Cellulose Nanocrystals Show Anti-Adherent and Anti-Biofilm Properties against Oral Microorganisms
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation and Characterization of CNCs
2.2. Procedures
2.3. Preparation of Enamel Disks
2.3.1. Saliva Preparation
2.3.2. Microorganisms
2.3.3. Adherence
2.3.4. Biofilm Formation
2.4. Biomass Viability Assay
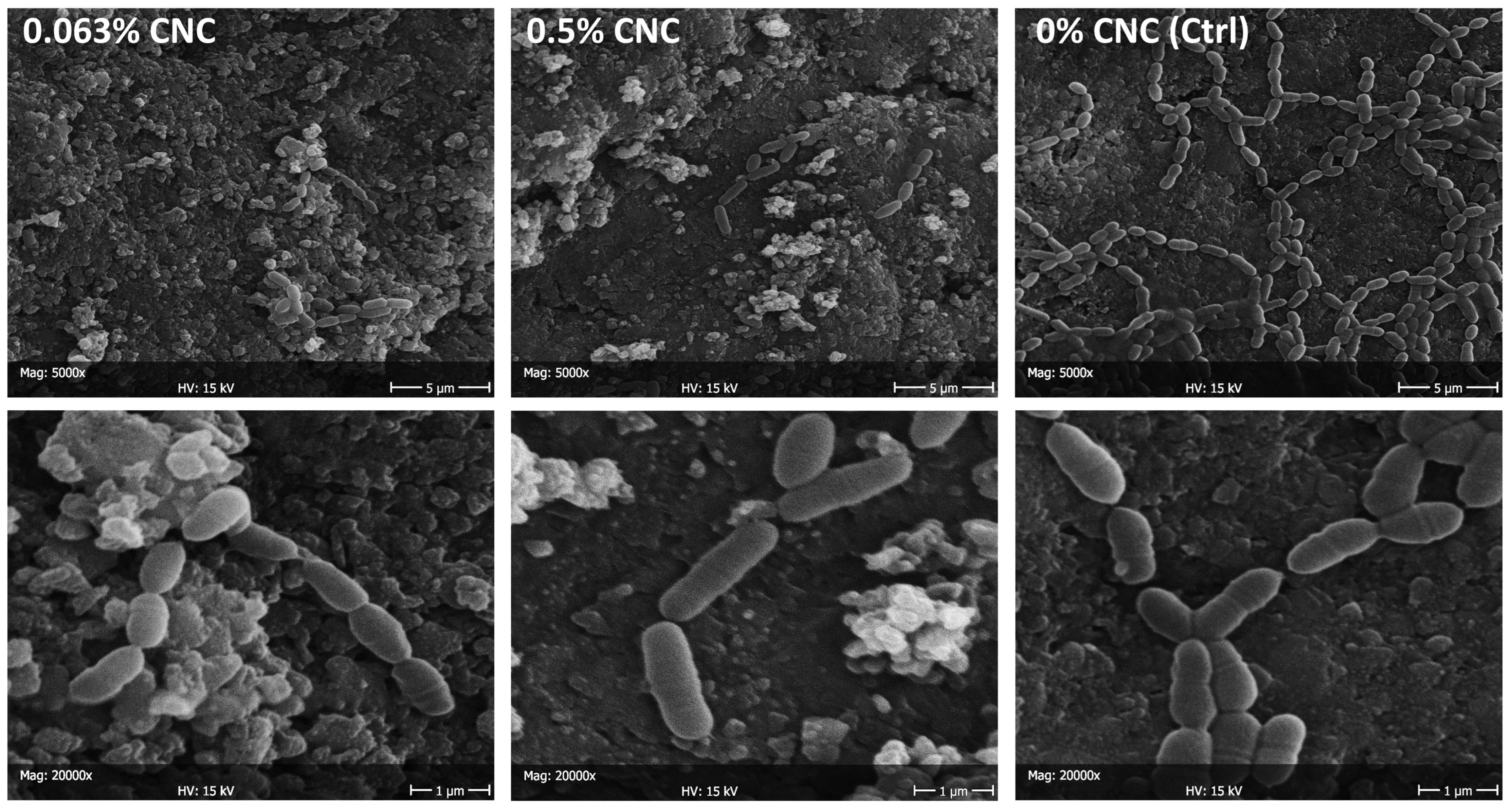
2.5. Scanning Electron Microscopy (SEM) Evaluation
2.6. Statistical Analysis
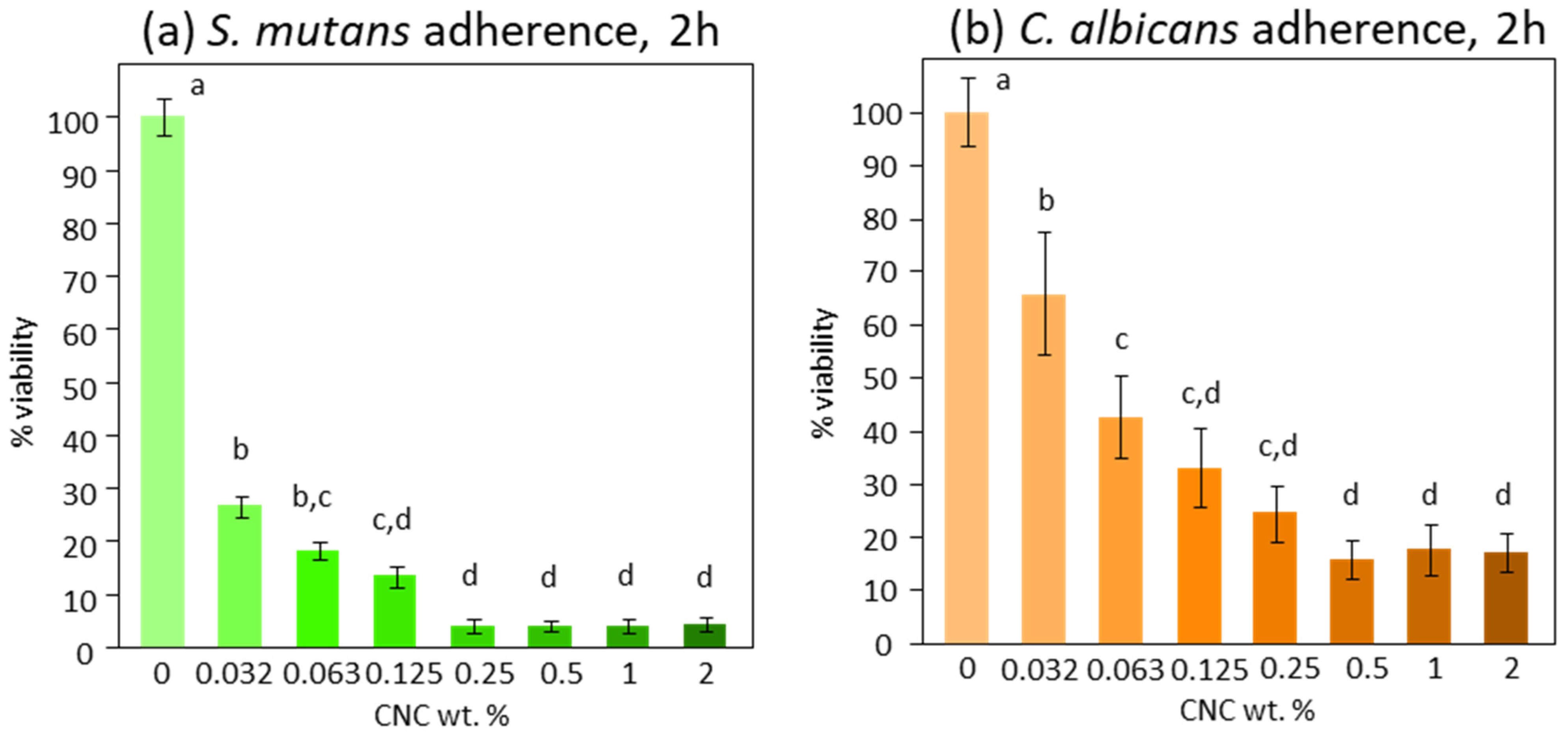
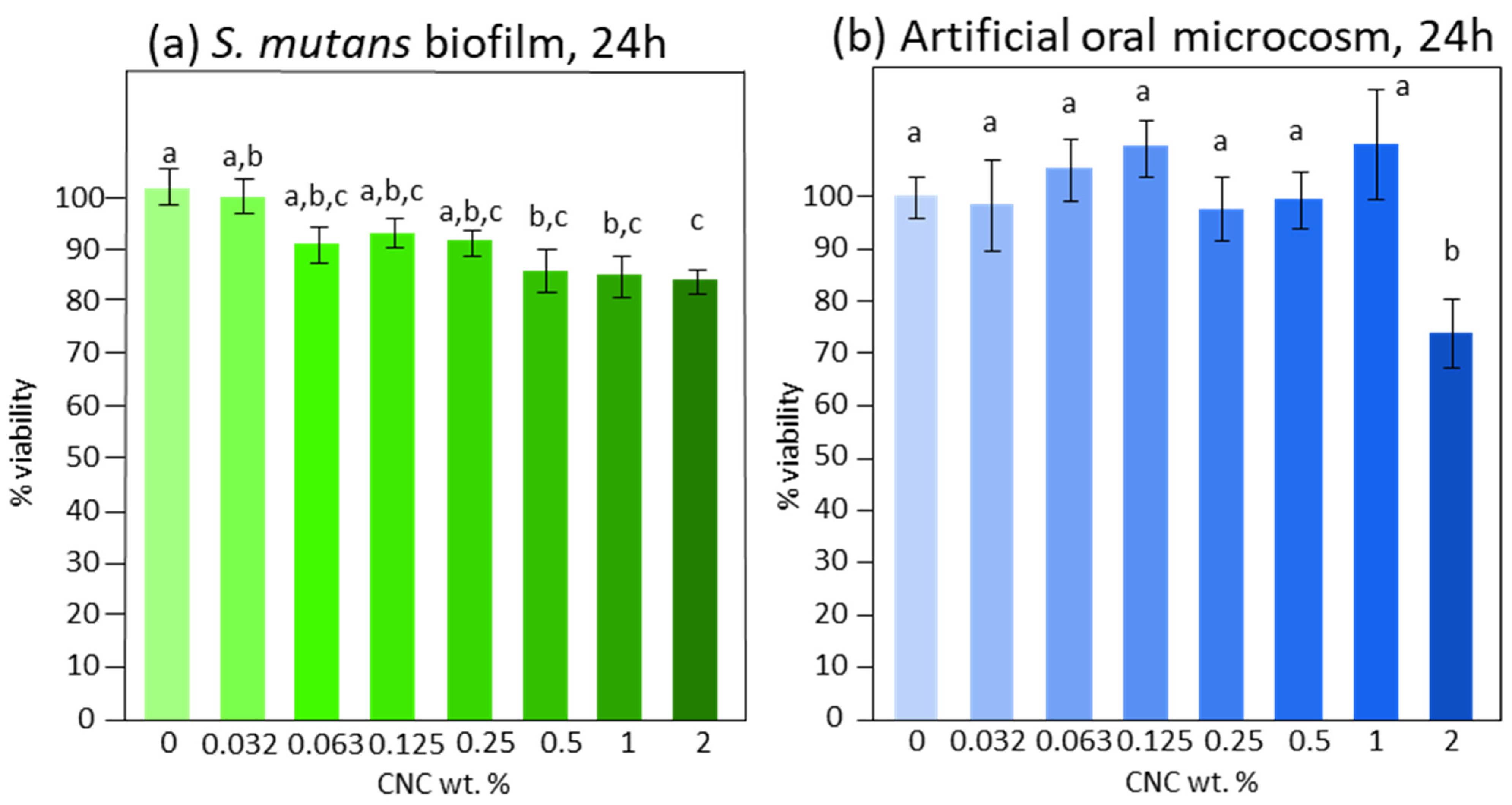
3. Results
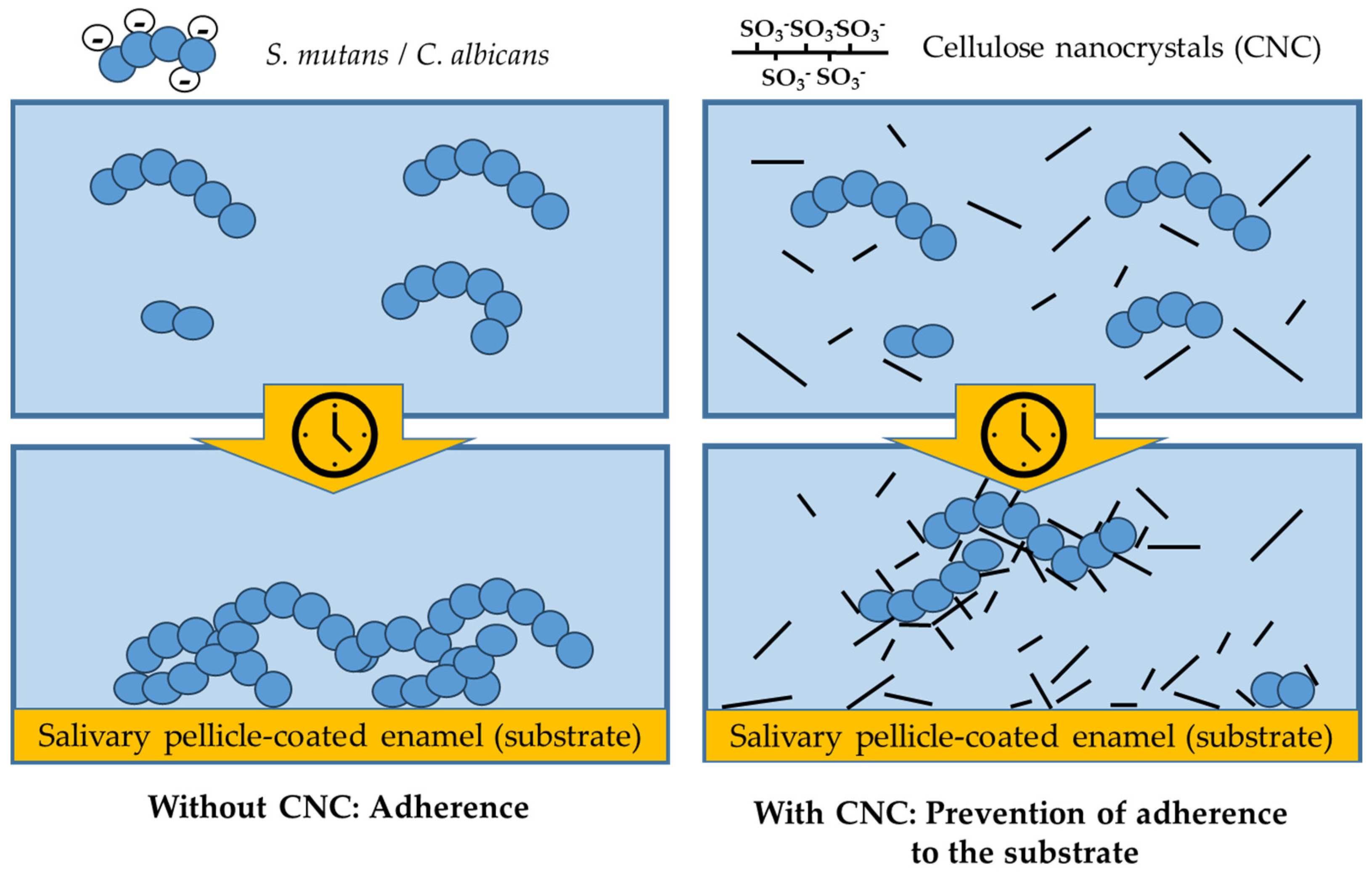
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sun, F.; Hung, H.C.; Yan, W.; Wu, K.; Shimchuk, A.A.; Gray, S.D.; He, W.; Huang, X.; Zhang, H. Inhibition of oral biofilm formation by zwitterionic nonfouling coating. J. Biomed. Mater. Res. B Appl. Biomater. 2021, 109, 1418–1425. [Google Scholar] [CrossRef]
- Butera, A.; Maiorani, C.; Morandini, A.; Simonini, M.; Morittu, S.; Trombini, J.; Scribante, A. Evaluation of children caries risk factors: A narrative review of nutritional aspects, oral hygiene habits, and bacterial alterations. Children 2022, 9, 262. [Google Scholar] [CrossRef] [PubMed]
- Butera, A.; Gallo, S.; Pascadopoli, M.; Taccardi, D.; Scribante, A. Home oral care of periodontal patients using antimicrobial gel with postbiotics, lactoferrin, and aloe barbadensis leaf juice powder vs. conventional chlorhexidine gel: A split-mouth randomized clinical trial. Antibiotics 2022, 11, 118. [Google Scholar] [CrossRef] [PubMed]
- Zaura, E.; Keijser, B.J.; Huse, S.M.; Crielaard, W. Defining the healthy “core microbiome” of oral microbial communities. BMC Microbiol. 2009, 9, 259. [Google Scholar] [CrossRef] [PubMed]
