Effects of Ankle Position While Performing One- and Two-Leg Floor Bridging Exercises on Core and Lower Extremity Muscle Recruitment
Abstract
1. Introduction
2. Methods
2.1. Participants
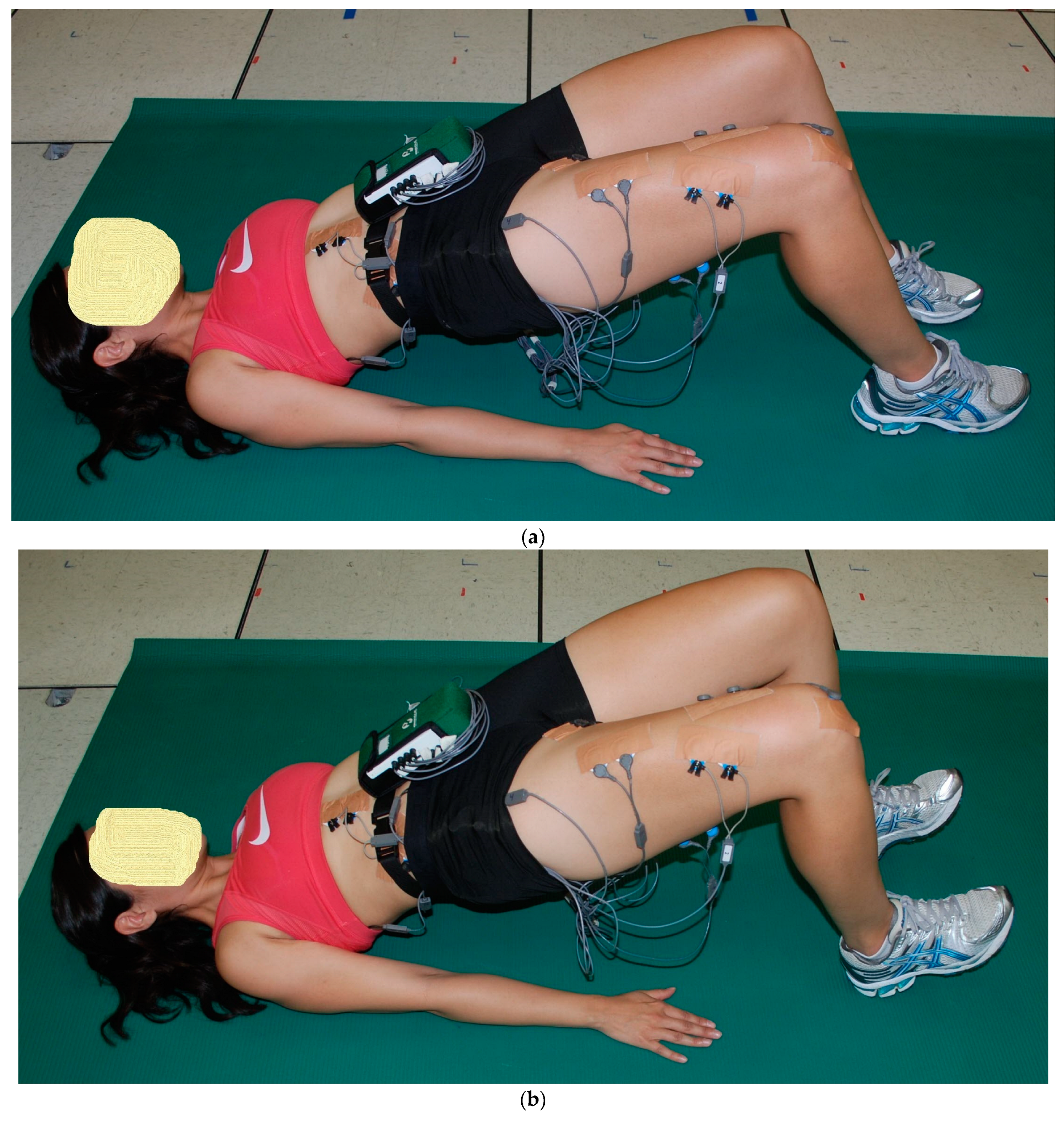
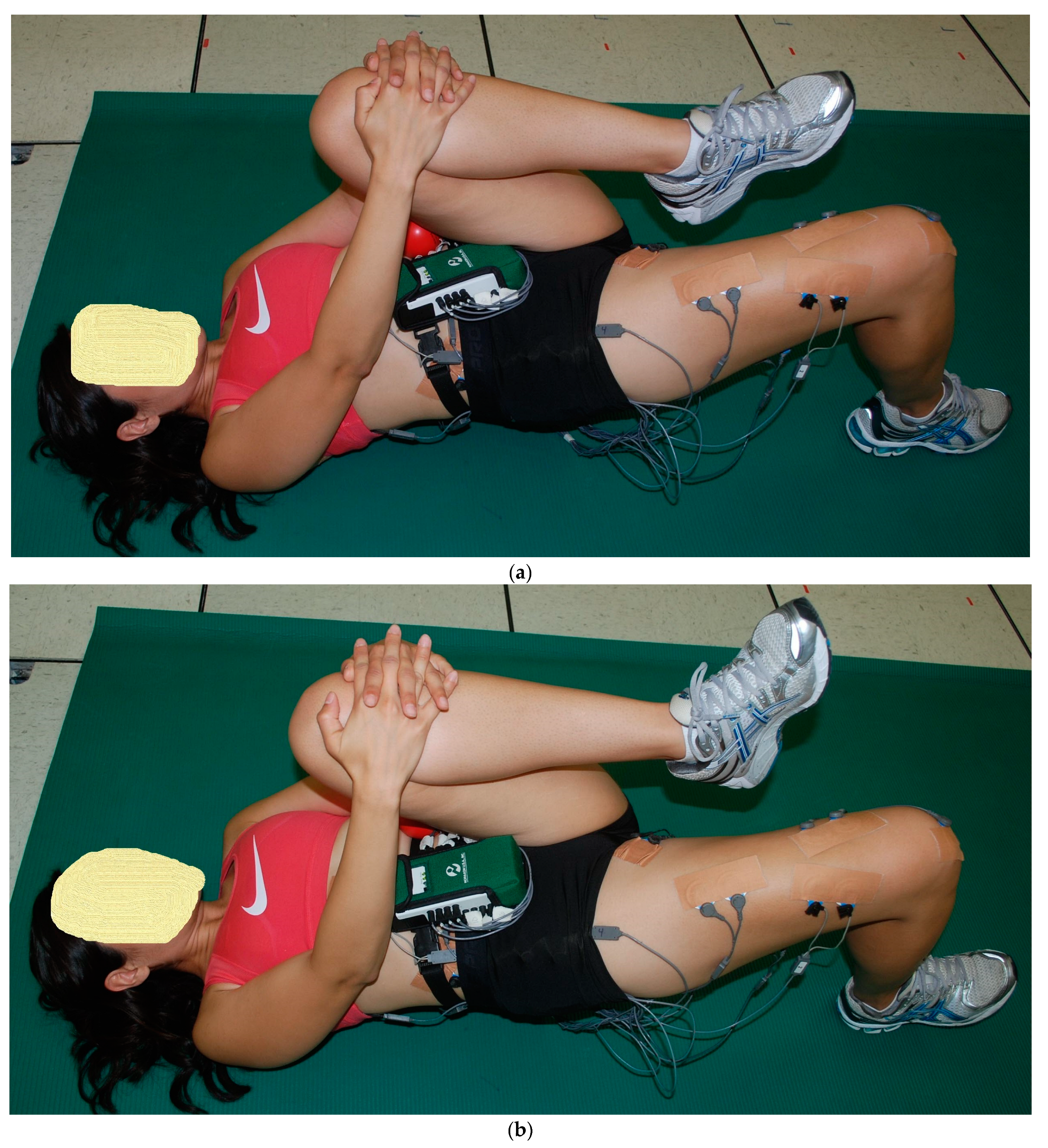
2.2. Exercise Descriptions
2.3. Procedures
2.4. Data Processing
2.5. Data Analysis
3. Results
4. Discussion
5. Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Axler, C.T.; McGill, S.M. Low back loads over a variety of abdominal exercises: Searching for the safest abdominal challenge. Med. Sci. Sports Exerc. 1997, 29, 804–811. [Google Scholar] [CrossRef] [PubMed]
- McGill, S.M. Low back stability: From formal description to issues for performance and rehabilitation. Exerc. Sport. Sci. Rev. 2001, 29, 26–31. [Google Scholar] [CrossRef] [PubMed]
- Hewett, T.E.; Myer, G.D.; Ford, K.R. Anterior cruciate ligament injuries in female athletes: Part 1, mechanisms and risk factors. Am. J. Sports Med. 2006, 34, 299–311. [Google Scholar] [CrossRef] [PubMed]
- Cowan, R.M.; Semciw, A.I.; Pizzari, T.; Cook, J.; Rixon, M.K.; Gupta, G.; Plass, L.M.; Ganderton, C.L. Muscle Size and Quality of the Gluteal Muscles and Tensor Fasciae Latae in Women with Greater Trochanteric Pain Syndrome. Clin. Anat. 2020, 33, 1082–1090. [Google Scholar] [CrossRef] [PubMed]
- Zacharias, A.; Pizzari, T.; Semciw, A.I.; English, D.J.; Kapakoulakis, T.; Green, R.A. Comparison of gluteus medius and minimus activity during gait in people with hip osteoarthritis and matched controls. Scand. J. Med. Sci. Sports 2019, 29, 696–705. [Google Scholar] [CrossRef] [PubMed]
- Lankhorst, N.E.; Bierma-Zeinstra, S.M.; van Middelkoop, M. Risk factors for patellofemoral pain syndrome: A systematic review. J. Orthop. Sports Phys. Ther. 2012, 42, 81–94. [Google Scholar] [CrossRef] [PubMed]
