Dimensionality Matters: Exploiting UV-Photopatterned 2D and Two-Photon-Printed 2.5D Contact Guidance Cues to Control Corneal Fibroblast Behavior and Collagen Deposition
Abstract
:1. Introduction
2. Materials and Methods
2.1. Scanning Electron Microscopy Imaging of Corneal Stroma
2.2. Design of Contact Guidance Patterns
2.3. 2D Contact Guidance Cues
2.4. 2.5D Contact Guidance Cues
2.5. Characterization of 2D and 2.5D Contact Guidance Substrates
2.6. Cell Isolation and Culture on Contact Guidance Substrates
2.7. Staining and Imaging of Keratocytes and Extracellular Matrix Proteins
2.8. Quantification of Cell Morphological Parameters
2.9. Verification of Keratocyte Phenotype Using Real-Time Polymerase Chain Reaction
2.10. Statistical Analysis
3. Results and Discussion
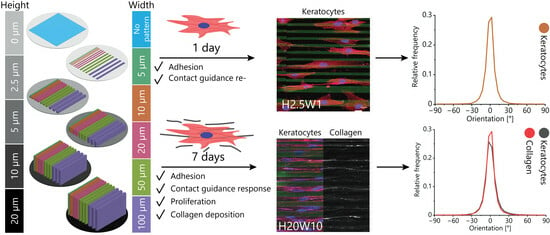
3.1. Fabrication of Contact Guidance Substrates
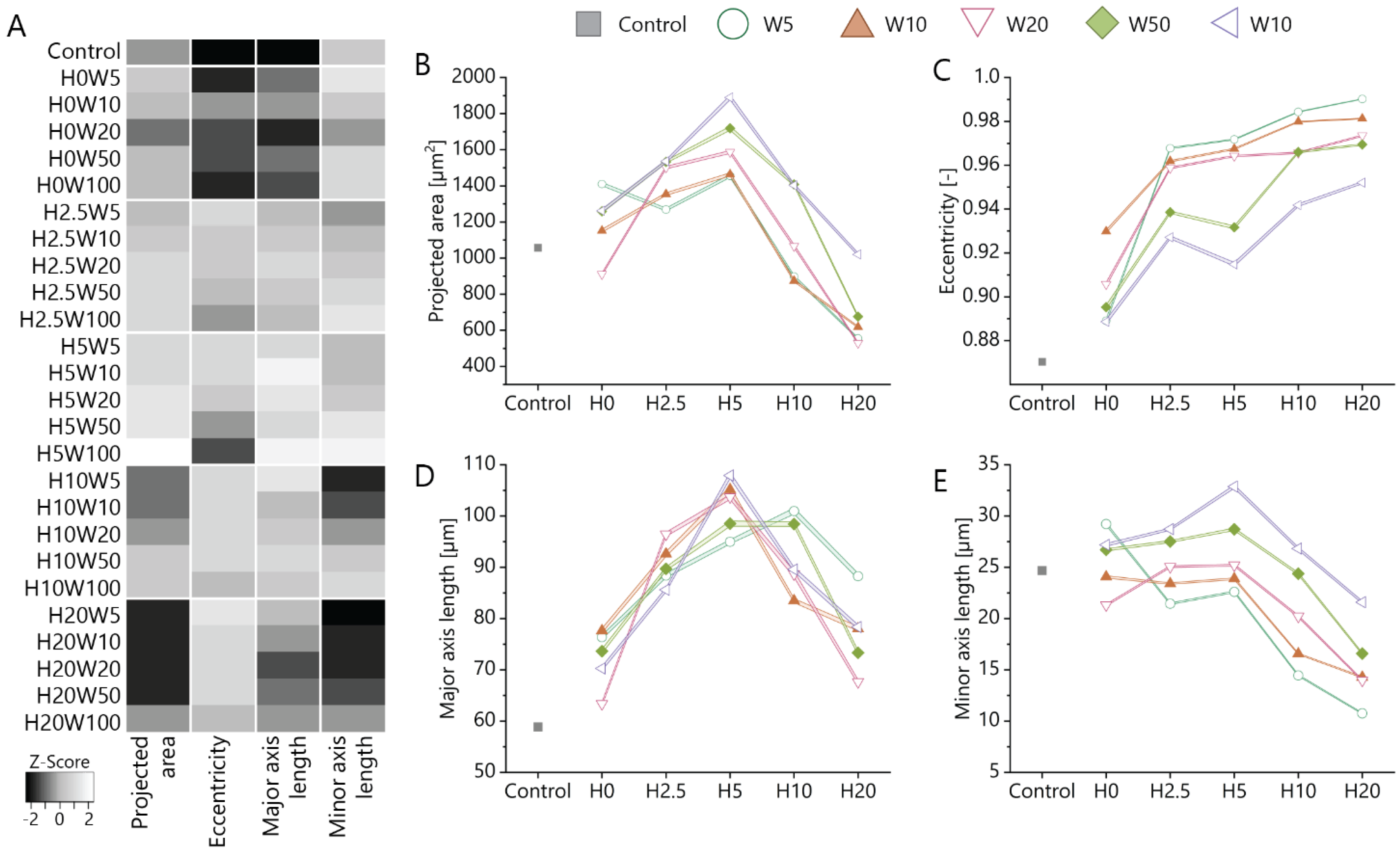
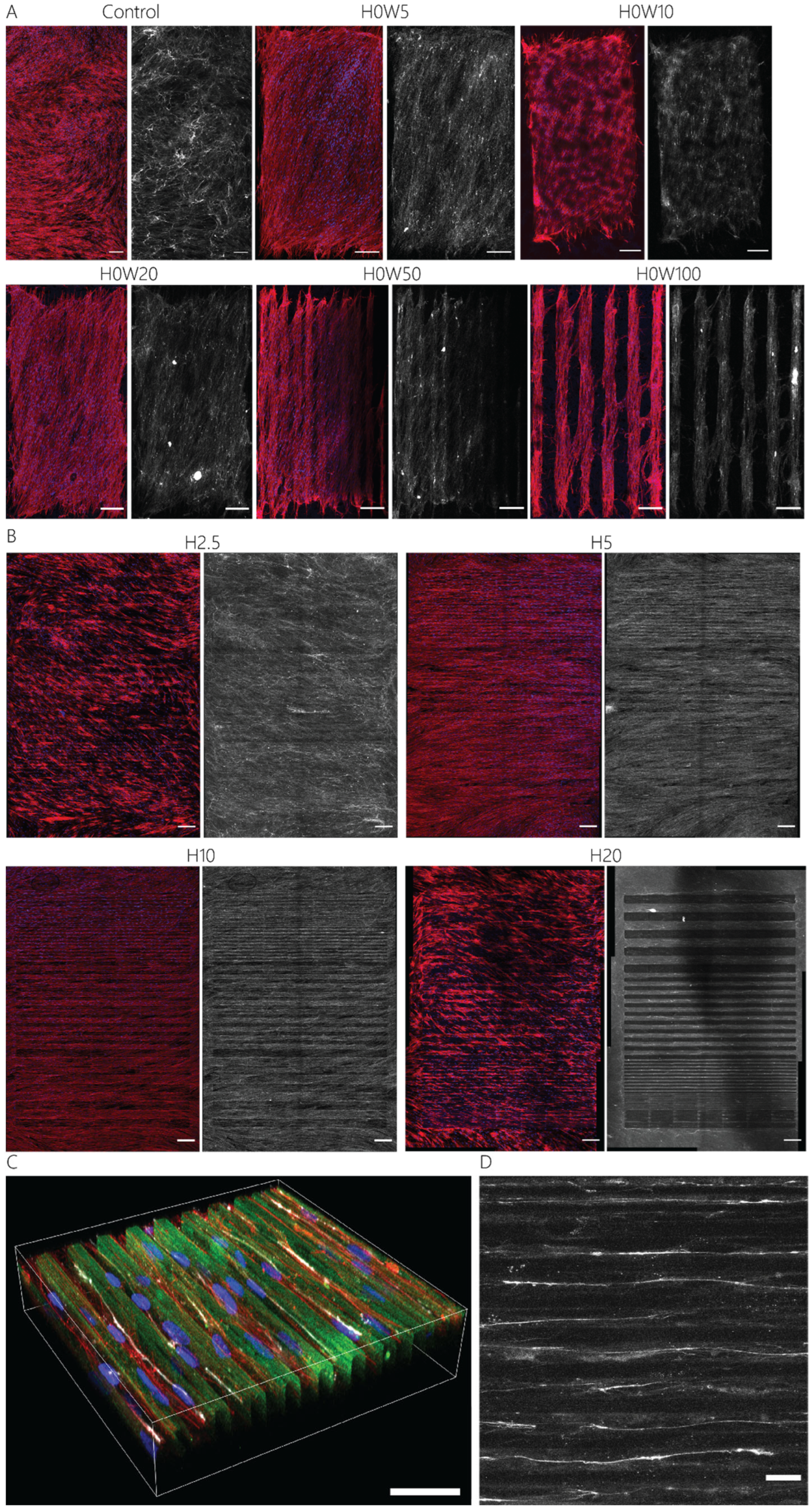
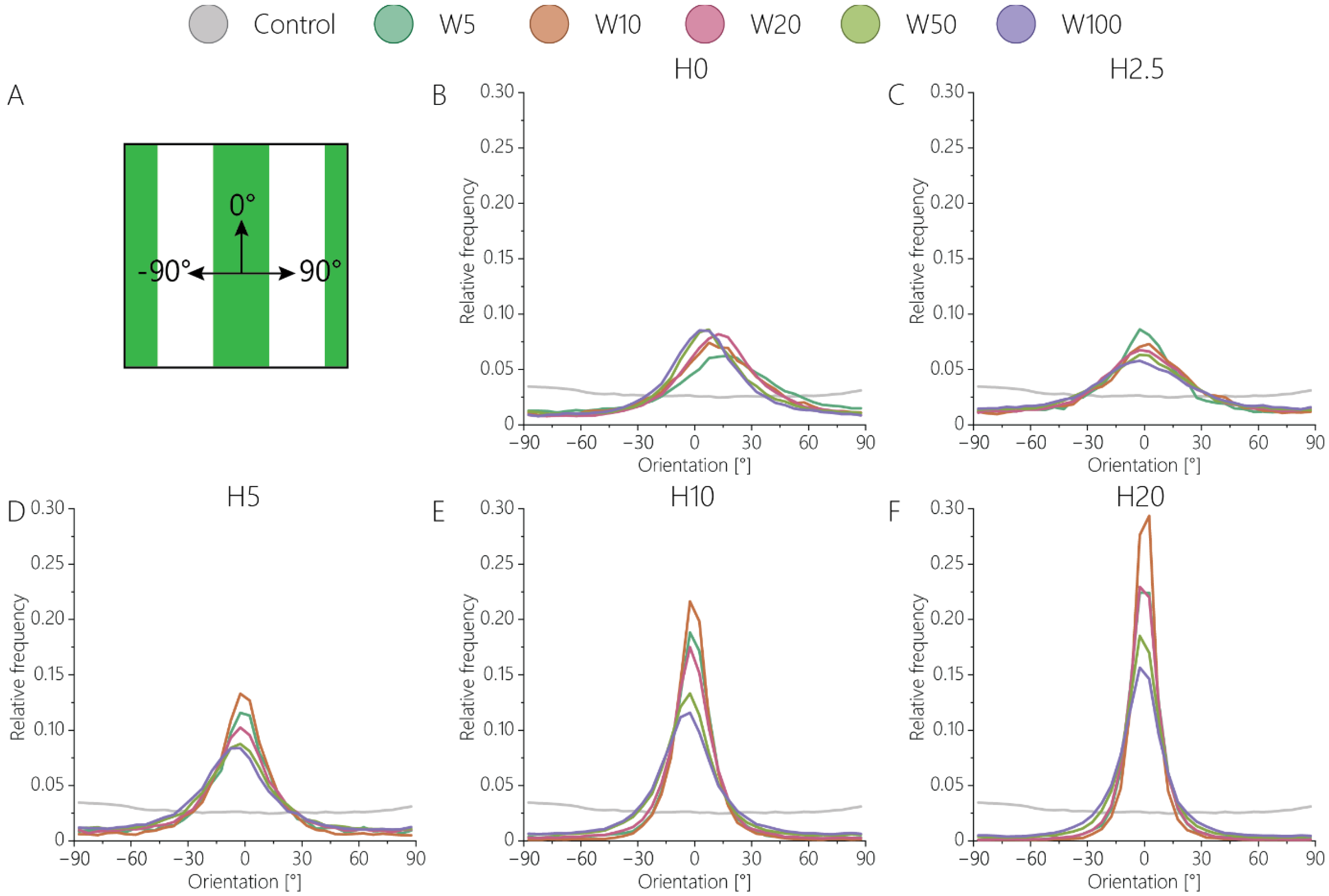
3.2. Orientation of Fibroblastic Keratocytes Is Affected by 2.5D and 2D Contact Guidance Cues
3.3. Cue Dimensionality Directly Influences Morphology and Focal Adhesions of Fibroblastic Keratocytes
3.4. Maintaining the Cellular Phenotype and Steering Collagen Formation Using Contact Guidance Cues
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gain, P.; Jullienne, R.; He, Z.; Aldossary, M.; Acquart, S.; Cognasse, F.; Thuret, G. Global survey of corneal transplantation and eye banking. JAMA Ophthalmol. 2016, 134, 167–173. [Google Scholar] [CrossRef] [PubMed]
- Meek, K.M.; Knupp, C. Corneal structure and transparency. Prog. Retin. Eye Res. 2015, 49, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Funderburgh, J.L. The Corneal Stroma. In Encyclopedia of the Eye; Elsevier: Amsterdam, The Netherlands, 2010; pp. 515–521. [Google Scholar] [CrossRef]
- West-Mays, J.A.; Dwivedi, D.J. The keratocyte: Corneal stromal cell with variable repair phenotypes. Int. J. Biochem. Cell Biol. 2006, 38, 1625–1631. [Google Scholar] [CrossRef] [PubMed]