- Kilian, M.; Chapple, I.L.; Hannig, M.; Marsh, P.D.; Meuric, V.; Pedersen, A.M.; Tonetti, M.S.; Wade, W.G.; Zaura, E. The oral microbiome—An update for oral healthcare professionals. Br. Dent. J. 2016, 221, 657–666. [Google Scholar] [CrossRef]
- Marsh, P.D.; Zaura, E. Dental biofilm: Ecological interactions in health and disease. J. Clin. Periodontol. 2017, 44 (Suppl. S18), S12–S22. [Google Scholar] [CrossRef] [PubMed]
- Allaker, R.P. The use of nanoparticles to control oral biofilm formation. J. Dent. Res. 2010, 89, 1175–1186. [Google Scholar] [CrossRef]
- Yu, H.-Y.; Qin, Z.-Y.; Yan, C.-F.; Yao, J.-M. Green nanocomposites based on functionalized cellulose nanocrystals: A study on the relationship between interfacial interaction and property enhancement. ACS Sustain. Chem. Eng. 2014, 2, 875–886. [Google Scholar] [CrossRef]
- Iakovlev, M.; You, X.; van Heiningen, A.; Sixta, H. SO2-ethanol-water (SEW) fractionation process: Production of dissolving pulp from spruce. Cellulose 2014, 21, 1419–1429. [Google Scholar] [CrossRef]
- Zhang, S.; Keshwani, D.R.; Xu, Y.; Hanna, M.A. Alkali combined extrusion pretreatment of corn stover to enhance enzyme saccharification. Ind. Crops Prod. 2012, 37, 352–357. [Google Scholar] [CrossRef]
- Yu, H.; Qin, Z.; Liang, B.; Liu, N.; Zhou, Z.; Chen, L. Facile extraction of thermally stable cellulose nanocrystals with a high yield of 93% through hydrochloric acid hydrolysis under hydrothermal conditions. J. Mater. Chem. A 2013, 1, 3938–3944. [Google Scholar] [CrossRef]