- Fredericson, M.; Moore, T. Muscular balance, core stability, and injury prevention for middle- and long-distance runners. Phys. Med. Rehabil. Clin. N. Am. 2005, 16, 669–689. [Google Scholar] [CrossRef] [PubMed]
- Friel, K.; McLean, N.; Myers, C.; Caceres, M. Ipsilateral hip abductor weakness after inversion ankle sprain. J. Athl. Train. 2006, 41, 74–78. [Google Scholar] [PubMed]
- Goubert, D.; Oosterwijck, J.V.; Meeus, M.; Danneels, L. Structural Changes of Lumbar Muscles in Non-specific Low Back Pain: A Systematic Review. Pain. Physician 2016, 19, E985–E1000. [Google Scholar] [PubMed]
- Stevens, V.K.; Bouche, K.G.; Mahieu, N.N.; Coorevits, P.L.; Vanderstraeten, G.G.; Danneels, L.A. Trunk muscle activity in healthy subjects during bridging stabilization exercises. BMC Musculoskelet. Disord. 2006, 7, 75. [Google Scholar] [CrossRef] [PubMed]
- Reiman, M.P.; Bolgla, L.A.; Loudon, J.K. A literature review of studies evaluating gluteus maximus and gluteus medius activation during rehabilitation exercises. Physiother. Theory Pract. 2012, 28, 257–268. [Google Scholar] [CrossRef]
- Neto, W.K.; Vieira, T.L.; Gama, E.F. Barbell Hip Thrust, Muscular Activation and Performance: A Systematic Review. J. Sports Sci. Med. 2019, 18, 198–206. [Google Scholar] [PubMed]
- Feldwieser, F.M.; Sheeran, L.; Meana-Esteban, A.; Sparkes, V. Electromyographic analysis of trunk-muscle activity during stable, unstable and unilateral bridging exercises in healthy individuals. Eur. Spine J. 2012, 21 (Suppl. S2), S171–S186. [Google Scholar] [CrossRef] [PubMed]
- Myer, G.D.; Chu, D.A.; Brent, J.L.; Hewett, T.E. Trunk and hip control neuromuscular training for the prevention of knee joint injury. Clin. Sports Med. 2008, 27, 425–448.ix. [Google Scholar] [CrossRef] [PubMed]
- Saragiotto, B.T.; Latimer, J. Prevention of low back pain (PEDro synthesis). Br. J. Sports Med. 2016, 50, 1345. [Google Scholar] [CrossRef] [PubMed]
- Verstegen, M.; Williams, P. Core Performance Essentials; Rodale: New York, NY, USA, 2006; p. 242. [Google Scholar]
