2 | [81] | |
| 6 | Chryseobacterium yeoncheonense sp. nov. | DCY67T | Soil | Rb1→F2→CK | [82] |
| 7 | Dekkera anomala YAE-1 | YAE-1 | Mongolian Fermented Milk | Rb1→Rd | [59] |
| 8 | Flavobacterium panaciterrae | DCY69T | Soil | Rb1→Rd/F2 | [83] |
| 9 | Fomitella fraxinea | KACC 42289 | Korean Agricultural Culture Collection | Rb1→Rd→F2→CK Rc→C-Mc1→C-Mc→CK Rb2→CO→CY | [78] |
| 10 | Intrasporangium sp. | GS603 | Soil | Rb1→F2 Rb1→GypXVII | [61] |
| 11 | Lactobacillus mesenteroides, Pediococcus pentosaceus | WiKim19 WiKim20 | Kimchi | Rb1→Rg3 | [14] |
| 12 | Lactobacillus rossiae | DC05 | Kimchi | Rb1→CK Re→Rg2 | [64] |
| 13 | Lentilactobacillus buchneri | URN103L | Korean fermented foods | Rb1→Rd→F2→CK Rb1→Rd→Rg3 | [84] |
| 14 | Leuconostoc citreum | LH1 | Kimchi | Rb1→Rd→F2→CK | [62] |
| 15 | Microbacterium sp. | GS514 | Soil | Rb1→Rd→Rg3 | [85] |
| 16 | Paecilomyces Bainier sp. | 229 | Soil | Rb1→Rd→Rg3 | [26] |
| 17 | Paenibacillus puernese | DCY97T | Pu’er tea | Rb1→CK | [86] |
| 18 | Penicillium decumbens | Rb1→GypXVII→F2→CK Rd→F2→CK Rg3→Rh2 | [40] | ||
| 19 | Penicillium sclerotiorum | Soil | Rg1→F1 | [29] | |
| 20 | Weissella hellenica | DC06 | Fermented food (radish and cabbage) | Rb1→Rg3 Rb1→Rd→F2→CK | [65] [87] |
6. Bioconversion of Ginsenoside by Recombinant β-Glucosidases
| No. | Microorganism | Gene | Gene/Protein ID | Family | Bioconversion Pathway | Ref. |
|---|---|---|---|---|---|---|
| 1 | Actinosynnema mirum KACC 20028T | bglAm | YP_003100744 | GH3 | Rb1→GypXVII→GypLXXV/F2 Rd→F2→Rh2(S) Re→Rh2(S) Rg1→Rh1(S) | [72] |
| 2 | Bifidobacterium adolescentis ATCC15703 | BaBgl1A BaBgl3A | Rb1→Gyp XVII Rd→F2 Rb1→Gyp XVII and F2 | [31] | ||
| 3 | Bifidobacterium breve ATCC 15700 | BbBgl | CP006715.1 (1366947 to 1368227) | GH1 | Rd→F2→CK | [48] |
| 4 | Caldicellulosiruptor bescii DSM 6725 | β-glucosidase | ACM59590 | GH1 | Rb1/Rb2/Rc→Rd→F2→CK | [52] |
| 5 | Flavobacterium chilense | BglFc | SHL96941.1 | GH3 | Rb1→Rd→Rg3(S)→Rh2(S) GypXVII →GypLXXV→CK F2→CK Rb2 →CO→CY Rb3→C-Mx1→C-Mx Rc→C-Mc1→C-Mc Re→Rg2(S) Rg1→Rh1(S) | [44] |
| 6 | Flavobacterium johnsoniae | bglF3 | ABQ03809 | GH3 | Rb1→Rd GypXVII→F2 | [63] |
| 7 | Lactobacillus brevis | Bgy1 | BAN07577 | GH3 | GypXVII→GypLXXV→CK | [56] |
| 8 | Lactobacillus brevis | bgy2 | BAN05876 | GH3 | Rb1→Rd F2→CK | [42] |
| 9 | Lactobacillus insenosidimutans EMML 3041T | GST-BglL. gin-952 | WP_053084464 | GH3 | Rb1→Rd→Rg3(S) | [68] |
| 10 | Microbacterium esteraromaticum | Bgp1 | AEX88466.1 | GH3 | Re→Rg2; Rg1→Rh1 Rb1→Rd→20(S)-Rg3 | [7,88,90] |
| 11 | Microbacterium esteraromaticum GS514 | Bgp2 | EU036992.1 | GH2 | Rb2→20(S)-Rg3 | [91] |
| 12 | Microbacterium esteraromaticum | bgp3 | JN 603821 | GH3 | Rb1→Rd→CK | [74] |
| 13 | Microbacterium sp. Gsoil 167 | BglG167b | ~WP_018187396 | GH3 | GypXVII→GypLXXV | [66] |
| 14 | Microbacterium testaceum ATCC 15829 | MT619 | Re→F1 Rb1→CK | [49] | ||
| 15 | Niabella ginsenosidivorans | BglNg-767 | CP015772 | GH3 | Rb1→GypXVII→F2 Rd→F2 Rb2→CO Rb3→C-Mx1 Rc→C-Mc1 | [73] |
| 16 | Paenibacillus mucilaginosus 3870T | BglPm | AEI42200 | GH1 | Rb1/Rd→F2 | [92] |
| 17 | Pseudonocardia sp. Gsoil 1536 | BglPC28 | JX960416 | GH3 | Re→Rg2(S) Rg1→Rh1(S) Rb1/Rd/Rb3→Rg3(S) | [41] |
| 18 | Thermotoga petrophila DSM 13995 | Tpexyl3 Tpebgl3 | CP000702.1 | GH3 | Ginsenoside extract→20(S)-Rg3 | [36,89] |
| 19 | Thermotoga thermarum DSM 5069T | Tt-BGL | YP 004660190.1 | GH1 | Rb1→Rd | [93] |
7. Other Methods for Ginsenoside Bioconversion
8. Potential Application of β-Glucosidases and Converted Ginsenosides
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lin, Y.; Hao, B.; Lu, Y.C.; Dong, Y.; Li, Y.; Zhang, G.H.; Yang, Z.J.; Xiang, G.S.; Liu, G.Z.; Li, X.J.; et al. PanaxGDB: A Comprehensive Platform for Panax. Front. Plant Sci. 2022, 13, 883818. [Google Scholar] [CrossRef]
