Validation and Estimation of Obesity-Induced Intervertebral Disc Degeneration through Subject-Specific Finite Element Modelling of Functional Spinal Units
Abstract
:1. Introduction
2. Materials and Methods
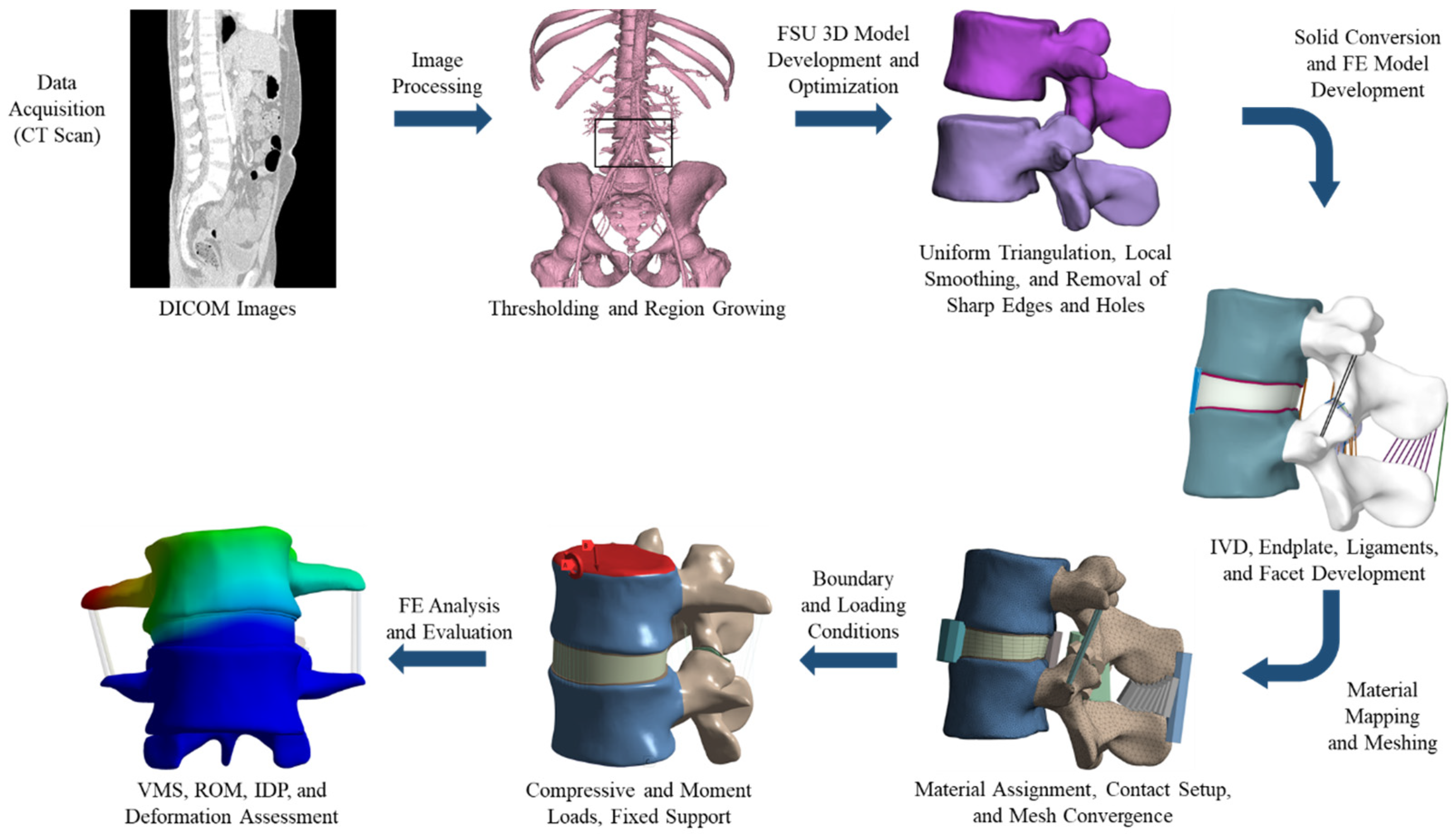
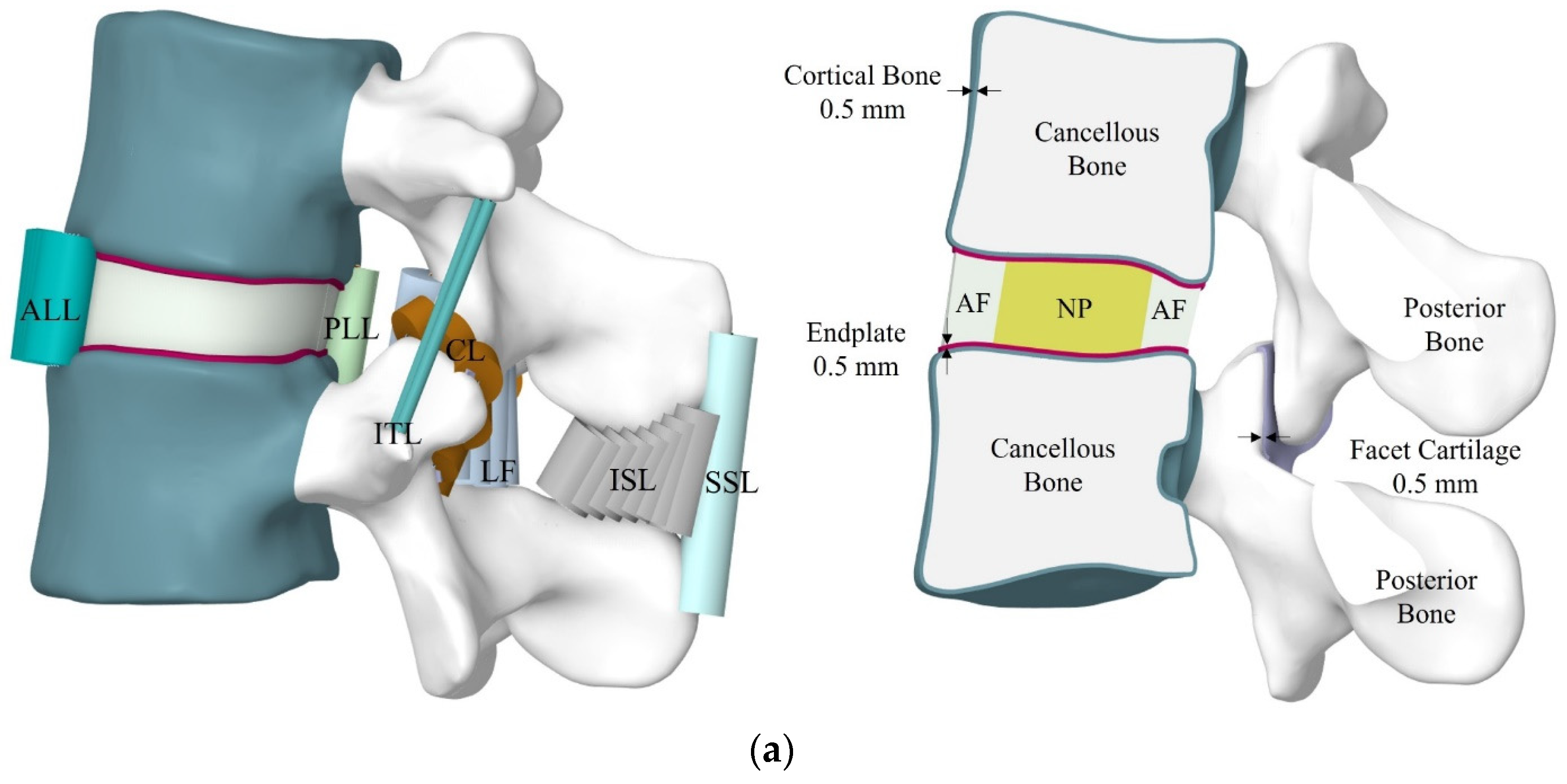
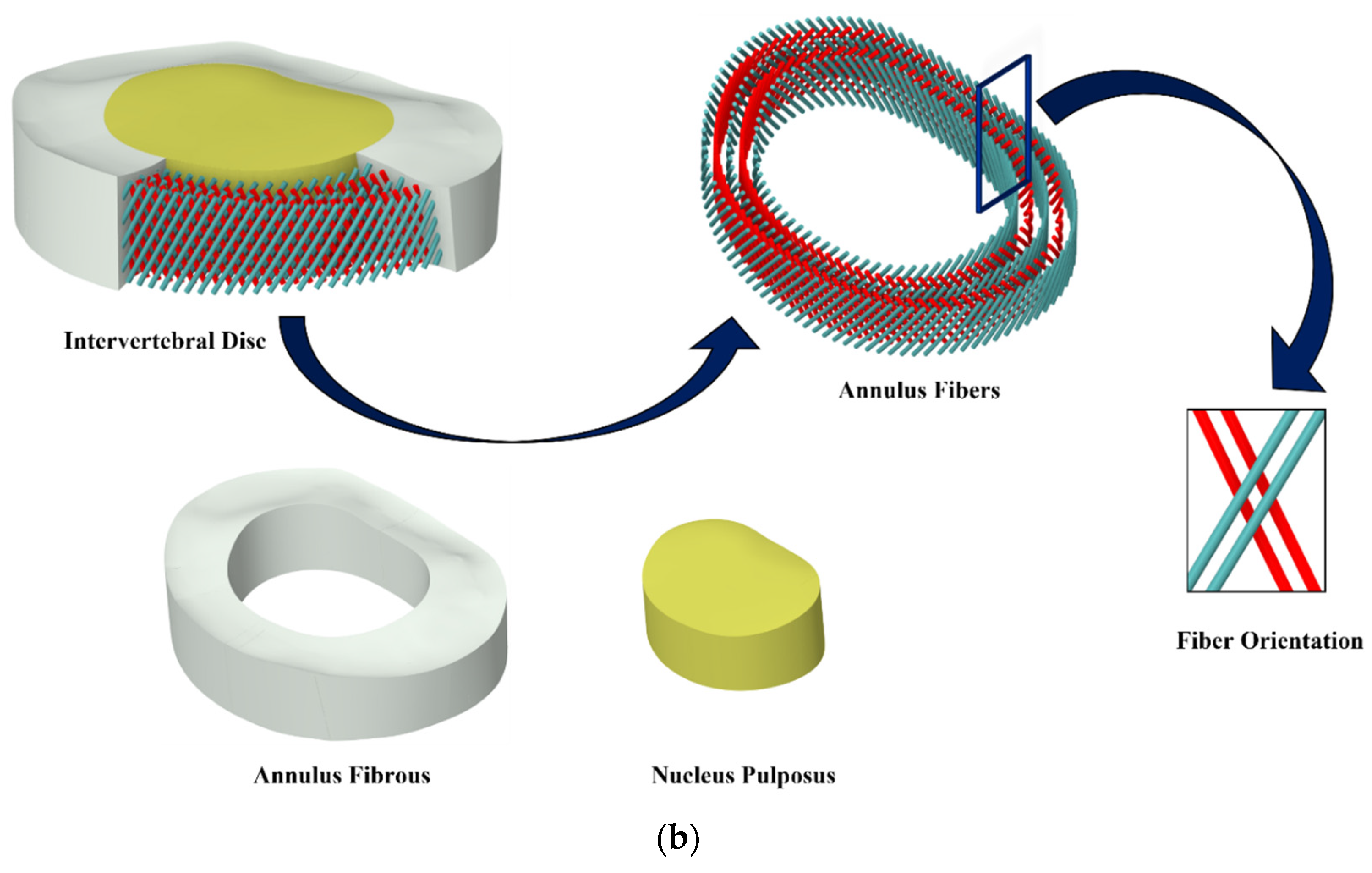
2.1. FE Model Development of FSU
| W | Strain Energy Function |
| I1 and I2 | First/second deviatoric strain invariants |
| c01 and c10 | Material constants |
| d | Incompressibility parameter of the material |
| J | Elastic volume ratio |
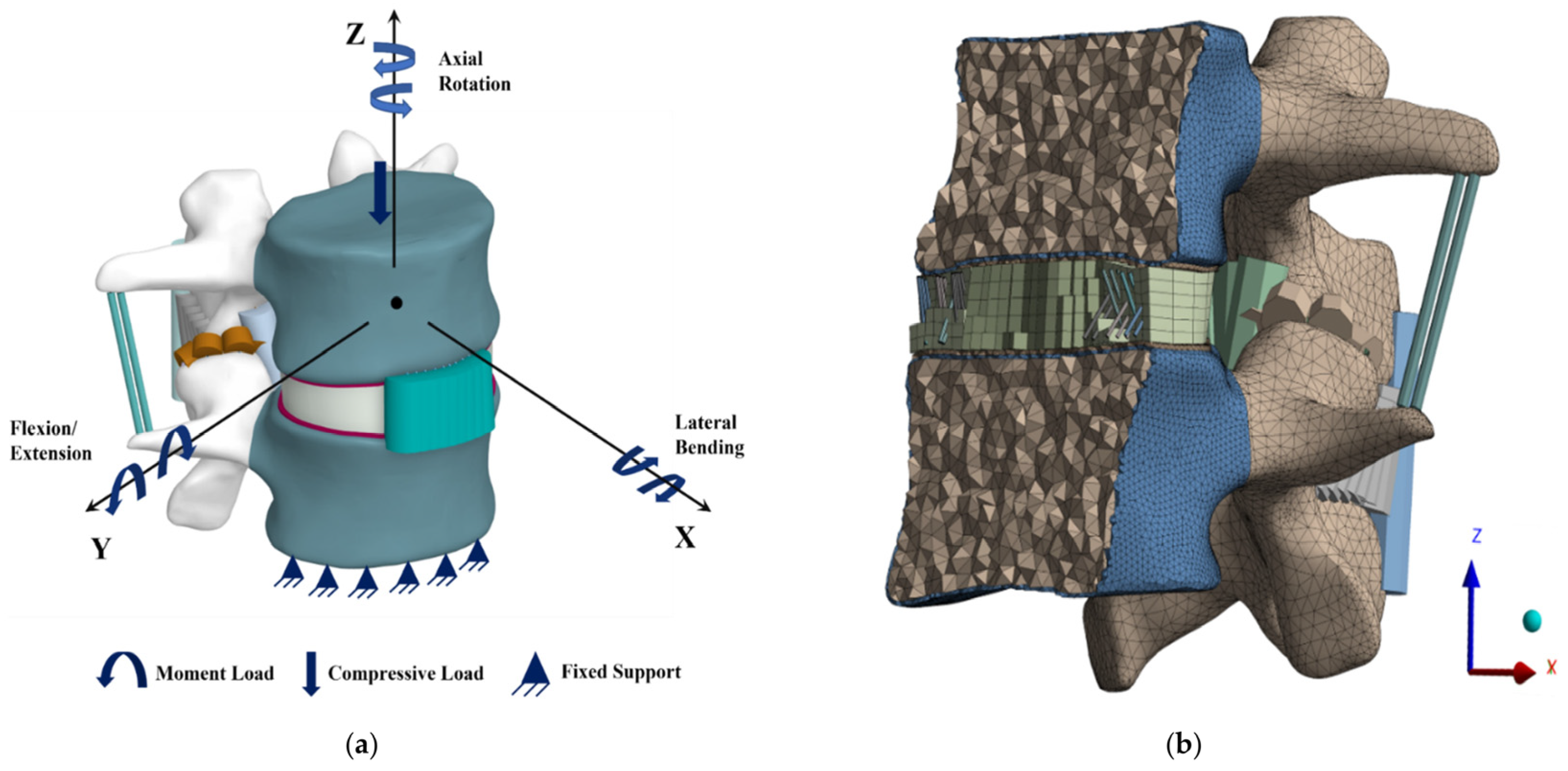
2.2. Boundary and Loading Conditions
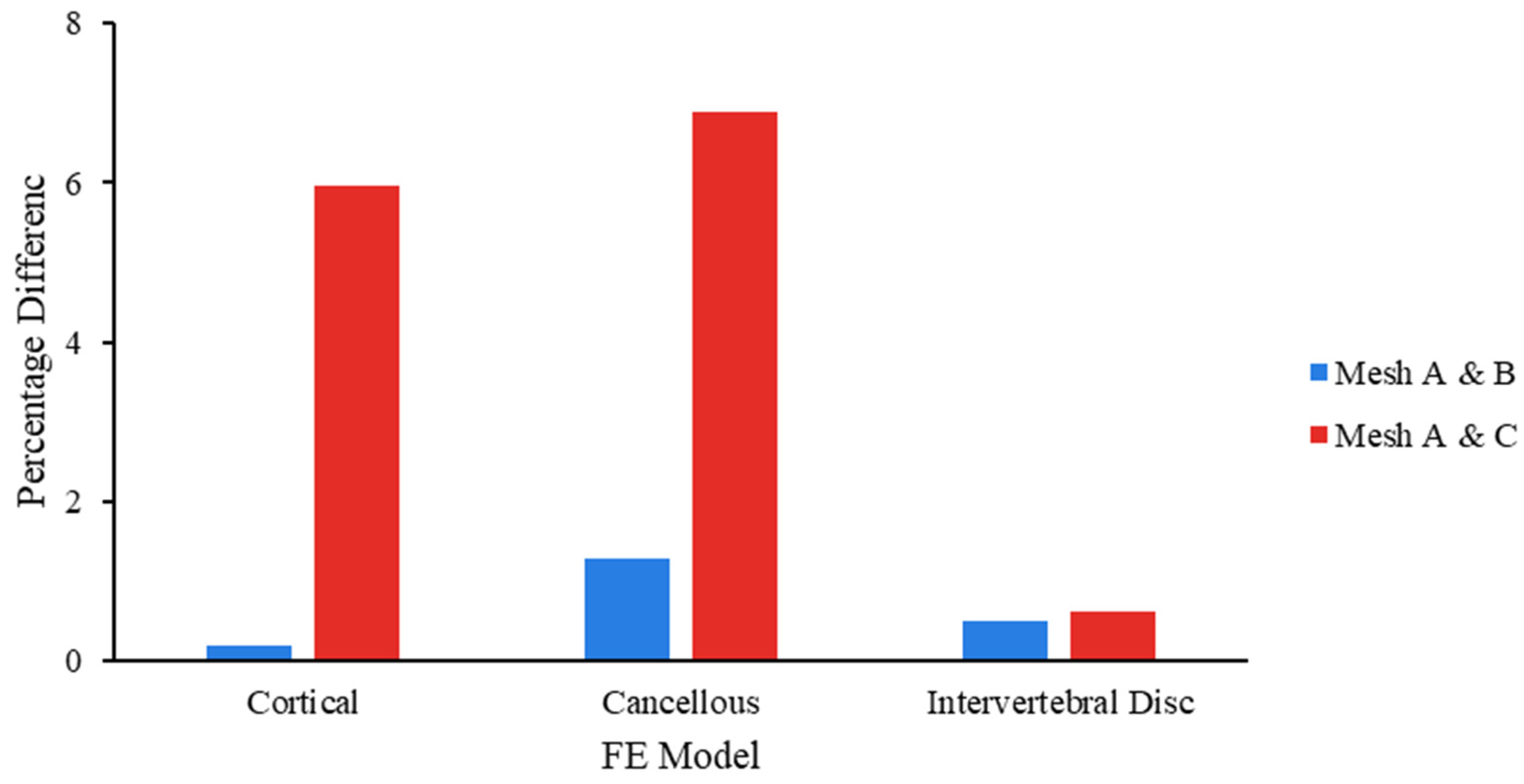
2.3. Mesh Convergence
3. Results
3.1. Validation of FSU-FE Model
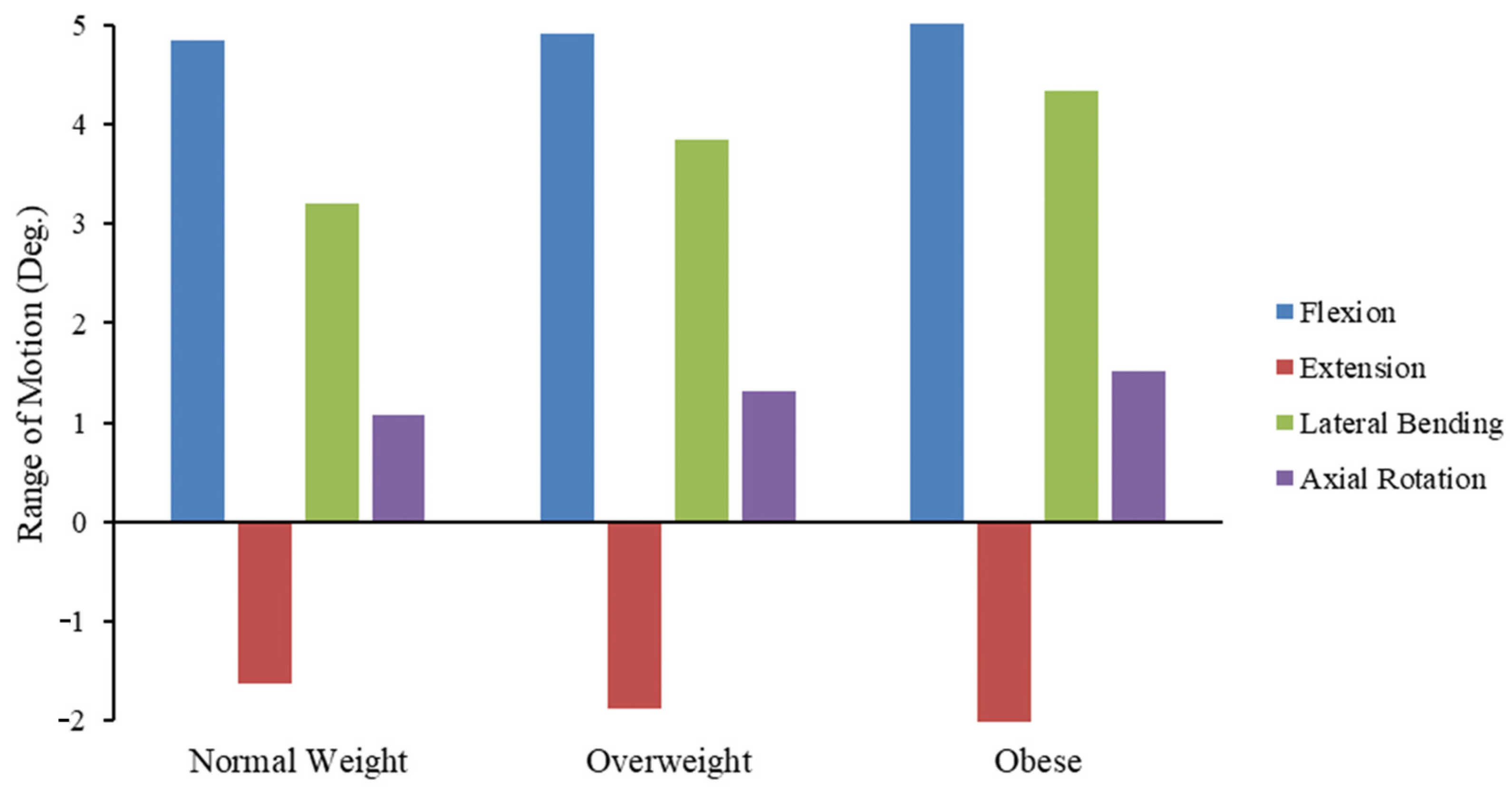
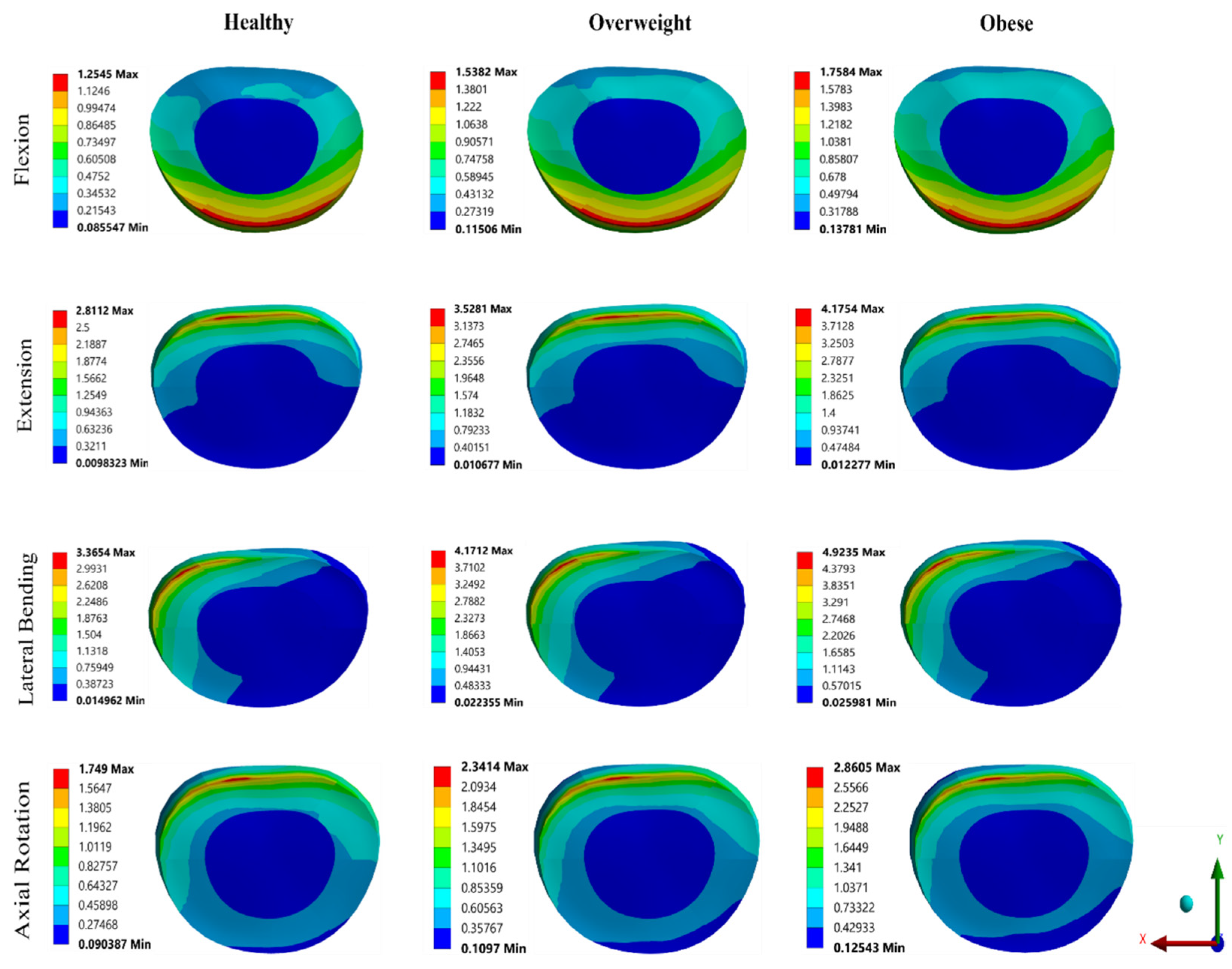
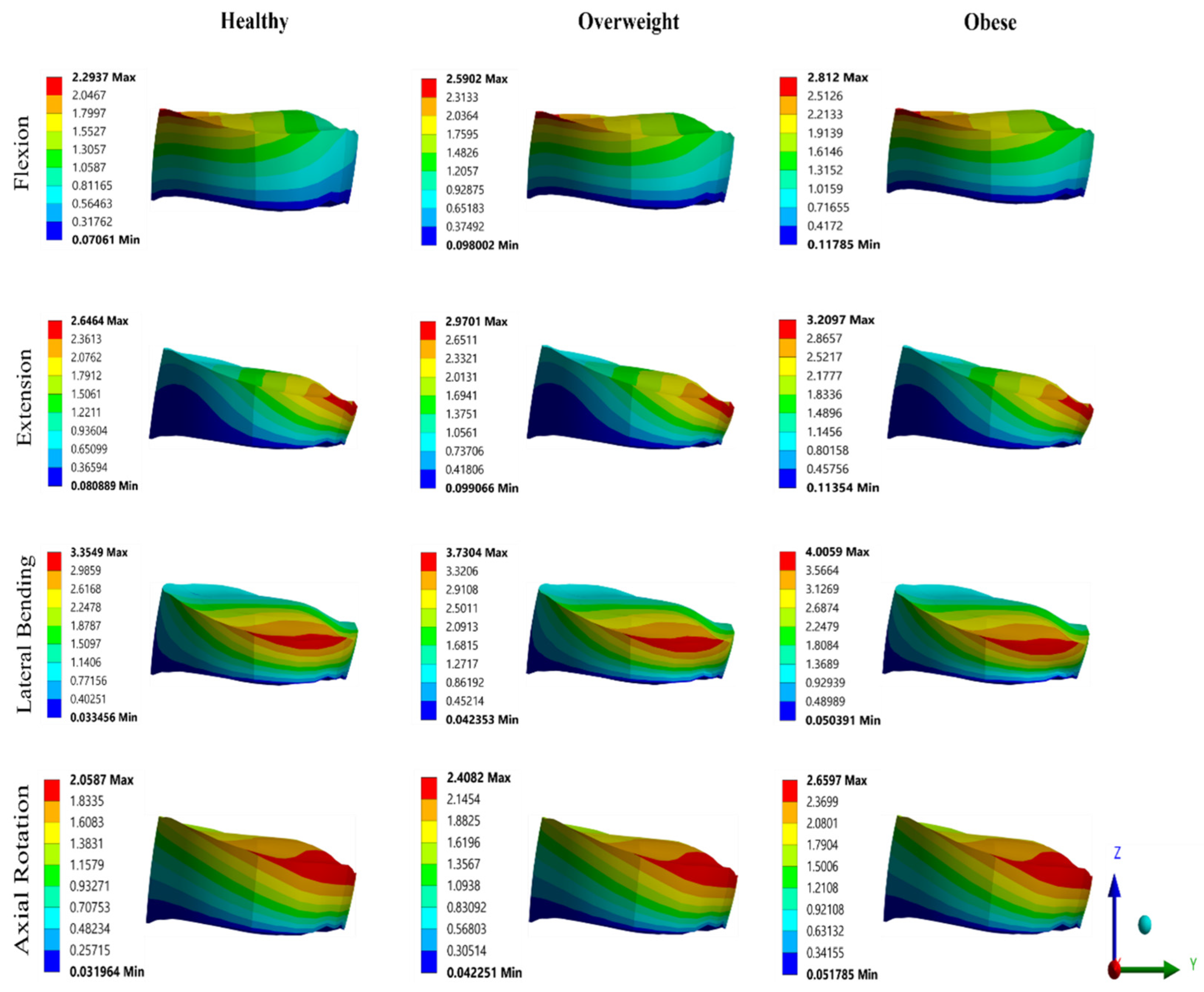
3.2. Effect of BMI on FSU