- Yam, G.H.F.; Riau, A.K.; Funderburgh, M.L.; Mehta, J.S.; Jhanji, V. Keratocyte biology. Exp. Eye Res. 2020, 196, 108062. [Google Scholar] [CrossRef] [PubMed]
- Medeiros, C.S.; Marino, G.K.; Santhiago, M.R.; Wilson, S.E. The corneal basement membranes and stromal fibrosis. Investig. Ophthalmol. Vis. Sci. 2018, 59, 4044–4053. [Google Scholar] [CrossRef] [PubMed]
- Matthyssen, S.; Van den Bogerd, B.; Dhubhghaill, S.N.; Koppen, C.; Zakaria, N. Corneal regeneration: A review of stromal replacements. Acta Biomater. 2018, 69, 31–41. [Google Scholar] [CrossRef] [PubMed]
- Gil, E.S.; Mandal, B.B.; Park, S.H.; Marchant, J.K.; Omenetto, F.G.; Kaplan, D.L. Helicoidal multi-lamellar features of RGD-functionalized silk biomaterials for corneal tissue engineering. Biomaterials 2010, 31, 8953–8963. [Google Scholar] [CrossRef]
- Pot, S.A.; Liliensiek, S.J.; Myrna, K.E.; Bentley, E.; Jester, J.V.; Nealey, P.F.; Murphy, C.J. Nanoscale topography-induced modulation of fundamental cell behaviors of rabbit corneal keratocytes, fibroblasts, and myofibroblasts. Investig. Ophthalmol. Vis. Sci. 2010, 51, 1373–1381. [Google Scholar] [CrossRef] [PubMed]
- Russell, P.; Gasiorowski, J.Z.; Nealy, P.F.; Murphy, C.J. Response of human trabecular meshwork cells to topographic cues on the nanoscale level. Investig. Ophthalmol. Vis. Sci. 2008, 49, 629–635. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, A.I.; Nealey, P.F.; Murphy, C.J. Responses of human keratocytes to micro- and nanostructured substrates. J. Biomed. Mater. Res. 2004, 71A, 369–376. [Google Scholar] [CrossRef]
- Brooks, S.; Mittler, S.; Hamilton, D.W. Contact Guidance of Connective Tissue Fibroblasts on Submicrometer Anisotropic Topographical Cues Is Dependent on Tissue of Origin, β1 Integrins, and Tensin-1 Recruitment. ACS Appl. Mater. Interfaces 2023, 15, 19817–19832. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharjee, P.; Cavanagh, B.L.; Ahearne, M. Influence of micropatterned substrates on keratocyte phenotype. Sci. Rep. 2020, 10, 6679. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharjee, P.; Cavanagh, B.L.; Ahearne, M. Effect of substrate topography on the regulation of human corneal stromal cells. Colloids Surf. B Biointerfaces 2020, 190, 110971. [Google Scholar] [CrossRef] [PubMed]