- Reddy, N.; Yang, Y. Completely biodegradable soyprotein-jute biocomposites developed using water without any chemicals as plasticizer. Ind. Crops Prod. 2011, 33, 35–41. [Google Scholar] [CrossRef][Green Version]
- Li, Y.; Liu, Y.; Chen, W.; Wang, Q.; Liu, Y.; Li, J.; Yu, H. Facile extraction of cellulose nanocrystals from wood using ethanol and peroxide solvothermal pretreatment followed by ultrasonic nanofibrillation. Green. Chem. 2016, 18, 1010–1018. [Google Scholar] [CrossRef]
- Mali, P.; Sherje, A.P. Cellulose nanocrystals: Fundamentals and biomedical applications. Carbohydr. Polym. 2022, 275, 118668. [Google Scholar] [CrossRef] [PubMed]
- Taheri, A.; Mohammadi, M. The Use of Cellulose Nanocrystals for Potential Application in Topical Delivery of Hydroquinone. Chem. Biol. Drug Des. 2015, 86, 102–106. [Google Scholar] [CrossRef] [PubMed]
- Sunasee, R.; Hemraz, U.D.; Ckless, K. Cellulose nanocrystals: A versatile nanoplatform for emerging biomedical applications. Expert Opin. Drug Deliv. 2016, 13, 1243–1256. [Google Scholar] [CrossRef] [PubMed]
- Shojaeiarani, J.; Bajwa, D.S.; Chanda, S. Cellulose Nanocrystals Based Composites: A review. Compos. Part C Open Access 2021, 5, 100164. [Google Scholar] [CrossRef]
- Roman, M.; Dong, S.; Hirani, A.; Lee, Y.W. Cellulose nanocrystals for drug delivery. ACS Symp. Ser. Am. Chem. Soc. 2009, 1017, 81–91. [Google Scholar] [CrossRef]
- Xiao, J.; Grier, A.; Faustoferri, R.C.; Alzoubi, S.; Gill, A.L.; Feng, C.; Liu, Y.; Quivey, R.G.; Kopycka-Kedzierawski, D.T.; Koo, H.; et al. Association between Oral Candida and Bacteriome in Children with Severe ECC. J. Dent. Res. 2018, 97, 1468–1476. [Google Scholar] [CrossRef]