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Masni-Azian; Tanaka, M. Biomechanical investigation on the influence of the regional material degeneration of an intervertebral disc in a lower lumbar spinal unit: A finite element study. Comput. Biol. Med. 2018, 98, 26–38. [Google Scholar] [CrossRef] [PubMed]
- Tavakoli, J.; Diwan, A.D.; Tipper, J.L. Advanced strategies for the regeneration of lumbar disc annulus fibrosus. Int. J. Mol. Sci. 2020, 21, 4889. [Google Scholar] [CrossRef] [PubMed]
- Whatley, B.R.; Wen, X. Intervertebral disc (IVD): Structure, degeneration, repair and regeneration. Mater. Sci. Eng. C Mater. Biol. Appl. 2012, 32, 61–77. [Google Scholar] [CrossRef]
- Dogru, S.C.; Arslan, Y.Z. Effect of model parameters on the biomechanical behavior of the finite element cervical spine model. Appl. Bionics Biomech. 2021, 2021, 5593037. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.E.; Hu, Y.J.; Dinescu, A.T. Bone Structure and Function. In Encyclopedia of Bone Biology; Zaidi, M., Ed.; Elsevier: San Diego, CA, USA, 2020; pp. 233–246. [Google Scholar]
- Pfeiffer, F. The use of finite element analysis to enhance research and clinical practice in orthopedics. J. Knee Surg. 2016, 29, 149–158. [Google Scholar] [CrossRef] [PubMed]
- Lavecchia, C.E.; Espino, D.M.; Moerman, K.M.; Tse, K.M.; Robinson, D.; Lee, P.V.S.; Shepherd, D.E.T. Lumbar model generator: A tool for the automated generation of a parametric scalable model of the lumbar spine. J. R. Soc. Interface 2018, 15, 20170829. [Google Scholar] [CrossRef] [PubMed]
- Campbell, J.Q.; Coombs, D.J.; Rao, M.; Rullkoetter, P.J.; Petrella, A.J. Automated finite element meshing of the lumbar spine: Verification and validation with 18 specimen-specific models. J. Biomech. 2016, 49, 2669–2676. [Google Scholar] [CrossRef] [PubMed]
- Adams, M.A.; Hutton, W.C. The Effect of Posture on the Lumbar Spine. J. Bone Joint Surg. Br. 1985, 67-B, 625–629. [Google Scholar] [CrossRef] [PubMed]
- White, A.A.; Panjabi, M.M. Clinical Biomechanics of the Spine, 2nd ed.; Lippincott Williams and Wilkins: Philadelphia, PA, USA, 1990. [Google Scholar]
- Schultz, A.B.; Warwick, D.N.; Berkson, M.H.; Nachemson, A.L. Mechanical Properties of Human Lumbar Spine Motion Segments—Part I: Responses in Flexion, Extension, Lateral Bending, and Torsion. J. Biomech. Eng. 1979, 101, 46–52. [Google Scholar] [CrossRef]
- Boden, S.D.; Davis, D.O.; Dina, T.S.; Patronas, N.J.; Wiesel, S.W. Abnormal Magnetic-Resonance Scans of the Lumbar Spine in Asymptomatic Subjects. A Prospective Investigation. J. Bone Joint Surg. Am. 1990, 72, 403–408. [Google Scholar] [CrossRef]
- WHO. Obesity and Overweight. Fact Sheet. WHO Media Cent. 2021. Available online: https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight (accessed on 5 January 2024).