- Ghezzi, C.E.; Marelli, B.; Omenetto, F.G.; Funderburgh, J.L.; Kaplan, D.L. 3D Functional Corneal Stromal Tissue Equivalent Based on Corneal Stromal Stem Cells and Multi-Layered Silk Film Architecture. PLoS ONE 2017, 12, e0169504. [Google Scholar] [CrossRef]
- Lawrence, B.D.; Marchant, J.K.; Pindrus, M.; Omenetto, F.; Kaplan, L. Silk film biomaterials for cornea tissue engineering. Biomaterials 2010, 30, 1299–1308. [Google Scholar] [CrossRef]
- Then, K.Y.; Yang, Y.; Ahearne, M.; El Haj, A.J. Effect of Microtopographical Cues on Human Keratocyte Orientation and Gene Expression. Curr. Eye Res. 2011, 36, 88–93. [Google Scholar] [CrossRef] [PubMed]
- Vrana, N.E.; Elsheikh, A.; Builles, N.; Damour, O.; Hasirci, V. Effect of human corneal keratocytes and retinal pigment epithelial cells on the mechanical properties of micropatterned collagen films. Biomaterials 2007, 28, 4303–4310. [Google Scholar] [CrossRef] [PubMed]
- Vrana, E.; Builles, N.; Hindie, M.; Damour, O.; Aydinli, A.; Hasirci, V. Contact guidance enhances the quality of a tissue engineered corneal stroma. J. Biomed. Mater. Res. A 2008, 84A, 454–463. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Rnjak-Kovacina, J.; Du, Y.; Funderburgh, M.L.; Kaplan, D.L.; Funderburgh, J.L. Corneal Stromal Bioequivalents Secreted on Patterned Silk Substrates. Biomaterials 2015, 25, 713–724. [Google Scholar] [CrossRef]
- Xiong, S.; Gao, H.C.; Qin, L.; Jia, Y.G.; Ren, L. Engineering topography: Effects on corneal cell behavior and integration into corneal tissue engineering. Bioact. Mater. 2019, 4, 293–302. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Chen, J.; Backman, L.J.; Malm, A.D.; Danielson, P. Surface Topography and Mechanical Strain Promote Keratocyte Phenotype and Extracellular Matrix Formation in a Biomimetic 3D Corneal Model. Adv. Healthc. Mater. 2017, 6, 1601238. [Google Scholar] [CrossRef] [PubMed]
