Abstract
This study aimed to determine the performance and characteristics of a synthetic barrier membrane of polylactic acid and acetyl butyl citrate (PLAB) for the lateral bone augmentation of peri-implant dehiscence defects (mean height × depth = 3 mm × 1 mm). In eight dogs, three treatment groups were randomly allocated at each chronic peri-implant dehiscence-type defect: (i) a deproteinized bovine bone mineral covered by a synthetic barrier membrane (test group), (ii) a deproteinized bovine bone mineral covered by a natural collagen membrane (positive control), and (iii) a synthetic barrier membrane (negative control). After 4 and 12 weeks of submerged healing, dissected tissue blocks were processed for calcified and decalcified histological analysis. Histometric measurements for tissue and bone width were performed, and bone-to-implant contact and alkaline phosphatase expression where measured. After 4 and 12 weeks of healing, no statistical differences between the groups were observed for the histometric measurements. The expression of alkaline phosphatase was higher in the positive control group after 4 weeks followed by the positive and negative controls (5.25 ± 4.09, 4.46 ± 3.03, and 4.35 ± 2.28%, p > 0.05) and 12 weeks followed by the negative and positive controls (4.3 ± 2.14, 3.21 ± 1.53, and 2.39 ± 1.03%, p > 0.05). Concerning the bone-to-implant contact, after 4 weeks, the test group obtained the highest results (39.54 ± 48.7) vs. (31.24 ± 42.6) and (20.23 ± 36.1), respectively, while after 12 weeks, the positive control group obtained the highest Bone to imaplant contact (BIC) results, followed by the test and negative controls, (35.91 ± 24.9) vs. (18.41 ± 20.5) and (24.3 ± 32.1), respectively; no statistically significant differences were obtained. Within the limitations of the study, new bone formation can be achieved in guided bone regeneration procedures simultaneously with implant placement either with the use of a PLAB membrane or a native collagen membrane, although these differences were not statistically significant.
1. Introduction
The rehabilitation of lost dentition with dental implants has demonstrated high long-term success rates [1,2]; this success, however, is dependent on the bone availability in the alveolar ridge. A sufficient bone volume is not only needed to obtain implant stability but to enable a correct prosthetically driven tridimensional implant position, thus providing an acceptable functional and aesthetic outcome [3]. Bone availability is frequently jeopardized by the causes of tooth loss, since frequently, the presence of chronic/acute infections or trauma results in bone defects in the healed ridge. Even the uneventful healing after tooth extraction results in significant dimensional changes in the alveolar ridge that may condition an appropriate implant placement [1,4,5].
To overcome these limitations in bone availability, which is more frequently in the horizontal dimension, simultaneous implant placement with different bone regeneration interventions for lateral bone augmentation [6] has demonstrated successful outcomes [7]. Among these interventions, guided bone regeneration (GBR) combining a bone replacement graft (BRP) and a barrier membrane (BM) has not only been the most evaluated procedure but the one demonstrating higher predictability [8]. Among the different biomaterials used as BRPs, deproteinized bovine bone mineral (DBBM) has shown the highest predictability, with it being the most used BRP in GBR for lateral bone augmentation procedures [8]. Among the available BMs, bio-absorbable membranes have become the gold standard for lateral bone augmentation, with those made out of natural native porcine collagen (NPCM) being the most frequently used due to their proven properties of biocompatibility and cellular exclusion, together with easy surgical handling [9]. However, these natural collagen BMs have shown fast bio-absorbability during postoperative healing, with a reduction to half of their thickness in the first 2–4 weeks and them being completely resorbed between 4 to 12 weeks [10,11,12]. Furthermore, these forms of NPCM lack structural stiffness, which makes them collapse under light mechanical forces [13].
To overcome limitations from NPCM, cross-linked collagen membranes (CM) has been proposed as an alternative. Cross-linked CM enhances stability and resistance to degradation, ensuring long-term structural support during the healing process [10,11,14,15]. Nevertheless, membrane cross-linking increases the risk of post-operative complications like foreign body reactions, slower vascularization, inflammation, and diminished tissue integration [16] and often leads to reduced bone regeneration [17].
To improve these properties, there has been a search for synthetic bio-absorbable barrier membranes made of different polymers, such as poly(lactic acid) (PLA), poly(glycolic acid) (PGA), poly(epsilon-caprolactone) (PCL), poly(hydroxyl Valerie acid), poly (hydroxyl butyric acid), and copolymers [18].
As part of this initiative, a synthetic barrier membrane was manufactured combining polylactic acid and acetyl butyl citrate (PLAB) with the goal of providing controlled bio-absorbability and greater stiffness [19]. Although the use of a barrier membrane with this composition has been widely used and documented for the periodontal regeneration of intrabony defects and furcations [20,21], there is no information on how a BM with the same composition but designed for bone regeneration would behave, especially in lateral augmentation procedures simultaneous with implant placement [22,23].
It was therefore the aim of this investigation to evaluate the histological behavior and healing under these synthetic barrier membranes when used in experimental dehiscence-type bone defects. Our primary outcome variables were histometric variation in bone and tissue and our secondary outcomes were bone-to-implant contact and the expression of alkaline phosphatase. With this aim, we designed a prospective randomized experimental in vivo investigation comparing a test membrane (PLAB) with a control (NPCM) membrane to reconstruct chronified dehiscence-type bone defects.
2. Materials and Methods
The present work is a complementary analysis to the previously published Micro CT and profilometric data analysis [24]. The present report provides the histological results of the same experimental study.
The present study reports the results of an experimental in vivo investigation comparing a test membrane (PLAB) with a control (NPCM) membrane in the early (4 weeks) and delayed (12 weeks) regeneration of peri-implant dehiscence defects, with the primary outcome being the newly formed bone thickness in the exposed buccal implant surface.
This experimental in vivo investigation was approved by the Regional Ethics Committee for Animal Research (EXP-20170327) and fulfilled the ARRIVE guidelines for animal experimentation [25]. All surgical procedures were conducted at the Minimally Invasive Surgery Centre Jesús Usón in Caceres, Spain, an internationally accredited center for experimental research where specialist veterinary doctors monitored and cared for the animal’s well-being during the entire investigation.
2.1. Study Sample
Eight female beagles (12–24 months in age), weighing between 10 and 15 kg, were selected and monitored 4 weeks before the study to assess their general health status. Each experimental animal was identified by a subcutaneous chip, maintained in an individual kennel under a light/darkness cycle of 12:12 and a controlled temperature of 21–22 °C, and monitored daily by an experienced veterinarian. They were fed with hard pellets specifically manufactured for this species and with free access to water.
2.2. Surgical Interventions
Three different GBR interventions were tested at both, early (4 weeks) and late (12 weeks), healing periods [9,24].
- The test group using deproteinized bovine bone mineral (DBBM) (BioOss®, Geistlich Pharma, Wolhusen, Switzerland) combined with a synthetic polylactic membrane (PLAB) (GUIDOR®, Sunstar, Schlieren, Switzerland)
- The positive control group using DBBM (BioOss®, Geistlich Pharma, Wolhusen, Switzerland) combined with a natural porcine collagen membrane (NPCM) (BioGide®, Geistlich Pharma, Wolhusen, Switzerland)
- The negative control group using only the synthetic polylactic membrane (PLAB) (GUIDOR®, Sunstar, Schlieren, Switzerland)
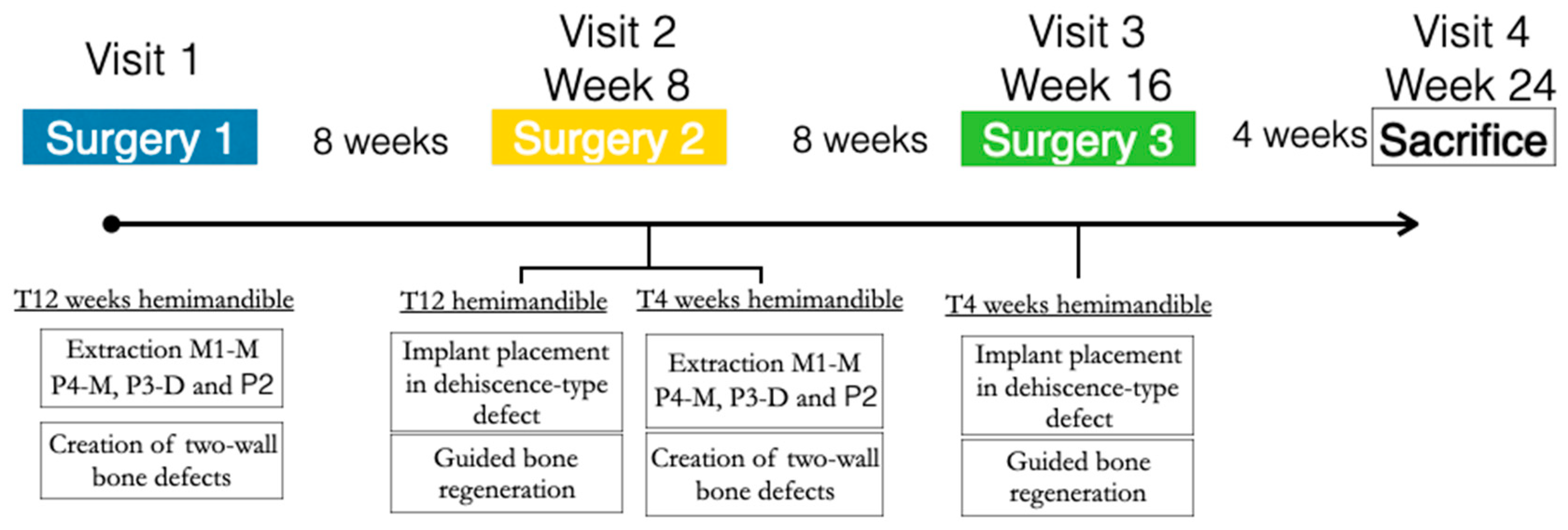
In the three similar peri-implant dehiscence-type defects created in each hemimandible, the three treatment groups were tested for either early or delayed healing (4 and 12 weeks, respectively). A block randomized allocation using a computer-generated list assigned the treatment groups depending on the defect position (mesial, medial, or distal) and the hemimandible side (left or right) (Figure 1).
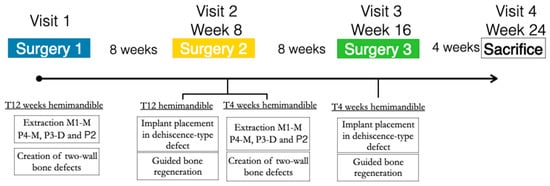
Figure 1.
Study flowchart.