- National Health Portal of India. Zahid, Obesity. 2016. Available online: https://www.nhp.gov.in/disease/non-communicable-disease/obesity (accessed on 5 January 2024).
- National Family Health Survey (NFHS-5). 2021. Available online: http://rchiips.org/nfhs/factsheet_NFHS-5.shtml (accessed on 5 January 2024).
- Luhar, S.; Timæus, I.M.; Jones, R.; Cunningham, S.; Patel, S.A.; Kinra, S.; Clarke, L.; Houben, R. Forecasting the prevalence of overweight and obesity in India to 2040. PLoS ONE 2020, 15, e0229438. [Google Scholar] [CrossRef] [PubMed]
- Weir, C.B.; Jan, A. BMI Classification Percentile and Cut Off Points; StatPearls Publishing: Treasure Island, FL, USA, 2024. Available online: http://www.ncbi.nlm.nih.gov/books/NBK541070/ (accessed on 31 January 2024).
- Dymond, T. Musculoskeletal Fitness and Body Composition of Newfoundland and Labrador Offshore Oil and Gas Workers. Master’s Thesis, Memorial University of Newfoundland, St. John’s, NL, Canada, May 2016; pp. 1–75. [Google Scholar]
- Vismara, L.; Menegoni, F.; Zaina, F.; Galli, M.; Negrini, S.; Capodaglio, P. Effect of obesity and low back pain on spinal mobility: A cross sectional study in women. J. Neuroeng. Rehabil. 2010, 7, 3. [Google Scholar] [CrossRef]
- Porto, H.C.D.; Pechak, C.M.; Smith, D.R.; Reed-Jones, R.J. Biomechanical effects of obesity on balance. Int. J. Exerc. Sci. 2012, 5, 301–320. [Google Scholar]
- Mellin, G.; Härkäpää, K.; Vanharanta, H.; Hupli, M.; Heinonen, R.; Järvikoski, A. Outcome of a multimodal treatment including intensive physical training of patients with chronic low back pain. Spine 1993, 18, 825–829. [Google Scholar] [CrossRef]
- Martin, K.; Fontaine, K.R.; Nicklas, B.J.; Dennis, K.E.; Goldberg, A.P.; Hochberg, M.C. Weight loss and exercise walking reduce pain and improve physical functioning in overweight postmenopausal women with knee osteoarthritis. J. Clin. Rheumatol. 2001, 7, 219–223. [Google Scholar] [CrossRef]
- Heuer, F.; Schmidt, H.; Wilke, H.-J. Stepwise reduction of functional spinal structures increase disc bulge and surface strains. J. Biomech. 2008, 41, 1953–1960. [Google Scholar] [CrossRef]
- Palepu, V.; Kodigudla, M.; Goel, V.K. Biomechanics of disc degeneration. Adv. Orthop. 2012, 2012, 726210. [Google Scholar] [CrossRef]
- Koremans, F.W.; Chen, X.; Das, A.; Diwan, A.D. Changes in back pain scores after bariatric surgery in obese patients: A systematic review and meta-analysis. J. Clin. Med. 2021, 10, 1443. [Google Scholar] [CrossRef] [PubMed]
- Coppock, J.A.; Danyluk, S.T.; Englander, Z.A.; Spritzer, C.E.; Goode, A.P.; DeFrate, L.E. Increasing BMI Increases Lumbar Intervertebral Disc Deformation Following a Treadmill Walking Stress Test. J. Biomech. 2021, 121, 110392. [Google Scholar] [CrossRef]
- Zahaf, S.; Mansouri, B.; Belarbi, A.; Azari, Z. Risks Which Caused by Obesity on the Spine. Eur. J. Mech. Eng. Res. 2017, 4, 49–68. [Google Scholar]
- Zahaf, S.; Mansouri, B.; Belarbi, A.; Azari, Z. Obesity Effect on the Spine. Adv. Biosci. Biotechnol. 2015, 6, 556–571. [Google Scholar] [CrossRef]
- Han, K.-S.; Rohlmann, A.; Zander, T.; Taylor, W.R. Lumbar Spinal Loads Vary with Body Height and Weight. Med. Eng. Phys. 2013, 35, 969–977. [Google Scholar] [CrossRef] [PubMed]
- Zahari, S.N.; Latif, M.J.A.; Rahim, N.R.A.; Kadir, M.R.A.; Kamarul, T. The Effects of Physiological Biomechanical Loading on Intradiscal Pressure and Annulus Stress in Lumbar Spine: A Finite Element Analysis. J. Healthc. Eng. 2017, 2017, 9618940. [Google Scholar] [CrossRef] [PubMed]
- Sato, Y.; Shiraga, N.; Nakajima, S.; Tamura, S.; Kikinis, R. Local Maximum Intensity Projection (LMIP: A New Rendering Method for Vascular Visualization. J. Comput. Assist. Tomogr. 1998, 22, 912–917. [Google Scholar] [CrossRef] [PubMed]
- Kanitsar, A.; Fleischmann, D.; Wegenkittl, R.; Felkel, P.; Groller, E. CPR–Curved Planar Reformation. In Proceedings of the IEEE Visualization, VIS 2002, 27 October–1 November 2002; IEEE: Piscataway, NJ, USA, 2008; pp. 37–44. [Google Scholar]
- Whitted, T. An Improved Illumination Model for Shaded Display. Commun. ACM 1980, 23, 343–349. [Google Scholar] [CrossRef]
- Heffernan, P.B.; Robb, R.A. A New Method for Shaded Surface Display of Biological and Medical Images. IEEE Trans. Med. Imaging 1985, 4, 26–38. [Google Scholar] [CrossRef] [PubMed]
- Kajiya, J.T. New Techniques for Ray Tracing Procedurally Defined Objects. In SIGGRAPH’83 Proceedings of the 10th Annual Conference on Computer Graphics and Interactive Techniques, Detroit, MI, USA, 25–29 July 1983; ACM: New York, NY, USA, 1983; pp. 91–102. [Google Scholar]
- Westover, L.A. Splatting: A Parallel, Feed-Forward Volume Rendering Algorithm; University of North Carolina at Chapel Hill: Chapel Hill, NC, USA, 1992; pp. 1–114. [Google Scholar]
- Lacroute, P.; Levoy, M. Fast volume rendering using a shear-warp factorization of the viewing transformation. In Proceedings of the 21st Annual Conference on Computer Graphics and Interactive Techniques-SIGGRAPH 94, Orlando, FL, USA, 24–29 July 1994; pp. 1–18. [Google Scholar]
- Lorensen, W.E.; Cline, H.E. Marching cubes: A high resolution 3D surface construction algorithm. ACM Siggraph Comput. Graph. 1987, 21, 163–169. [Google Scholar] [CrossRef]
- Arena, P.; Bucolo, M.; Fazzino, S.; Fortuna, L.; Frasca, M. The CNN Paradigm: Shapes and Complexity. Int. J. Bifurcat. Chaos 2005, 15, 2063–2090. [Google Scholar] [CrossRef]
- An, G.; Hong, L.; Zhou, X.-B.; Yang, Q.; Li, M.-Q.; Tang, X.-Y. Accuracy and Efficiency of Computer-Aided Anatomical Analysis Using 3D Visualization Software Based on Semi-Automated and Automated Segmentations. Ann. Anat. 2017, 210, 76–83. [Google Scholar] [CrossRef]
- Eltes, P.E.; Bartos, M.; Hajnal, B.; Pokorni, A.J.; Kiss, L.; Lacroix, D.; Varga, P.P.; Lazary, A. Development of a computer-aided design and finite element analysis combined method for affordable spine surgical navigation with 3D-printed customized template. Front. Surg. 2020, 7, 583386. [Google Scholar] [CrossRef]
- Manickam, P.S.; Roy, S. The biomechanical study of cervical spine: A Finite Element Analysis. Int. J. Artif. Organs 2022, 45, 89–95. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, H.; Kettler, A.; Heuer, F.; Simon, U.; Claes, L.; Wilke, H.-J. Intradiscal pressure, shear strain, and fiber strain in the intervertebral disc under combined loading. Spine 2007, 32, 748–755. [Google Scholar] [CrossRef] [PubMed]
- Schileo, E.; Dall’Ara, E.; Taddei, F.; Malandrino, A.; Schotkamp, T.; Baleani, M.; Viceconti, M. An accurate estimation of bone density improves the accuracy of subject-specific finite element models. J. Biomech. 2008, 41, 2483–2491. [Google Scholar] [CrossRef] [PubMed]
- Morgan, E.F.; Bayraktar, H.H.; Keaveny, T.M. Trabecular bone modulus-density relationships depend on anatomic site. J. Biomech. 2003, 36, 897–904. [Google Scholar] [CrossRef] [PubMed]