- Rojas-Rodríguez, M.; Fiaschi, T.; Mannelli, M.; Mortati, L.; Celegato, F.; Wiersma, D.S.; Parmeggiani, C.; Martella, D. Cellular Contact Guidance on Liquid Crystalline Networks with Anisotropic Roughness. ACS Appl. Mater. Interfaces 2023, 15, 14122–14130. [Google Scholar] [CrossRef] [PubMed]
- Phu, D.; Wray, L.S.; Warren, R.V.; Haskell, R.C.; Orwin, E.J. Effect of substrate composition and alignment on corneal cell phenotype. Tissue Eng. Part A 2011, 17, 799–807. [Google Scholar] [CrossRef] [PubMed]
- Wilson, S.L.; Wimpenny, I.; Ahearne, M.; Rauz, S.; El Haj, A.J.; Yang, Y. Chemical and topographical effects on cell differentiation and matrix elasticity in a corneal stromal layer model. Adv. Funct. Mater. 2012, 22, 3641–3649. [Google Scholar] [CrossRef]
- Wray, L.S.; Orwin, E.J. Recreating the microenvironment of the native cornea for tissue engineering applications. Tissue Eng. Part A 2009, 15, 1463–1472. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Du, Y.; Watkins, S.C.; Funderburgh, J.L.; Wagner, W.R. The engineering of organized human corneal tussue through the spatial guidance of corneal stromal stem cells. Biomaterials 2012, 33, 1343–1352. [Google Scholar] [CrossRef]
- Wu, Z.; Kong, B.; Liu, R.; Sun, W.; Mi, S. Engineering of Corneal Tissue through an Aligned PVA/Collagen Composite Nanofibrous Electrospun Scaffold. Nanomaterials 2018, 8, 124. [Google Scholar] [CrossRef]
- Yan, J.; Qiang, L.; Gao, Y.; Cui, X.; Zhou, H.; Zhong, S.; Wang, Q.; Wang, H. Effect of fiber alignment in electrospun scaffolds on keratocytes and corneal epithelial cells behavior. J. Biomed. Mater. Res. A 2012, 100A, 527–535. [Google Scholar] [CrossRef] [PubMed]