- Xiao, J.; Moon, Y.; Li, L.; Rustchenko, E.; Wakabayashi, H.; Zhao, X.; Feng, C.; Gill, S.R.; McLaren, S.; Malmstrom, H.; et al. Candida albicans Carriage in Children with Severe Early Childhood Caries (S-ECC) and Maternal Relatedness. PLoS ONE 2016, 11, e0164242. [Google Scholar] [CrossRef]
- Yawata, Y.; Nguyen, J.; Stocker, R.; Rusconi, R. Microfluidic Studies of Biofilm Formation in Dynamic Environments. J. Bacteriol. 2016, 198, 2589–2595. [Google Scholar] [CrossRef] [PubMed]
- Colombo, L.; Zoia, L.; Violatto, M.B.; Previdi, S.; Talamini, L.; Sitia, L.; Nicotra, F.; Orlandi, M.; Salmona, M.; Recordati, C.; et al. Organ Distribution and Bone Tropism of Cellulose Nanocrystals in Living Mice. Biomacromolecules 2015, 16, 2862–2871. [Google Scholar] [CrossRef] [PubMed]
- Ionescu, A.C.; Cazzaniga, G.; Ottobelli, M.; Garcia-Godoy, F.; Brambilla, E. Substituted nano-hydroxyapatite toothpastes reduce biofilm formation on enamel and resin-based composite surfaces. J. Funct. Biomater. 2020, 11, 36. [Google Scholar] [CrossRef] [PubMed]
- Guggenheim, B.; Giertsen, E.; Schüpbach, P.; Shapiro, S. Validation of an in vitro biofilm model of supragingival plaque. J. Dent. Res. 2001, 80, 363–370. [Google Scholar] [CrossRef] [PubMed]
- Ionescu, A.C.; Cazzaniga, G.; Ottobelli, M.; Ferracane, J.L.; Paolone, G.; Brambilla, E. In vitro biofilm formation on resin-based composites cured under different surface conditions. J. Dent. 2018, 77, 78–86. [Google Scholar] [CrossRef] [PubMed]
- Brambilla, E.; Ionescu, A.C.; Cazzaniga, G.; Ottobelli, M.; Samaranayake, L.P. Levorotatory carbohydrates and xylitol subdue Streptococcus mutans and Candida albicans adhesion and biofilm formation. J. Basic Microbiol. 2016, 56, 480–492. [Google Scholar] [CrossRef] [PubMed]