- Verstegen, M.; Williams, P. Core Performance Endurance; Rodale: New York, NY, USA, 2007; p. 238. [Google Scholar]
- Yoo, W.G. Effects of bridging plus exercises with heel lift on lower extremity muscles. J. Phys. Ther. Sci. 2016, 28, 1582–1583. [Google Scholar] [CrossRef] [PubMed]
- Balady, G.; Berra, K.; LA, G.; Gordon, N.; Mahler, D.; Myers, J.; Sheldahl, L. Physical fitness testing and interpretation. In ACSM’s Guidelines for Exercise Testing and Prescription; Lippincott Williams & Wilkins: Baltimore, MD, USA, 2000; pp. 59–67. [Google Scholar]
- Escamilla, R.F.; Lewis, C.; Bell, D.; Bramblet, G.; Daffron, J.; Lambert, S.; Pecson, A.; Imamura, R.; Paulos, L.; Andrews, J.R. Core muscle activation during Swiss ball and traditional abdominal exercises. J. Orthop. Sports Phys. Ther. 2010, 40, 265–276. [Google Scholar] [CrossRef] [PubMed]
- Escamilla, R.F.; McTaggart, M.S.; Fricklas, E.J.; DeWitt, R.; Kelleher, P.; Taylor, M.K.; Hreljac, A.; Moorman, C.T. An electromyographic analysis of commercial and common abdominal exercises: Implications for rehabilitation and training. J. Orthop. Sports Phys. Ther. 2006, 36, 45–57. [Google Scholar] [CrossRef]
- McGill, S.; Juker, D.; Kropf, P. Appropriately placed surface EMG electrodes reflect deep muscle activity (psoas, quadratus lumborum, abdominal wall) in the lumbar spine. J. Biomech. 1996, 29, 1503–1507. [Google Scholar] [CrossRef] [PubMed]
- Ng, J.K.; Kippers, V.; Richardson, C.A. Muscle fibre orientation of abdominal muscles and suggested surface EMG electrode positions. Electromyogr. Clin. Neurophysiol. 1998, 38, 51–58. [Google Scholar] [PubMed]
- Ekstrom, R.A.; Osborn, R.W.; Hauer, P.L. Surface electromyographic analysis of the low back muscles during rehabilitation exercises. J. Orthop. Sports Phys. Ther. 2008, 38, 736–745. [Google Scholar] [CrossRef] [PubMed]