- Hasenzahl, M.; Müsken, M.; Mertsch, S.; Schrader, S.; Reichl, S. Cell sheet technology: Influence of culture conditions on in vitro-cultivated corneal stromal tissue for regenerative therapies of the ocular surface. J. Biomed. Mater. Res. B Appl. Biomater. 2021, 109, 1488–1504. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Chen, X.; Ma, Y.; Liu, Y.; Li, J.; Peng, K.; Dai, Y.; Chen, X. Effect of Anisotropic Structural Depth on Orientation and Differentiation Behavior of Skeletal Muscle Cells. ACS Omega 2023, 8, 41374–41382. [Google Scholar] [CrossRef] [PubMed]
- Leclech, C.; Krishnamurthy, A.; Muller, L.; Barakat, A.I. Distinct Contact Guidance Mechanisms in Single Endothelial Cells and in Monolayers. Adv. Mater. Interfaces 2023, 10, 2202421. [Google Scholar] [CrossRef]
- Robitaille, M.C.; Kim, C.; Christodoulides, J.A.; Calhoun, P.J.; Kang, W.; Liu, J.; Byers, J.M.; Raphael, M.P. Topographical depth reveals contact guidance mechanism distinct from focal adhesion confinement. Cytoskeleton 2024. online ahead of print. [Google Scholar] [CrossRef]
- Fraser, S.A.; Ting, Y.; Mallon, K.S.; Wendt, A.E.; Murphy, C.J.; Nealey, P.F. Sub-micron and nanoscale feature depth modulates alignment of stromal fibroblasts and corneal epithelial cells in serum-rich and serum-free media. J. Biomed. Mater. Res. A 2008, 86A, 725–735. [Google Scholar] [CrossRef] [PubMed]
- Crouch, A.S.; Miller, D.; Luebke, K.J.; Hu, W. Correlation of anisotropic cell behaviors with topographic aspect ratio. Biomaterials 2009, 30, 1560–1567. [Google Scholar] [CrossRef] [PubMed]
- Barata, D.; Dias, P.; Wieringa, P.; Van Blitterswijk, C.; Habibovic, P. Cell-instructive high-resolution micropatterned polylactic acid surfaces. Biofabrication 2017, 9, 035004. [Google Scholar] [CrossRef] [PubMed]
- Jeon, H.; Hidai, H.; Hwang, D.J.; Grigoropoulos, C.P. Fabrication of arbitrary polymer patterns for cell study by two-photon polymerization process. J. Biomed. Mater. Res. A 2009, 93, 56–66. [Google Scholar] [CrossRef] [PubMed]