- Ionescu, A.; Brambilla, E.; Hahnel, S. Does recharging dental restorative materials with fluoride influence biofilm formation? Dent. Mater. 2019, 35, 1450–1463. [Google Scholar] [CrossRef] [PubMed]
- Siqueira, G.; Abdillahi, H.; Bras, J.; Dufresne, A. High reinforcing capability cellulose nanocrystals extracted from Syngonanthus nitens (Capim Dourado). Cellulose 2010, 17, 289–298. [Google Scholar] [CrossRef]
- Abitbol, T.; Kloser, E.; Gray, D.G. Estimation of the surface sulfur content of cellulose nanocrystals prepared by sulfuric acid hydrolysis. Cellulose 2013, 20, 785–794. [Google Scholar] [CrossRef]
- D’Orazio, G.; Munizza, L.; Zampolli, J.; Forcella, M.; Zoia, L.; Fusi, P.; Di Gennaro, P.; La Ferla, B. Cellulose nanocrystals are effective in inhibiting host cell bacterial adhesion. J. Mater. Chem. B 2017, 5, 7018–7020. [Google Scholar] [CrossRef]
- Imiete, I.E.; Giannini, L.; Tadiello, L.; Orlandi, M.; Zoia, L. The effect of sulfate half-ester groups on the mechanical performance of cellulose nanocrystal-natural rubber composites. Cellulose 2023, 30, 8929–8940. [Google Scholar] [CrossRef]
- Zoia, L.; Morelli, A.; Talamini, L.; Violatto, M.B.; Lovati, A.B.; Lopa, S.; Recordati, C.; Toffanin, C.; Salanti, A.; Russo, L.; et al. Cellulose nanocrystals: A multimodal tool to enhance the targeted drug delivery against bone disorders. Nanomedicine 2020, 15, 2271–2285. [Google Scholar] [CrossRef] [PubMed]
- Zoppe, J.O.; Johansson, L.S.; Seppälä, J. Manipulation of cellulose nanocrystal surface sulfate groups toward biomimetic nanostructures in aqueous media. Carbohydr. Polym. 2015, 126, 23–31. [Google Scholar] [CrossRef]