- Escamilla, R.F.; Babb, E.; DeWitt, R.; Jew, P.; Kelleher, P.; Burnham, T.; Busch, J.; D’Anna, K.; Mowbray, R.; Imamura, R.T. Electromyographic analysis of traditional and nontraditional abdominal exercises: Implications for rehabilitation and training. Phys. Ther. 2006, 86, 656–671. [Google Scholar] [CrossRef] [PubMed]
- Croce, R.V.; Miller, J.P.; St Pierre, P. Effect of ankle position fixation on peak torque and electromyographic activity of the knee flexors and extensors. Electromyogr. Clin. Neurophysiol. 2000, 40, 365–373. [Google Scholar] [PubMed]
- Kim, D.H.; Lee, J.H.; Yu, S.M.; An, C.M. The Effects of Ankle Position on Torque and Muscle Activity of the Knee Extensor During Maximal Isometric Contraction. J. Sport Rehabil. 2020, 29, 37–42. [Google Scholar] [CrossRef]
- Chon, S.C.; Chang, K.Y.; You, J.S. Effect of the abdominal draw-in manoeuvre in combination with ankle dorsiflexion in strengthening the transverse abdominal muscle in healthy young adults: A preliminary, randomised, controlled study. Physiotherapy 2010, 96, 130–136. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.H.; Huang, M.H.; Chen, T.W.; Weng, M.C.; Lee, C.L.; Wang, G.J. Relationship between ankle position and pelvic floor muscle activity in female stress urinary incontinence. Urology 2005, 66, 288–292. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.L.; Lin, Y.C.; Chien, W.J.; Huang, W.C.; Lin, H.Y.; Chen, P.L. The effect of ankle position on pelvic floor muscle contraction activity in women. J. Urol. 2009, 181, 1217–1223. [Google Scholar] [CrossRef]
- Voss, D.E. Proprioceptive neuromuscular facilitation. Am. J. Phys. Med. 1967, 46, 838–899. [Google Scholar] [PubMed]
- Gontijo, L.B.; Pereira, P.D.; Neves, C.D.; Santos, A.P.; Machado Dde, C.; Bastos, V.H. Evaluation of strength and irradiated movement pattern resulting from trunk motions of the proprioceptive neuromuscular facilitation. Rehabil. Res. Pract. 2012, 2012, 281937. [Google Scholar] [CrossRef] [PubMed]