- Eom, S.J.; Kim, K.T.; Paik, H.D. Microbial bioconversion of ginsenosides in Panax ginseng and their improved bioactivities. Food Rev. Int. 2018, 34, 698–712. [Google Scholar] [CrossRef]
- Yun, T.K. Brief introduction of Panax ginseng C.A. Meyer. J. Korean Med. Sci. 2001, 16, 16–18. [Google Scholar] [CrossRef]
- Piao, X.; Zhang, H.; Kang, J.P.; Yang, D.U.; Li, Y.; Pang, S.; Jin, Y.; Yang, D.C.; Wang, Y. Advances in saponin diversity of panax ginseng. Molecules 2020, 25, 3452. [Google Scholar] [CrossRef] [PubMed]
- Shin, K.C.; Oh, D.K. Classification of glycosidases that hydrolyze the specific positions and types of sugar moieties in ginsenosides. Crit. Rev. Biotechnol. 2016, 36, 1036–1049. [Google Scholar] [CrossRef]
- An, C.; Ma, S.; Shi, X.; Liu, C.; Ding, H.; Xue, W. Diversity and Ginsenoside Biotransformation Potential of Cultivable Endophytic Fungi Associated With Panax bipinnatifidus var. bipinnatifidus in Qinling Mountains, China. Front. Pharmacol. 2022, 13, 762862. [Google Scholar] [CrossRef] [PubMed]
- Huq, A.; Siraj, F.M.; Kim, Y.-J.; Yang, D.-C. Enzymatic transformation of ginseng leaf saponin by recombinant β-glucosidase (bgp1) and its efficacy in an adipocyte cell line. Biotechnol. Appl. Biochem. 2015, 63, 532–538. [Google Scholar] [CrossRef]
- Kim, Y.J.; Zhang, D.; Yang, D.C. Biosynthesis and biotechnological production of ginsenosides. Biotechnol. Adv. 2015, 33, 717–735. [Google Scholar] [CrossRef]
- Chu, L.L.; Bae, H. Bacterial endophytes from ginseng and their biotechnological application. J. Ginseng Res. 2022, 46, 1–10. [Google Scholar] [CrossRef]
- Cairns, J.R.K.; Esen, A. β-Glucosidases. Cell Mol. Life Sci. 2010, 67, 3389–3405. [Google Scholar] [CrossRef]
- Kim, D.; Kim, M.; Raña, G.S.; Han, J. Seasonal variation and possible biosynthetic pathway of ginsenosides in korean ginseng Panax ginseng meyer. Molecules 2018, 23, 1824. [Google Scholar] [CrossRef] [PubMed]
- Piao, X.M.; Huo, Y.; Kang, J.P.; Mathiyalagan, R.; Zhang, H. Diversity of Ginsenoside Profiles Produced by Various Processing Technologies. Molecules 2020, 25, 4390. [Google Scholar] [CrossRef] [PubMed]
- Ying, Z.; Awais, M.; Akter, R.; Xu, F.; Baik, S.; Jung, D.; Yang, D.C.; Kwak, G.Y.; Wenying, Y. Discrimination of Panax ginseng from counterfeits using single nucleotide polymorphism: A focused review. Front. Plant Sci. 2022, 13, 903306. [Google Scholar] [CrossRef] [PubMed]
- Park, B.; Hwang, H.; Lee, J.; Sohn, S.O.; Lee, S.H.; Jung, M.Y.; Lim, H.I.; Park, H.W.; Lee, J.H. Evaluation of ginsenoside bioconversion of lactic acid bacteria isolated from kimchi. J. Ginseng Res. 2017, 41, 524–530. [Google Scholar] [CrossRef]
- Upadhyaya, J.; Yoon, M.S.; Kim, M.J.; Ryu, N.S.; Song, Y.E.; Kim, Y.H.; Kim, M.K. Purification and characterization of a novel ginsenoside Rc-hydrolyzing β-glucosidase from Armillaria mellea mycelia. AMB Express 2016, 6, 112. [Google Scholar] [CrossRef] [PubMed]
- Jung, J.; Paik, N.L.H. Bioconversion, health benefits, and application of ginseng and red ginseng in dairy products. Food Sci. Biotechnol. 2017, 26, 1155–1168. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Ma, N.; Xia, F.; Li, P.; He, C.; Wu, Z.; Wan, J. Preparative separation of minor saponins from Panax notoginseng leaves using biotransformation, macroporous resins, and preparative high-performance liquid chromatography. J. Ginseng Res. 2019, 43, 105–115. [Google Scholar] [CrossRef]
- Rebecca, M.C.; Jorge, F.S.; Ferreira, S.D.E.; Murphy, L.L. Simplified Extraction of Ginsenosides from American Ginseng (Panax quinquefolius L.) for High-Performance Liquid Chromatography—Ultraviolet Analysis. J. Agric. Food Chem. 2005, 53, 9867–9873. [Google Scholar]
- Lee, J.; Ha, J. Effective Purification of Ginsenosides from Cultured Wild Ginseng Roots, Red Ginseng, and White Ginseng with Macroporous Resins. J. Microbiol. Biotechnol. 2008, 18, 1789–1791. [Google Scholar] [CrossRef]
