Mesenchymal Stem Cells and Their Extracellular Vesicles Are a Promising Alternative to Antibiotics for Treating Sepsis
Abstract
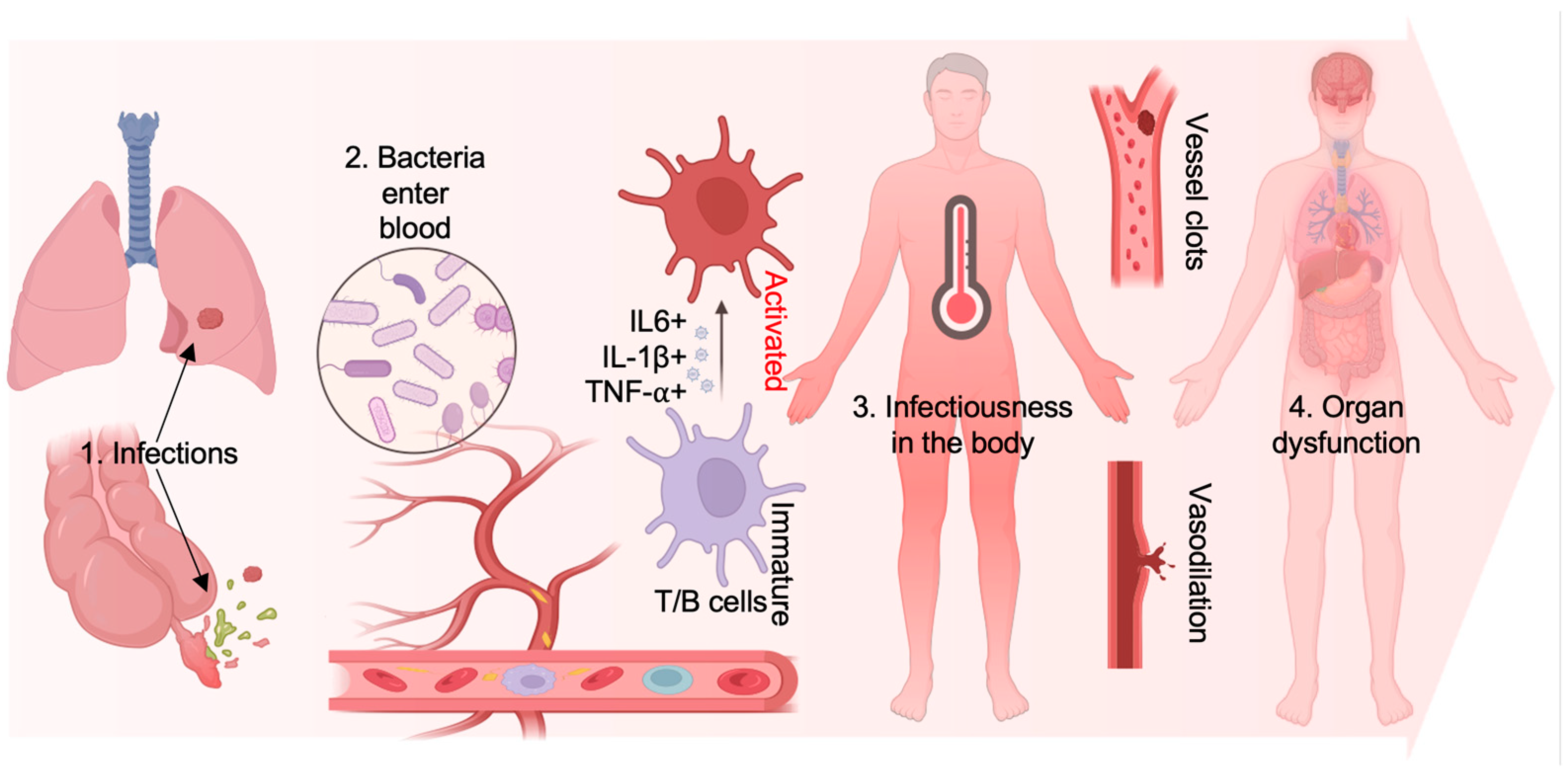
:1. Introduction
2. The Administration of Sepsis and Emerged Antibiotic Resistance
2.1. Generation of Antibiotic Resistance in Microbes During Sepsis
2.2. The Conventional Treatments for Sepsis
2.3. The Shortages of the Conventional Treatment for Sepsis
3. Mesenchymal Stem Cells Serve as a Novel Avenue to Attenuate Sepsis
3.1. Origins and Characters of Mesenchymal Stem Cells
3.2. MSCs Exert Multiple Bioactivities for Sepsis Administration
3.3. Current Application of MSCs for Diseases
4. The Beneficial Characteristics of MSCs for Sepsis Treatment
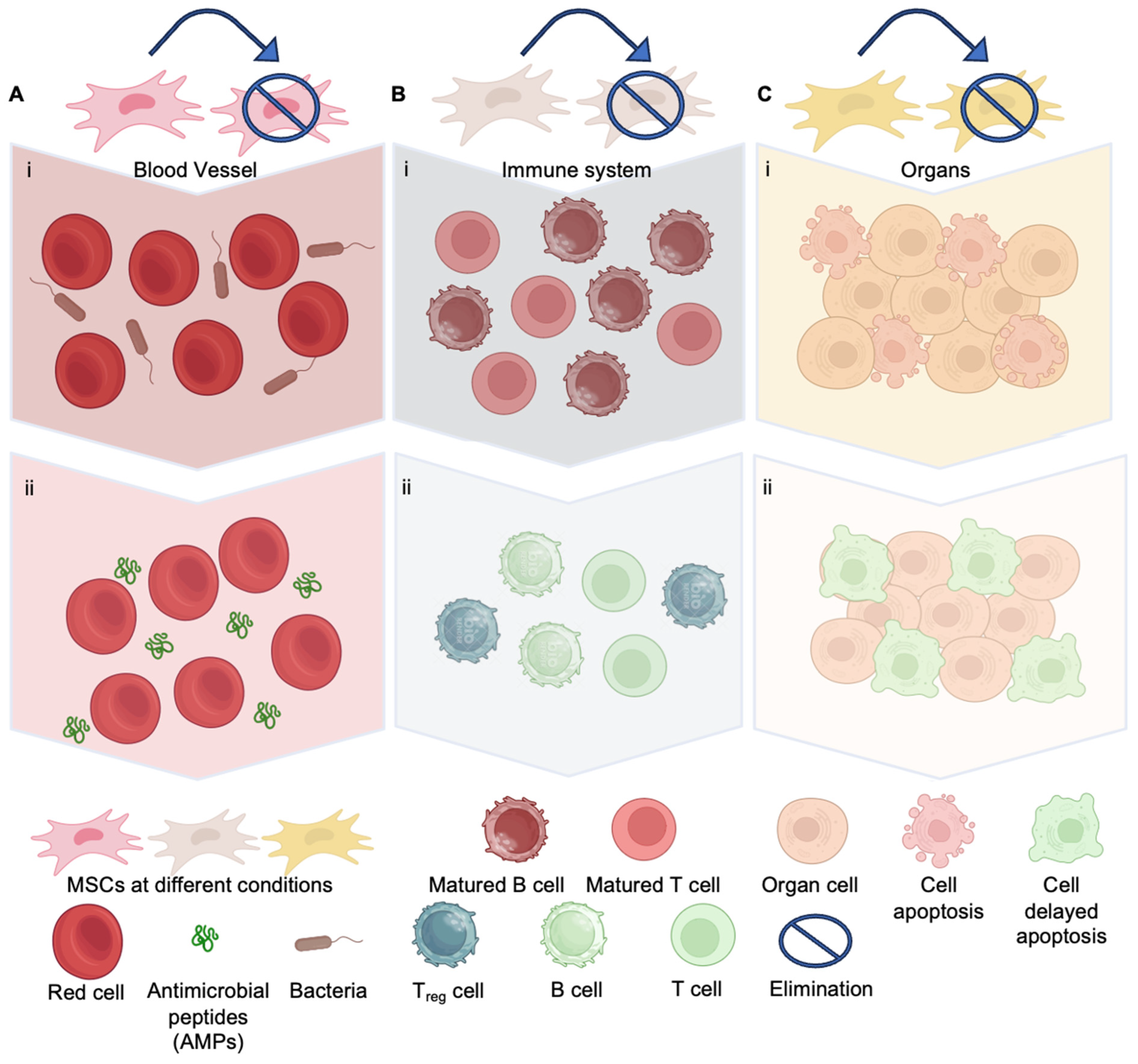
4.1. Anti-Bacteria Effect of MSCs
4.2. Immunomodulatory Effect of MSCs for Sepsis
4.3. Trophism of MSCs Prevents the Organs from Septic Shock
4.4. The High Safety Profile of MSCs In Vivo for Sepsis Treatment
4.5. MSCs Having a Promising Performance for Sepsis Treatment in Preclinical Research
5. Limitations of MSC for Sepsis Treatment
5.1. Variety of Cell Quality of MSCs from the Origins
5.2. MSCs Are Administrated In Vivo and Are Easily Affected by Drugs
5.3. Safety Concern of MSCs on Pre-Tumor Growth
6. The Avenue of Increasing MSC Function for Sepsis Treatment
6.1. MSC Priming Enhances Its Capability of Immunomodulation and Trophism
6.2. Exosomes Derived from MSCs May Achieve Non-Cell Treatment for Sepsis
6.3. Genetic Engineering on MSCs May Enhance Its Performance in Applications
7. Conclusion and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ferrer, R.; Martin-Loeches, I.; Phillips, G.; Osborn, T.M.; Townsend, S.; Dellinger, R.P.; Artigas, A.; Schorr, C.; Levy, M.M. Empiric antibiotic treatment reduces mortality in severe sepsis and septic shock from the first hour: Results from a guideline-based performance improvement program. Crit. Care Med. 2014, 42, 1749–1755. [Google Scholar] [CrossRef] [PubMed]
- Kuehn, B.M. Guideline promotes early, aggressive sepsis treatment to boost survival. JAMA 2013, 309, 969–970. [Google Scholar] [CrossRef] [PubMed]
- Zhu, M.; Wang, L.; Zhuge, Z.; Li, W.; Zheng, Y.; Mai, J.; Lin, Z.; Lin, J. Risk Factors Associated with Multi-Drug Resistance in Neonatal Sepsis Caused by Escherichia coli. Infect. Drug Resist. 2023, 16, 2097–2106. [Google Scholar] [CrossRef] [PubMed]
- Zilberberg, M.D.; Shorr, A.F.; Micek, S.T.; Vazquez-Guillamet, C.; Kollef, M.H. Multi-drug resistance, inappropriate initial antibiotic therapy and mortality in Gram-negative severe sepsis and septic shock: A retrospective cohort study. Crit. Care 2014, 18, 596. [Google Scholar] [CrossRef]
- Albabtain, I.T.; Almohanna, R.S.; Alkhuraiji, A.A.; Alsalamah, R.K.; Almasoud, N.A.; AlBaqmi, K.H.; Althubaiti, A.M. Risk factors for the systemic inflammatory response syndrome and sepsis following surgical management of acute intestinal obstruction. Int. J. Health Sci. 2021, 15, 28–33. [Google Scholar]
- Miksa, M. Beyond Survival: Insights From the Phenotyping Sepsis-Induced Multiple Organ Failure Study on the Neurological Impact of Pediatric Sepsis. Pediatr. Crit. Care Med. 2023, 24, 877–880. [Google Scholar] [CrossRef]
- Karbian, N.; Abutbul, A.; El-Amore, R.; Eliaz, R.; Beeri, R.; Reicher, B.; Mevorach, D. Apoptotic cell therapy for cytokine storm associated with acute severe sepsis. Cell Death Dis. 2020, 11, 535. [Google Scholar] [CrossRef]
- Chousterman, B.G.; Swirski, F.K.; Weber, G.F. Cytokine storm and sepsis disease pathogenesis. Semin. Immunopathol. 2017, 39, 517–528. [Google Scholar] [CrossRef]
- Otto, G.P.; Busch, M.; Sossdorf, M.; Claus, R.A. Impact of sepsis-associated cytokine storm on plasma NGAL during acute kidney injury in a model of polymicrobial sepsis. Crit. Care 2013, 17, 419. [Google Scholar] [CrossRef]
- Reddy, H.; Javvaji, C.K.; Malali, S.; Kumar, S.; Acharya, S.; Toshniwal, S. Navigating the Cytokine Storm: A Comprehensive Review of Chemokines and Cytokines in Sepsis. Cureus 2024, 16, e54275. [Google Scholar] [CrossRef]
- Wang, C.; Zhao, D.; Zheng, L.; Bao, X.; Yang, Q.; Jiang, S.; Zhou, X.; Tang, L.; Liu, Z. Safety and efficacy of human umbilical cord mesenchymal stem cells for the treatment of sepsis induced by pneumonia: Study protocol for a single-centre, randomised single-blind parallel group trial. BMJ Open 2022, 12, e058444. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.T.; Liu, K.J.; Sytwu, H.K.; Yen, M.L.; Yen, B.L. Advances in mesenchymal stem cell therapy for immune and inflammatory diseases: Use of cell-free products and human pluripotent stem cell-derived mesenchymal stem cells. Stem Cells Transl. Med. 2021, 10, 1288–1303. [Google Scholar] [CrossRef] [PubMed]
- Jiang, B.; Fu, X.; Yan, L.; Li, S.; Zhao, D.; Wang, X.; Duan, Y.; Yan, Y.; Li, E.; Wu, K.; et al. Transplantation of human ESC-derived mesenchymal stem cell spheroids ameliorates spontaneous osteoarthritis in rhesus macaques. Theranostics 2019, 9, 6587–6600. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Wu, W.Q.; Ding, L.; Liu, Y.L.; Mao, N.; Zhang, Y.; Zhu, H.; Ning, S.B. Modulatory Effect of Mouse Compact Bone-derived Suspending MSC on T Cells and It’s Related Mechanisms. Zhongguo Shi Yan Xue Ye Xue Za Zhi 2016, 24, 584–589. [Google Scholar] [CrossRef]
- Wang, H.; Luo, J.; Zhong, Y.; Ma, L. Protein Phosphorylation Mechanism of Mesenchymal Stem Cells in the Treatment of Sepsis: A Systematic Review and Meta-analysis. Curr. Mol. Med. 2023, 23, 1087–1094. [Google Scholar] [CrossRef]
- van der Weijden, B.M.; van Dorth, J.R.; Achten, N.B.; Plotz, F.B. Factors Associated with Prolonged Antibiotic Therapy in Neonates with Suspected Early-Onset Sepsis. Antibiotics 2024, 13, 388. [Google Scholar] [CrossRef]
- Harwood, A.; Pearson, S.; Howard, J.; Jones, N.; Greenlees, R.; Broms, C.; Gardiner, S.J.; Dalton, S.C. Pre-hospital, pre-antibiotic blood cultures for patients with suspected sepsis-a feasibility study. N. Z. Med. J. 2024, 137, 108–112. [Google Scholar] [CrossRef]
- Uddin, T.M.; Chakraborty, A.J.; Khusro, A.; Zidan, B.R.M.; Mitra, S.; Emran, T.B.; Dhama, K.; Ripon, M.K.H.; Gajdacs, M.; Sahibzada, M.U.K.; et al. Antibiotic resistance in microbes: History, mechanisms, therapeutic strategies and future prospects. J. Infect. Public Health 2021, 14, 1750–1766. [Google Scholar] [CrossRef]
- Coculescu, B.I. Antimicrobial resistance induced by genetic changes. J. Med. Life 2009, 2, 114–123. [Google Scholar]
- Talan, D.A.; Takhar, S.S.; Krishnadasan, A.; Abrahamian, F.M.; Mower, W.R.; Moran, G.J.; Group, E.M.I.N.S. Fluoroquinolone-Resistant and Extended-Spectrum beta-Lactamase-Producing Escherichia coli Infections in Patients with Pyelonephritis, United States. Emerg. Infect. Dis. 2016, 22, 1594–1603. [Google Scholar] [CrossRef]
- Ubeda, C.; Taur, Y.; Jenq, R.R.; Equinda, M.J.; Son, T.; Samstein, M.; Viale, A.; Socci, N.D.; van den Brink, M.R.; Kamboj, M.; et al. Vancomycin-resistant Enterococcus domination of intestinal microbiota is enabled by antibiotic treatment in mice and precedes bloodstream invasion in humans. J. Clin. Investig. 2010, 120, 4332–4341. [Google Scholar] [CrossRef] [PubMed]