| Exercise | Ankle Position | VM | VL | RF | TFL | GMED | GMAX | ST | BF | ADD | ES | LATS | RA | EO | IO |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 2LB | PF | 3 ± 1% | 2 ± 1% | 2 ± 1% | 15 ± 8% | 14 ± 8% | 17 ± 12% | 22 ± 8% | 18 ± 12% | 8 ± 4% | 25 ± 7% | 14 ± 10% | 5 ± 4% | 5 ± 5% | 7 ± 2% |
| 2LB | DF | 4 ± 2% | 3 ± 2% | 2 ± 1% | 15 ± 12% | 14 ± 8% | 19 ± 14% | 14 ± 5% | 11 ± 7% | 11 ± 4% | 25 ± 7% | 13 ± 8% | 5 ± 4% | 6 ± 5% | 7 ± 3% |
| Two-tailed p-value | 0.191 | 0.290 | 0.097 | 0.792 | 0.536 | 0.149 | 0.0006 * | 0.009 * | 0.035 | 0.693 | 0.477 | 0.683 | 0.259 | 0.786 | |
| 2LB-ABD | PF | 2 ± 1% | 2 ± 1% | 1 ± 1% | 22 ± 16% | 21 ± 8% | 19 ± 10% | 19 ± 8% | 16 ± 11% | 5 ± 2% | 23 ± 8% | 13 ± 9% | 5 ± 4% | 5 ± 4% | 6 ± 2% |
| 2LB-ABD | DF | 3 ± 4% | 2 ± 2% | 1 ± 1% | 21 ± 12% | 22 ± 8% | 19 ± 12% | 11 ± 7% | 11 ± 9% | 5 ± 2% | 23 ± 8% | 14 ± 12% | 5 ± 3% | 8 ± 6% | 6 ± 4% |
| Two-tailed p-value | 0.362 | 0.351 | 0.606 | 0.675 | 0.342 | 0.619 | 0.001 * | 0.001 * | 0.589 | 0.839 | 0.494 | 0.406 | 0.046 | 0.827 | |
| 2LB-ADD | PF | 8 ± 5% | 5 ± 3% | 5 ± 3% | 22 ± 16% | 20 ± 11% | 23 ± 13% | 36 ± 12% | 30 ± 12% | 35 ± 16% | 34 ± 10% | 21 ± 12% | 7 ± 5% | 11 ± 9% | 14 ± 4% |
| 2LB-ADD | DF | 9 ± 5% | 6 ± 3% | 6 ± 3% | 21 ± 14% | 18 ± 10% | 23 ± 13% | 30 ± 10% | 26 ± 9% | 36 ± 15% | 35 ± 14% | 23 ± 11% | 8 ± 7% | 12 ± 9% | 14 ± 4% |
| Two-tailed p-value | 0.469 | 0.091 | 0.116 | 0.459 | 0.073 | 0.977 | 0.009 * | 0.048 | 0.521 | 0.318 | 0.413 | 0.268 | 0.233 | 0.544 | |
| 1LB-LFlex | PF | 8 ± 5% | 5 ± 3% | 3 ± 2% | 64 ± 28% | 57 ± 27% | 28 ± 11% | 38 ± 13% | 30 ± 14% | 18 ± 6% | 33 ± 9% | 16 ± 10% | 5 ± 3% | 8 ± 4% | 13 ± 6% |
| 1LB-LFlex | DF | 13 ± 7% | 10 ± 6% | 6 ± 3% | 64 ± 28% | 58 ± 27% | 28 ± 14% | 32 ± 14% | 25 ± 12% | 22 ± 8% | 32 ± 7% | 17 ± 12% | 6 ± 3% | 12 ± 4% | 17 ± 8% |