- Cui, L.; Yan, H.; Wang, D.; Chen, Q. An efficient isolation and purification of broad partition coefficient range ginsenosides from roots of Panax quinquefolium L. by linear gradient counter-current chromatography coupled with preparative high-performance liquid chromatography. J. Sep. Sci. 2023, 2300046. [Google Scholar] [CrossRef]
- Wang, J.; Bai, H.; Liu, C.; Li, L. Isolation and Purification of Ginsenosides from Plant Extract of Panax quinquefolium L. by High Performance Centrifugal Partition Chromatography Coupled with ELSD. Chromatographia 2010, 71, 267–271. [Google Scholar] [CrossRef]
- Li, K.; Xu, F.; Gong, X. Isolation, Purification and Quantification of Ginsenoside F5 and F3 Isomeric Compounds from Crude Extracts of Flower Buds of Panax ginseng. Molecules 2016, 21, 315. [Google Scholar] [CrossRef] [PubMed]
- Berrnard, H.; Gideon, D. Structure and sequence-base classification of glycoside hydrolases. Curr. Opin. Struct. Biol. 1997, 7, 637–644. [Google Scholar] [CrossRef]
- Henrissat, B.; Vegetales, M.; Grenoble, F. A classification of glycosyl hydrolases based sequence similarities amino acid. Biochem. J. 1991, 280, 309–316. [Google Scholar] [CrossRef] [PubMed]
- Liew, K.J.; Lim, L.; Woo, H.Y.; Chan, K.G.; Shamsir, M.S.; Goh, K.M. Purification and characterization of a novel GH1 beta-glucosidase from Jeotgalibacillus malaysiensis. Int. J. Biol. Macromol. 2018, 115, 1094–1102. [Google Scholar] [CrossRef]
- Yan, Q.; Zhou, W.; Li, X.; Feng, M.; Zhou, P. Purification method improvement and characterization of a novel ginsenoside-hydrolyzing β-glucosidase from Paecilomyces bainier sp. 229. Biosci. Biotechnol. Biochem. 2008, 72, 352–359. [Google Scholar] [CrossRef]
- Nallamsetty, S.; Waugh, D.S. A generic protocol for the expression and purification of recombinant proteins in Escherichia coli using a combinatorial His6-maltose binding protein fusion tag. Nat. Protoc. 2007, 2, 383–391. [Google Scholar] [CrossRef]
- Chang, K.H.; Jo, M.N.; Kim, K.T.; Paik, H.D. Purification and characterization of a ginsenoside Rb1-hydrolyzing β-glucosidase from Aspergillus niger KCCM 11239. Int. J. Mol. Sci. 2012, 13, 12140–12152. [Google Scholar] [CrossRef]
- Wei, Y.; Zhao, W.; Zhang, Q.; Zhao, Y.; Zhang, Y. Purification and characterization of a novel and unique ginsenoside Rg 1-hydrolyzing β-D-Glucosidase from Penicillium sclerotiorum. Acta Biochim. Biophys. Sin. (Shanghai) 2011, 43, 226–231. [Google Scholar] [CrossRef]
- Singhania, R.R.; Patel, A.K.; Pandey, A.; Ganansounou, E. Genetic modification: A tool for enhancing beta-glucosidase production for biofuel application. Bioresour. Technol. 2017, 245, 1352–1361. [Google Scholar] [CrossRef]
- Hu, Y.; Zhai, L.; Hong, H.; Shi, Z.; Zhao, J.; Liu, D. Study on the Biochemical Characterization and Selectivity of Three β-Glucosidases From Bifidobacterium adolescentis ATCC15703. Front. Microbiol. 2022, 13, 860014. [Google Scholar] [CrossRef] [PubMed]
- Hati, S.; Vij, S.; Singh, B.P.; Mandal, S. β-Glucosidase activity and bioconversion of isoflavones during fermentation of soymilk. J. Sci. Food Agric. 2015, 95, 216–220. [Google Scholar] [CrossRef]
- Costa, R.; Domínguez, A.; Choupina, A. Cloning and expression analysis of an endo-1,3-β-d-glucosidase from Phytophthora cinnamomi. Mol. Biol. Rep. 2020, 47, 935–942. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.S.; Lee, C.J.; Ma, J.Y. Enhancement of active compound, genipin, from Gardeniae Fructus using immobilized glycosyl hydrolase family 3 β-glucosidase from Lactobacillus antri. AMB Express 2017, 7, 64. [Google Scholar] [CrossRef] [PubMed]