- Silva, M.J.; Wang, C.; Keaveny, T.M.; Hayes, W.C. Direct and computed tomography thickness measurements of the human, lumbar vertebral shell and endplate. Bone 1994, 15, 409–414. [Google Scholar] [CrossRef] [PubMed]
- Thomas, E.W.; Zheng, Y.; Ferrara, L.A.; Yuan, H.A. Structural features and thickness of the vertebral cortex in the thoracolumbar spine. Spine 2001, 26, 218–225. [Google Scholar] [CrossRef]
- Cvijanovic, O.; Bobinac, D.; Zoricic, S.; Ostojic, Z.; Maric, I.; Crncevic-orlic, Z.; Kristofic, I.; Oatojic, L. Age and region dependent changes in human lumbar vertebral bone: A histomorphometric study. Spine 2004, 29, 2370. [Google Scholar] [CrossRef]
- Du, C.-F.; Yang, N.; Guo, J.-C.; Huang, Y.-P.; Zhang, C. Biomechanical response of lumbar facet joints under follower preload: A finite element study. BMC Musculoskelet. Disord. 2016, 17, 126. [Google Scholar] [CrossRef] [PubMed]
- Du, H.-G.; Liao, S.-H.; Jiang, Z.; Huang, H.-M.; Ning, X.-T.; Jiang, N.-Y.; Pei, J.-W.; Huang, Q.; Wei, H. Biomechanical analysis of press-extension technique on degenerative lumbar with disc herniation and staggered facet joint. Saudi Pharm. J. 2016, 24, 305–311. [Google Scholar] [CrossRef]
- Remus, R.; Lipphaus, A.; Neumann, M.; Bender, B. Calibration and validation of a novel hybrid model of the lumbosacral spine in ArtiSynth-The passive structures. PLoS ONE 2021, 16, e0250456. [Google Scholar] [CrossRef]
- Zhu, H.; Zhong, W.; Zhang, P.; Liu, X.; Huang, J.; Liu, F.; Li, J. Biomechanical evaluation of autologous bone-cage in posterior lumbar interbody fusion: A finite element analysis. BMC Musculoskelet. Disord. 2020, 21, 379. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Tang, H.; Guan, X.; Jiang, F.; Xu, N.; Ju, W.; Zhu, X.; Zhang, X.; Zhang, Q.; Li, M. Biomechanical comparison of posterior lumbar interbody fusion and transforaminal lumbar interbody fusion by finite element analysis. Neurosurgery 2013, 72 (Suppl. S1 Operative), 21–26. [Google Scholar] [CrossRef] [PubMed]
- Glaeser, J.D.; Tawackoli, W.; Ju, D.G.; Yang, J.H.; Kanim, L.E.A.; Salehi, K.; Yu, V.; Saidara, E.; Vit, J.-P.; Khnkoyan, Z.; et al. Optimization of a rat lumbar IVD degeneration model for low back pain. JOR Spine 2020, 3, e1092. [Google Scholar] [CrossRef] [PubMed]
- Newman, H.R.; DeLucca, J.F.; Peloquin, J.M.; Vresilovic, E.J.; Elliott, D.M. Multiaxial validation of a finite element model of the intervertebral disc with multigenerational fibers to establish residual strain. JOR Spine 2021, 4, e1145. [Google Scholar] [CrossRef] [PubMed]
- White, A.A., III. Clinical biomechanics of cervical spine implants. Spine 1989, 14, 1040–1045. [Google Scholar] [CrossRef] [PubMed]
- Goel, V.K.; Monroe, B.T.; Gilbertson, L.G.; Brinckmann, P. Interlaminar shear stresses and laminae separation in a disc. Finite element analysis of the L3-L4 motion segment subjected to axial compressive loads. Spine 1995, 20, 689–698. [Google Scholar] [CrossRef] [PubMed]
- Shirazi-Adl, A.; Ahmed, A.M.; Shrivastava, S.C. A finite element study of a lumbar motion segment subjected to pure sagittal plane moments. J. Biomech. 1986, 19, 331–350. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Z.; Wang, L.; Gong, P.H.; Gao, J.; Zhang, X. Establishment and verification of a non-linear finite element model for human L4-L5 lumbar segment. In Proceedings of the 2010 3rd International Conference on Biomedical Engineering and Informatics, BMEI 2010, Yantai, China, 16–18 October 2010; Volume 3, pp. 1171–1175. [Google Scholar]
- Kosalishkwaran, G.; Parasuraman, S.; Singh, D.K.J.; Natarajan, E.; Elamvazuthi, I.; George, J. Measurement of range of motions of L3-L4 healthy spine through offsetting reflective markers and in silico analysis of meshed model. Med. Biol. Eng. Comput. 2019, 57, 2305–2318. [Google Scholar] [CrossRef]
- Kiapour, A.; Ambati, D.; Hoy, R.W.; Goel, V.K. Effect of graded facetectomy on biomechanics of Dynesys dynamic stabilization system. Spine 2012, 37, E581–E589. [Google Scholar] [CrossRef]
- Kiapour, A.; Anderson, D.G.; Spenciner, D.B.; Ferrara, L.; Goel, V.K. Kinematic effects of a pedicle-lengthening osteotomy for the treatment of lumbar spinal stenosis: Laboratory investigation. J. Neurosurg. Spine 2012, 17, 314–320. [Google Scholar] [CrossRef]
- Lu, Y.M.; Hutton, W.C.; Gharpuray, V.M. Do bending, twisting, and diurnal fluid changes in the disc affect the propensity to prolapse? A viscoelastic finite element model. Spine 1996, 21, 2570–2579. [Google Scholar] [CrossRef]
- Schmidt, H.; Heuer, F.; Simon, U.; Kettler, A.; Rohlmann, A.; Claes, L.; Wilke, H.-J. Application of a new calibration method for a three-dimensional finite element model of a human lumbar annulus fibrosus. Clin. Biomech. (Bristol Avon.) 2006, 21, 337–344. [Google Scholar] [CrossRef] [PubMed]
- Ibarz, E.; Más, Y.; Mateo, J.; Lobo-Escolar, A.; Herrera, A.; Gracia, L. Instability of the lumbar spine due to disc degeneration. A finite element simulation. Adv. Biosci. Biotechnol. 2013, 04, 548–556. [Google Scholar] [CrossRef]
- Li, Q.Y.; Kim, H.-J.; Son, J.; Kang, K.-T.; Chang, B.-S.; Lee, C.-K.; Seok, H.S.; Yeom, J.S. Biomechanical analysis of lumbar decompression surgery in relation to degenerative changes in the lumbar spine–Validated finite element analysis. Comput. Biol. Med. 2017, 89, 512–519. [Google Scholar] [CrossRef] [PubMed]
- Dreischarf, M.; Rohlmann, A.; Bergmann, G.; Zander, T. Optimised in vitro applicable loads for the simulation of lateral bending in the lumbar spine. Med. Eng. Phys. 2012, 34, 777–780. [Google Scholar] [CrossRef] [PubMed]
- Dreischarf, M.; Rohlmann, A.; Bergmann, G.; Zander, T. Optimised loads for the simulation of axial rotation in the lumbar spine. J. Biomech. 2011, 44, 2323–2327. [Google Scholar] [CrossRef]
- Zander, T.; Rohlmann, A.; Bergmann, G. Influence of different artificial disc kinematics on spine biomechanics. Clin. Biomech. 2009, 24, 135–142. [Google Scholar] [CrossRef]
- Dreischarf, M.; Zander, T.; Shirazi-Adl, A.; Puttlitz, C.M.; Adam, C.J.; Chen, C.S.; Goel, V.K.; Kiapour, A.; Kim, Y.H.; Labus, K.M.; et al. Comparison of eight published static finite element models of the intact lumbar spine: Predictive power of models improves when combined together. J. Biomech. 2014, 47, 1757–1766. [Google Scholar] [CrossRef] [PubMed]
- Panjabi, M.M.; Kifune, M.; Liu, W.; Arand, M.; Vasavada, A.; Oxland, T.R. Graded thoracolumbar spinal injuries: Development of multidirectional instability. Eur. Spine J. 1998, 7, 332–339. [Google Scholar] [CrossRef]
- ICMR-NIN Expert Group on Requirements for Indians, Recommended Dietary Allowancs and Estimated Average Reqiurment; National Institute of Nutrition: Hyderabad, India. Available online: https://www.nin.res.in/RDA_Full_Report_2020.html (accessed on 5 January 2024).