| Two-tailed p-value | 0.001 * | 0.001 * | 0.004 * | 0.861 | 0.783 | 0.821 | 0.006 * | 0.007 * | 0.001 * | 0.278 | 0.578 | 0.200 | 0.007 * | 0.009 * | |
| 1LB-LExt | PF | 6 ± 4% | 5 ± 3% | 3 ± 2% | 38 ± 17% | 41 ± 17% | 29 ± 15% | 33 ± 12% | 29 ± 12% | 15 ± 6% | 24 ± 7% | 14 ± 7% | 8 ± 6% | 11 ± 8% | 10 ± 2% |
| 1LB-LExt | DF | 9 ± 5% | 9 ± 5% | 5 ± 2% | 39 ± 18% | 41 ± 18% | 30 ± 16% | 28 ± 11% | 24 ± 12% | 19 ± 7% | 24 ± 9% | 15 ± 8% | 8 ± 6% | 12 ± 8% | 11 ± 4% |
| Two-tailed p-value | 0.031 | 0.009 * | 0.048 | 0.553 | 0.987 | 0.523 | 0.009 * | 0.004 * | 0.001 * | 0.785 | 0.579 | 0.882 | 0.145 | 0.315 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Escamilla, R.F.; Thompson, I.S.; Carinci, J.; MacLean, D.; MacLean, L.; Aguinaldo, A.L. Effects of Ankle Position While Performing One- and Two-Leg Floor Bridging Exercises on Core and Lower Extremity Muscle Recruitment. Bioengineering 2024, 11, 356. https://doi.org/10.3390/bioengineering11040356
Escamilla RF, Thompson IS, Carinci J, MacLean D, MacLean L, Aguinaldo AL. Effects of Ankle Position While Performing One- and Two-Leg Floor Bridging Exercises on Core and Lower Extremity Muscle Recruitment. Bioengineering. 2024; 11(4):356. https://doi.org/10.3390/bioengineering11040356
Chicago/Turabian StyleEscamilla, Rafael F., Irwin S. Thompson, Joe Carinci, Daniel MacLean, Lisa MacLean, and Arnel L. Aguinaldo. 2024. "Effects of Ankle Position While Performing One- and Two-Leg Floor Bridging Exercises on Core and Lower Extremity Muscle Recruitment" Bioengineering 11, no. 4: 356. https://doi.org/10.3390/bioengineering11040356
APA StyleEscamilla, R. F., Thompson, I. S., Carinci, J., MacLean, D., MacLean, L., & Aguinaldo, A. L. (2024). Effects of Ankle Position While Performing One- and Two-Leg Floor Bridging Exercises on Core and Lower Extremity Muscle Recruitment. Bioengineering, 11(4), 356. https://doi.org/10.3390/bioengineering11040356