- Llàcer Navarro, S.; Nakayama, K.; Idström, A.; Evenäs, L.; Ström, A.; Nypelö, T. The effect of sulfate half-ester groups on cellulose nanocrystal periodate oxidation. Cellulose 2021, 28, 9633–9644. [Google Scholar] [CrossRef]
- Lin, N.; Dufresne, A. Surface chemistry, morphological analysis and properties of cellulose nanocrystals with gradiented sulfation degrees. Nanoscale 2014, 6, 5384–5393. [Google Scholar] [CrossRef]
- Amoroso, L.; Muratore, G.; Ortenzi, M.A.; Gazzotti, S.; Limbo, S.; Piergiovanni, L. Fast production of cellulose nanocrystals by hydrolytic-oxidative microwave-assisted treatment. Polymers 2020, 12, 68. [Google Scholar] [CrossRef]
- Barana, D.; Salanti, A.; Orlandi, M.; Ali, D.S.; Zoia, L. Biorefinery process for the simultaneous recovery of lignin, hemicelluloses, cellulose nanocrystals and silica from rice husk and Arundo donax. Ind. Crops Prod. 2016, 86, 31–39. [Google Scholar] [CrossRef]
- Habibi, Y.; Lucia, L.A.; Rojas, O.J. Cellulose nanocrystals: Chemistry, self-assembly, and applications. Chem. Rev. 2010, 110, 3479–3500. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Danumah, C.; Liu, Y.; Boluk, Y. Flocculation of bacteria by depletion interactions due to rod-shaped cellulose nanocrystals. Chem. Eng. J. 2012, 198–199, 476–481. [Google Scholar] [CrossRef]
- Sun, X.; Shao, Y.; Boluk, Y.; Liu, Y. The impact of cellulose nanocrystals on the aggregation and initial adhesion to a solid surface of Escherichia coli K12: Role of solution chemistry. Colloids Surf. B Biointerfaces 2015, 136, 570–576. [Google Scholar] [CrossRef]
- Rashid, A.B.; Hoque, M.E.; Kabir, N.; Rifat, F.F.; Ishrak, H.; Alqahtani, A.; Chowdhury, M.E.H. Synthesis, Properties, Applications, and Future Prospective of Cellulose Nanocrystals. Polymers 2023, 15, 4070. [Google Scholar] [CrossRef] [PubMed]