- Kim, W.Y.; Kim, J.M.; Han, S.B.; Lee, S.K.; Kim, N.D.; Park, M.K.; Kim, C.K.; Park, J.H. Steaming of ginseng at high temperature enhances biological activity. J. Nat. Prod. 2000, 63, 1702–1704. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Xie, J.; Zhao, L.; Pei, J.; Su, E.; Xiao, W.; Wang, Z. Cloning, overexpression and characterization of a thermostable β-xylosidase from Thermotoga petrophila and cooperated transformation of ginsenoside extract to ginsenoside 20(S)-Rg3 with a β-glucosidase. Bioorg. Chem. 2019, 85, 159–167. [Google Scholar] [CrossRef]
- Amin, K.; Tranchimand, S.; Benvegnu, T.; Abdel-Razzak, Z.; Chamieh, H. Glycoside hydrolases and glycosyltransferases from hyperthermophilic archaea: Insights on their characteristics and applications in biotechnology. Biomolecules 2021, 11, 1557. [Google Scholar] [CrossRef]
- Wang, D.D.; Kim, Y.J.; Beak, N.I.; Mathiyalagan, R.; Wang, C.; Jin, Y.; Xu, X.Y.; Yang, D.C. Glycosyltransformation of ginsenoside Rh2 into two novel ginsenosides using recombinant glycosyltransferase from Lactobacillus rhamnosus and its in vitro applications. Ginseng Res. 2021, 45, 48–57. [Google Scholar] [CrossRef]
- Zhang, Y.; Yao, L.; Tang, C.; Jiang, J.; Ye, Y.; Liu, J. Qualitatively and quantitatively investigating the metabolism of 20(S)-protopanaxadiol-type ginsenosides by gut microbiota of different species. Biomed. Chromatogr. 2021, 35, e5219. [Google Scholar] [CrossRef]
- Kim, S.Y.; Lee, H.N.; Hong, S.J.; Kang, H.J.; Cho, J.Y.; Kim, D.; Ameer, K.; Kim, Y.M. Enhanced biotransformation of the minor ginsenosides in red ginseng extract by Penicillium decumbens β-glucosidase. Enzym. Microb. Technol. 2022, 153, 109941. [Google Scholar] [CrossRef]
- Du, J.; Cui, C.H.; Park, S.C.; Kim, J.K.; Yu, H.S.; Jin, F.X.; Sun, C.; Kim, S.C.; Im, W.T. Identification and characterization of a ginsenoside-transforming β-glucosidase from Pseudonocardia sp. Gsoil 1536 and its application for enhanced production of minor ginsenoside Rg2(S). PLoS ONE 2014, 9, e96914. [Google Scholar] [CrossRef] [PubMed]
- Zhong, F.L.; Ma, R.; Jiang, M.; Dong, W.W.; Jiang, J.; Wu, S.; Li, D.; Quan, L.H. Cloning and characterization of ginsenoside-hydrolyzing β-glucosidase from lactobacillus brevis that transforms ginsenosides Rb1 and F2 into ginsenoside Rd and compound K. J. Microbiol. Biotechnol. 2016, 26, 1661–1667. [Google Scholar] [CrossRef]
- Siddiqi, M.Z.; Cui, C.H.; Park, S.K.; Han, N.S.; Kim, S.C.; Im, W.T. Comparative analysis of the expression level of recombinant ginsenoside-transforming β-glucosidase in GRAS hosts and mass production of theginsenoside Rh2-Mix. PLoS ONE 2017, 12, e0176098. [Google Scholar] [CrossRef] [PubMed]
- Siddiqi, M.Z.; Medjebouri, S.; Liu, Q.; Park, H.Y.; Kim, G.R.; Im, W.T. Efficient Production of Various Minor Ginsenosides from PPD- and PPT-type Major Ginsenosides Using a Single Recombinant BglFc Isolated from Flavobacterium chilense. Biotechnol. Bioprocess Eng. 2021, 26, 232–246. [Google Scholar] [CrossRef]
- Wang, D.D.; Jin, Y.; Wang, C.; Kim, Y.J.; Zuly, E.J.P.; Baek, N.I.; Mathiyalagan, R.; Josua, M.; Yang, D.C. Rare ginsenoside Ia synthesized from F1 by cloning and overexpression of the UDP-glycosyltransferase gene from Bacillus subtilis: Synthesis, characterization, and in vitro melanogenesis inhibition activity in BL6B16 cells. J. Ginseng Res. 2018, 42, 42–49. [Google Scholar] [CrossRef]
- Li, L.; Lee, S.J.; Yuan, Q.P.; Im, W.T.; Kim, S.C.; Han, N.S. Production of bioactive ginsenoside Rg3(S) and compound K using recombinant Lactococcus lactis. J. Ginseng Res. 2018, 42, 412–418. [Google Scholar] [CrossRef]
- Li, W.N.; Fan, D. Di Biocatalytic strategies for the production of ginsenosides using glycosidase: Current state and perspectives. Appl. Microbiol. Biotechnol. 2020, 104, 3807–3823. [Google Scholar] [CrossRef]
- Zhang, R.; Huang, X.M.; Yan, H.J.; Liu, X.Y.; Zhou, Q.; Luo, Z.Y.; Tan, X.N.; Zhang, B.L. Highly selective production of compound k from ginsenoside rd by hydrolyzing glucose at c-3 glycoside using β-glucosidase of bifidobacterium breve atcc 15700. J. Microbiol. Biotechnol. 2019, 29, 410–418. [Google Scholar] [CrossRef]