In all of the surgical interventions, the animals were sedated with propofol (2 mg/kg/i.v., Propovet, Abbott Laboratories, Kent, UK), and then, general anesthesia was administrated with 2.5–4% of isoflurane (Isoba-vet, Schering-Plough, Madrid, Spain). Local anesthesia with vasoconstriction (lidocaine 2% with epinephrine 1:100,000) (2% Xylocaine Dental, Dentsply, York, PA, USA) was then infiltrated in the surgical area during the surgery.
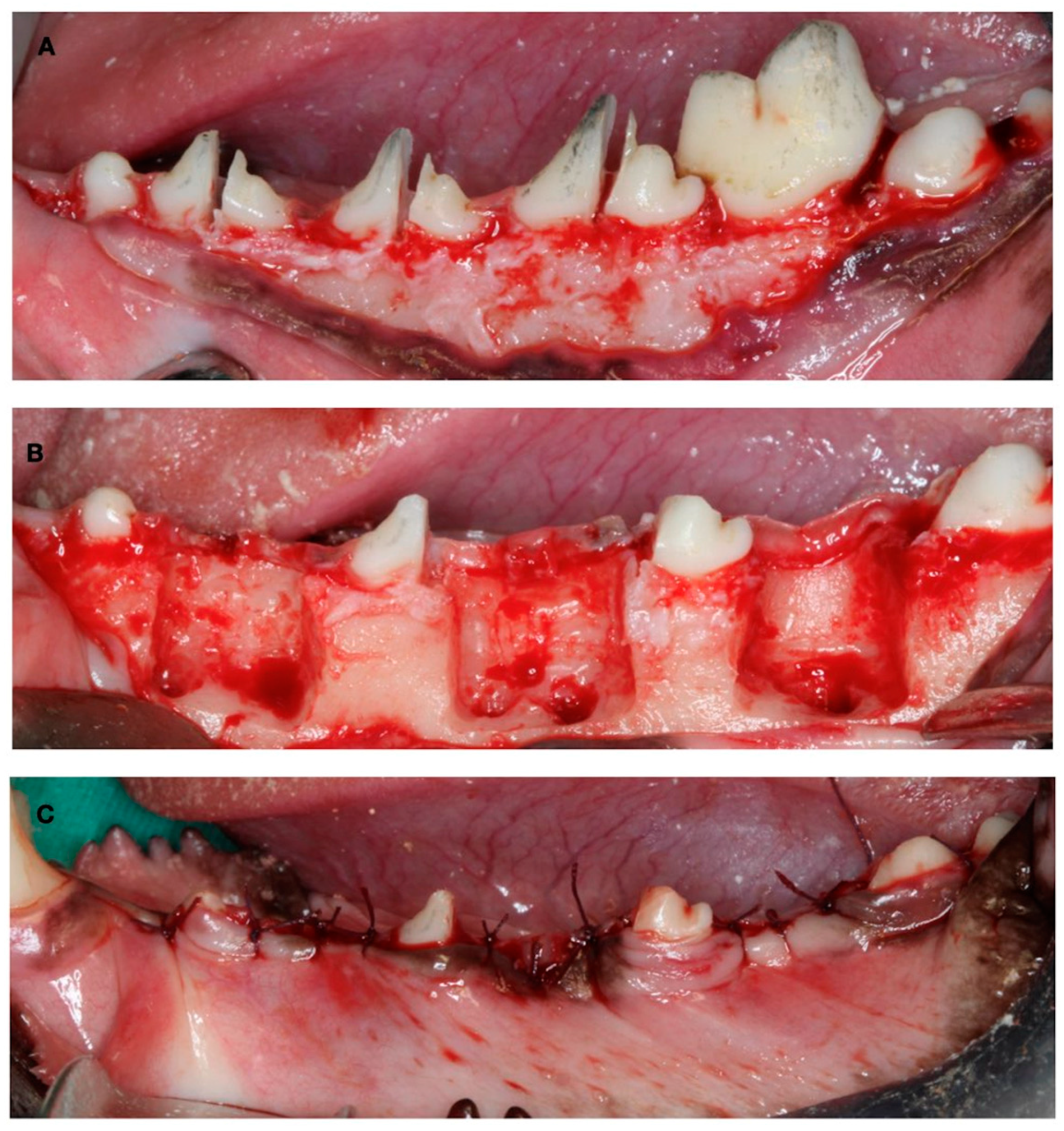
The first surgical Intervention consisted of the creation of experimental defects for the late-healing group. The mucoperiostal flaps were elevated, and the mesial roots of the first molar and fourth premolar and the distal roots of the second and third premolar were carefully extracted. Then, the remaining roots were pulp capped with calcium hydroxide (Dycal, Dentsply, York, PA, USA), and the buccal bone plate of the fresh extraction sockets was removed, thus creating three two-wall bone defects per hemimandible (10 mm × 10 mm × 5 mm) to experimentally induce a narrow ridge. The flaps were then sutured with bio-absorbable sutures to ensure primary soft tissue healing (Figure 2).
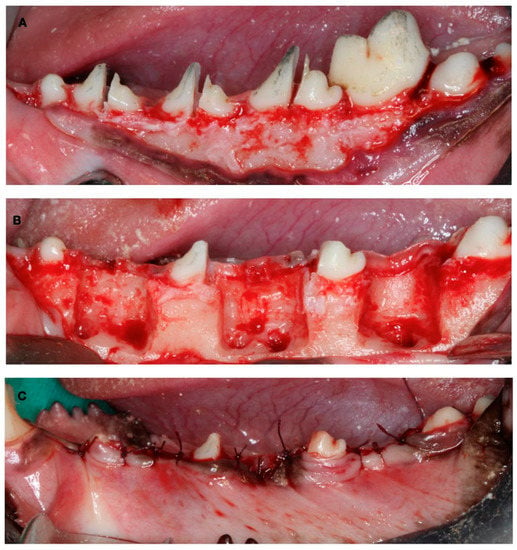
Figure 2.
Surgical defect creation. (A) Tooth hemisection. (B) Tooth extraction and two-wall bone defect creation. (C) Suture.
After 8 weeks of undisturbed healing, the second surgery in this hemimandible was aimed at the simultaneous implant placement and bone regenerative intervention for the delayed healing group (12 weeks), while in the contralateral hemimandible, identical defects were created for the early healing group (4 weeks).
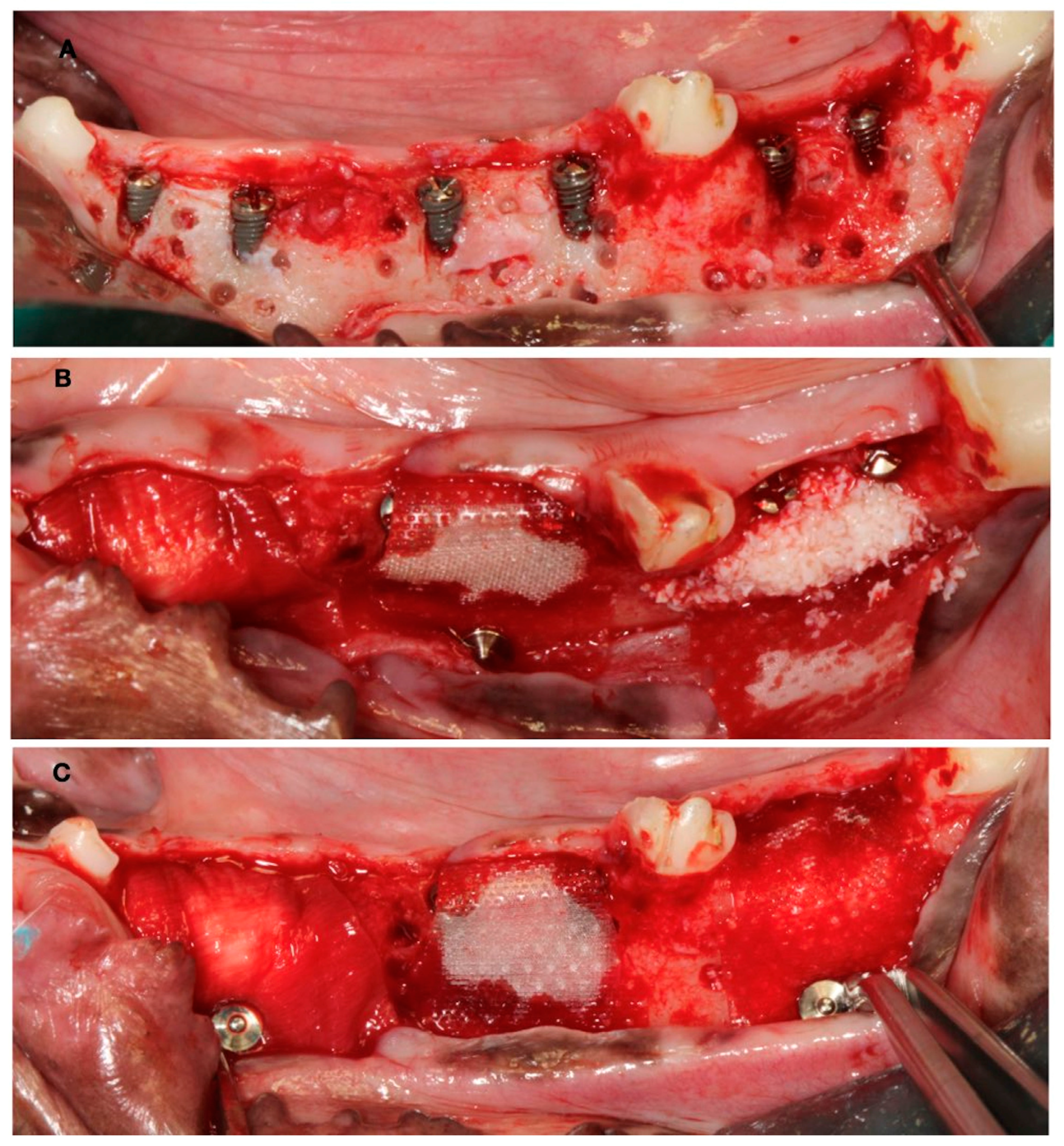
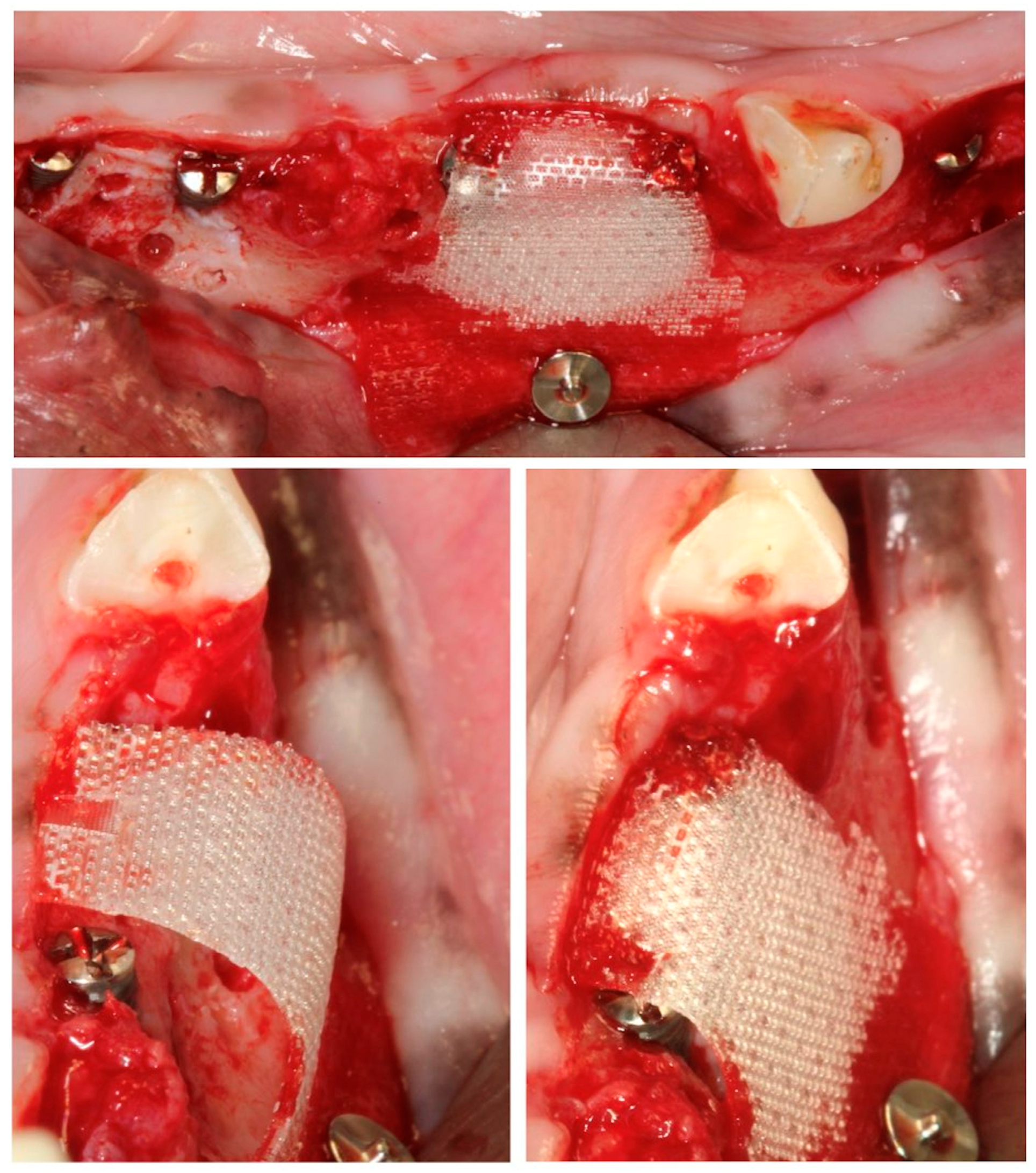
In these chronified bone defects (two-wall narrow ridges), two dental implants of 2.5 mm in diameter and 7 or 9 mm in length (Dentium NR®, Suwon, Republic of Korea) were placed in each defect, resulting in a buccal bone dehiscence around each implant. After measuring the height and width of the dehiscence with a periodontal probe, bone cortical perforations with a small round burr under profuse saline irrigation were made around the dehiscence defects. Then, the experimental treatment groups were randomly allocated, and the corresponding bone replacement grafts and barrier membranes were customized and adapted to properly fill the defects. The membranes were fixed with two metal tacks (Dentium, Seoul, Republic of Korea) to avoid displacement during healing; then, the flaps were closed with bio-absorbable sutures, after resealing the periosteum to assure a passive primary closure (Figure 3).
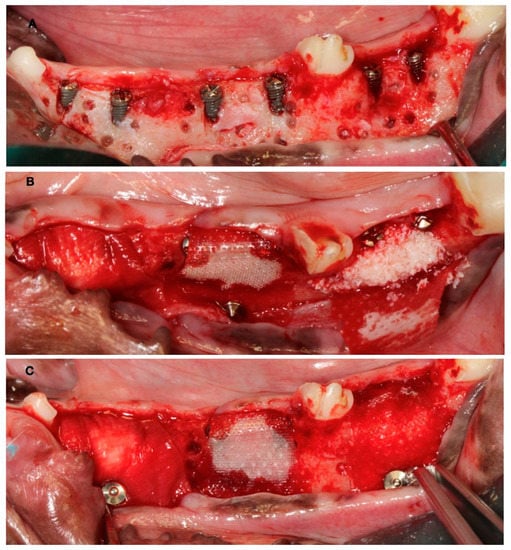
Figure 3.
(A) Implant placement with vestibular dehiscence and bone perforations. (B) Detail of the three regeneration approaches. (C) Membrane stabilization with pins.
After 8 weeks, the same GBR surgical intervention was only carried out on the contralateral side for the 4-week healing group. The contralateral hemimandible remained in the healing process.
Four weeks after this third surgery, the experimental animals were euthanized with a lethal dose of sodium pentothal. Then, the mandibles were extracted, and the tissue blocks were dissected and placed in 10% buffered formalin for histological analysis.
2.3. Histological Analysis