- Mancuso, G.; Midiri, A.; Gerace, E.; Biondo, C. Bacterial Antibiotic Resistance: The Most Critical Pathogens. Pathogens 2021, 10, 1310. [Google Scholar] [CrossRef] [PubMed]
- Ankawi, G.; Neri, M.; Zhang, J.; Breglia, A.; Ricci, Z.; Ronco, C. Extracorporeal techniques for the treatment of critically ill patients with sepsis beyond conventional blood purification therapy: The promises and the pitfalls. Crit. Care 2018, 22, 262. [Google Scholar] [CrossRef] [PubMed]
- Tsantes, A.G.; Parastatidou, S.; Tsantes, E.A.; Bonova, E.; Tsante, K.A.; Mantzios, P.G.; Vaiopoulos, A.G.; Tsalas, S.; Konstantinidi, A.; Houhoula, D.; et al. Sepsis-Induced Coagulopathy: An Update on Pathophysiology, Biomarkers, and Current Guidelines. Life 2023, 13, 350. [Google Scholar] [CrossRef] [PubMed]
- Jarczak, D.; Kluge, S.; Nierhaus, A. Sepsis-Pathophysiology and Therapeutic Concepts. Front. Med. 2021, 8, 628302. [Google Scholar] [CrossRef]
- Al-Kader, D.A.; Anwar, S.; Hussaini, H.; Jones Amaowei, E.E.; Rasuli, S.F.; Hussain, N.; Kaddo, S.; Memon, A. Systematic Review on the Effects of Prompt Antibiotic Treatment on Survival in Septic Shock and Sepsis Patients in Different Hospital Settings. Cureus 2022, 14, e32405. [Google Scholar] [CrossRef]
- Vaeli Zadeh, A.; Wong, A.; Crawford, A.C.; Collado, E.; Larned, J.M. Guideline-based and restricted fluid resuscitation strategy in sepsis patients with heart failure: A systematic review and meta-analysis. Am. J. Emerg. Med. 2023, 73, 34–39. [Google Scholar] [CrossRef]
- Scheeren, T.W.L.; Bakker, J.; De Backer, D.; Annane, D.; Asfar, P.; Boerma, E.C.; Cecconi, M.; Dubin, A.; Dunser, M.W.; Duranteau, J.; et al. Current use of vasopressors in septic shock. Ann. Intensive Care 2019, 9, 20. [Google Scholar] [CrossRef]
- Singer, M. Management of multiple organ failure: Guidelines but no hard-and-fast rules. J. Antimicrob. Chemother. 1998, 41 (Suppl. A), 103–112. [Google Scholar] [CrossRef]
- Sun, G.D.; Zhang, Y.; Mo, S.S.; Zhao, M.Y. Multiple Organ Dysfunction Syndrome Caused by Sepsis: Risk Factor Analysis. Int. J. Gen. Med. 2021, 14, 7159–7164. [Google Scholar] [CrossRef]
- Aguilar, J.L.; Varshney, A.K.; Pechuan, X.; Dutta, K.; Nosanchuk, J.D.; Fries, B.C. Monoclonal antibodies protect from Staphylococcal Enterotoxin K (SEK) induced toxic shock and sepsis by USA300 Staphylococcus aureus. Virulence 2017, 8, 741–750. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Qiang, X.; Wang, Y.; Zhu, S.; Li, J.; Babaev, A.; Yang, H.; Gong, J.; Becker, L.; Wang, P.; et al. Identification of tetranectin-targeting monoclonal antibodies to treat potentially lethal sepsis. Sci. Transl. Med. 2020, 12, eaaz3833. [Google Scholar] [CrossRef] [PubMed]
- Daniel, M.; Bedoui, Y.; Vagner, D.; Raffray, L.; Ah-Pine, F.; Doray, B.; Gasque, P. Pathophysiology of Sepsis and Genesis of Septic Shock: The Critical Role of Mesenchymal Stem Cells (MSCs). Int. J. Mol. Sci. 2022, 23, 9274. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.; Li, J.; Wang, J.; Jiang, Q.; Yang, J.; Dou, H.; Liang, H.; Li, K.; Hou, Y. Ferroptotic MSCs protect mice against sepsis via promoting macrophage efferocytosis. Cell Death Dis. 2022, 13, 825. [Google Scholar] [CrossRef]
- Sherif, M.; Abera, D.; Desta, K. Prevalence and antibiotic resistance pattern of bacteria from sepsis suspected neonates at St. Paul’s Hospital Millennium Medical College, Addis Ababa, Ethiopia. BMC Pediatr. 2023, 23, 575. [Google Scholar] [CrossRef]
- Zhang, L.; Feng, Y.; Fu, P. Blood purification for sepsis: An overview. Precis. Clin. Med. 2021, 4, 45–55. [Google Scholar] [CrossRef]
- Minotti, C.; Di Caprio, A.; Facchini, L.; Bedetti, L.; Miselli, F.; Rossi, C.; Della Casa Muttini, E.; Lugli, L.; Luppi, L.; Ferrari, F.; et al. Antimicrobial Resistance Pattern and Empirical Antibiotic Treatments in Neonatal Sepsis: A Retrospective, Single-Center, 12-Year Study. Antibiotics 2023, 12, 1488. [Google Scholar] [CrossRef]
- Iredell, J.; Brown, J.; Tagg, K. Antibiotic resistance in Enterobacteriaceae: Mechanisms and clinical implications. BMJ 2016, 352, h6420. [Google Scholar] [CrossRef]
- Atif, M.; Zia, R.; Malik, I.; Ahmad, N.; Sarwar, S. Treatment outcomes, antibiotic use and its resistance pattern among neonatal sepsis patients attending Bahawal Victoria Hospital, Pakistan. PLoS ONE 2021, 16, e0244866. [Google Scholar] [CrossRef]
- Curren, E.J.; Lutgring, J.D.; Kabbani, S.; Diekema, D.J.; Gitterman, S.; Lautenbach, E.; Morgan, D.J.; Rock, C.; Salerno, R.M.; McDonald, L.C. Advancing Diagnostic Stewardship for Healthcare-Associated Infections, Antibiotic Resistance, and Sepsis. Clin. Infect. Dis. 2022, 74, 723–728. [Google Scholar] [CrossRef]
- Kim, H.I.; Park, S. Sepsis: Early Recognition and Optimized Treatment. Tuberc. Respir. Dis. 2019, 82, 6–14. [Google Scholar] [CrossRef] [PubMed]
- Pant, A.; Mackraj, I.; Govender, T. Advances in sepsis diagnosis and management: A paradigm shift towards nanotechnology. J. Biomed. Sci. 2021, 28, 6. [Google Scholar] [CrossRef] [PubMed]
- Leong, K.; Gaglani, B.; Khanna, A.K.; McCurdy, M.T. Novel Diagnostics and Therapeutics in Sepsis. Biomedicines 2021, 9, 311. [Google Scholar] [CrossRef] [PubMed]
- Rhodes, A.; Evans, L.E.; Alhazzani, W.; Levy, M.M.; Antonelli, M.; Ferrer, R.; Kumar, A.; Sevransky, J.E.; Sprung, C.L.; Nunnally, M.E.; et al. Surviving Sepsis Campaign: International Guidelines for Management of Sepsis and Septic Shock: 2016. Intensive Care Med. 2017, 43, 304–377. [Google Scholar] [CrossRef]
- Trzeciak, A.; Pietropaoli, A.P.; Kim, M. Biomarkers and Associated Immune Mechanisms for Early Detection and Therapeutic Management of Sepsis. Immune Netw. 2020, 20, e23. [Google Scholar] [CrossRef]
- Cartwright, M.; Rottman, M.; Shapiro, N.I.; Seiler, B.; Lombardo, P.; Gamini, N.; Tomolonis, J.; Watters, A.L.; Waterhouse, A.; Leslie, D.; et al. A Broad-Spectrum Infection Diagnostic that Detects Pathogen-Associated Molecular Patterns (PAMPs) in Whole Blood. EBioMedicine 2016, 9, 217–227. [Google Scholar] [CrossRef]
- Lipcsey, M.; Hanslin, K.; Stalberg, J.; Smekal, D.; Larsson, A. The time course of calprotectin liberation from human neutrophil granulocytes after Escherichia coli and endotoxin challenge. Innate Immun. 2019, 25, 369–373. [Google Scholar] [CrossRef]
- Havelka, A.; Sejersen, K.; Venge, P.; Pauksens, K.; Larsson, A. Calprotectin, a new biomarker for diagnosis of acute respiratory infections. Sci. Rep. 2020, 10, 4208. [Google Scholar] [CrossRef]
- Deshmane, S.L.; Kremlev, S.; Amini, S.; Sawaya, B.E. Monocyte chemoattractant protein-1 (MCP-1): An overview. J. Interferon Cytokine Res. 2009, 29, 313–326. [Google Scholar] [CrossRef]
- Qiu, L.; Li, J.; Bai, H.; Wang, L.; Zeng, Q.; Wu, S.; Li, P.; Mu, L.; Yin, X.; Ye, J. Long-chain pentraxin 3 possesses agglutination activity and plays a role in host defense against bacterial infection in Oreochromis niloticus. Dev. Comp. Immunol. 2023, 149, 105053. [Google Scholar] [CrossRef]
- Porte, R.; Silva-Gomes, R.; Theroude, C.; Parente, R.; Asgari, F.; Sironi, M.; Pasqualini, F.; Valentino, S.; Asselta, R.; Recordati, C.; et al. Regulation of inflammation and protection against invasive pneumococcal infection by the long pentraxin PTX3. eLife 2023, 12, e78601. [Google Scholar] [CrossRef] [PubMed]