- Cui, C.H.; Jeon, B.M.; Fu, Y.; Im, W.T.; Kim, S.C. High-density immobilization of a ginsenoside-transforming β-glucosidase for enhanced food-grade production of minor ginsenosides. Appl. Microbiol. Biotechnol. 2019, 103, 7003–7015. [Google Scholar] [CrossRef]
- Yang, W.; Zhou, J.; Harindintwali, J.D.; Yu, X. Production of minor ginsenosides by combining Stereum hirsutum and cellulase. PLoS ONE 2021, 16, e0255899. [Google Scholar] [CrossRef]
- Qi, G.; Ji, B.; Zhang, Y.; Huang, L.; Wang, J.; Gao, W. Microbiome-based screening and co-fermentation of rhizospheric microorganisms for highly ginsenoside Rg3 production. Microbiol. Res. 2022, 261, 127054. [Google Scholar] [CrossRef] [PubMed]
- Shin, K.C.; Kim, T.H.; Choi, J.H.; Oh, D.K. Complete Biotransformation of Protopanaxadiol-Type Ginsenosides to 20-O-β-Glucopyranosyl-20(S)-protopanaxadiol Using a Novel and Thermostable β-Glucosidase. J. Agric. Food Chem. 2018, 66, 2822–2829. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Li, W.; Ma, X.; Fan, D. Enzymatic hydrolysis and extraction of ginsenoside recovered from deep eutectic solvent-salt aqueous two-phase system. J. Biosci. Bioeng. 2020, 130, 390–396. [Google Scholar] [CrossRef]
- An, D.S.; Cui, C.H.; Siddiqi, M.Z.; Yu, H.S.; Jin, F.X.; Kim, S.G.; Im, W.T. Gram-scale production of ginsenoside F1 using a recombinant bacterial β-glucosidase. J. Microbiol. Biotechnol. 2017, 27, 1559–1565. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.W.; Han, S.H.; Lee, S.W.; Choi, H.S.; Suh, H.J.; Hong, K.B. Enzymatic hydrolysis increases ginsenoside content in Korean red ginseng (Panax ginseng CA Meyer) and its biotransformation under hydrostatic pressure. J. Sci. Food Agric. 2019, 99, 6806–6813. [Google Scholar] [CrossRef] [PubMed]
- Zhong, F.L.; Dong, W.W.; Wu, S.; Jiang, J.; Yang, D.C.; Li, D.; Quan, L.H. Biotransformation of gypenoside XVII to compound K by a recombinant b-glucosidase. Biotechnol. Lett. 2016, 38, 1187–1193. [Google Scholar] [CrossRef] [PubMed]
- Yan, S.; Wei, P.C.; Chen, Q.; Chen, X.; Wang, S.C.; Li, J.R.; Gao, C. Functional and structural characterization of a β-glucosidase involved in saponin metabolism from intestinal bacteria. Biochem. Biophys. Res. Commun. 2018, 496, 1349–1356. [Google Scholar] [CrossRef]
- Jung, I.H.; Lee, J.H.; Hyun, Y.J.; Kim, D.H. Metabolism of ginsenoside Rb1 by human intestinal microflora and cloning of its metabolizing β-D-glucosidase from Bifidobacterium longum H-1. Biol. Pharm. Bull. 2012, 35, 573–581. [Google Scholar] [CrossRef]
- Renchinkhand, G.; Cho, S.H.; Park, Y.W.; Song, G.Y.; Nam, M.S. Biotransformation of major ginsenoside Rb1 to Rd by Dekkera anomala YAE-1 from mongolian fermented milk (Airag). J. Microbiol. Biotechnol. 2020, 30, 1536–1542. [Google Scholar] [CrossRef]
- Liang, Z.Q.; Srinivasan, S.; Kim, Y.J.; Kim, H.B.; Wang, H.T.; Yang, D.C. Lactobacillus kimchicus sp. nov., a β-glucosidase producing bacterium isolated from kimchi. Int. J. Syst. Evol. Microbiol. 2011, 61, 894–987. [Google Scholar] [CrossRef]
- Cheng, L.Q.; Na, J.R.; Kim, M.K.; Bang, M.H.; Yang, D.C. Microbial Conversion of Ginsenoside Rb1 to Minor Ginsenoside F2 and Gypenoside XVII by Intrasporangium sp. GS603 Isolated from Soil. J. Microbiol. 2007, 17, 1937–1943. [Google Scholar]
- Quan, L.H.; Piao, J.Y.; Min, I.W.; Yang, D.U.; Lee, H.N.; Yang, D.C. Bioconversion Of Ginsenoside Rb1 Into Compound K By Leuconostoc Citreum Lh1 Isolated From Kimchi. Braz. J. Microbiol. 2011, 42, 1227–1237. [Google Scholar] [CrossRef] [PubMed]
- Hong, H.; Cui, C.H.; Kim, J.K.; Jin, F.X.; Kim, S.C.; Im, W.T. Enzymatic biotransformation of ginsenoside Rb1 and gypenoside XVII into ginsenosides Rd and F2 by recombinant β-glucosidase from Flavobacterium johnsoniae. J. Ginseng Res. 2012, 36, 418–424. [Google Scholar] [CrossRef] [PubMed]