Each fixed tissue block contained one experimental defect that included two implants. These blocks were sectioned into two halves, each containing one implant and one dehiscence defect, and were processed for either decalcified histology or undecalcified ground sectioning. The undecalcified ground sections were processed using the methodology described by Donath and Breuner [26]. In brief, the fixed specimens were first dehydrated in a graded series of ethanol solutions and embedded in a light-curing resin (Technovit 7200 VLC; Heraeus-Kulzer GMBH, Werheim, Germany). From each specimen, one buccolingual section representing the central implant area was micro-ground and polished using a band saw (Exakt® Apparatebau, Norderstedt, Germany) and 1200 and 4000 grit silicon carbide paper (Struers, Copenhagen, Denmark) until achieving a thickness of approximately 30 μm. The obtained sections were then stained using Mason Goldner’s trichrome staining. These histological sections were analyzed using a Leica DMRBE microscope equipped with a Leitz DMRD micro-photographic unit (Leica Microsystems GmbH, Wetzlar, Germany) connected to a digital camera and a computer. High-resolution images were acquired and measured using a dedicated image analysis software (Image Pro Premiere 9.1; Media Cybernetic Inc., Rockville, Maryland). The histometric measurements were performed at 10× magnification using a calibrated digital tool (“smart segmentation tool” (SST)) incorporated into the image analysis software (Image pro premier 9.1).
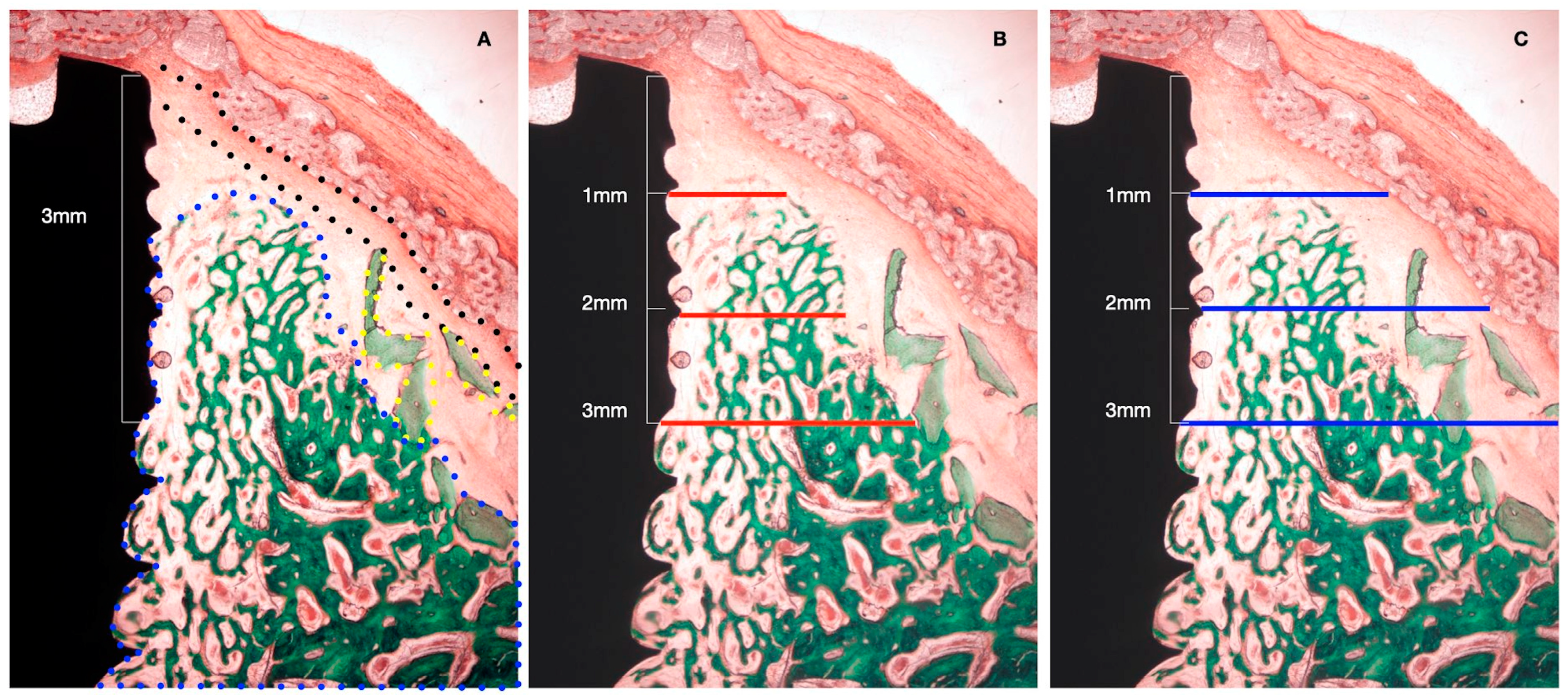
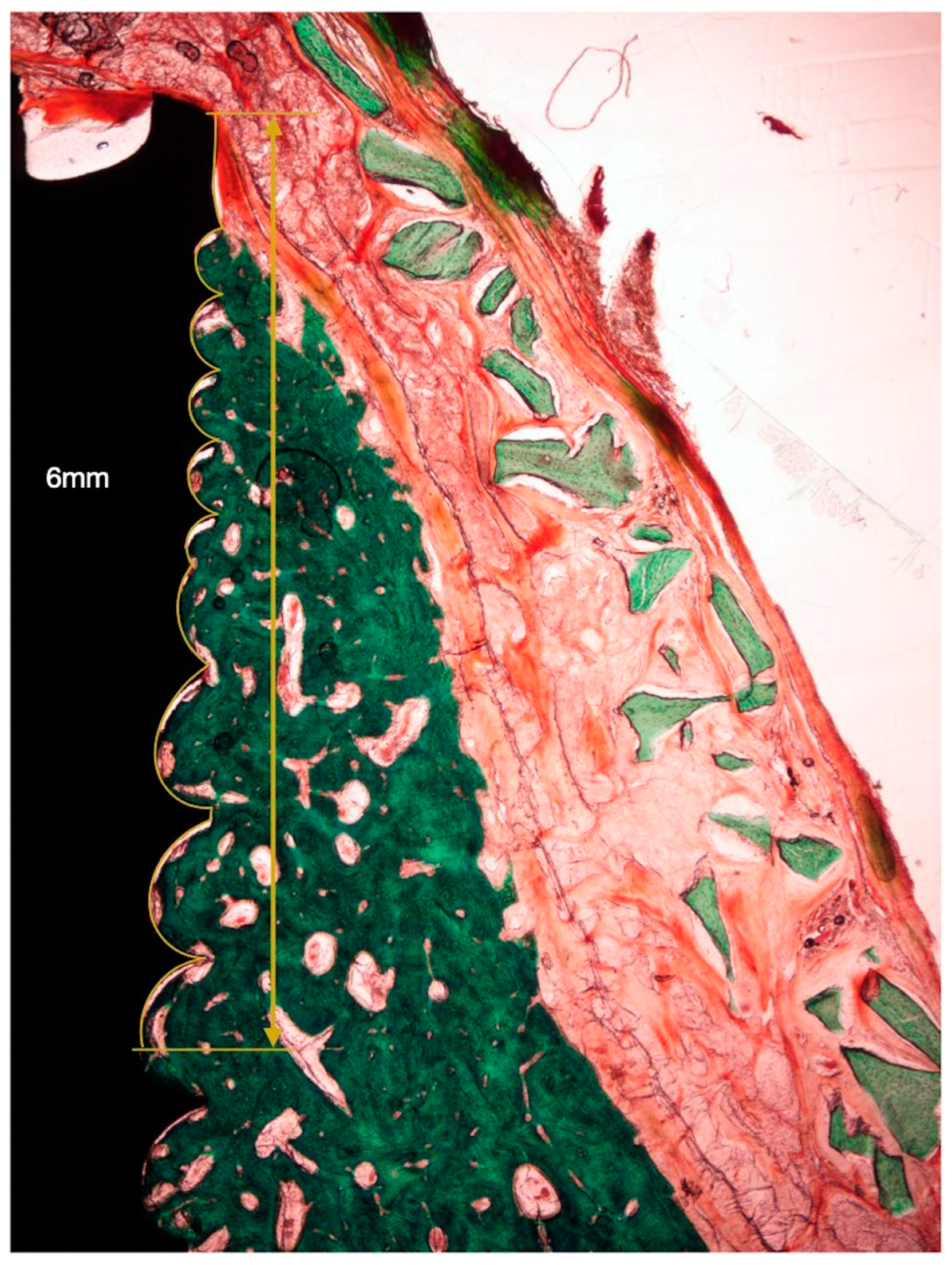
The following horizontal linear measurements were obtained at the buccal aspect of the implant, perpendicular to its long axis (Figure 4):
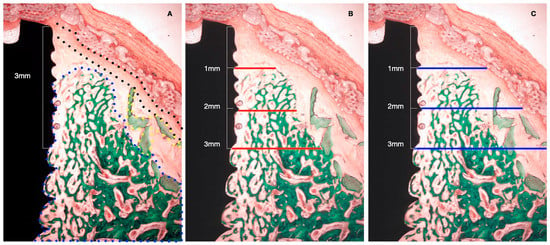
Figure 4.
Histometric measurements of bone width and membrane width at 1, 2, and 3 mm. (A) Histological tissue identification (NB: newly formed bone, blue dots. XG: xenograft particles, yellow dots. MB: barrier membrane, black dots). (B) Histometrical measurement: newly formed bone. (C) Histometrical measurement: barrier space maintenance (augmented tissue thickness).
- NBT: Newly formed bone thickness at 1, 2, and 3 mm apical to the implant shoulder, described as the distance from the implant surface and the most buccal bone tissue.
- AGT: Augmented tissue thickness at 1, 2, and 3 mm apical to the implant shoulder (barrier space maintenance capacity), described as the distance from the implant surface to the inner part of the identified barrier membrane.
The percentage of bone-to-implant contact (BIC) was also calculated along the buccal aspect of the implants.
2.4. Decalcified Histology
For the immunohistochemical preparation, we processed the samples according to the fracture technique [27]. In brief, the tissue blocks were decalcified with EDTA (12.5%, pH = 7), which was replaced every week for 8 months. Once decalcified, the tissue blocks were split into vestibular and lingual sides, with the dental implant being removed very carefully. The resultant vestibular pieces were decalcified for two extra months with a different decalcifier (Osteosoft ®, Merck; Branchburg, New Jersey, USA) and then embedded in paraffin. The resulting blocks were cut into 7 µm-thick sections and stained with hematoxylin and eosin (PanReac AppliChem; Darmstadt, Germany). The remaining slides were used for immunohistochemical staining.
Immunohistochemical staining was carried out with the “Master Polymer Plus Detection System (Peroxidase)” kit (Master Diagnóstica; Granada, Spain), which uses rabbit monoclonal and polyclonal primary antibodies and DAB (3,3′-diaminobenzidine) as visualization solution. Specific antibodies for alkaline phosphatase (1:200 Santa Cruz Biotechnology Inc.; Santa Cruz, CA, USA) were used to measure bone metabolism.
2.5. Immunohistochemical Analysis
All slides were observed using a light microscope (Leica Geosystems AG; Heerbrugg, Switzerland) and photographed by ×2.5 magnification with a digital camera (Leica Geosystems AG, Heerbrugg, Switzerland) in a standardized light condition. A 3 mm × 3 mm square region of interest (ROI) was delimited, taking the implant shoulder as a reference [28]. The stained intensity (%) of the ALP was measured using an IHC profiler and Image J (NIH, Bethesda, Bethesda, Maryland, USA), for the quantitative analysis [29,30]. The results were divided into four categories according to the intensity of staining: high positive, positive, low positive, and negative. To reduce false positives, only high positive and positive stained values were analyzed.