- Li, J.; An, Z.; Wu, J.; Gao, Y.; Lu, S.; He, D.; Zhao, Y. Construction of the adjusted scoliosis 3D finite element model and biomechanical analysis under gravity. Orthop. Surg. 2023, 15, 606–616. [Google Scholar] [CrossRef]
- Ayturk, U.M.; Puttlitz, C.M. Parametric convergence sensitivity and validation of a finite element model of the human lumbar spine. Comput. Methods Biomech. Biomed. Eng. 2011, 14, 695–705. [Google Scholar] [CrossRef] [PubMed]
- Jones, A.C.; Wilcox, R.K. Finite element analysis of the spine: Towards a framework of verification, validation and sensitivity analysis. Med. Eng. Phys. 2008, 30, 1287–1304. [Google Scholar] [CrossRef] [PubMed]
- Somovilla-Gómez, F.; Lostado-Lorza, R.; Corral-Bobadilla, M.; Escribano-García, R. Improvement in determining the risk of damage to the human lumbar functional spinal unit considering age, height, weight and sex using a combination of FEM and RSM. Biomech. Model. Mechanobiol. 2020, 19, 351–387. [Google Scholar] [CrossRef] [PubMed]
- Anderson, A.E.; Ellis, B.J.; Weiss, J.A. Verification, validation and sensitivity studies in computational biomechanics. Comput. Methods Biomech. Biomed. Eng. 2007, 10, 171–184. [Google Scholar] [CrossRef] [PubMed]
- Henninger, H.B.; Reese, S.P.; Anderson, A.E.; Weiss, J.A. Validation of computational models in biomechanics. Proc. Inst. Mech. Eng. H 2010, 224, 801–812. [Google Scholar] [CrossRef] [PubMed]
- Viceconti, M.; Olsen, S.; Nolte, L.-P.; Burton, K. Extracting clinically relevant data from finite element simulations. Clin. Biomech. (Bristol Avon.) 2005, 20, 451–454. [Google Scholar] [CrossRef] [PubMed]
- Pearcy, M.; Portek, I.; Shepherd, J. Three-dimensional x-ray analysis of normal movement in the lumbar spine. Spine 1984, 9, 294–297. [Google Scholar] [CrossRef] [PubMed]
- Pearcy, M.J.; Tibrewal, S.B. Axial rotation and lateral bending in the normal lumbar spine measured by three-dimensional radiography. Spine 1984, 9, 582–587. [Google Scholar] [CrossRef] [PubMed]
- Pearcy, M.J. Stereo radiography of lumbar spine motion. Acta Orthop. Scand. Suppl. 1985, 212, 1–45. [Google Scholar] [CrossRef]
- Wilke, H.; Neef, P.; Hinz, B.; Seidel, H.; Claes, L. Intradiscal pressure together with anthropometric data–a data set for the validation of models. Clin. Biomech. (Bristol Avon.) 2001, 16 (Suppl. S1), S111–S126. [Google Scholar] [CrossRef]
- Park, W.M.; Kim, K.; Kim, Y.H. Effects of degenerated intervertebral discs on intersegmental rotations, intradiscal pressures, and facet joint forces of the whole lumbar spine. Comput. Biol. Med. 2013, 43, 1234–1240. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.-L.; Zhong, Z.-C.; Hsu, H.-W.; Shih, S.-L.; Wang, S.-T.; Hung, C.; Chen, C.-S. Effect of the cord pretension of the Dynesys dynamic stabilisation system on the biomechanics of the lumbar spine: A finite element analysis. Eur. Spine J. 2011, 20, 1850–1858. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, H.; Galbusera, F.; Rohlmann, A.; Zander, T.; Wilke, H.-J. Effect of multilevel lumbar disc arthroplasty on spine kinematics and facet joint loads in flexion and extension: A finite element analysis. Eur. Spine J. 2012, 21 (Suppl. S5), S663–S674. [Google Scholar] [CrossRef] [PubMed]
- Shirazi-Adl, A. Biomechanics of the lumbar spine in sagittal/lateral moments. Spine 1994, 19, 2407–2414. [Google Scholar] [CrossRef] [PubMed]
- Heo, M.; Yun, J.; Kim, H.; Lee, S.-S.; Park, S. Optimization of a Lumbar Interspinous Fixation Device for the Lumbar Spine with Degenerative Disc Disease. PLoS ONE 2022, 17, e0265926. [Google Scholar] [CrossRef] [PubMed]
- Fada, R.; Shahgholi, M.; Azimi, R.; Babadi, N.F. Estimation of Porosity Effect on Mechanical Properties in Calcium Phosphate Cement Reinforced by Strontium Nitrate Nanoparticles: Fabrication and FEM Analysis. Arab. J. Sci. Eng. 2024, 49, 1815–1825. [Google Scholar] [CrossRef]
- Panzer, M.B.; Cronin, D.S. C4-C5 segment finite element model development, validation, and load-sharing investigation. J. Biomech. 2009, 42, 480–490. [Google Scholar] [CrossRef] [PubMed]
- Warren, J.M.; Hey, L.A.; Mazzoleni, A.P. A finite element study of the relationship between upper body weight and the loads experienced by the human lumbosacral spine, and fusion instrumentation, in a standing upright posture. Biomed. Eng. Adv. 2021, 2, 100023. [Google Scholar] [CrossRef]
- Markolf, K.L.; Morris, J.M. The structural components of the intervertebral disc. A study of their contributions to the ability of the disc to withstand compressive forces. J. Bone Joint Surg. Am. 1974, 56, 675–687. [Google Scholar] [CrossRef]
- Johnstone, B.; Urban, J.P.; Roberts, S.; Menage, J. The fluid content of the human intervertebral disc. Comparison between fluid content and swelling pressure profiles of discs removed at surgery and those taken postmortem. Spine 1992, 17, 412–416. [Google Scholar] [CrossRef]
- Wilder, D.G.; Pope, M.H.; Frymoyer, J.W. The biomechanics of lumbar disc herniation and the effect of overload and instability. J. Spinal Disord. 1988, 1, 16–32. [Google Scholar] [CrossRef]
- Diwan, A.D.; Melrose, J. Intervertebral disc degeneration and how it leads to low back pain. JOR Spine 2023, 6, e1231. [Google Scholar] [CrossRef] [PubMed]
- Beattie, P.F.; Meyers, S.P.; Stratford, P.; Millard, R.W.; Hollenberg, G.M. Associations between patient report of symptoms and anatomic impairment visible on lumbar magnetic resonance imaging. Spine 2000, 25, 819–828. [Google Scholar] [CrossRef] [PubMed]
- Goode, A.P.; Carey, T.S.; Jordan, J.M. Low back pain and lumbar spine osteoarthritis: How are they related? Curr. Rheumatol. Rep. 2013, 15, 305. [Google Scholar] [CrossRef]
- Taylor, J.B.; Goode, A.P.; George, S.Z.; Cook, C.E. Incidence and risk factors for first-time incident low back pain: A systematic review and meta-analysis. Spine J. 2014, 14, 2299–2319. [Google Scholar] [CrossRef] [PubMed]