- Huq, M.A.; Kim, Y.J.; Min, J.W.; Bae, K.S.; Yang, D.C. Use of Lactobacillus rossiae DC05 for Bioconversion of the Major Ginsenosides Rb1 and Re into the Pharmacologically Active Ginsenosides C-K and Rg2. Food Sci. Biotechnol. 2014, 23, 1561–1567. [Google Scholar] [CrossRef]
- Huq, M.A.; Kim, Y.J.; Min, J.W.; Siraj, F.M.; Siddiqi, M.Z.; Yang, D.C. Enzymatic transformation of the major ginsenoside Rb1 to compound K by Weissella hellenica DC06. Indian J. Biotechnol. 2015, 14, 270–275. [Google Scholar]
- Cui, C.H.; Kim, D.J.; Jung, S.C.; Kim, S.C.; Im, W.T. Enhanced production of gypenoside LXXV using a novel ginsenoside-transforming β-glucosidase from ginseng-cultivating soil bacteria and its anti-cancer property. Molecules 2017, 22, 844. [Google Scholar] [CrossRef]
- Zheng, Y.; Zheng, Z.; Ming, Y.; Bai, Y.; Chen, L.; Huang, W.; Lin, M.; Liu, S.; Xiao, J.; Lin, H. Compound K producing from the enzymatic conversion of gypenoside by naringinase. Food Chem. Toxicol. 2019, 130, 253–261. [Google Scholar] [CrossRef]
- Siddiqi, M.Z.; Srinivasan, S.; Park, H.Y.; Im, W.T. Exploration and characterization of novel glycoside hydrolases from the whole genome of lactobacillus ginsenosidimutans and enriched production of minor ginsenoside Rg3(S) by a recombinant enzymatic process. Biomolecules 2020, 10, 288. [Google Scholar] [CrossRef]
- Jeong, E.B.; Kim, S.A.; Shin, K.C.; Oh, D.K. Biotransformation of protopanaxadiol-type ginsenosides in Korean ginseng extract into food-available compound K by an extracellular enzyme from aspergillus niger. J. Microbiol. Biotechnol. 2020, 30, 1560–1567. [Google Scholar] [CrossRef]
- Wu, X.; Qu, B.; Liu, Y.; Ren, X.; Wang, S.; Quan, Y. Highly enhanced activity and stability via affinity induced immobilization β-glucosidase from Aspergillus niger onto amino-based silica for the biotransformation of ginsenoside Rb1. J. Chromatogr. A 2021, 1653, 462388. [Google Scholar] [CrossRef]
- Jiang, Y.; Li, W.; Fan, D. Biotransformation of Ginsenoside Rb1 to Ginsenoside CK by Strain XD101: A Safe Bioconversion Strategy. Appl. Biochem. Biotechnol. 2021, 193, 2110–2127. [Google Scholar] [CrossRef] [PubMed]
- Cui, C.H.; Kim, S.C.; Im, W.T. Characterization of the ginsenoside-transforming recombinant β-glucosidase from Actinosynnema mirum and bioconversion of major ginsenosides into minor ginsenosides. Appl. Microbiol. Biotechnol. 2013, 97, 649–659. [Google Scholar] [CrossRef] [PubMed]
- Siddiqi, M.Z.; Hashmi, M.S.; Oh, J.M.; Chun, S.; Im, W.T. Identification of novel glycoside hydrolases via whole genome sequencing of Niabella ginsenosidivorans for production of various minor ginsenosides. 3 Biotech. 2019, 9, 258. [Google Scholar] [CrossRef] [PubMed]
- Quan, L.H.; Min, J.W.; Jin, Y.; Wang, C.; Kim, Y.J.; Yang, D.C. Enzymatic Biotransformation of Ginsenoside Rb1 to Compound K by Recombinant β-Glucosidase from Microbacterium esteraromaticum. Agric. Food Chem. 2012, 60, 3776–3781. [Google Scholar] [CrossRef]
- Sathiyaraj, S.; Kim, M.K.; Gayathri, S.; Kim, Y.J.; Jung, S.K.; In, J.G.; Yang, D.C. Microbacterium soli sp. nov., an a-glucosidase- producing bacterium isolated from soil of a ginseng field. Int. J. Syst. Evol. Microbiol. 2010, 60, 478–483. [Google Scholar] [CrossRef]
- Pérez, G.; Fariña, L.; Barquet, M.; Boido, E.; Gaggero, C.; Dellacassa, E.; Carrau, F. A quick screening method to identify β-glucosidase activity in native wine yeast strains: Application of Esculin Glycerol Agar (EGA) medium. World J. Microbiol. Biotechnol. 2011, 27, 47–55. [Google Scholar] [CrossRef]
- Fu, Y.; Yin, Z.; Wu, L.; Yin, C. Diversity of cultivable b -glycosidase-producing micro-organisms isolated from the soil of a ginseng field and their ginsenosides-hydrolysing activity. Lett. Appl. Microbiol. 2013, 58, 138–144. [Google Scholar] [CrossRef]