2.6. Data Analysis
Data from continuous outcome variables were expressed as means and standard deviations. Shapiro-Wilk normality tests were used to assess the data distribution. Whenever normality could be assumed, ANOVA tests were used for the intergroup comparisons in each of the healing time point groups (4 weeks and 12 weeks). When the data did not accomplish normality, the Kruskall-Wallis test was performed to assess the intergroup comparisons. Bonferroni corrections were performed for multiple comparisons. The alpha error was set at 0.05, and all tests were performed with the statistical software package (IBM SPSS Statistics® V20 JM.Domenech).
3. Results
The healing after the surgical interventions was uneventful without the advent of any wound dehiscence or other local adverse events. All implants and the grafted materials healed uneventfully and resulted in 48 defects being prepared for ground undecalcified sections and analyses (16 tests, 16 positives, and 16 negative control groups), with 24 for each healing period (4 and 12 weeks). Differences in the surgical handling of the tested BMs were noticeable, with the PLAB membrane being stiffer and better suited to maintain the space underneath, even without the use of a bone replacement graft (Figure 5).
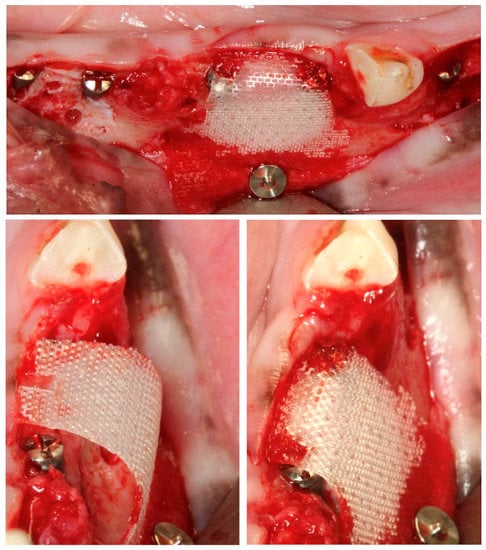
Figure 5.
Space maintenance properties of the PLAB membrane when used without the mechanical support of a biomaterial underneath.
3.1. Histological Observations
Bone regeneration up to the implant platform was never observed in any of the treatment groups. In the groups using a bone replacement graft, DBBM particles were found surrounded by new bone, although in the outer parts of the regenerated area, the granules were sometimes encapsulated by connective tissue. In the test group, the PLAB membrane remained basically unaltered in most of the samples during both healing periods, while in the positive test group, although the membrane was recognizable at 4 weeks of healing, it was partially resorbed or even undetectable in the late healing period at 12 weeks.
In the negative control group (the PLAB membrane alone), newly formed bone was observed in contact with the implant surface, and the proportion of new mineralized tissue was higher when compared with the tested groups using DBBM, although its thickness was considerably inferior. In all groups, a space between the bone and the membrane was frequently observed, which may correspond to either immature bone not detectable in the histological preparations or a dense periosteum-like tissue.
After 4 weeks of healing, new bone in contact with the implant surface at the dehiscence area could be identified in both the test and positive control group. In both groups, the presence of bone substitute particles occupied most of the space under the membrane, with them being surrounded by bone. In the negative control group, the apical area of the dehiscence was filled with new bone; in the coronal area, the membrane had collapsed into the implant surface, with the space between the membrane and implant occupied by soft tissue at this level.
In the PLAB membrane groups, the membrane structure and space were preserved, while in the sections with a native collagen membrane, its presence was frequently disintegrated, exhibiting tissue interstices between the membrane segments. In all groups, periosteum-like tissue was observed underneath the barrier membrane (Figure 6).
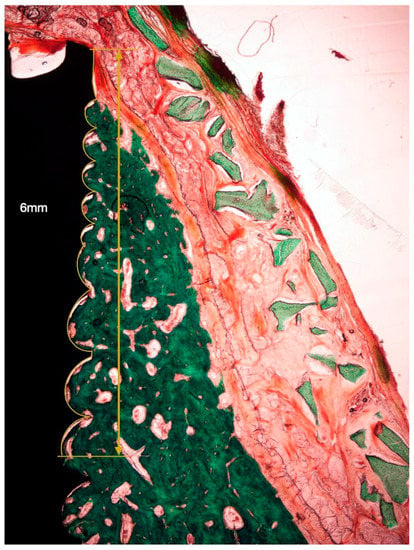
Figure 6.