- Wright, C.J.; Burns, L.H.; Jack, A.A.; Back, C.R.; Dutton, L.C.; Nobbs, A.H.; Lamont, R.J.; Jenkinson, H.F. Microbial interactions in building of communities. Mol. Oral Microbiol. 2013, 28, 83–101. [Google Scholar] [CrossRef] [PubMed]
- Koo, H.; Allan, R.N.; Howlin, R.P.; Stoodley, P.; Hall-Stoodley, L. Targeting microbial biofilms: Current and prospective therapeutic strategies. Nat. Rev. Microbiol. 2017, 15, 740–755. [Google Scholar] [CrossRef]
- Gunaratnam, G.; Dudek, J.; Jung, P.; Becker, S.L.; Jacobs, K.; Bischoff, M.; Hannig, M. Quantification of the Adhesion Strength of Candida albicans to Tooth Enamel. Microorganisms 2021, 9, 2213. [Google Scholar] [CrossRef]
- Berger, D.; Rakhamimova, A.; Pollack, A.; Loewy, Z. Oral Biofilms: Development, Control, and Analysis. High-Throughput 2018, 7, 24. [Google Scholar] [CrossRef]
- Marsh, P.D. In Sickness and in Health-What Does the Oral Microbiome Mean to Us? An Ecological Perspective. Adv. Dent. Res. 2018, 29, 60–65. [Google Scholar] [CrossRef] [PubMed]
- Bechinger, B.; Gorr, S.U. Antimicrobial Peptides: Mechanisms of Action and Resistance. J. Dent. Res. 2017, 96, 254–260. [Google Scholar] [CrossRef]
- Nemeş, N.S.; Ardean, C.; Davidescu, C.M.; Negrea, A.; Ciopec, M.; Duteanu, N.; Negrea, P.; Paul, C.; Duda-Seiman, D.; Muntean, D. Antimicrobial Activity of Cellulose Based Materials. Polymers 2022, 14, 735. [Google Scholar] [CrossRef]
- Noronha, V.T.; Camargos, C.H.M.; Jackson, J.C.; Souza Filho, A.G.; Paula, A.J.; Rezende, C.A.; Faria, A.F. Physical Membrane-Stress-Mediated Antimicrobial Properties of Cellulose Nanocrystals. ACS Sustain. Chem. Eng. 2021, 9, 3203–3212. [Google Scholar] [CrossRef]