- Kim, D.W.; Lee, W.J.; Gebru, Y.A.; Upadhyaya, J.; Ko, S.R.; Kim, Y.H.; Kim, M.K. Production of minor ginsenosides c-k and c-y from naturally occurring major ginsenosides using crude β-glucosidase preparation from submerged culture of fomitella fraxinea. Molecules 2021, 26, 4820. [Google Scholar] [CrossRef]
- Michlmayr, H.; Schümann, C.; Barreira Braz Da Silva, N.M.; Kulbe, K.D.; Del Hierro, A.M. Isolation and basic characterization of a β-glucosidase from a strain of Lactobacillus brevis isolated from a malolactic starter culture. J. Appl. Microbiol. 2010, 108, 550–559. [Google Scholar] [CrossRef]
- Keum, D.H.; Yeon, J.M.; Yun, C.S.; Lee, S.Y.; Im, W.T. Chryseobacterium panacisoli sp. Nov., isolated from ginseng-cultivation soil with ginsenoside-converting activity. Int. J. Syst. Evol. Microbiol. 2021, 71, 005086. [Google Scholar] [CrossRef]
- Noh, J.H.; Hoang, V.A.; Kim, Y.J.; Kang, J.P.; Yang, D.C. Chryseobacterium ginsengiterrae sp. nov., with Beta-Glucosidase Activity Isolated from Soil of a Ginseng Field. Curr. Microbiol. 2017, 74, 1417–1424. [Google Scholar] [CrossRef] [PubMed]
- Hoang, V.A.; Kim, Y.J.; Nguyen, N.L.; Yang, D.C. Chryseobacterium yeoncheonense sp. nov., with ginsenoside converting activity isolated from soil of a ginseng field. Arch. Microbiol. 2013, 195, 463–471. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.; Kim, Y.J.; Hoang, V.A.; Jung, S.Y.; Nguyen, N.L.; Min, J.W.; Wang, C.; Yang, D.C. Flavobacterium panaciterrae sp. nov., a β-glucosidase producing bacterium with ginsenoside-converting activity isolated from the soil of a ginseng field. J. Gen. Appl. Microbiol. 2014, 60, 59–64. [Google Scholar] [CrossRef] [PubMed]
- Renchinkhand, G.; Magsar, U.; Bae, H.C.; Choi, S.H.; Nam, M.S. Identification of β-Glucosidase Activity of Lentilactobacillus buchneri URN103L and Its Potential to Convert Ginsenoside Rb1 from Panax ginseng. Foods 2022, 11, 529. [Google Scholar] [CrossRef] [PubMed]
- Cheng, L.Q.; Na, J.R.; Bang, M.H.; Kim, M.K.; Yang, D.C. Conversion of major ginsenoside Rb1 to 20(S)-ginsenoside Rg3 by Microbacterium sp. GS514. Phytochemistry 2008, 69, 218–224. [Google Scholar] [CrossRef]
- Wang, D.D.; Kim, Y.J.; Hoang, V.A.; Nguyen, N.L.; Singh, P.; Wang, C.; Yang, D.C. Paenibacillus puernese sp. nov., a β-glucosidase-producing bacterium isolated from Pu’er tea. Arch Microbiol. 2016, 198, 211–217. [Google Scholar] [CrossRef] [PubMed]
- Huq, M.A.; Akter, S.; Kim, Y.J.; Farh, M.E.A.; Yang, D.C. Biotransformation of major ginsenoside Rb1 to pharmacologically active ginsenoside Rg3 through fermentation byWeissella hellenica DC06 in newly developed medium Md. Bangladesh J. Sci. Ind. Res. 2016, 51, 271–278. [Google Scholar] [CrossRef]
- Quan, L.H.; Min, J.W.; Subramaniyam, S.; Yang, D.U.; Kim, Y.J.; Yang, D.C. Biotransformation of ginsenosides Re and Rg1 into ginsenosides Rg2 and Rh1 by recombinant b-glucosidase. Biotechnol. Lett. 2012, 34, 913–917. [Google Scholar] [CrossRef]
- Xie, J.; Zhao, D.; Zhao, L.; Pei, J.; Xiao, W.; Ding, G.; Wang, Z. Overexpression and characterization of a Ca2+ activated thermostable β-glucosidase with high ginsenoside Rb1 to ginsenoside 20(S)-Rg3 bioconversion productivity. J. Ind. Microbiol. Biotechnol. 2015, 42, 839–850. [Google Scholar] [CrossRef]
- Quan, L.H.; Min, J.W.; Yang, D.U.; Kim, Y.J.; Yang, D.C. Enzymatic biotransformation of ginsenoside Rb1 to 20(S)-Rg3 by recombinant β-glucosidase from Microbacterium esteraromaticum. Appl. Microbiol. Biotechnol. 2012, 94, 377–384. [Google Scholar] [CrossRef]
- Quan, L.H.; Wang, C.; Jin, Y.; Wang, T.R.; Kim, Y.J.; Yang, D.C. Isolation and characterization of novel ginsenoside-hydrolyzing glycosidase from Microbacterium esteraromaticum that transforms ginsenoside Rb2 to rare ginsenoside 20(S)-Rg3. Antonie Leeuwenhoek 2013, 104, 129–137. [Google Scholar] [CrossRef] [PubMed]