- Tonosu, J.; Oka, H.; Higashikawa, A.; Okazaki, H.; Tanaka, S.; Matsudaira, K. The associations between magnetic resonance imaging findings and low back pain: A 10-year longitudinal analysis. PLoS ONE 2017, 12, e0188057. [Google Scholar] [CrossRef] [PubMed]
- Steffens, D.; Hancock, M.J.; Maher, C.G.; Williams, C.; Jensen, T.S.; Latimer, J. Does magnetic resonance imaging predict future low back pain? A systematic review: MRI predicting future LBP. Eur. J. Pain 2014, 18, 755–765. [Google Scholar] [CrossRef] [PubMed]
- Ye, Y.; Jin, S.; Zou, Y.; Fang, Y.; Xu, P.; Zhang, Z.; Wu, N.; Zhang, C. Biomechanical evaluation of lumbar spondylolysis repair with various fixation options: A finite element analysis. Front. Bioeng. Biotechnol. 2022, 10, 1024159. [Google Scholar] [CrossRef]
- Turbucz, M.; Pokorni, A.J.; Szőke, G.; Hoffer, Z.; Kiss, R.M.; Lazary, A.; Eltes, P.E. Development and validation of two intact lumbar spine finite element models for in silico investigations: Comparison of the bone modelling approaches. Appl. Sci. 2022, 12, 10256. [Google Scholar] [CrossRef]
- Rao, M. Explicit Finite Element Modeling of the Human Lumbar Spine. Ph.D. Thesis, University of Denver, Denver, CO, USA, 2012; pp. 1–164. [Google Scholar]
- Schlager, B.; Niemeyer, F.; Galbusera, F.; Volkheimer, D.; Jonas, R.; Wilke, H.-J. Uncertainty Analysis of Material Properties and Morphology Parameters in Numerical Models Regarding the Motion of Lumbar Vertebral Segments. Comput. Methods Biomech. Biomed. Eng. 2018, 21, 673–683. [Google Scholar] [CrossRef]
- Doulgeris, J.; Lin, M.; Lee, W.; Aghayev, K.; Papanastassiou, I.D.; Tsai, C.-T.; Vrionis, F.D. Inter-Specimen Analysis of Diverse Finite Element Models of the Lumbar Spine. Bioengineering 2023, 11, 24. [Google Scholar] [CrossRef] [PubMed]
- Mangado, N.; Piella, G.; Noailly, J.; Pons-Prats, J.; Ballester, M.Á.G. Analysis of Uncertainty and Variability in Finite Element Computational Models for Biomedical Engineering: Characterization and Propagation. Front. Bioeng. Biotechnol. 2016, 4, 85. [Google Scholar] [CrossRef] [PubMed]
- Laville, A.; Laporte, S.; Skalli, W. Parametric and Subject-Specific Finite Element Modelling of the Lower Cervical Spine. Influence of Geometrical Parameters on the Motion Patterns. J. Biomech. 2009, 42, 1409–1415. [Google Scholar] [CrossRef] [PubMed]

| BMI Category | BMI (kg/m2) |
|---|---|
| Underweight | <18.5 |
| Normal weight | 18.5 to 24.9 |
| Overweight | 25 to 29.9 |
| Obesity | ≥30 |
| Components | Element Type | Elements | Nodes | Density (g/cm3) | Young’s Modulus (MPa) | Poisson’s Ratio | Cross-Sectional Area (mm2) | References |
|---|---|---|---|---|---|---|---|---|
| Bone | ||||||||
| Cortical Bone | Solid 185 | 33,934 | 101,066 | 1.91 | 12,000 | 0.3 | - | [61,62] |
| Cancellous Bone | Solid 187 | 76,347 | 112,474 | 1.87 | 100 | 0.2 | - | [61,62] |
| Posterior Bone | Solid 187 | 68,897 | 109,181 | 1.87 | 3500 | 0.25 | - | [63] |
| Intervertebral Disc | ||||||||
| Nucleus Pulposus | Solid 185 | 965 | 1290 | 1.0003 | C10 = 0.12, C01 = 0.09 D1 = 1 | [64] | ||
| Annulus Fibrosus | Solid 185 | 1440 | 1960 | 1.0003 | C10 = 0.56, C01 = 0.14 D1 = 1 | [64] | ||
| Annulus Fibers | ||||||||
| Outermost | Link 180 | 100 | 400 | 1.0003 | 550 | 0.3 | 0.196 | [65] |
| Second | Link 180 | 92 | 368 | 1.0003 | 503 | 0.3 | 0.196 | [65] |
| Third | Link 180 | 85 | 340 | 1.0003 | 455 | 0.3 | 0.196 | [65] |
| Fourth | Link 180 | 79 | 316 | 1.0003 | 408 | 0.3 | 0.196 | [65] |
| Fifth | Link 180 | 71 | 284 | 1.0003 | 360 | 0.3 | 0.196 | [65] |
| Endplate | Solid 185 | 6962 | 19,077 | 1.0003 | 23.8 | 0.4 | - | [63] |
| Facet Cartilage | Solid 185 | 1964 | 5216 | 1.0003 | 35 | 0.4 | - | [66] |
| Ligaments | ||||||||
| Anterior Longitudinal | Link 180 | 9 | 36 | 1.0003 | 7.8 | 0.3 | 63.7 | [66] |
| Posterior Longitudinal | Link 180 | 5 | 20 | 1.0003 | 10.0 | 0.3 | 20 | [66] |
| Ligamentum Flavum | Link 180 | 11 | 44 | 1.0003 | 15.0 | 0.3 | 40 | [66] |
| Inter Transverse | Link 180 | 4 | 16 | 1.0003 | 10.0 | 0.3 | 1.8 | [66] |
| Inter Spinous | Link 180 | 8 | 32 | 1.0003 | 10.0 | 0.3 | 40 | [66] |
| Supra Spinous | Link 180 | 1 | 4 | 1.0003 | 8.0 | 0.3 | 30 | [66] |
| Capsular | Link 180 | 20 | 80 | 1.0003 | 7.5 | 0.3 | 30 | [66] |
| Direction of Loading | Compressive Force (N) | Moment (Nm) |
|---|---|---|
| Lateral Bending | 700 | 7.8 |
| Axial Rotation | 720 | 5.5 |
| Extension | 500 | 7.5 |
| Flexion | 1175 | 7.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Singh, N.K.; Singh, N.K.; Verma, R.; Diwan, A.D. Validation and Estimation of Obesity-Induced Intervertebral Disc Degeneration through Subject-Specific Finite Element Modelling of Functional Spinal Units. Bioengineering 2024, 11, 344. https://doi.org/10.3390/bioengineering11040344
Singh NK, Singh NK, Verma R, Diwan AD. Validation and Estimation of Obesity-Induced Intervertebral Disc Degeneration through Subject-Specific Finite Element Modelling of Functional Spinal Units. Bioengineering. 2024; 11(4):344. https://doi.org/10.3390/bioengineering11040344
Chicago/Turabian StyleSingh, Nitesh Kumar, Nishant K. Singh, Rati Verma, and Ashish D. Diwan. 2024. "Validation and Estimation of Obesity-Induced Intervertebral Disc Degeneration through Subject-Specific Finite Element Modelling of Functional Spinal Units" Bioengineering 11, no. 4: 344. https://doi.org/10.3390/bioengineering11040344
APA StyleSingh, N. K., Singh, N. K., Verma, R., & Diwan, A. D. (2024). Validation and Estimation of Obesity-Induced Intervertebral Disc Degeneration through Subject-Specific Finite Element Modelling of Functional Spinal Units. Bioengineering, 11(4), 344. https://doi.org/10.3390/bioengineering11040344