- Kuramitsu, H.K.; He, X.; Lux, R.; Anderson, M.H.; Shi, W. Interspecies interactions within oral microbial communities. Microbiol. Mol. Biol. Rev. 2007, 71, 653–670. [Google Scholar] [CrossRef]
- Ionescu, A.C.; Brambilla, E. Bioreactors: How to Study Biofilms in Vitro. In Oral Biofilms and Modern Dental Materials; Springer: Berlin/Heidelberg, Germany, 2021; pp. 37–54. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Panio, A.; Ionescu, A.C.; La Ferla, B.; Zoia, L.; Savadori, P.; Tartaglia, G.M.; Brambilla, E. Cellulose Nanocrystals Show Anti-Adherent and Anti-Biofilm Properties against Oral Microorganisms. Bioengineering 2024, 11, 355. https://doi.org/10.3390/bioengineering11040355
Panio A, Ionescu AC, La Ferla B, Zoia L, Savadori P, Tartaglia GM, Brambilla E. Cellulose Nanocrystals Show Anti-Adherent and Anti-Biofilm Properties against Oral Microorganisms. Bioengineering. 2024; 11(4):355. https://doi.org/10.3390/bioengineering11040355
Chicago/Turabian StylePanio, Antonella, Andrei C. Ionescu, Barbara La Ferla, Luca Zoia, Paolo Savadori, Gianluca M. Tartaglia, and Eugenio Brambilla. 2024. "Cellulose Nanocrystals Show Anti-Adherent and Anti-Biofilm Properties against Oral Microorganisms" Bioengineering 11, no. 4: 355. https://doi.org/10.3390/bioengineering11040355
APA StylePanio, A., Ionescu, A. C., La Ferla, B., Zoia, L., Savadori, P., Tartaglia, G. M., & Brambilla, E. (2024). Cellulose Nanocrystals Show Anti-Adherent and Anti-Biofilm Properties against Oral Microorganisms. Bioengineering, 11(4), 355. https://doi.org/10.3390/bioengineering11040355