- Tamayo, E.; Fernandez, A.; Almansa, R.; Carrasco, E.; Heredia, M.; Lajo, C.; Goncalves, L.; Gomez-Herreras, J.I.; de Lejarazu, R.O.; Bermejo-Martin, J.F. Pro- and anti-inflammatory responses are regulated simultaneously from the first moments of septic shock. Eur. Cytokine Netw. 2011, 22, 82–87. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Sun, Y.; Xu, W.; Chang, F.; Wang, Y.; Ding, J. Mesenchymal Stem Cells-Involved Strategies for Rheumatoid Arthritis Therapy. Adv. Sci. 2024, 11, e2305116. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.S.; Xin, Z.C.; Dai, J.; Lue, T.F. Commonly used mesenchymal stem cell markers and tracking labels: Limitations and challenges. Histol. Histopathol. 2013, 28, 1109–1116. [Google Scholar] [CrossRef] [PubMed]
- Turner, L. ISSCR’s Guidelines for Stem Cell Research and Clinical Translation: Supporting development of safe and efficacious stem cell-based interventions. Stem Cell Rep. 2021, 16, 1394–1397. [Google Scholar] [CrossRef]
- Schu, S.; Nosov, M.; O’Flynn, L.; Shaw, G.; Treacy, O.; Barry, F.; Murphy, M.; O’Brien, T.; Ritter, T. Immunogenicity of allogeneic mesenchymal stem cells. J. Cell. Mol. Med. 2012, 16, 2094–2103. [Google Scholar] [CrossRef]
- Diez-Tejedor, E.; Gutierrez-Fernandez, M.; Martinez-Sanchez, P.; Rodriguez-Frutos, B.; Ruiz-Ares, G.; Lara, M.L.; Gimeno, B.F. Reparative therapy for acute ischemic stroke with allogeneic mesenchymal stem cells from adipose tissue: A safety assessment: A phase II randomized, double-blind, placebo-controlled, single-center, pilot clinical trial. J. Stroke Cerebrovasc. Dis. 2014, 23, 2694–2700. [Google Scholar] [CrossRef]
- Sharma, A.K.; Gokulchandran, N.; Kulkarni, P.P.; Sane, H.M.; Sharma, R.; Jose, A.; Badhe, P.B. Cell transplantation as a novel therapeutic strategy for autism spectrum disorders: A clinical study. Am. J. Stem Cells 2020, 9, 89–100. [Google Scholar]
- Gu, J.; Huang, L.; Zhang, C.; Wang, Y.; Zhang, R.; Tu, Z.; Wang, H.; Zhou, X.; Xiao, Z.; Liu, Z.; et al. Therapeutic evidence of umbilical cord-derived mesenchymal stem cell transplantation for cerebral palsy: A randomized, controlled trial. Stem Cell Res. Ther. 2020, 11, 43. [Google Scholar] [CrossRef]
- Levy, M.L.; Crawford, J.R.; Dib, N.; Verkh, L.; Tankovich, N.; Cramer, S.C. Phase I/II Study of Safety and Preliminary Efficacy of Intravenous Allogeneic Mesenchymal Stem Cells in Chronic Stroke. Stroke 2019, 50, 2835–2841. [Google Scholar] [CrossRef]
- Yang, Y.; Pang, M.; Du, C.; Liu, Z.Y.; Chen, Z.H.; Wang, N.X.; Zhang, L.M.; Chen, Y.Y.; Mo, J.; Dong, J.W.; et al. Repeated subarachnoid administrations of allogeneic human umbilical cord mesenchymal stem cells for spinal cord injury: A phase 1/2 pilot study. Cytotherapy 2021, 23, 57–64. [Google Scholar] [CrossRef] [PubMed]
- Dahbour, S.; Jamali, F.; Alhattab, D.; Al-Radaideh, A.; Ababneh, O.; Al-Ryalat, N.; Al-Bdour, M.; Hourani, B.; Msallam, M.; Rasheed, M.; et al. Mesenchymal stem cells and conditioned media in the treatment of multiple sclerosis patients: Clinical, ophthalmological and radiological assessments of safety and efficacy. CNS Neurosci. Ther. 2017, 23, 866–874. [Google Scholar] [CrossRef] [PubMed]
- Matthay, M.A.; Calfee, C.S.; Zhuo, H.; Thompson, B.T.; Wilson, J.G.; Levitt, J.E.; Rogers, A.J.; Gotts, J.E.; Wiener-Kronish, J.P.; Bajwa, E.K.; et al. Treatment with allogeneic mesenchymal stromal cells for moderate to severe acute respiratory distress syndrome (START study): A randomised phase 2a safety trial. Lancet Respir. Med. 2019, 7, 154–162. [Google Scholar] [CrossRef] [PubMed]
- Ahn, S.Y.; Chang, Y.S.; Lee, M.H.; Sung, S.I.; Lee, B.S.; Kim, K.S.; Kim, A.R.; Park, W.S. Stem cells for bronchopulmonary dysplasia in preterm infants: A randomized controlled phase II trial. Stem Cells Transl. Med. 2021, 10, 1129–1137. [Google Scholar] [CrossRef] [PubMed]
- Le Thi Bich, P.; Nguyen Thi, H.; Dang Ngo Chau, H.; Phan Van, T.; Do, Q.; Dong Khac, H.; Le Van, D.; Nguyen Huy, L.; Mai Cong, K.; Ta Ba, T.; et al. Allogeneic umbilical cord-derived mesenchymal stem cell transplantation for treating chronic obstructive pulmonary disease: A pilot clinical study. Stem Cell Res. Ther. 2020, 11, 60. [Google Scholar] [CrossRef]
- Gentile, P.; Sterodimas, A.; Pizzicannella, J.; Calabrese, C.; Garcovich, S. Research progress on Mesenchymal Stem Cells (MSCs), Adipose-Derived Mesenchymal Stem Cells (AD-MSCs), Drugs, and Vaccines in Inhibiting COVID-19 Disease. Aging Dis. 2020, 11, 1191–1201. [Google Scholar] [CrossRef]
- Lanzoni, G.; Linetsky, E.; Correa, D.; Messinger Cayetano, S.; Alvarez, R.A.; Kouroupis, D.; Alvarez Gil, A.; Poggioli, R.; Ruiz, P.; Marttos, A.C.; et al. Umbilical cord mesenchymal stem cells for COVID-19 acute respiratory distress syndrome: A double-blind, phase 1/2a, randomized controlled trial. Stem Cells Transl. Med. 2021, 10, 660–673. [Google Scholar] [CrossRef]
- Kouroupis, D.; Lanzoni, G.; Linetsky, E.; Messinger Cayetano, S.; Wishnek Metalonis, S.; Lenero, C.; Stone, L.D.; Ruiz, P.; Correa, D.; Ricordi, C. Umbilical Cord-derived Mesenchymal Stem Cells modulate TNF and soluble TNF Receptor 2 (sTNFR2) in COVID-19 ARDS patients. Eur. Rev. Med. Pharmacol. Sci. 2021, 25, 4435–4438. [Google Scholar] [CrossRef]
- Averyanov, A.; Koroleva, I.; Konoplyannikov, M.; Revkova, V.; Lesnyak, V.; Kalsin, V.; Danilevskaya, O.; Nikitin, A.; Sotnikova, A.; Kotova, S.; et al. First-in-human high-cumulative-dose stem cell therapy in idiopathic pulmonary fibrosis with rapid lung function decline. Stem Cells Transl. Med. 2020, 9, 6–16. [Google Scholar] [CrossRef]
- Dantas, J.R.; Araujo, D.B.; Silva, K.R.; Souto, D.L.; de Fatima Carvalho Pereira, M.; Luiz, R.R.; Dos Santos Mantuano, M.; Claudio-da-Silva, C.; Gabbay, M.A.L.; Dib, S.A.; et al. Adipose tissue-derived stromal/stem cells + cholecalciferol: A pilot study in recent-onset type 1 diabetes patients. Arch. Endocrinol. Metab. 2021, 65, 342–351. [Google Scholar] [CrossRef]
- Nguyen, L.T.; Hoang, D.M.; Nguyen, K.T.; Bui, D.M.; Nguyen, H.T.; Le, H.T.A.; Hoang, V.T.; Bui, H.T.H.; Dam, P.T.M.; Hoang, X.T.A.; et al. Type 2 diabetes mellitus duration and obesity alter the efficacy of autologously transplanted bone marrow-derived mesenchymal stem/stromal cells. Stem Cells Transl. Med. 2021, 10, 1266–1278. [Google Scholar] [CrossRef] [PubMed]
- Abo-Elkheir, W.; Hamza, F.; Elmofty, A.M.; Emam, A.; Abdl-Moktader, M.; Elsherefy, S.; Gabr, H. Role of cord blood and bone marrow mesenchymal stem cells in recent deep burn: A case-control prospective study. Am. J. Stem Cells 2017, 6, 23–35. [Google Scholar] [PubMed]
- Qayyum, A.A.; Mathiasen, A.B.; Helqvist, S.; Jorgensen, E.; Haack-Sorensen, M.; Ekblond, A.; Kastrup, J. Autologous adipose-derived stromal cell treatment for patients with refractory angina (MyStromalCell Trial): 3-years follow-up results. J. Transl. Med. 2019, 17, 360. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.W.; Fang, X.; Gupta, N.; Serikov, V.; Matthay, M.A. Allogeneic human mesenchymal stem cells for treatment of E. coli endotoxin-induced acute lung injury in the ex vivo perfused human lung. Proc. Natl. Acad. Sci. USA 2009, 106, 16357–16362. [Google Scholar] [CrossRef]