- Strale, P.O.; Azioune, A.; Bugnicourt, G.; Lecomte, Y.; Chahid, M.; Studer, V. Multiprotein Printing by Light-Induced Molecular Adsorption. Adv. Mater. 2016, 28, 2024–2029. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Jiang, W.; Zhou, X.; Long, Y.; Sun, Y.; Zeng, Y.; Yao, X. Focus: Cell Fate: Advances in Regulating Cellular Behavior Using Micropatterns. Yale J. Biol. Med. 2023, 96, 527. [Google Scholar] [CrossRef] [PubMed]
- Formisano, N.; van der Putten, C.; Grant, R.; Sahin, G.; Truckenmüller, R.K.; Bouten, C.V.C.; Kurniawan, N.A.; Giselbrecht, S. Mechanical Properties of Bioengineered Corneal Stroma. Adv. Healthc. Mater. 2021, 10, 2100972. [Google Scholar] [CrossRef] [PubMed]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An open-source platform for biological-image analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef] [PubMed]
- Feliciano, A.J.; Grant, R.; Fernández-Pérez, J.; Giselbrecht, S.; Baker, M.B. Introducing Dynamicity: Engineering Stress Relaxation Into Hydrogels Via Thiol-Ene Modified Alginate for Mechanobiological in vitro Modeling of the Cornea. Macromol. Biosci. 2023, 24, 2300109. [Google Scholar] [CrossRef] [PubMed]
- Aper, S.J.A.; Van Spreeuwel, A.C.C.; Van Turnhout, M.C.; Van Der Linden, A.J.; Pieters, P.A.; Van Der Zon, N.L.L.; De La Rambelje, S.L.; Bouten, C.V.C.; Merkx, M. Colorful protein-based fluorescent probes for collagen imaging. PLoS ONE 2014, 9, e114983. [Google Scholar] [CrossRef] [PubMed]
- Carpenter, A.E.; Jones, T.R.; Lamprecht, M.R.; Clarke, C.; Kang, I.H.; Friman, O.; Guertin, D.A.; Chang, J.H.; Lindquist, R.A.; Moffat, J.; et al. CellProfiler: Image analysis software for identifying and quantifying cell phenotypes. Genome Biol. 2006, 7, R100. [Google Scholar] [CrossRef] [PubMed]
- Frangi, A.F.; Niessen, W.J.; Vincken, K.L.; Viergever, M.A. Multiscale vessel enhancement filtering. In Lecture Notes in Computer Science (Including Subseries Lecture Notes in Artificial Intelligence and Lecture Notes in Bioinformatics); Springer: Berlin/Heidelberg, Germany, 1998; pp. 130–137. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Ye, J.; Coulouris, G.; Zaretskaya, I.; Cutcutache, I.; Rozen, S.; Madden, T.L. Primer-BLAST: A tool to design target-specific primers for polymerase chain reaction. BMC Bioinform. 2012, 13, 134. [Google Scholar] [CrossRef] [PubMed]