Histological bone-to-implant contact measurement. BIC: bone in contact with the implant surface within 6 coronal millimeters from the implant shoulder.
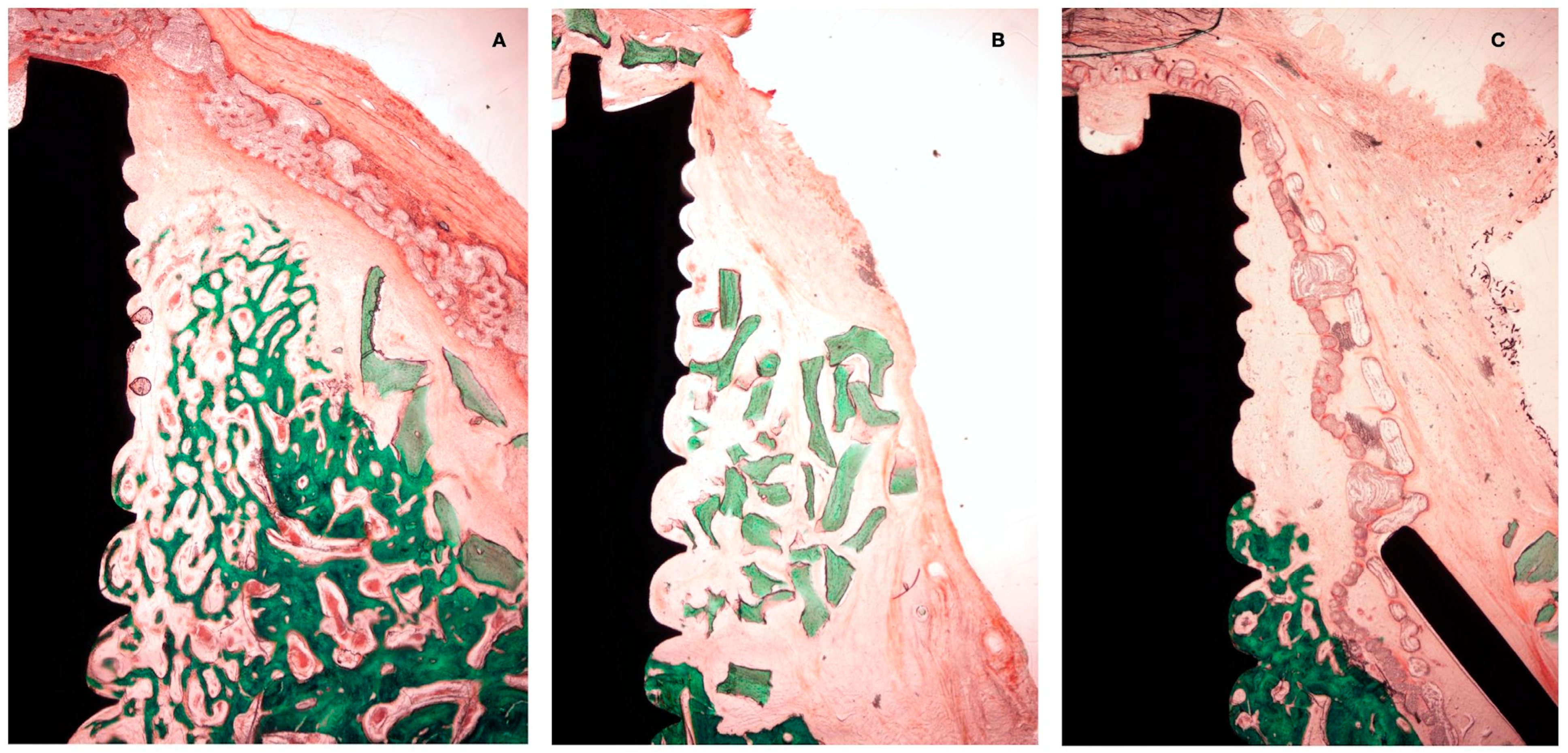
A net reduction in thickness was noted in all groups between 4 and 12 weeks. Hard tissue modeling was observed in most of the specimens using a bone replacement graft, especially bone apposition around the xenograft particles. In the negative control group, there was scarce additional bone modeling. At 12 weeks, the collagen membrane appeared in most of the sections as completely resorbed, while the PLAB membrane was still present, although with a reduced thickness (Figure 7). In most of the sections, there was an apical displacement of the bone replacement graft.
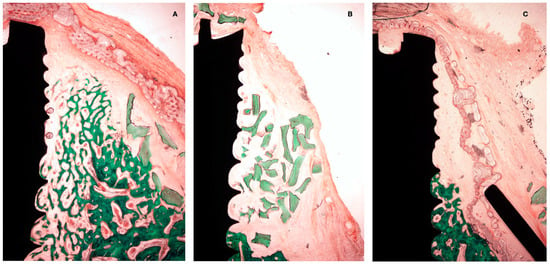
Figure 7.
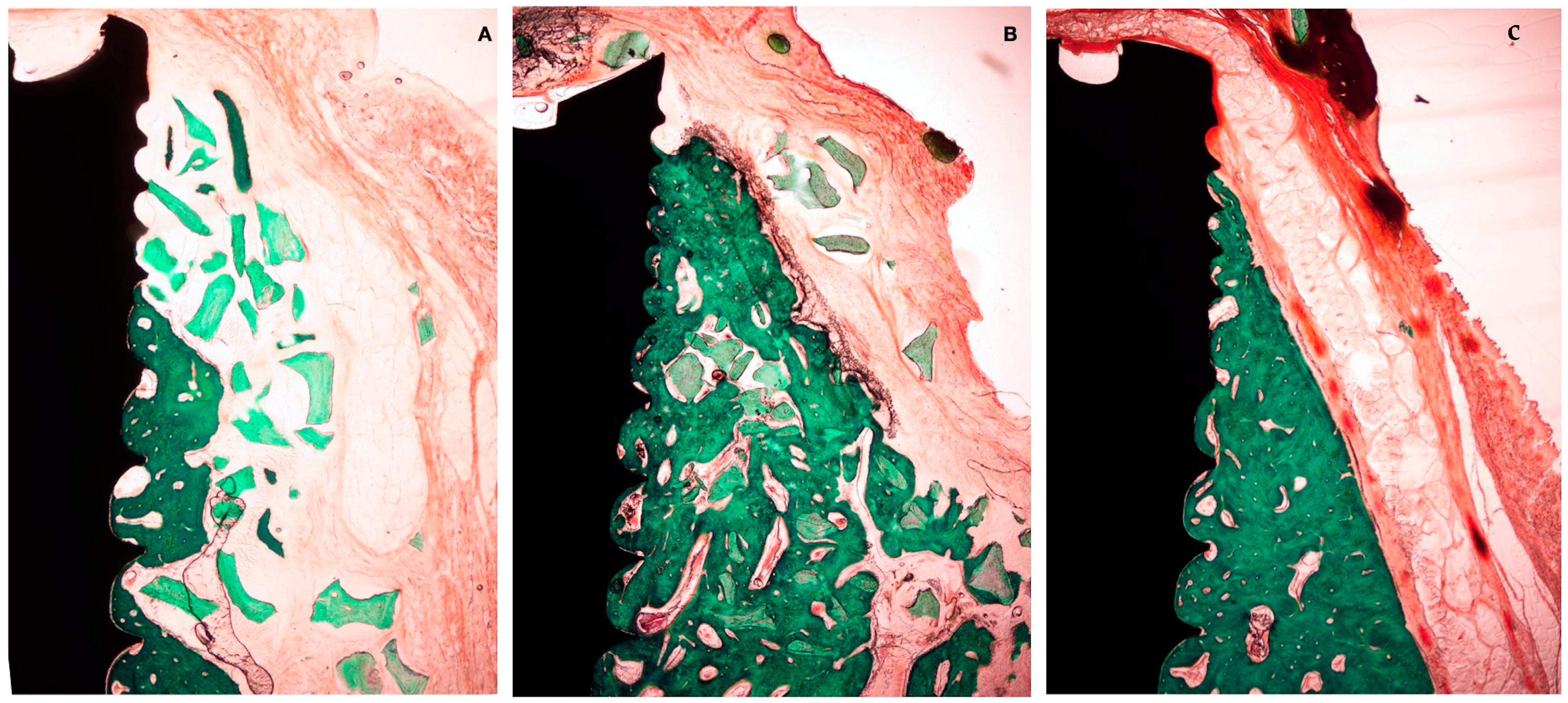
Histological preparation at 4 weeks of healing. (A) Test group. (B) +C group. (C) -C group.