- Lee, J.W.; Krasnodembskaya, A.; McKenna, D.H.; Song, Y.; Abbott, J.; Matthay, M.A. Therapeutic effects of human mesenchymal stem cells in ex vivo human lungs injured with live bacteria. Am. J. Respir. Crit. Care Med. 2013, 187, 751–760. [Google Scholar] [CrossRef]
- McIntyre, L.A.; Stewart, D.J.; Mei, S.H.J.; Courtman, D.; Watpool, I.; Granton, J.; Marshall, J.; Dos Santos, C.; Walley, K.R.; Winston, B.W.; et al. Cellular Immunotherapy for Septic Shock. A Phase I Clinical Trial. Am. J. Respir. Crit. Care Med. 2018, 197, 337–347. [Google Scholar] [CrossRef]
- Alp, E.; Gonen, Z.B.; Gundogan, K.; Esmaoglu, A.; Kaynar, L.; Cetin, A.; Karakukcu, M.; Cetin, M.; Kalin, G.; Doganay, M. The Effect of Mesenchymal Stromal Cells on the Mortality of Patients with Sepsis and Septic Shock: A Promising Therapy. Emerg. Med. Int. 2022, 2022, 9222379. [Google Scholar] [CrossRef]
- Wheeler, D.S.; Zingarelli, B.; Wheeler, W.J.; Wong, H.R. Novel pharmacologic approaches to the management of sepsis: Targeting the host inflammatory response. Recent. Pat. Inflamm. Allergy Drug Discov. 2009, 3, 96–112. [Google Scholar] [CrossRef]
- Uccelli, A.; Moretta, L.; Pistoia, V. Mesenchymal stem cells in health and disease. Nat. Rev. Immunol. 2008, 8, 726–736. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, X.; Cao, W.; Shi, Y. Plasticity of mesenchymal stem cells in immunomodulation: Pathological and therapeutic implications. Nat. Immunol. 2014, 15, 1009–1016. [Google Scholar] [CrossRef]
- Kadri, N.; Amu, S.; Iacobaeus, E.; Boberg, E.; Le Blanc, K. Current perspectives on mesenchymal stromal cell therapy for graft versus host disease. Cell. Mol. Immunol. 2023, 20, 613–625. [Google Scholar] [CrossRef] [PubMed]
- Rivers, E.; Nguyen, B.; Havstad, S.; Ressler, J.; Muzzin, A.; Knoblich, B.; Peterson, E.; Tomlanovich, M.; Early Goal-Directed Therapy Collaborative, G. Early goal-directed therapy in the treatment of severe sepsis and septic shock. N. Engl. J. Med. 2001, 345, 1368–1377. [Google Scholar] [CrossRef] [PubMed]
- Kode, J.A.; Mukherjee, S.; Joglekar, M.V.; Hardikar, A.A. Mesenchymal stem cells: Immunobiology and role in immunomodulation and tissue regeneration. Cytotherapy 2009, 11, 377–391. [Google Scholar] [CrossRef] [PubMed]
- Gao, S.; Zhang, Y.; Liang, K.; Bi, R.; Du, Y. Mesenchymal Stem Cells (MSCs): A Novel Therapy for Type 2 Diabetes. Stem Cells Int. 2022, 2022, 8637493. [Google Scholar] [CrossRef] [PubMed]
- Suchanek, J.; Nasry, S.A.; Soukup, T. The Differentiation Potential of Human Natal Dental Pulp Stem Cells into Insulin-Producing Cells. Folia Biol. 2017, 63, 132–138. [Google Scholar] [CrossRef]
- Xu, B.; Fan, D.; Zhao, Y.; Li, J.; Wang, Z.; Wang, J.; Wang, X.; Guan, Z.; Niu, B. Three-Dimensional Culture Promotes the Differentiation of Human Dental Pulp Mesenchymal Stem Cells Into Insulin-Producing Cells for Improving the Diabetes Therapy. Front. Pharmacol. 2019, 10, 1576. [Google Scholar] [CrossRef]
- Karantalis, V.; Hare, J.M. Use of mesenchymal stem cells for therapy of cardiac disease. Circ. Res. 2015, 116, 1413–1430. [Google Scholar] [CrossRef]
- Singh, A.; Singh, A.; Sen, D. Mesenchymal stem cells in cardiac regeneration: A detailed progress report of the last 6 years (2010–2015). Stem Cell Res. Ther. 2016, 7, 82. [Google Scholar] [CrossRef]
- Bagno, L.; Hatzistergos, K.E.; Balkan, W.; Hare, J.M. Mesenchymal Stem Cell-Based Therapy for Cardiovascular Disease: Progress and Challenges. Mol. Ther. 2018, 26, 1610–1623. [Google Scholar] [CrossRef]
- Hmadcha, A.; Martin-Montalvo, A.; Gauthier, B.R.; Soria, B.; Capilla-Gonzalez, V. Therapeutic Potential of Mesenchymal Stem Cells for Cancer Therapy. Front. Bioeng. Biotechnol. 2020, 8, 43. [Google Scholar] [CrossRef]
- Bahman Soufiani, K.; Pourfathollah, A.A.; Nikougoftar Zarif, M.; Arefian, E. Tumor Microenvironment Changing through Application of MicroRNA-34a Related Mesenchymal Stem Cells Conditioned Medium: Modulation of Breast Cancer Cells toward Non-aggressive Behavior. Iran. J. Allergy Asthma Immunol. 2021, 20, 221–232. [Google Scholar] [CrossRef] [PubMed]
- Sung, D.K.; Chang, Y.S.; Sung, S.I.; Yoo, H.S.; Ahn, S.Y.; Park, W.S. Antibacterial effect of mesenchymal stem cells against Escherichia coli is mediated by secretion of beta- defensin- 2 via toll- like receptor 4 signalling. Cell. Microbiol. 2016, 18, 424–436. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.W.; Gupta, N.; Serikov, V.; Matthay, M.A. Potential application of mesenchymal stem cells in acute lung injury. Expert. Opin. Biol. Ther. 2009, 9, 1259–1270. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Dong, Y.; Wu, X.; Xu, X.; Niu, J. The assessment of mesenchymal stem cells therapy in acute on chronic liver failure and chronic liver disease: A systematic review and meta-analysis of randomized controlled clinical trials. Stem Cell Res. Ther. 2022, 13, 204. [Google Scholar] [CrossRef]
- Liu, H.M.; Liu, Y.T.; Zhang, J.; Ma, L.J. Bone marrow mesenchymal stem cells ameliorate lung injury through anti-inflammatory and antibacterial effect in COPD mice. J. Huazhong Univ. Sci. Technol. Med. Sci. 2017, 37, 496–504. [Google Scholar] [CrossRef]
- Chen, Y.; Yang, L.; Li, X. Advances in Mesenchymal stem cells regulating macrophage polarization and treatment of sepsis-induced liver injury. Front. Immunol. 2023, 14, 1238972. [Google Scholar] [CrossRef]
- Ramos Maia, D.R.; Otsuki, D.A.; Rodrigues, C.E.; Zboril, S.; Sanches, T.R.; Neto, A.N.D.; Andrade, L.; Auler, J.O.C., Jr. Treatment with Human Umbilical Cord-Derived Mesenchymal Stem Cells in a Pig Model of Sepsis-Induced Acute Kidney Injury: Effects on Microvascular Endothelial Cells and Tubular Cells in the Kidney. Shock 2023, 60, 469–477. [Google Scholar] [CrossRef]
- Xing, J.; Wang, R.; Cui, F.; Song, L.; Ma, Q.; Xu, H. Role of the regulation of mesenchymal stem cells on macrophages in sepsis. Int. J. Immunopathol. Pharmacol. 2023, 37, 3946320221150722. [Google Scholar] [CrossRef]
- Laroye, C.; Gibot, S.; Reppel, L.; Bensoussan, D. Concise Review: Mesenchymal Stromal/Stem Cells: A New Treatment for Sepsis and Septic Shock? Stem Cells 2017, 35, 2331–2339. [Google Scholar] [CrossRef]
- Khosrojerdi, A.; Soudi, S.; Hosseini, A.Z.; Eshghi, F.; Shafiee, A.; Hashemi, S.M. Immunomodulatory and Therapeutic Effects of Mesenchymal Stem Cells on Organ Dysfunction in Sepsis. Shock. 2021, 55, 423–440. [Google Scholar] [CrossRef]
- Eshghi, F.; Tahmasebi, S.; Alimohammadi, M.; Soudi, S.; Khaligh, S.G.; Khosrojerdi, A.; Heidari, N.; Hashemi, S.M. Study of immunomodulatory effects of mesenchymal stem cell-derived exosomes in a mouse model of LPS induced systemic inflammation. Life Sci. 2022, 310, 120938. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Ai, S.; Guo, W.; Yang, Y.; Wang, Z.; Jiang, D.; Xu, X. Umbilical cord-derived mesenchymal stem (stromal) cells for treatment of severe sepsis: Aphase 1 clinical trial. Transl. Res. 2018, 199, 52–61. [Google Scholar] [CrossRef] [PubMed]