- Babicki, S.; Arndt, D.; Marcu, A.; Liang, Y.; Grant, J.R.; Maciejewski, A.; Wishart, D.S. Heatmapper: Web-enabled heat mapping for all. Nucleic Acids Res. 2016, 44, W147–W153. [Google Scholar] [CrossRef] [PubMed]
- Buskermolen, A.B.C.; Ristori, T.; Mostert, D.; van Turnhout, M.C.; Shishvan, S.S.; Loerakker, S.; Kurniawan, N.A.; Deshpande, V.S.; Bouten, C.V.C. Cellular Contact Guidance Emerges from Gap Avoidance. Cell Rep. Phys. Sci. 2020, 1, 100055. [Google Scholar] [CrossRef] [PubMed]
- Dalby, M.J.; Riehle, M.O.; Yarwood, S.J.; Wilkinson, C.D.W.; Curtis, A.S.G. Nucleus alignment and cell signaling in fibroblasts: Response to a micro-grooved topography. Exp. Cell Res. 2003, 284, 272–280. [Google Scholar] [CrossRef] [PubMed]
- Guo, Q.; Phillip, J.M.; Majumdar, S.; Wu, P.H.; Chen, J.; Calderón-Colón, X.; Schein, O.; Smith, B.J.; Trexler, M.M.; Wirtz, D.; et al. Modulation of keratocyte phenotype by collagen fibril nanoarchitecture in membranes for corneal repair. Biomaterials 2013, 34, 9365–9372. [Google Scholar] [CrossRef] [PubMed]
- Koo, S.; Ahn, S.J.; Zhang, H.; Wang, J.C.; Yim, E.K.F. Human corneal keratocyte response to micro-and nano-gratings on chitosan and PDMS. Cell Mol. Bioeng. 2011, 4, 399–410. [Google Scholar] [CrossRef]
- Beales, M.P.; Funderburgh, J.L.; Jester, J.V.; Hassell, J.R. Proteoglycan synthesis by bovine keratocytes and corneal fibroblasts: Maintenance of the keratocyte phenotype in culture. Investig. Ophthalmol. Vis. Sci. 1999, 40, 1658–1663. [Google Scholar]
- Canty, E.G.; Lu, Y.; Meadows, R.S.; Shaw, M.K.; Holmes, D.F.; Kadler, K.E. Coalignment of plasma membrane channels and protrusions (fibropositors) specifies the parallelism of tendon. J. Cell Biol. 2004, 165, 553–563. [Google Scholar] [CrossRef] [PubMed]