- Cui, C.H.; Kim, J.K.; Kim, S.C.; Im, W.T. Characterization of a ginsenoside-transforming β-glucosidase from Paenibacillus mucilaginosus and its application for enhanced production of minor ginsenoside F2. PLoS ONE 2014, 9, e85727. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Xie, J.; Zhang, X.; Cao, F.; Pei, J. Overexpression and characterization of a glucose-tolerant β-glucosidase from Thermotoga thermarum DSM 5069T with high catalytic efficiency of ginsenoside Rb1 to Rd. J. Mol. Catal. B Enzym. 2013, 95, 62–69. [Google Scholar] [CrossRef]
- Park, J.K.; Yang, D.U.; Arunkumar, L.; Han, Y.; Lee, S.J.; Arif, M.H.; Li, J.F.; Huo, Y.; Kang, J.P.; Hoang, V.A.; et al. Cumulative production of bioactive RG3, RG5, RK1, and CK from fermented black ginseng using novel aspergillus niger KHNT-1 strain isolated from Korean traditional food. Processes 2021, 9, 227. [Google Scholar] [CrossRef]
- Kim, S.A.; Shin, K.C.; Oh, D.K. Complete biotransformation of protopanaxadiol-type ginsenosides into 20-O-β-glucopyranosyl-20(S)-protopanaxadiol by permeabilized recombinant Escherichia coli cells coexpressing β-glucosidase and chaperone genes. J. Agric. Food Chem. 2019, 67, 8393–8401. [Google Scholar] [CrossRef]
- Ke, Y.; Huang, L.; Song, Y.; Liu, Z.; Liang, L. Preparation and pharmacological effects of minor ginsenoside nanoparticles: A review. Front. Pharmacol. 2022, 13, 974274. [Google Scholar] [CrossRef]
- Murugesan, M.; Mathiyalagan, R.; Boopathi, V.; Kong, B.M.; Choi, S.; Lee, C.; Yang, D.C.; Kang, S.C.; Thambi, T. Production of Minor Ginsenoside CK from Major Ginsenosides by Biotransformation and Its Advances in Targeted Delivery to Tumor Tissues Using Nanoformulations. Nanomaterials 2022, 12, 3427. [Google Scholar] [CrossRef]
- Srivastava, N.; Rathour, R.; Jha, S.; Pandey, K.; Srivastava, M.; Thakur, V.K.; Sengar, R.S.; Gupta, V.K.; Mazumder, P.B.; Khan, A.F.; et al. Microbial beta glucosidase enzymes: Recent advances in biomass conversation for biofuels application. Biomolecules 2019, 9, 220. [Google Scholar] [CrossRef]
- Yang, J.; Wang, C.; Guo, Q.; Deng, W.; Du, G.; Li, R. Isolation of the Thermostable β-Glucosidase-Secreting Strain Bacillus altitudinis JYY-02 and Its Application in the Production of Gardenia Blue. Microbiol. Spectr. 2022, 10, e01535-22. [Google Scholar] [CrossRef]
- Almeida, L.E.d.S.; Ribeiro, G.C.A.; Aparecida de Assis, S. β-Glucosidase produced by Moniliophthora perniciosa: Characterization and application in the hydrolysis of sugarcane bagasse. Biotechnol. Appl. Biochem. 2022, 69, 963–973. [Google Scholar] [CrossRef]
- Bi, Y.F.; Wang, X.Z.; Jiang, S.; Liu, J.S.; Zheng, M.Z.; Chen, P. Enzymatic transformation of ginsenosides Re, Rg1, and Rf to ginsenosides Rg2 and aglycon PPT by using β-glucosidase from Thermotoga neapolitana. Biotechnol. Lett. 2019, 41, 613–623. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tran, T.N.A.; Son, J.-S.; Awais, M.; Ko, J.-H.; Yang, D.C.; Jung, S.-K. β-Glucosidase and Its Application in Bioconversion of Ginsenosides in Panax ginseng. Bioengineering 2023, 10, 484. https://doi.org/10.3390/bioengineering10040484
Tran TNA, Son J-S, Awais M, Ko J-H, Yang DC, Jung S-K. β-Glucosidase and Its Application in Bioconversion of Ginsenosides in Panax ginseng. Bioengineering. 2023; 10(4):484. https://doi.org/10.3390/bioengineering10040484
Chicago/Turabian StyleTran, Thi Ngoc Anh, Jin-Sung Son, Muhammad Awais, Jae-Heung Ko, Deok Chun Yang, and Seok-Kyu Jung. 2023. "β-Glucosidase and Its Application in Bioconversion of Ginsenosides in Panax ginseng" Bioengineering 10, no. 4: 484. https://doi.org/10.3390/bioengineering10040484
APA StyleTran, T. N. A., Son, J.-S., Awais, M., Ko, J.-H., Yang, D. C., & Jung, S.-K. (2023). β-Glucosidase and Its Application in Bioconversion of Ginsenosides in Panax ginseng. Bioengineering, 10(4), 484. https://doi.org/10.3390/bioengineering10040484