- Weiss, A.R.R.; Dahlke, M.H. Immunomodulation by Mesenchymal Stem Cells (MSCs): Mechanisms of Action of Living, Apoptotic, and Dead MSCs. Front. Immunol. 2019, 10, 1191. [Google Scholar] [CrossRef] [PubMed]
- Zheng, G.; Huang, R.; Qiu, G.; Ge, M.; Wang, J.; Shu, Q.; Xu, J. Mesenchymal stromal cell-derived extracellular vesicles: Regenerative and immunomodulatory effects and potential applications in sepsis. Cell Tissue Res. 2018, 374, 1–15. [Google Scholar] [CrossRef]
- Wu, K.H.; Li, J.P.; Chao, W.R.; Lee, Y.J.; Yang, S.F.; Cheng, C.C.; Chao, Y.H. Immunomodulation via MyD88-NFkappaB Signaling Pathway from Human Umbilical Cord-Derived Mesenchymal Stem Cells in Acute Lung Injury. Int. J. Mol. Sci. 2022, 23, 5295. [Google Scholar] [CrossRef]
- Deng, H.; Zhu, L.; Zhang, Y.; Zheng, L.; Hu, S.; Zhou, W.; Zhang, T.; Xu, W.; Chen, Y.; Zhou, H.; et al. Differential Lung Protective Capacity of Exosomes Derived from Human Adipose Tissue, Bone Marrow, and Umbilical Cord Mesenchymal Stem Cells in Sepsis-Induced Acute Lung Injury. Oxid. Med. Cell. Longev. 2022, 2022, 7837837. [Google Scholar] [CrossRef]
- Caplan, A.I.; Correa, D. The MSC: An injury drugstore. Cell Stem Cell 2011, 9, 11–15. [Google Scholar] [CrossRef]
- Kouroupis, D.; Sanjurjo-Rodriguez, C.; Jones, E.; Correa, D. Mesenchymal Stem Cell Functionalization for Enhanced Therapeutic Applications. Tissue Eng. Part B Rev. 2019, 25, 55–77. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, L.; Liu, M.; Zhao, J.; Xu, X.; Wei, D.; Chen, J. Mechanism of exosomes from adipose-derived mesenchymal stem cells on sepsis-induced acute lung injury by promoting TGF-beta secretion in macrophages. Surgery 2023, 174, 1208–1219. [Google Scholar] [CrossRef]
- Lalu, M.M.; Sullivan, K.J.; Mei, S.H.; Moher, D.; Straus, A.; Fergusson, D.A.; Stewart, D.J.; Jazi, M.; MacLeod, M.; Winston, B.; et al. Evaluating mesenchymal stem cell therapy for sepsis with preclinical meta-analyses prior to initiating a first-in-human trial. eLife 2016, 5, e17850. [Google Scholar] [CrossRef]
- Hum, C.; Tahir, U.; Mei, S.H.J.; Champagne, J.; Fergusson, D.A.; Lalu, M.; Stewart, D.J.; Walley, K.; Marshall, J.; Dos Santos, C.C.; et al. Efficacy and Safety of Umbilical Cord-Derived Mesenchymal Stromal Cell Therapy in Preclinical Models of Sepsis: A Systematic Review and Meta-analysis. Stem Cells Transl. Med. 2024, 13, 346–361. [Google Scholar] [CrossRef] [PubMed]
- Abdolmohammadi, K.; Mahmoudi, T.; Alimohammadi, M.; Tahmasebi, S.; Zavvar, M.; Hashemi, S.M. Mesenchymal stem cell-based therapy as a new therapeutic approach for acute inflammation. Life Sci. 2023, 312, 121206. [Google Scholar] [CrossRef] [PubMed]
- Marti-Chillon, G.J.; Muntion, S.; Preciado, S.; Osugui, L.; Navarro-Bailon, A.; Gonzalez-Robledo, J.; Sagredo, V.; Blanco, J.F.; Sanchez-Guijo, F. Therapeutic potential of mesenchymal stromal/stem cells in critical-care patients with systemic inflammatory response syndrome. Clin. Transl. Med. 2023, 13, e1163. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Yi, H.; Song, Y. The safety of MSC therapy over the past 15 years: A meta-analysis. Stem Cell Res. Ther. 2021, 12, 545. [Google Scholar] [CrossRef]
- Jiang, B.; Yan, L.; Shamul, J.G.; Hakun, M.; He, X. Stem cell therapy of myocardial infarction: A promising opportunity in bioengineering. Adv. Ther. 2020, 3, 1900182. [Google Scholar] [CrossRef]
- Bartosh, T.J.; Ullah, M.; Zeitouni, S.; Beaver, J.; Prockop, D.J. Cancer cells enter dormancy after cannibalizing mesenchymal stem/stromal cells (MSCs). Proc. Natl. Acad. Sci. USA 2016, 113, E6447–E6456. [Google Scholar] [CrossRef]
- Terry, A.R.; Hay, N. Fuelling cancer cells. Nat. Rev. Endocrinol. 2019, 15, 71–72. [Google Scholar] [CrossRef]
- Jiang, B.; Yan, L.; Wang, X.; Li, E.; Murphy, K.; Vaccaro, K.; Li, Y.; Xu, R.H. Concise Review: Mesenchymal Stem Cells Derived from Human Pluripotent Cells, an Unlimited and Quality-Controllable Source for Therapeutic Applications. Stem Cells 2019, 37, 572–581. [Google Scholar] [CrossRef]
- Mazine, A.; Rushani, D.; Yau, T.M. Clinical mesenchymal stem cell therapy in ischemic cardiomyopathy. JTCVS Open 2021, 8, 135–141. [Google Scholar] [CrossRef]
- Tan, N.; Xin, W.; Huang, M.; Mao, Y. Mesenchymal stem cell therapy for ischemic stroke: Novel insight into the crosstalk with immune cells. Front. Neurol. 2022, 13, 1048113. [Google Scholar] [CrossRef]
- Wang, X.; Jiang, B.; Sun, H.; Zheng, D.; Zhang, Z.; Yan, L.; Li, E.; Wu, Y.; Xu, R.H. Noninvasive application of mesenchymal stem cell spheres derived from hESC accelerates wound healing in a CXCL12-CXCR4 axis-dependent manner. Theranostics 2019, 9, 6112–6128. [Google Scholar] [CrossRef] [PubMed]
- Luo, F.; Jiang, W.; Xu, Y.; Liu, X.M.; Wang, W.; Zhang, W.; Luo, C. The Mechanisms Involved in Mesenchymal Stem Cell Alleviation of Sepsis-Induced Acute Lung Injury in Mice: A Pilot Study. Curr. Ther. Res. Clin. Exp. 2020, 93, 100593. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Wang, T.; Li, R.; Xue, F.; Zeng, G.; Zhang, J.; Ma, Y.; Feng, L.; Kang, Y.J. Dose-specific efficacy of adipose-derived mesenchymal stem cells in septic mice. Stem Cell Res. Ther. 2023, 14, 32. [Google Scholar] [CrossRef] [PubMed]
- Sari, M.I.; Jusuf, N.K.; Munir, D.; Putra, A.; Bisri, T.; Ilyas, S.; Farhat, F.; Muhar, A.M.; Rusda, M.; Amin, M.M. The Role of Mesenchymal Stem Cell Secretome in the Inflammatory Mediators and the Survival Rate of Rat Model of Sepsis. Biomedicines 2023, 11, 2325. [Google Scholar] [CrossRef]
- Ge, L.; Zhao, J.; Deng, H.; Chen, C.; Hu, Z.; Zeng, L. Effect of Bone Marrow Mesenchymal Stromal Cell Therapies in Rodent Models of Sepsis: A Meta-Analysis. Front. Immunol. 2021, 12, 792098. [Google Scholar] [CrossRef]
- Liang, H.; Ding, X.; Yu, Y.; Zhang, H.; Wang, L.; Kan, Q.; Ma, S.; Guan, F.; Sun, T. Adipose-derived mesenchymal stem cells ameliorate acute liver injury in rat model of CLP induced-sepsis via sTNFR1. Exp. Cell Res. 2019, 383, 111465. [Google Scholar] [CrossRef]
- Horak, J.; Nalos, L.; Martinkova, V.; Tegl, V.; Vistejnova, L.; Kuncova, J.; Kohoutova, M.; Jarkovska, D.; Dolejsova, M.; Benes, J.; et al. Evaluation of Mesenchymal Stem Cell Therapy for Sepsis: A Randomized Controlled Porcine Study. Front. Immunol. 2020, 11, 126. [Google Scholar] [CrossRef]
- Brozovich, A.; Sinicrope, B.J.; Bauza, G.; Niclot, F.B.; Lintner, D.; Taraballi, F.; McCulloch, P.C. High Variability of Mesenchymal Stem Cells Obtained via Bone Marrow Aspirate Concentrate Compared with Traditional Bone Marrow Aspiration Technique. Orthop. J. Sports Med. 2021, 9, 23259671211058459. [Google Scholar] [CrossRef]
- Bowles-Welch, A.C.; Jimenez, A.C.; Stevens, H.Y.; Frey Rubio, D.A.; Kippner, L.E.; Yeago, C.; Roy, K. Mesenchymal stromal cells for bone trauma, defects, and disease: Considerations for manufacturing, clinical translation, and effective treatments. Bone Rep. 2023, 18, 101656. [Google Scholar] [CrossRef]
- Debnath, T.; Chelluri, L.K. Standardization and quality assessment for clinical grade mesenchymal stem cells from human adipose tissue. Hematol. Transfus. Cell Ther. 2019, 41, 7–16. [Google Scholar] [CrossRef]