3.2. Histo-Morphometric Measurements
3.2.1. Newly Formed Bone at 4 Weeks
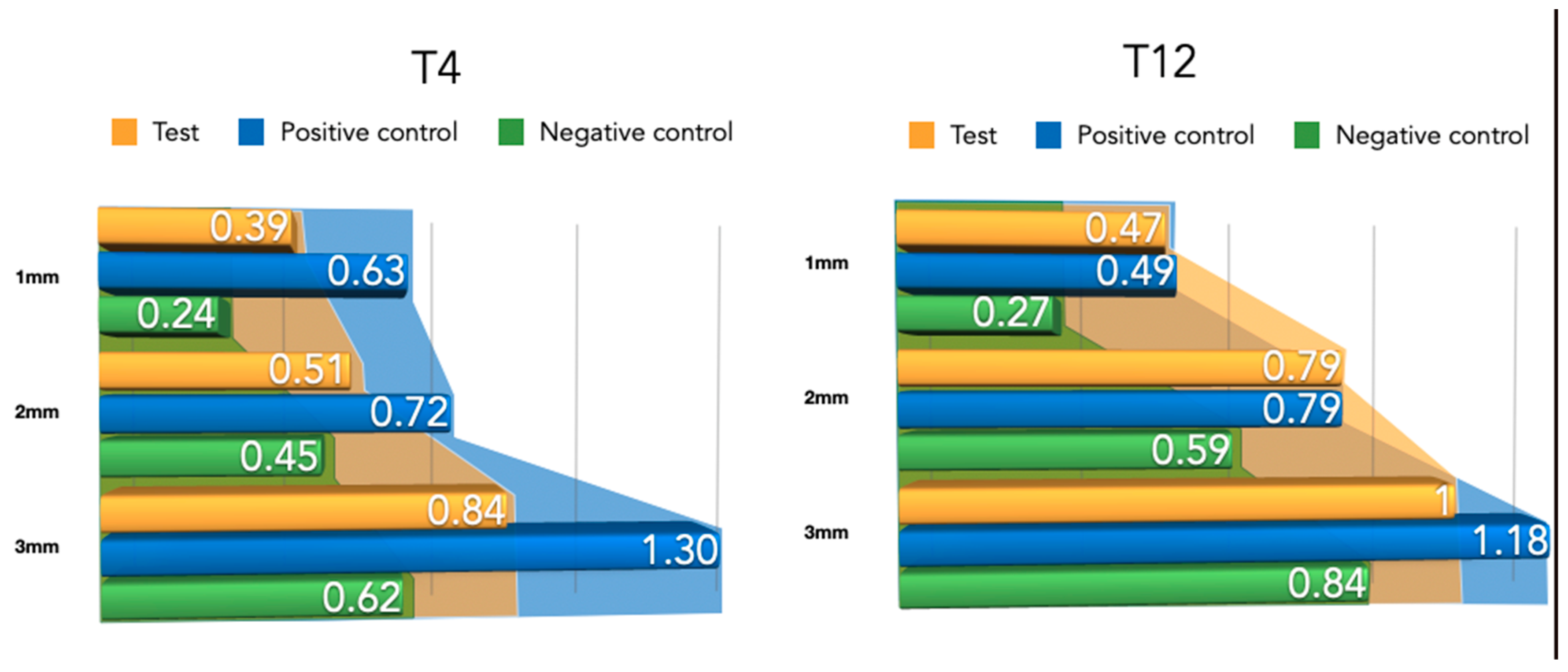
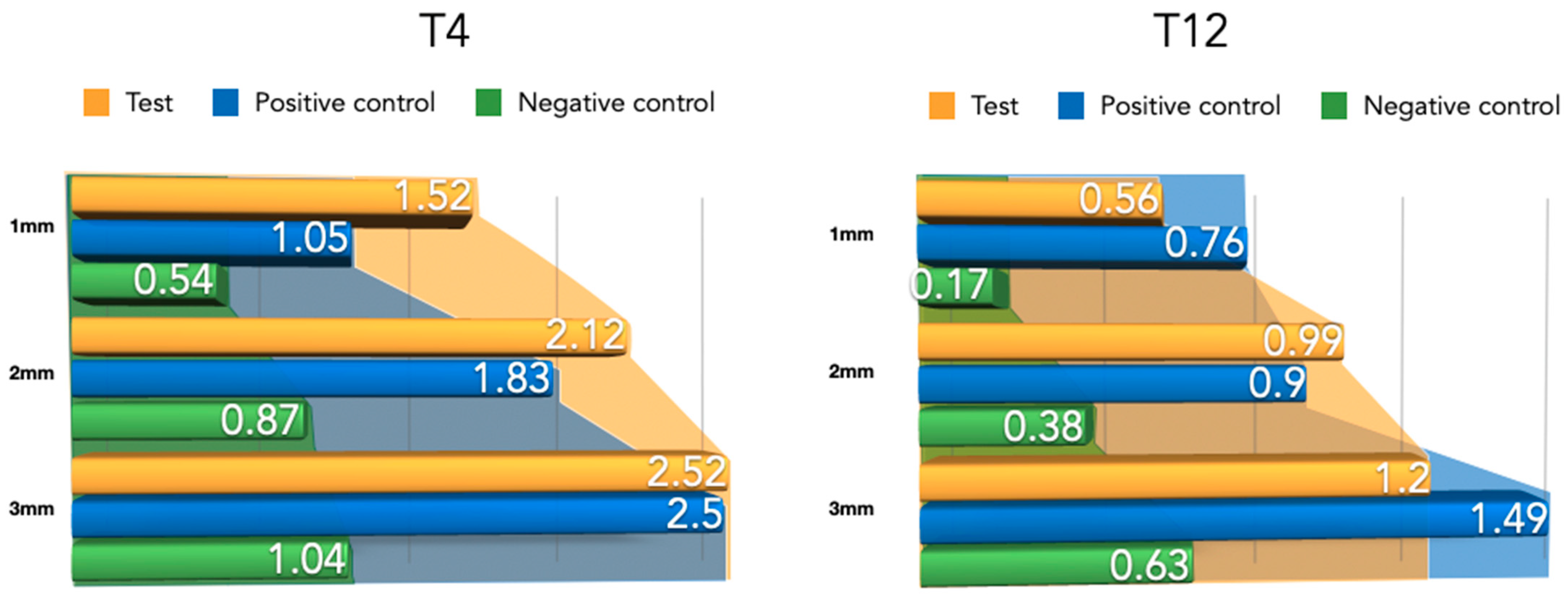
The width of the newly formed buccal bone width at 1–2 and 3 mm from the implant platform is presented in Table 1 for the three tested groups. Although the differences were not statistically significant, the positive control group (NPCM) attained a higher width of new bone compared with the test group (PLAB) and the membrane-only group.

Table 1.
Newly formed bone thickness measurements for both healing periods (4–12 weeks) at 1 mm, 2 mm, and 3 mm apico-coronal levels. All measurements are expressed in millimeters (mean ± SD values).
3.2.2. Newly Formed Tissue Thickness at 4 Weeks
Differently from the results for the newly formed buccal bone width, during this healing period, the PLAB membrane group attained wider tissue thickness compared with the positive and negative control groups (Figure 8). At 3 mm from the implant platform, higher values were obtained by the test group, followed by the positive control and the negative control, (2.52 ± 0.9) vs. (2.50 ± 0.54) and (1.04 ± 0.63), with the difference between the test and negative control group being statistically significant (p = 0.03) (Table 2).
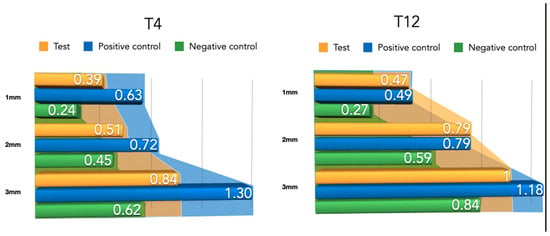
Figure 8.
Newly formed bone thickness after 4 and 12 weeks of healing.

Table 2.
Augmented tissue thickness (barrier space maintenance) measurements for both healing periods (4–12 weeks) at 1 mm, 2 mm, and 3 mm apico-coronal levels. All measurements are expressed in millimeters (mean ± SD values).
3.2.3. Newly Formed Buccal Bone at 12 Weeks
During this healing period, the differences among the groups regarding the width of newly formed bone were reduced (Figure 9). An apical displacement of the bone replacement graft was clearly noticeable in both the test and positive control group. Table 2 depicts the linear measurements of increased width at the three height levels, with consistently higher widths in the positive control group, although very similar to the test group, followed by the negative control group. These differences were not statistically significant.
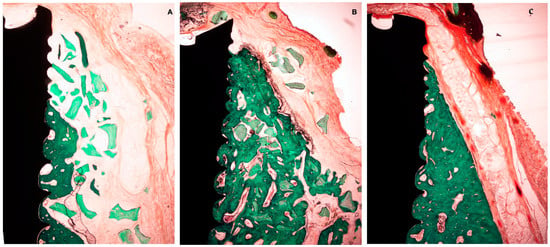
Figure 9.
Histological preparation at 12 weeks of healing. (A) Test group. (B) +C group. (C) -C group.
3.2.4. Newly Formed Tissue Thickness at 12 Weeks
Compared to the 4-week measurements, clear tissue shrinkage had occurred in the three groups at all measured levels (Figure 10). The attained increased tissue thickness was similar between the test and positive control group (Table 2).
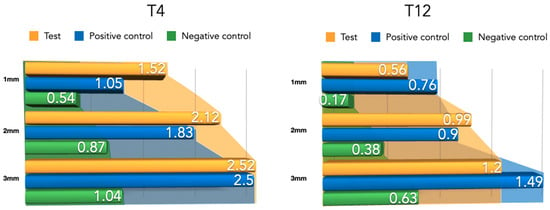
Figure 10.
Barrier space maintenance (augmented tissue thickness) after 4 and 12 weeks of healing.
3.3. Bone to Implant Contact
At 4 weeks of healing, the highest BIC was obtained by the test group (39.54 ± 48.7) and the negative control (31.24 ± 42.6), while the more discrete results were obtained by the positive control (20.23 ± 36.1).
After 12 weeks of healing, the positive control group experienced an improvement in BIC, obtaining the best results (35.91 ± 24.9) while test and negative control groups experienced a decrease in BIC, (18.41 ± 20.5) and (24.3 ± 32.1) respectively.
3.4. Immunohistochemical Analysis
ALP was detected mainly around newly formed bone and bone substitutes. In all the groups the stain was more intense after 4 weeks of healing, while it decreased after 12 weeks of healing. The positive control group showed higher ALP reactivity than the test and negative controls at 4 weeks, although the differences were not statistically significant (5.25 ± 4.09, 4.46 ± 3.03, and 4.35 ± 2.28%, respectively, p > 0.05). After 12 weeks of healing the highest results were obtained in the positive control group followed by the test group and positive control (4.3 ± 2.14, 3.21 ± 1.53, and 2.39 ± 1.03%, respectively, p > 0.05).
4. Discussion
The present pre-clinical investigation evaluated the performance of three different guided bone regenerative procedures aimed at lateral bone augmentation using an experimental in vivo model of peri-implant dehiscence. The use of the DBBM + PLAB membrane showed a trend towards higher performance in terms of space maintenance capacity at the early healing stage, while, after 12 weeks of healing, these differences were lost with even a slightly better result in terms of newly formed bone and space maintenance with the collagen membrane + DBBM, although these differences were not statistically significant.
The available scientific evidence reports that native collagen membranes have a fast resorption rate, albeit maintaining their structural integrity between 4–8 weeks [31,32]. These results have been corroborated in the present investigation where the collagen membrane structure could be identified at 4 weeks but was fully or partially disintegrated at 12 weeks. Another potential disadvantage of native collagen membranes is their low stiffness and, hence, their lack of maintenance-keeping ability [10,33,34]. This fact was also corroborated in this investigation since the tissue thickness attained in this group at 4 weeks was clearly inferior to the tested synthetic membrane.
The synthetic polymer membrane tested (PLAB) has been reported to start its degradation after 6 weeks, with membrane remnants still being identifiable even after 12 months of healing [19]. Preclinical studies comparing the resorption rate of PLA and collagen membranes have concluded that PLA membranes will maintain their integrity for longer [35,36]. In the present study, complete integrity of the PLAB membrane was observed in the histological sections after 4 weeks of healing, while the rest of the membrane was still present in the 12-week sections. These synthetic bio-absorbable membranes (PLAB) have reported superior space maintenance properties [20,21], which is congruent with the results from the present investigation at the early healing stage, PLAB membranes demonstrated better results in bone and augmented tissue thickness, while the integrity of the membrane was maintained (4 weeks). However, after 12 weeks of healing, the PLA membrane lost its integrity, probably due to its mechanism of biodegradation. While collagen membranes are bio-absorbed by enzymatic degradation [3], synthetic polymer membranes like PLAB, when inserted into an aqueous environment, are degraded by hydrolysis [20,23]. This hydrolysis process leads to reduced PH levels because of the release of acidic degradation products, which may induce an inflammatory response [37,38]. This possible inflammatory response could explain the reason why after 12 weeks of healing, the group of the DBBM + PLAB membrane experienced a remarkable decrease in bone width, compared with the DBBM + collagen membrane group.