- Koudouna, E.; Mikula, E.; Brown, D.J.; Young, R.D.; Quantock, A.J.; Jester, J.V. Cell regulation of collagen fibril macrostructure during corneal morphogenesis. Acta Biomater. 2018, 79, 96–112. [Google Scholar] [CrossRef] [PubMed]




| Width (μm) | Height (μm) | ||||
|---|---|---|---|---|---|
| 2D | 2.5D | ||||
| 0 | 2.5 | 5 | 10 | 20 | |
| 0 | Control | ||||
| 5 | H0W5 | H2.5W5 | H5W5 | H10W5 | H20W5 |
| 10 | H0W10 | H2.5W10 | H5W10 | H10W10 | H20W10 |
| 20 | H0W20 | H2.5W20 | H5W20 | H10W20 | H20W20 |
| 50 | H0W50 | H2.5W50 | H5W50 | H10W50 | H20W50 |
| 100 | H0W100 | H2.5W100 | H5W100 | H10W100 | H20W100 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
van der Putten, C.; Sahin, G.; Grant, R.; D’Urso, M.; Giselbrecht, S.; Bouten, C.V.C.; Kurniawan, N.A. Dimensionality Matters: Exploiting UV-Photopatterned 2D and Two-Photon-Printed 2.5D Contact Guidance Cues to Control Corneal Fibroblast Behavior and Collagen Deposition. Bioengineering 2024, 11, 402. https://doi.org/10.3390/bioengineering11040402
van der Putten C, Sahin G, Grant R, D’Urso M, Giselbrecht S, Bouten CVC, Kurniawan NA. Dimensionality Matters: Exploiting UV-Photopatterned 2D and Two-Photon-Printed 2.5D Contact Guidance Cues to Control Corneal Fibroblast Behavior and Collagen Deposition. Bioengineering. 2024; 11(4):402. https://doi.org/10.3390/bioengineering11040402
Chicago/Turabian Stylevan der Putten, Cas, Gozde Sahin, Rhiannon Grant, Mirko D’Urso, Stefan Giselbrecht, Carlijn V. C. Bouten, and Nicholas A. Kurniawan. 2024. "Dimensionality Matters: Exploiting UV-Photopatterned 2D and Two-Photon-Printed 2.5D Contact Guidance Cues to Control Corneal Fibroblast Behavior and Collagen Deposition" Bioengineering 11, no. 4: 402. https://doi.org/10.3390/bioengineering11040402
APA Stylevan der Putten, C., Sahin, G., Grant, R., D’Urso, M., Giselbrecht, S., Bouten, C. V. C., & Kurniawan, N. A. (2024). Dimensionality Matters: Exploiting UV-Photopatterned 2D and Two-Photon-Printed 2.5D Contact Guidance Cues to Control Corneal Fibroblast Behavior and Collagen Deposition. Bioengineering, 11(4), 402. https://doi.org/10.3390/bioengineering11040402