- Awad, M.E.; Hussein, K.A.; Helwa, I.; Abdelsamid, M.F.; Aguilar-Perez, A.; Mohsen, I.; Hunter, M.; Hamrick, M.W.; Isales, C.M.; Elsalanty, M.; et al. Meta-Analysis and Evidence Base for the Efficacy of Autologous Bone Marrow Mesenchymal Stem Cells in Knee Cartilage Repair: Methodological Guidelines and Quality Assessment. Stem Cells Int. 2019, 2019, 3826054. [Google Scholar] [CrossRef] [PubMed]
- Jeyaraman, M.; Muthu, S.; Ganie, P.A. Does the Source of Mesenchymal Stem Cell Have an Effect in the Management of Osteoarthritis of the Knee? Meta-Analysis of Randomized Controlled Trials. Cartilage 2021, 13 (Suppl. 1), 1532S–1547S. [Google Scholar] [CrossRef] [PubMed]
- Duggal, S.; Brinchmann, J.E. Importance of serum source for the in vitro replicative senescence of human bone marrow derived mesenchymal stem cells. J. Cell. Physiol. 2011, 226, 2908–2915. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Huang, B.; Jin, T.; Ocansey, D.K.W.; Jiang, J.; Mao, F. Intestinal Fibrosis in Inflammatory Bowel Disease and the Prospects of Mesenchymal Stem Cell Therapy. Front. Immunol. 2022, 13, 835005. [Google Scholar] [CrossRef]
- Zhao, Q.; Han, Z.; Wang, J.; Han, Z. Development and investigational new drug application of mesenchymal stem/stromal cells products in China. Stem Cells Transl. Med. 2021, 10 (Suppl. 2), S18–S30. [Google Scholar] [CrossRef]
- Marinas-Pardo, L.; Garcia-Castro, J.; Rodriguez-Hurtado, I.; Rodriguez-Garcia, M.I.; Nunez-Naveira, L.; Hermida-Prieto, M. Allogeneic Adipose-Derived Mesenchymal Stem Cells (Horse Allo 20) for the Treatment of Osteoarthritis-Associated Lameness in Horses: Characterization, Safety, and Efficacy of Intra-Articular Treatment. Stem Cells Dev. 2018, 27, 1147–1160. [Google Scholar] [CrossRef]
- Wang, H.; Jiang, H.Y.; Zhang, Y.X.; Jin, H.Y.; Fei, B.Y.; Jiang, J.L. Mesenchymal stem cells transplantation for perianal fistulas: A systematic review and meta-analysis of clinical trials. Stem Cell Res. Ther. 2023, 14, 103. [Google Scholar] [CrossRef]
- Shao, Y.; Wichern, E.; Childress, P.J.; Adaway, M.; Misra, J.; Klunk, A.; Burr, D.B.; Wek, R.C.; Mosley, A.L.; Liu, Y.; et al. Loss of Nmp4 optimizes osteogenic metabolism and secretion to enhance bone quality. Am. J. Physiol. Endocrinol. Metab. 2019, 316, E749–E772. [Google Scholar] [CrossRef]
- Klopp, A.H.; Gupta, A.; Spaeth, E.; Andreeff, M.; Marini, F., 3rd. Concise review: Dissecting a discrepancy in the literature: Do mesenchymal stem cells support or suppress tumor growth? Stem Cells 2011, 29, 11–19. [Google Scholar] [CrossRef]
- Spaeth, E.L.; Dembinski, J.L.; Sasser, A.K.; Watson, K.; Klopp, A.; Hall, B.; Andreeff, M.; Marini, F. Mesenchymal stem cell transition to tumor-associated fibroblasts contributes to fibrovascular network expansion and tumor progression. PLoS ONE 2009, 4, e4992. [Google Scholar] [CrossRef]
- Le Blanc, K.; Mougiakakos, D. Multipotent mesenchymal stromal cells and the innate immune system. Nat. Rev. Immunol. 2012, 12, 383–396. [Google Scholar] [CrossRef] [PubMed]
- Roodhart, J.M.; Daenen, L.G.; Stigter, E.C.; Prins, H.J.; Gerrits, J.; Houthuijzen, J.M.; Gerritsen, M.G.; Schipper, H.S.; Backer, M.J.; van Amersfoort, M.; et al. Mesenchymal stem cells induce resistance to chemotherapy through the release of platinum-induced fatty acids. Cancer Cell 2011, 20, 370–383. [Google Scholar] [CrossRef] [PubMed]
- Andrews, S.H.; Klinker, M.W.; Bauer, S.R.; Marklein, R.A. Morphological landscapes from high content imaging reveal cytokine priming strategies that enhance mesenchymal stromal cell immunosuppression. Biotechnol. Bioeng. 2022, 119, 361–375. [Google Scholar] [CrossRef] [PubMed]
- Pierce, L.M.; Kurata, W.E. Priming with Toll-Like Receptor 3 Agonist Poly(I:C) Enhances Content of Innate Immune Defense Proteins but Not MicroRNAs in Human Mesenchymal Stem Cell-Derived Extracellular Vesicles. Front. Cell Dev. Biol. 2021, 9, 676356. [Google Scholar] [CrossRef]
- Noronha, N.C.; Mizukami, A.; Caliari-Oliveira, C.; Cominal, J.G.; Rocha, J.L.M.; Covas, D.T.; Swiech, K.; Malmegrim, K.C.R. Priming approaches to improve the efficacy of mesenchymal stromal cell-based therapies. Stem Cell Res. Ther. 2019, 10, 131. [Google Scholar] [CrossRef]
- Hezam, K.; Wang, C.; Fu, E.; Zhou, M.; Liu, Y.; Wang, H.; Zhu, L.; Han, Z.; Han, Z.C.; Chang, Y.; et al. Superior protective effects of PGE2 priming mesenchymal stem cells against LPS-induced acute lung injury (ALI) through macrophage immunomodulation. Stem Cell Res. Ther. 2023, 14, 48. [Google Scholar] [CrossRef]
- Yasan, G.T.; Gunel-Ozcan, A. Hypoxia and Hypoxia Mimetic Agents As Potential Priming Approaches to Empower Mesenchymal Stem Cells. Curr. Stem Cell Res. Ther. 2024, 19, 33–54. [Google Scholar] [CrossRef]
- Shin, J.Y.; Kim, D.Y.; Lee, J.; Shin, Y.J.; Kim, Y.S.; Lee, P.H. Priming mesenchymal stem cells with alpha-synuclein enhances neuroprotective properties through induction of autophagy in Parkinsonian models. Stem Cell Res. Ther. 2022, 13, 483. [Google Scholar] [CrossRef]
- Zolfaghari, S.; Milan, P.B.; Dehpour, A.R.; Fomeshi, M.R.; Eskandari, F.; Ebrahimi, L.; Hashemi, S.M.; Joghataei, M.T. The effect of poly I:C or LPS priming on the therapeutic efficacy of mesenchymal stem cells in an adjuvant-induced arthritis rat model. Pharmacol. Rep. 2022, 74, 654–668. [Google Scholar] [CrossRef]
- Martinez Villegas, K.; Rasouli, R.; Tabrizian, M. Enhancing metabolic activity and differentiation potential in adipose mesenchymal stem cells via high-resolution surface-acoustic-wave contactless patterning. Microsyst. Nanoeng. 2022, 8, 79. [Google Scholar] [CrossRef]
- Ebrahim, N.; Al Saihati, H.A.; Shaman, A.; Dessouky, A.A.; Farid, A.S.; Hussien, N.I.; Mostafa, O.; Seleem, Y.; Sabry, D.; Saad, A.S.; et al. Bone marrow-derived mesenchymal stem cells combined with gonadotropin therapy restore postnatal oogenesis of chemo-ablated ovaries in rats via enhancing very small embryonic-like stem cells. Stem Cell Res. Ther. 2021, 12, 517. [Google Scholar] [CrossRef] [PubMed]
- Kubota, K.; Nakano, M.; Kobayashi, E.; Mizue, Y.; Chikenji, T.; Otani, M.; Nagaishi, K.; Fujimiya, M. An enriched environment prevents diabetes-induced cognitive impairment in rats by enhancing exosomal miR-146a secretion from endogenous bone marrow-derived mesenchymal stem cells. PLoS ONE 2018, 13, e0204252. [Google Scholar] [CrossRef] [PubMed]
- Senamontree, S.; Lakthan, T.; Charoenpanich, P.; Chanchao, C.; Charoenpanich, A. Betulinic acid decreases lipid accumulation in adipogenesis-induced human mesenchymal stem cells with upregulation of PGC-1alpha and UCP-1 and post-transcriptional downregulation of adiponectin and leptin secretion. PeerJ 2021, 9, e12321. [Google Scholar] [CrossRef] [PubMed]
- Jammes, M.; Casse, F.; Velot, E.; Bianchi, A.; Audigie, F.; Contentin, R.; Galera, P. Pro-Inflammatory Cytokine Priming and Purification Method Modulate the Impact of Exosomes Derived from Equine Bone Marrow Mesenchymal Stromal Cells on Equine Articular Chondrocytes. Int. J. Mol. Sci. 2023, 24, 14169. [Google Scholar] [CrossRef]