However, in both tested groups, there was a reduction in the width of bone and tissue thickness between 4–12 weeks. This finding may be explained by the tissue shrinkage that usually occurs in the late healing phases when the treated defects are over-contoured during the bone regenerative intervention with the bone replacement graft outside the bony envelope. The fact that the test group demonstrated greater shrinkage compared with the positive control group using the same bone replacement graft and a similar degree of over-contouring may be explained by differences in the membrane resorption process [19,31,38].
This tissue shrinkage between T4 and T12 was more pronounced coronally, with displacement of the BRG granules apically. This result is also consistent with previous similar pre-clinical investigations [24] and can be explained by the prolonged action of the masticatory forces, with them being more intense in the coronal aspect, thus pushing the biomaterial to the apical and lateral directions [39,40].
When the PLAB membrane was used without a bone replacement graft (negative control group), there was marked new bone formation after 4 and 12 weeks of healing, which corroborates both the barrier membrane and enhanced space-maintaining properties of this synthetic membrane. In fact, in some sections, the amount of new bone formation was superior in the membrane-only group, compared with the groups using a BRG. Similar results have been reported in other pre-clinical investigations where more pronounced bone formation with a membrane alone occurred mainly in distal defects [41], thus implying that a more favorable defect morphology with thicker buccal bone plates may enhance GBR with only a BM since the space maintenance is improved. Also, similar heterogeneous results have been reported in clinical studies using a BM with enhanced space-keeping properties alone in GBR simultaneous with implant placement. This study reported enhanced bone regeneration in some cases, while in others, the space under the membrane was fully collapsed onto the implant surface, thus recommending the use of a BRG for more consistent results [42].
Although in this investigation we did not test the behavior of the use of collagen membranes without a bone replacement graft, a previous report from our research group using a similar experimental model clearly demonstrated their barrier membrane effect, by demonstrating new bone formation under the membrane [9]. Conversely, results from a similar pre-clinical in vivo experiment, where no membrane and no bone replacement graft were used, the dehiscence defects remained unrepaired in the negative control group, with minimal natural bone healing occurring at the base of the defect [9]. The results from these reports, together with those reported in the present study, strengthen the importance of membrane barrier function to allow new bone formation by preventing the ingrowth of connective tissue into the defect.
In the immunohistochemical analysis, we used ALP as a recognized biomarker of osteoblast metabolism [43]. Previous investigations have reported that ALP expression after implant placement reaches its peak after 5–20 days [44], which correlates with the primary mineralization around dental implants. In a similar study performed by our research group [28], higher values of ALP expression were shown after 8 weeks of healing, and the ALP staining was still present after 16 weeks of healing. These results have been corroborated in the present investigation, where ALP activity was still present at 12 weeks, albeit reduced compared with the 4-week healing time.
The recent formulation of non-cross-linked collagen membranes demonstrates notable improvements in degradation time, mechanical properties, and an extended degradation period which enhanced the mechanical characteristics, including higher tensile strength and suture pullout strength [45]. Nevertheless, no differences were determined when compared with the standard collagen membranes in dehiscence-type models [46].
In a similar study to the present one, Al-Hazmi [46] evaluated different regenerative strategies in dehiscence-type defects in dogs by means of micro-CT analysis, obtaining the best results when a collagen membrane was used over the biomaterial. In another study, evaluating dehiscence-type defects in dogs, Jung 2017 obtained higher histometric results when a cross-linked collagen membrane was used (1.22 ± 0.53 mm) than in the positive and negative controls (0.42 ± 0.51 and 0.36 ± 0.50 mm, respectively) [9].
Although this in vivo preclinical investigation has clearly corroborated previous reports on the importance of using a BM in GBR simultaneous with implant placement, its results should be taken with caution in part due to the inherent limitations of the experimental model used, mainly in relation to its translation to humans. Nevertheless, it is important to highlight that the bone defects were left to chronified, thus mimicking a two-wall bone defect, which is frequently found in clinical human conditions. Another limitation in this experimental model is due to the possible influence of the bone defect position (mesial, medial, or distal), since distal defects are usually surrounded by thicker buccal bone plates, while mesial defects are contained in thinner bonny envelopes. It has been demonstrated that the thickness of the base of the defect may influence the level of bone regeneration [42]. Although randomization also accounted for the defect position, this is an intrasubject condition that may influence the results in part.
In spite of these limitations, we can conclude that new bone formation can be achieved in GBR procedures simultaneous with implant placement either with the use of a PLAB membrane or a native collagen membrane. Although in the early healing stages, the PLAB membrane seemed to attain higher bone and tissue width when compared to native collagen membranes, the increased shrinkage that occurred in the PLAB group reversed the results in the late healing period, although these differences were not statistically significant.
Author Contributions
Methodology, J.S.-E.; investigation, R.P., J.S.-E., F.N., F.V., P.G., and M.S.; writing—original draft, R.P.; writing—review and editing, J.S.-E., F.V., and M.S.; supervision, M.S. All authors have read and agreed to the published version of the manuscript.
Funding
This study was partially funded through a research contract between the Sunstar Group (Switzerland) and the University Complutense of Madrid.
Institutional Review Board Statement
The animal study protocol was approved by the ethic committee for Animal research from Minimally Invasive Institute Jesus Usón, Cáceres, Spain, (EXP-20170327).
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
We would like to thank Fernando Luengo and Riccardo di Raimondo who actively collaborated on the surgical procedure. We would like to thank all of the staff from the Minimally Invasive Surgery Center, Caceres, Spain, for their care of the animals and their help during the surgical phases.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Moraschini, V.; Poubel, L.A.; Ferreira, V.F.; Barboza Edos, S. Evaluation of survival and success rates of dental implants reported in longitudinal studies with a follow-up period of at least 10 years: A systematic review. Int. J. Oral Maxillofac. Surg. 2015, 44, 377–388. [Google Scholar] [CrossRef] [PubMed]
- Pjetursson, B.E.; Thoma, D.; Jung, R.; Zwahlen, M.; Zembic, A. A systematic review of the survival and complication rates of implant-supported fixed dental prostheses (FDPs) after a mean observation period of at least 5 years. Clin. Oral Implant. Res. 2012, 23 (Suppl. S6), 22–38. [Google Scholar] [CrossRef] [PubMed]
- Spray, J.R.; Black, C.G.; Morris, H.F.; Ochi, S. The influence of bone thickness on facial marginal bone response: Stage 1 placement through stage 2 uncovering. Ann. Periodontol. 2000, 5, 119–128. [Google Scholar] [CrossRef] [PubMed]
- Araujo, M.G.; Lindhe, J. Dimensional ridge alterations following tooth extraction. An experimental study in the dog. J. Clin. Periodontol. 2005, 32, 212–218. [Google Scholar] [CrossRef]
- Araújo, M.G.; Silva, C.O.; Misawa, M.; Sukekava, F. Alveolar socket healing: What can we learn? Periodontology 2000 2015, 68, 122–134. [Google Scholar] [CrossRef]
- Donos, N.; Mardas, N.; Chadha, V. Clinical outcomes of implants following lateral bone augmentation: Systematic assessment of available options (barrier membranes, bone grafts, split osteotomy). J. Clin. Periodontol. 2008, 35, 173–202. [Google Scholar] [CrossRef]
- Kuchler, U.; von Arx, T. Horizontal ridge augmentation in conjunction with or prior to implant placement in the anterior maxilla: A systematic review. Int. J. Oral Maxillofac. Implant. 2014, 29, 14–24. [Google Scholar] [CrossRef]
- Sanz-Sanchez, I.; Ortiz-Vigon, A.; Sanz-Martin, I.; Figuero, E.; Sanz, M. Effectiveness of Lateral Bone Augmentation on the Alveolar Crest Dimension: A Systematic Review and Meta-analysis. J. Dent. Res. 2015, 94, 128S–142S. [Google Scholar] [CrossRef]
- Jung, U.-W.; Cha, J.-K.; Vignoletti, F.; Nuñez, J.; Sanz, J.; Sanz, M. Simultaneous lateral bone augmentation and implant placement using a particulated synthetic bone substitute around chronic peri-implant dehiscence defects in dogs. J. Clin. Periodontol. 2017, 44, 1172–1180. [Google Scholar] [CrossRef]
- Rothamel, D.; Schwarz, F.; Sager, M.; Herten, M.; Sculean, A.; Becker, J. Biodegradation of differently cross-linked collagen membranes: An experimental study in the rat. Clin. Oral Implant. Res. 2005, 16, 369–378. [Google Scholar] [CrossRef]