- Bulati, M.; Gallo, A.; Zito, G.; Busa, R.; Iannolo, G.; Cuscino, N.; Castelbuono, S.; Carcione, C.; Centi, C.; Martucci, G.; et al. 3D Culture and Interferon-gamma Priming Modulates Characteristics of Mesenchymal Stromal/Stem Cells by Modifying the Expression of Both Intracellular and Exosomal microRNAs. Biology 2023, 12, 1063. [Google Scholar] [CrossRef]
- Miceli, V.; Zito, G.; Bulati, M.; Gallo, A.; Busa, R.; Iannolo, G.; Conaldi, P.G. Different priming strategies improve distinct therapeutic capabilities of mesenchymal stromal/stem cells: Potential implications for their clinical use. World J. Stem Cells 2023, 15, 400–420. [Google Scholar] [CrossRef]
- Ma, Y.; Liu, X.; Long, Y.; Chen, Y. Emerging Therapeutic Potential of Mesenchymal Stem Cell-Derived Extracellular Vesicles in Chronic Respiratory Diseases: An Overview of Recent Progress. Front. Bioeng. Biotechnol. 2022, 10, 845042. [Google Scholar] [CrossRef]
- Zhang, S.; Chuah, S.J.; Lai, R.C.; Hui, J.H.P.; Lim, S.K.; Toh, W.S. MSC exosomes mediate cartilage repair by enhancing proliferation, attenuating apoptosis and modulating immune reactivity. Biomaterials 2018, 156, 16–27. [Google Scholar] [CrossRef]
- Toh, W.S.; Lai, R.C.; Zhang, B.; Lim, S.K. MSC exosome works through a protein-based mechanism of action. Biochem. Soc. Trans. 2018, 46, 843–853. [Google Scholar] [CrossRef]
- Heidari, N.; Abbasi-Kenarsari, H.; Namaki, S.; Baghaei, K.; Zali, M.R.; Ghaffari Khaligh, S.; Hashemi, S.M. Adipose-derived mesenchymal stem cell-secreted exosome alleviates dextran sulfate sodium-induced acute colitis by Treg cell induction and inflammatory cytokine reduction. J. Cell. Physiol. 2021, 236, 5906–5920. [Google Scholar] [CrossRef]
- Kita, S.; Shimomura, I. Stimulation of exosome biogenesis by adiponectin, a circulating factor secreted from adipocytes. J. Biochem. 2021, 169, 173–179. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.H.; Liang, J.P.; Zhu, C.J.; Lian, Y.J. Mesenchymal Stem Cell-Derived Extracellular Vesicles Therapy for Pulmonary Hypertension: A Comprehensive Review of Preclinical Studies. J. Interv. Cardiol. 2022, 2022, 5451947. [Google Scholar] [CrossRef] [PubMed]
- Elahi, F.M.; Farwell, D.G.; Nolta, J.A.; Anderson, J.D. Preclinical translation of exosomes derived from mesenchymal stem/stromal cells. Stem Cells 2020, 38, 15–21. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Han, Y.; Lu, M.; Liu, Z.; Jin, J.; Guo, Q.; Wang, Y.; Liu, H. Mesenchymal stem cell-exosome-mediated matrix metalloproteinase 1 participates in oral leukoplakia and carcinogenesis by inducing angiogenesis. J. Oral. Pathol. Med. 2022, 51, 638–648. [Google Scholar] [CrossRef]
- Hao, X.; Guo, Y.; Wang, R.; Yu, X.; He, L.; Shu, M. Exosomes from adipose-derived mesenchymal stem cells promote survival of fat grafts by regulating macrophage polarization via let-7c. Acta Biochim. Biophys. Sin. 2021, 53, 501–510. [Google Scholar] [CrossRef]
- Zhuang, X.M.; Zhou, B. Exosome secreted by human gingival fibroblasts in radiation therapy inhibits osteogenic differentiation of bone mesenchymal stem cells by transferring miR-23a. Biomed. Pharmacother. 2020, 131, 110672. [Google Scholar] [CrossRef]
- Yoon, J.; Lee, S.K.; Park, A.; Lee, J.; Jung, I.; Song, K.B.; Choi, E.J.; Kim, S.; Yu, J. Exosome from IFN-gamma-Primed Induced Pluripotent Stem Cell-Derived Mesenchymal Stem Cells Improved Skin Inflammation and Barrier Function. Int. J. Mol. Sci. 2023, 24, 11635. [Google Scholar] [CrossRef]
- Kim, Y.; Shin, S.; Lee, K.A. Exosome-based detection of EGFR T790M in plasma and pleural fluid of prospectively enrolled non-small cell lung cancer patients after first-line tyrosine kinase inhibitor therapy. Cancer Cell Int. 2021, 21, 50. [Google Scholar] [CrossRef]
- Pawitan, J.A.; Bui, T.A.; Mubarok, W.; Antarianto, R.D.; Nurhayati, R.W.; Dilogo, I.H.; Oceandy, D. Enhancement of the Therapeutic Capacity of Mesenchymal Stem Cells by Genetic Modification: A Systematic Review. Front. Cell Dev. Biol. 2020, 8, 587776. [Google Scholar] [CrossRef]
- Thakor, D.K.; Teng, Y.D.; Obata, H.; Nagane, K.; Saito, S.; Tabata, Y. Nontoxic genetic engineering of mesenchymal stem cells using serum-compatible pullulan-spermine/DNA anioplexes. Tissue Eng. Part C Methods 2011, 17, 131–144. [Google Scholar] [CrossRef]
- Ebrahim, N.; James, V.; Rizvanov, A.A.; Mukhamedshina, Y. Genetic Modification of Mesenchymal Stem Cells for Neurological Disease Therapy: What Effects Does it Have on Phenotype/Cell Behavior, Determining Their Effectiveness? Mol. Diagn. Ther. 2020, 24, 683–702. [Google Scholar] [CrossRef] [PubMed]
- Ball, A.N.; Phillips, J.N.; McIlwraith, C.W.; Kawcak, C.E.; Samulski, R.J.; Goodrich, L.R. Genetic modification of scAAV-equine-BMP-2 transduced bone-marrow-derived mesenchymal stem cells before and after cryopreservation: An “off-the-shelf” option for fracture repair. J. Orthop. Res. 2019, 37, 1310–1317. [Google Scholar] [CrossRef] [PubMed]
- Margiana, R. Enhancing Spermatogenesis in Non-obstructive Azoospermia through Mesenchymal Stem Cell Therapy. Curr. Stem Cell Res. Ther. 2024, 19, 1429–1441. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Hu, G.; Chen, X.; Zhang, L.; Guo, L.; Li, C.; Zhao, H.; Cui, Z.; Guo, X.; Sun, F.; et al. Excessive branched-chain amino acid accumulation restricts mesenchymal stem cell-based therapy efficacy in myocardial infarction. Signal Transduct. Target. Ther. 2022, 7, 171. [Google Scholar] [CrossRef]
- Damasceno, P.K.F.; de Santana, T.A.; Santos, G.C.; Orge, I.D.; Silva, D.N.; Albuquerque, J.F.; Golinelli, G.; Grisendi, G.; Pinelli, M.; Ribeiro Dos Santos, R.; et al. Genetic Engineering as a Strategy to Improve the Therapeutic Efficacy of Mesenchymal Stem/Stromal Cells in Regenerative Medicine. Front. Cell Dev. Biol. 2020, 8, 737. [Google Scholar] [CrossRef]
- Cheng, J.; Sun, Y.; Ma, Y.; Ao, Y.; Hu, X.; Meng, Q. Engineering of MSC-Derived Exosomes: A Promising Cell-Free Therapy for Osteoarthritis. Membranes 2022, 12, 739. [Google Scholar] [CrossRef]
- Laroye, C.; Gibot, S.; Huselstein, C.; Bensoussan, D. Mesenchymal stromal cells for sepsis and septic shock: Lessons for treatment of COVID-19. Stem Cells Transl. Med. 2020, 9, 1488–1494. [Google Scholar] [CrossRef]

| Biomarkers | Category | Function | Ref. |
|---|---|---|---|
| PAMPs | Innate response | Microbial motifs recognized by host cell pattern-recognition receptors | [45] |
| DAMPs | Innate response | Molecular alerts of host system damage | [46] |
| Calprotectin | Innate response | Released from neutrophils in response to bacterial infections, increasing within hours | [47,48] |
| MCP1 | Chemokine | Endothelial cells and monocytes secrete MCP-1 (i.e., C-C motif chemokine ligand (2) to trigger inflammation. | [49] |
| PTX-3 | Cytokine | Triggers early inflammation by activating the classical complement pathway and aiding pathogen recognition by macrophages and dendritic cells. | [50,51] |
| sTNFR | Cytokine | Early pro-inflammatory cytokines were studied to assess their correlation with sepsis mortality, typically linked to an exaggerated innate immune response | [52] |
| MSC Type | Disease | Year | Patient No. | Conclusion |
|---|---|---|---|---|
| ADSC | Acute ischemic stroke | 2014 [57] | 10 | Potential time window for the intravenous administration of allogeneic ADSC and improved efficacy when performed within 2 weeks after stroke. |