- Schwarz, F.; Rothamel, D.; Herten, M.; Sager, M.; Becker, J. Angiogenesis pattern of native and cross-linked collagen membranes: An immunohistochemical study in the rat. Clin. Oral Implant. Res. 2006, 17, 403–409. [Google Scholar] [CrossRef]
- Schwarz, F.; Sahm, N.; Bieling, K.; Becker, J. Surgical regenerative treatment of peri-implantitis lesions using a nanocrystalline hydroxyapatite or a natural bone mineral in combination with a collagen membrane: A four-year clinical follow-up report. J. Clin. Periodontol. 2009, 36, 807–814. [Google Scholar] [CrossRef] [PubMed]
- Jung, R.E.; Glauser, R.; Scharer, P.; Hammerle, C.H.; Sailer, H.F.; Weber, F.E. Effect of rhBMP-2 on guided bone regeneration in humans. Clin. Oral Implant. Res. 2003, 14, 556–568. [Google Scholar] [CrossRef] [PubMed]
- Friedmann, A.; Dehnhardt, J.; Kleber, B.M.; Bernimoulin, J.P. Cytobiocompatibility of collagen and ePTFE membranes on osteoblast-like cells in vitro. J. Biomed. Mater. Res. A 2008, 86, 935–941. [Google Scholar] [CrossRef] [PubMed]
- Sbricoli, L.; Guazzo, R.; Annunziata, M.; Gobbato, L.; Bressan, E.; Nastri, L. Selection of Collagen Membranes for Bone Regeneration: A Literature Review. Materials 2020, 13, 786. [Google Scholar] [CrossRef] [PubMed]
- Lim, H.-C.; Jung, U.-W.; You, H.; Lee, J.-S. Randomized clinical trial of ridge preservation using porcine bone/cross-linked collagen vs. bovine bone/non-cross-linked collagen: Cone beam computed tomographic analysis. Clin. Oral Implant. Res. 2017, 28, 1492–1500. [Google Scholar] [CrossRef]
- Jiménez Garcia, J.; Berghezan, S.; Caramês, J.M.M.; Dard, M.M.; Marques, D.N.S. Effect of cross-linked vs non-cross-linked collagen membranes on bone: A systematic review. J. Periodontal Res. 2017, 52, 955–964. [Google Scholar] [CrossRef]
- Gentile, P.; Chiono, V.; Tonda-Turo, C.; Ferreira, A.M.; Ciardelli, G. Polymeric membranes for guided bone regeneration. Biotechnol. J. 2011, 6, 1187–1197. [Google Scholar] [CrossRef]
- Wang, J.; Wang, L.; Zhou, Z.; Lai, H.; Xu, P.; Liao, L.; Wei, J. Biodegradable Polymer Membranes Applied in Guided Bone/Tissue Regeneration: A Review. Polymers 2016, 8, 115. [Google Scholar] [CrossRef]
- Gottlow, J.; Laurell, L.; Teiwik, A.; Genon, P. Guided tissue regeneration using a bioresorbable matrix barrier. Pract. Periodontics Aesthet. Dent. 1994, 6, 71–78; quiz 80. [Google Scholar]
- Lundgren, D.; Laurell, L.; Gottlow, J.; Rylander, H.; Mathisen, T.; Nyman, S.; Rask, M. The influence of the design of two different bioresorbable barriers on the results of guided tissue regeneration therapy. An intra-individual comparative study in the monkey. J. Periodontol. 1995, 66, 605–612. [Google Scholar] [CrossRef] [PubMed]
- Di Raimondo, R.; Sanz-Esporrin, J.; Pla, R.; Sanz-Martin, I.; Luengo, F.; Vignoletti, F.; Nunez, J.; Sanz, M. Alveolar crest contour changes after guided bone regeneration using different biomaterials: An experimental in vivo investigation. Clin. Oral Investig. 2020, 24, 2351–2361. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, G.; Hoshino, J.; Kinoshita, Y.; Sugita, Y.; Kubo, K.; Maeda, H.; Ikada, Y.; Kinoshita, Y. Alveolar bone regeneration using poly-(lactic acid-co-glycolic acid-co-epsilon-caprolactone) porous membrane with collagen sponge containing basic fibroblast growth factor: An experimental study in the dog. J. Biomater. Appl. 2012, 27, 485–493. [Google Scholar] [CrossRef]
- Di Raimondo, R.; Sanz-Esporrin, J.; Sanz-Martin, I.; Pla, R.; Luengo, F.; Vignoletti, F.; Nunez, J.; Sanz, M. Hard and soft tissue changes after guided bone regeneration using two different barrier membranes: An experimental in vivo investigation. Clin. Oral Investig. 2021, 25, 2213–2227. [Google Scholar] [CrossRef]
- Vignoletti, F.; Abrahamsson, I. Quality of reporting of experimental research in implant dentistry. Critical aspects in design, outcome assessment and model validation. J. Clin. Periodontol. 2012, 39, 6–27. [Google Scholar] [CrossRef] [PubMed]
- Donath, K.; Breuner, G. A method for the study of undecalcified bones and teeth with attached soft tissues. The Sage-Schliff (sawing and grinding) technique. J. Oral Pathol. 1982, 11, 318–326. [Google Scholar] [CrossRef] [PubMed]
- Berglundh, T.; Lindhe, J.; Jonsson, K.; Ericsson, I. The topography of the vascular systems in the periodontal and peri-implant tissues in the dog. J. Clin. Periodontol. 1994, 21, 189–193. [Google Scholar] [CrossRef]
- Cha, J.K.; Pla, R.; Vignoletti, F.; Jung, U.W.; Sanz-Esporrin, J.; Sanz, M. Immunohistochemical characteristics of lateral bone augmentation using different biomaterials around chronic peri-implant dehiscence defects: An experimental in vivo study. Clin. Oral Implant. Res. 2021, 32, 569–580. [Google Scholar] [CrossRef]
- Varghese, F.; Bukhari, A.B.; Malhotra, R.; De, A. IHC Profiler: An open source plugin for the quantitative evaluation and automated scoring of immunohistochemistry images of human tissue samples. PLoS ONE 2014, 9, e96801. [Google Scholar] [CrossRef]
- Owens, K.W.; Yukna, R.A. Collagen membrane resorption in dogs: A comparative study. Implant. Dent. 2001, 10, 49–58. [Google Scholar] [CrossRef]
- Rothamel, D.; Schwarz, F.; Fienitz, T.; Smeets, R.; Dreiseidler, T.; Ritter, L.; Happe, A.; Zoller, J. Biocompatibility and biodegradation of a native porcine pericardium membrane: Results of in vitro and in vivo examinations. Int. J. Oral Maxillofac. Implant. 2012, 27, 146–154. [Google Scholar]
- Bornert, F.; Herber, V.; Sandgren, R.; Witek, L.; Coelho, P.G.; Pippenger, B.E.; Shahdad, S. Comparative barrier membrane degradation over time: Pericardium versus dermal membranes. Clin. Exp. Dent. Res. 2021, 7, 711–718. [Google Scholar] [CrossRef] [PubMed]
- Schwarz, F.; Rothamel, D.; Herten, M.; Ferrari, D.; Sager, M.; Becker, J. Lateral ridge augmentation using particulated or block bone substitutes biocoated with rhGDF-5 and rhBMP-2: An immunohistochemical study in dogs. Clin. Oral Implant. Res. 2008, 19, 642–652. [Google Scholar] [CrossRef] [PubMed]
- Hoornaert, A.; D’Arros, C.; Heymann, M.-F.; Layrolle, P. Biocompatibility, resorption and biofunctionality of a new synthetic biodegradable membrane for guided bone regeneration. Biomed. Mater. 2016, 11, 045012. [Google Scholar] [CrossRef]
- Won, J.Y.; Park, C.Y.; Bae, J.H.; Ahn, G.; Kim, C.; Lim, D.H.; Cho, D.W.; Yun, W.S.; Shim, J.H.; Huh, J.B. Evaluation of 3D printed PCL/PLGA/ β -TCP versus collagen membranes for guided bone regeneration in a beagle implant model. Biomed. Mater. 2016, 11, 055013. [Google Scholar] [CrossRef]
- Sui, G.; Yang, X.; Mei, F.; Hu, X.; Chen, G.; Deng, X.; Ryu, S. Poly-L-lactic acid/hydroxyapatite hybrid membrane for bone tissue regeneration. J. Biomed. Mater. Res. A 2007, 82, 445–454. [Google Scholar] [CrossRef]
- von Arx, T.; Broggini, N.; Jensen, S.S.; Bornstein, M.M.; Schenk, R.K.; Buser, D. Membrane durability and tissue response of different bioresorbable barrier membranes: A histologic study in the rabbit calvarium. Int. J. Oral Maxillofac. Implant. 2005, 20, 843–853. [Google Scholar]
- Elnayef, B.; Porta, C.; Suarez-Lopez Del Amo, F.; Mordini, L.; Gargallo-Albiol, J.; Hernandez-Alfaro, F. The Fate of Lateral Ridge Augmentation: A Systematic Review and Meta-Analysis. Int. J. Oral Maxillofac. Implant. 2018, 33, 622–635. [Google Scholar] [CrossRef]
- Thoma, D.S.; Cha, J.-K.; Sapata, V.M.; Jung, R.E.; Hüsler, J.; Jung, U.-W. Localized bone regeneration around dental implants using recombinant bone morphogenetic protein-2 and platelet-derived growth factor-BB in the canine. Clin. Oral Implant. Res. 2017, 28, 1334–1341. [Google Scholar] [CrossRef]
- Sanz, M.; Ferrantino, L.; Vignoletti, F.; de Sanctis, M.; Berglundh, T. Guided bone regeneration of non-contained mandibular buccal bone defects using deproteinized bovine bone mineral and a collagen membrane: An experimental in vivo investigation. Clin. Oral Implant. Res. 2017, 28, 1466–1476. [Google Scholar] [CrossRef]
- Wikesjo, U.M.; Qahash, M.; Thomson, R.C.; Cook, A.D.; Rohrer, M.D.; Wozney, J.M.; Hardwick, W.R. rhBMP-2 significantly enhances guided bone regeneration. Clin. Oral Implant. Res. 2004, 15, 194–204. [Google Scholar] [CrossRef] [PubMed]
- Halling Linder, C.; Ek-Rylander, B.; Krumpel, M.; Norgard, M.; Narisawa, S.; Millan, J.L.; Andersson, G.; Magnusson, P. Bone Alkaline Phosphatase and Tartrate-Resistant Acid Phosphatase: Potential Co-regulators of Bone Mineralization. Calcif. Tissue Int. 2017, 101, 92–101. [Google Scholar] [CrossRef] [PubMed]
- Stucki, U.; Schmid, J.; Hammerle, C.F.; Lang, N.P. Temporal and local appearance of alkaline phosphatase activity in early stages of guided bone regeneration. A descriptive histochemical study in humans. Clin. Oral Implant. Res. 2001, 12, 121–127. [Google Scholar] [CrossRef] [PubMed]
- Bozkurt, A.; Apel, C.; Sellhaus, B.; Van Neerven, S.; Wessing, B.; Hilgers, R.-D.; Pallua, N. Differences in degradation behavior of two non-cross-linked collagen barrier membranes: An in vitro and in vivo study. Clin. Oral Implant. Res. 2014, 25, 1403–1411. [Google Scholar] [CrossRef]
- Urban, I.A.; Wessing, B.; Alández, N.; Meloni, S.; González-Martin, O.; Polizzi, G.; Sanz-Sanchez, I.; Montero, E.; Zechner, W. A multicenter randomized controlled trial using a novel collagen membrane for guided bone regeneration at dehisced single implant sites: Outcome at prosthetic delivery and at 1-year follow-up. Clin. Oral Implant. Res. 2019, 30, 487–497. [Google Scholar] [CrossRef]
- Al-Hazmi, B.A.; Al-Hamdan, K.S.; Al-Rasheed, A.; Babay, N.; Wang, H.-L.; Al-Hezaimi, K. Efficacy of Using PDGF and Xenograft With or Without Collagen Membrane for Bone Regeneration Around Immediate Implants With Induced Dehiscence-Type Defects: A Microcomputed Tomographic Study in Dogs. J. Periodontol. 2013, 84, 371–378. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).