| BM-MSC | Autism spectrum disorders | 2020 [58] | 254 | After the transplantation, the change in score: ISAA was positive (94.48% of patients); CARS improved (95.27% of patients); brain activity improved (86/86 in FDG-PET CT) |
| UC-MSC | Cerebral palsy | 2020 [59] | 19 | The activities of daily living, comprehensive functional assessment, and Gross motor function measurement—88 scores were significantly improved compared to pretransplant and control groups. |
| BM-MSCs | Chronic stroke | 2019 [60] | 36 | Based on serial examination, electrogram, laboratory, and computed tomography scans of chest/abdomen/pelvis, the therapy was safe and well-tolerated. |
| BM-MSCs | Spinal cord injury | 2021 [61] | 41 | There was a notable enhancement in the ASIA total score, pinprick score, and light touch, as well as the IANR-SCIFRS total score and sphincter score following transplantation when compared to pre-transplantation assessments. |
| BM-MSCs | Multiple sclerosis | 2017 [62] | 10 | There has been a general trend of enhancement observed in the Expanded Disability Status Scale and secondary clinical tests, which encompassed mobility, cognitive function (Mini-Mental Status Examination), and ophthalmological assessments. |
| BM-MSCs | Acute respiratory distress syndrome | 2019 [63] | 60 | Elevate the acute physiology and chronic health evaluation III score, enhance minute ventilation, and achieve a reduction in inflammation. |
| UC-MSC | Bronchopulmonary dysplasia | 2021 [64] | 33 | Transplantation of MSCs markedly enhanced the condition of patients with severe Bronchopulmonary Dysplasia. |
| UC-MSC | COPD | 2020 [65] | 20 | All patients experienced enhancements in their Modified Medical Research Council scores and COPD Assessment Test outcomes. |
| UC-MSC and ADSC | COVID-19 | 2021 [66] | 210 | Decrease in inflammatory responses and enhancement in survival rates. |
| UC-MSC | COVID-19 | 2021 [67] | 24 | UC-MSC infusions have demonstrated safety and may offer therapeutic benefits for individuals with ARDS in the context of COVID-19. |
| UC-MSC | COVID-19 | 2021 [68] | 24 | Recipients of UC-MSCs exhibit a marked increase in plasma sTNFR2 levels, along with a significant reduction in tumor necrosis factor α and β levels, when compared to control subjects. |
| BM-MSC | Idiopathic pulmonary fibrosis | 2019 [69] | 10 | Enhanced performance in carbon monoxide diffusing capacity, six-minute walk distance, and increased forced vital capacity. |
| ADSC | Type 1 diabetes | 2021 [70] | 7 | Marked enhancement in basal C-peptide levels and HbA1C measurements following transplantation relative to pre-transplantation values. |
| BM-MSCs | Type 2 diabetes | 2021 [71] | 25 | There was a minor decrease in HbA1c levels within the initial three months post-administration; however, the levels normalized after six months and subsequently rose. |
| BM-MSCs | Skin wound | 2017 [72] | 40 | BM-MSC group exhibited a notably higher healing rate compared to the group receiving traditional treatment in terms of percentage of burn coverage and duration of hospital stay. |
| ADSC | Refractory angina | 2019 [73] | 41 | Enhanced cardiac symptoms were observed, yet there was no improvement in exercise capacity. |
| BM-MSC | Sepsis | 2009 [74] | NA | Restoration of alveolar fluid clearance via sodium-dependent transport mechanisms (LPS induced on ex vivo human lung model). |
| BM-MSC | Sepsis | 2013 [75] | NA | Restore alveolar fluid clearance (AFC), reduce inflammation, and demonstrate antimicrobial effects, partly through the secretion of keratinocyte growth factor (E. coli induced on ex vivo human lung model). |
| MSCs (undefined) | Sepsis | 2013 | 30 | Improved the survival rate of 28-day period. However, its beneficial effect shows no significancy to control on day 90. ClinicalTrials.gov: NCT01849237. |
| BM-MSC | Sepsis | 2018 [76] | 9 | The infusion of allogenic BM-MSCs, up to 3 million cells/kg, appears safe in septic shock patients. ClinicalTrials.gov: NCT02421484. |
| ADSC | Sepsis | 2022 [77] | 10 | Improved early survival rates in sepsis for 10 patients, but larger randomized controlled studies are needed. ClinicalTrials.gov: NCT05283317. |
| Cell Type | Brand | Indication | Company | Approved Area | Approved Date |
|---|---|---|---|---|---|
| Allogeneic Bone Marrow-Derived Mesenchymal Stem Cells | Alofisel | Crohn’s Disease with Anal Fistula | TiGenix NV | Europa | 2018 |
| Allogeneic Bone Marrow-Derived Mesenchymal Stem Cells | Alofisel | Crohn’s Disease with Anal Fistula | TiGenix NV | Japan | 2021 |
| Autologous Adipose-Derived Mesenchymal Stem Cells | Guepistem | Crohn’s Disease with Anal Fistula | Anterogen | Korea | 2012 |
| Autologous Bone Marrow-Derived Mesenchymal Stem Cells | Hearticellgram-AMI | Acute Myocardial Infarction | FCB-Pharmicell | Korea | 2011 |
| Autologous Limbal Stem Cells | Holoclar | Burn-induced Limbal Stem Cell Deficiency | Chiesi Farmaceutici | Europa | 2015 |
| Autologous Mesenchymal Progenitor Cells | MPC | Damaged Bone Tissue Repair | Mesoblast | Australia | 2010 |
| Bone Marrow-Derived Mesenchymal Stem Cells | MultiStem | Ischemic stroke | America Stem Cell | United States | 2012 |
| Bone Marrow-Derived Mesenchymal Stem Cells | Prochymal | Graft-Versus-Host Disease | Osiris Therapeutics | United States | 2005 |
| Bone Marrow-Derived Mesenchymal Stem Cells | Prochymal | Crohn’s Disease | Osiris Therapeutics | United States | 2009 |
| Bone Marrow-Derived Mesenchymal Stem Cells | Prochymal | Type 1 Diabetes | 0siris Therapeutics | United States | 2010 |
| Bone Marrow-Derived Mesenchymal Stem Cells | Ryoncil (Prochymal) | Graft-Versus-Host Disease | Osiris Therapeutics | Canada | 2012 |
| Bone Marrow-Derived Mesenchymal Stem Cells | Stempeucel | Buerger’s disease | Stempeutics | India | 2020 |
| Bone Marrow-Derived Mesenchymal Stem Cells | Temcell | Graft-Versus-Host Disease | Mesoblast | Japan | 2016 |
| IPSC-Derived Mesenchymal Stem Cells | Cymerus | Graft-Versus-Host Disease | Cyanta Therapeutics | United States | 2018 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiang, Y.; Song, Y.; Zeng, Q.; Jiang, B. Mesenchymal Stem Cells and Their Extracellular Vesicles Are a Promising Alternative to Antibiotics for Treating Sepsis. Bioengineering 2024, 11, 1160. https://doi.org/10.3390/bioengineering11111160
Jiang Y, Song Y, Zeng Q, Jiang B. Mesenchymal Stem Cells and Their Extracellular Vesicles Are a Promising Alternative to Antibiotics for Treating Sepsis. Bioengineering. 2024; 11(11):1160. https://doi.org/10.3390/bioengineering11111160
Chicago/Turabian StyleJiang, Yu, Yunjuan Song, Qin Zeng, and Bin Jiang. 2024. "Mesenchymal Stem Cells and Their Extracellular Vesicles Are a Promising Alternative to Antibiotics for Treating Sepsis" Bioengineering 11, no. 11: 1160. https://doi.org/10.3390/bioengineering11111160
APA StyleJiang, Y., Song, Y., Zeng, Q., & Jiang, B. (2024). Mesenchymal Stem Cells and Their Extracellular Vesicles Are a Promising Alternative to Antibiotics for Treating Sepsis. Bioengineering, 11(11), 1160. https://doi.org/10.3390/bioengineering11111160







