Modulation of Canine Adipose-Derived Mesenchymal Stem/Medicinal Signalling Cells with Ascorbic Acid: Effect on Proliferation and Chondrogenic Differentiation on Standard Plastic and Silk Fibroin Surfaces
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Adipose Tissue Collection
2.2. Isolation of cAMSCs
2.3. Flow Cytometry for Cell Surface Markers
2.4. Trilineage Differentiation Potential
2.5. Cell Viability and Proliferation Potential Assay
2.6. Cell Culturing on SF Films
2.7. Alcian Blue Staining and Analysis
2.8. Light Microscopy and ImageJ Analysis
2.9. RNA Isolation
2.10. Reverse Transcription Quantitative Polymerase Chain Reaction
2.11. Statistical Analysis
3. Results
3.1. Isolation and Characterization of cAMSCs
3.2. Flow Cytometry for Surface Marker Expression
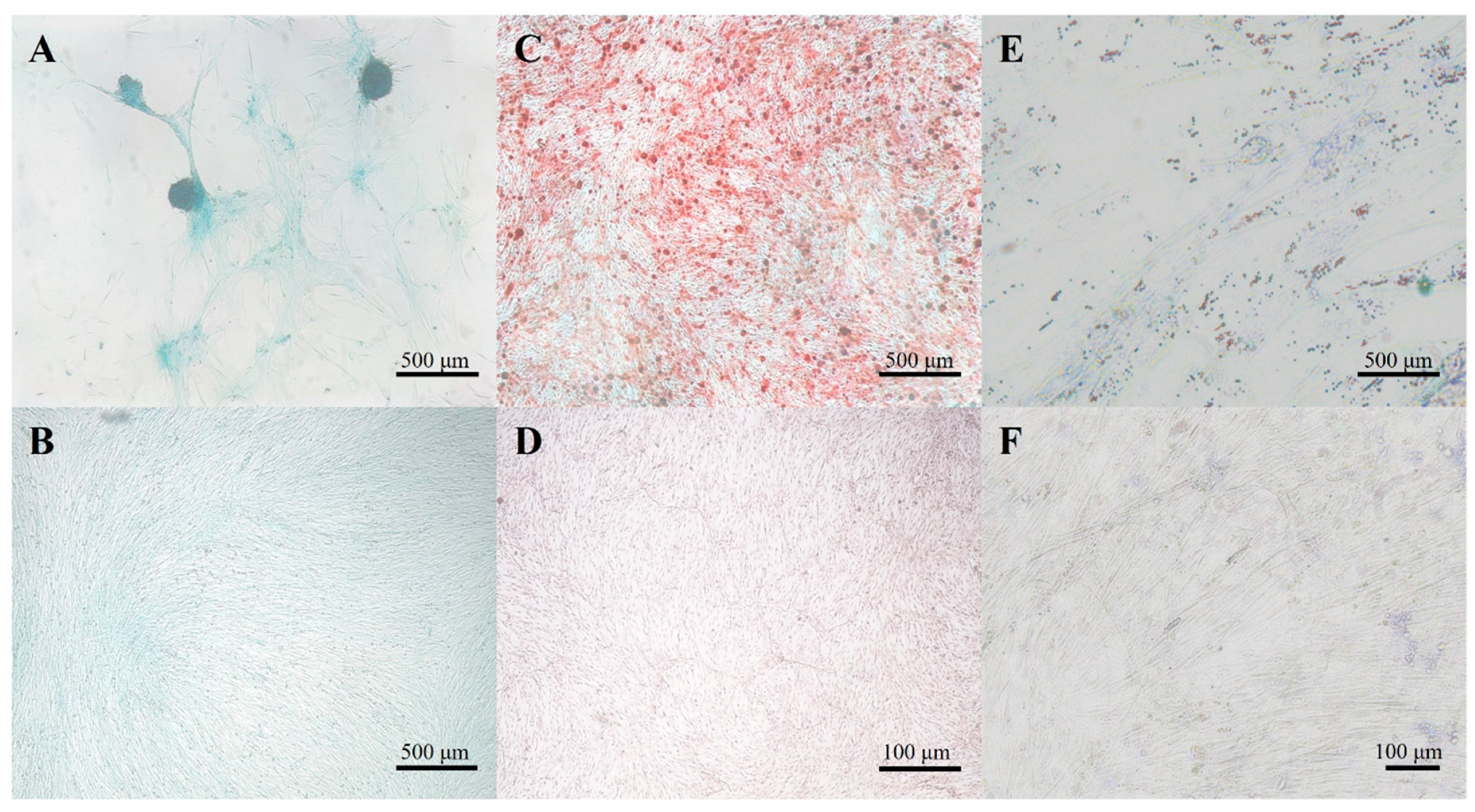
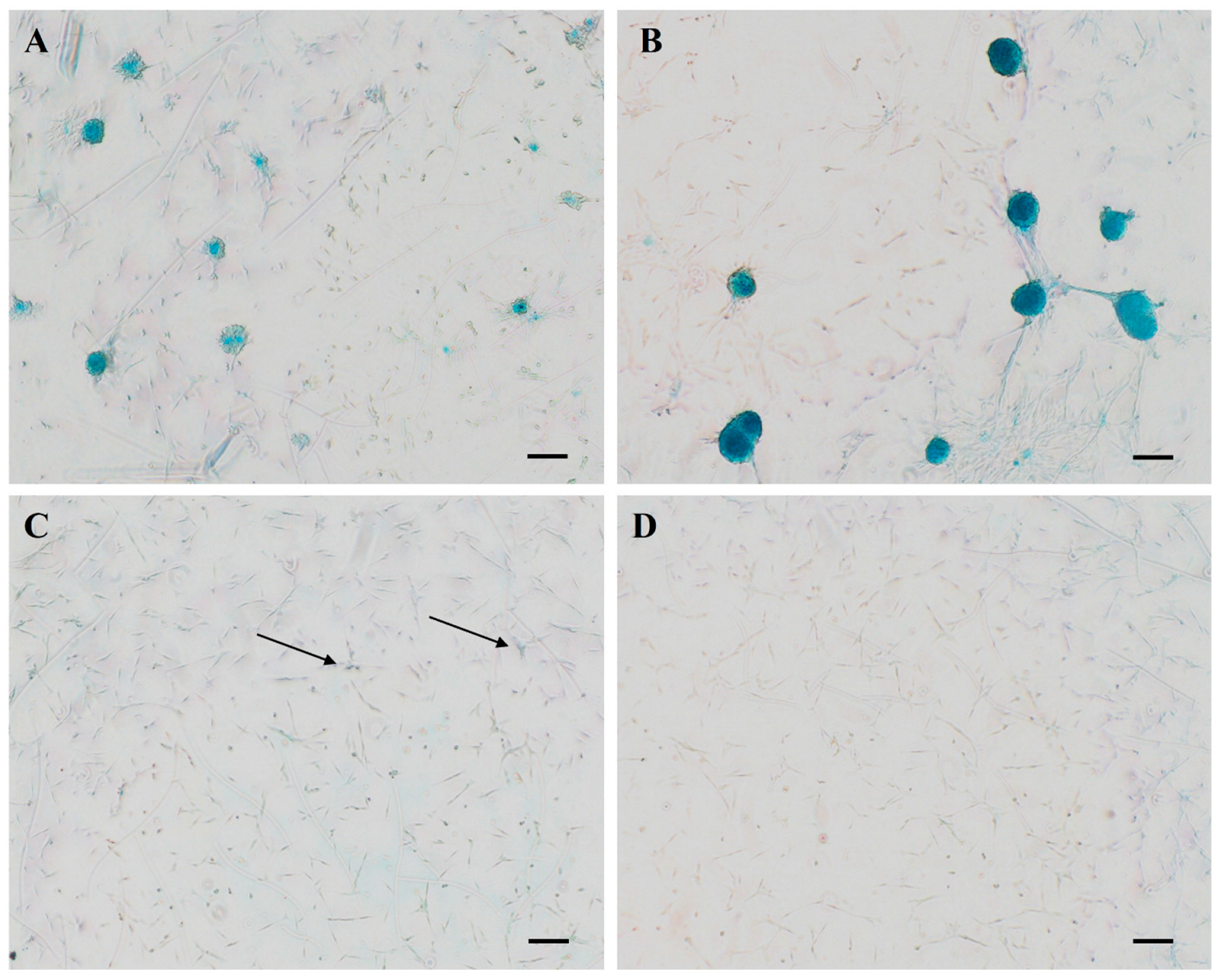
3.3. Multilineage Differentiation Potential
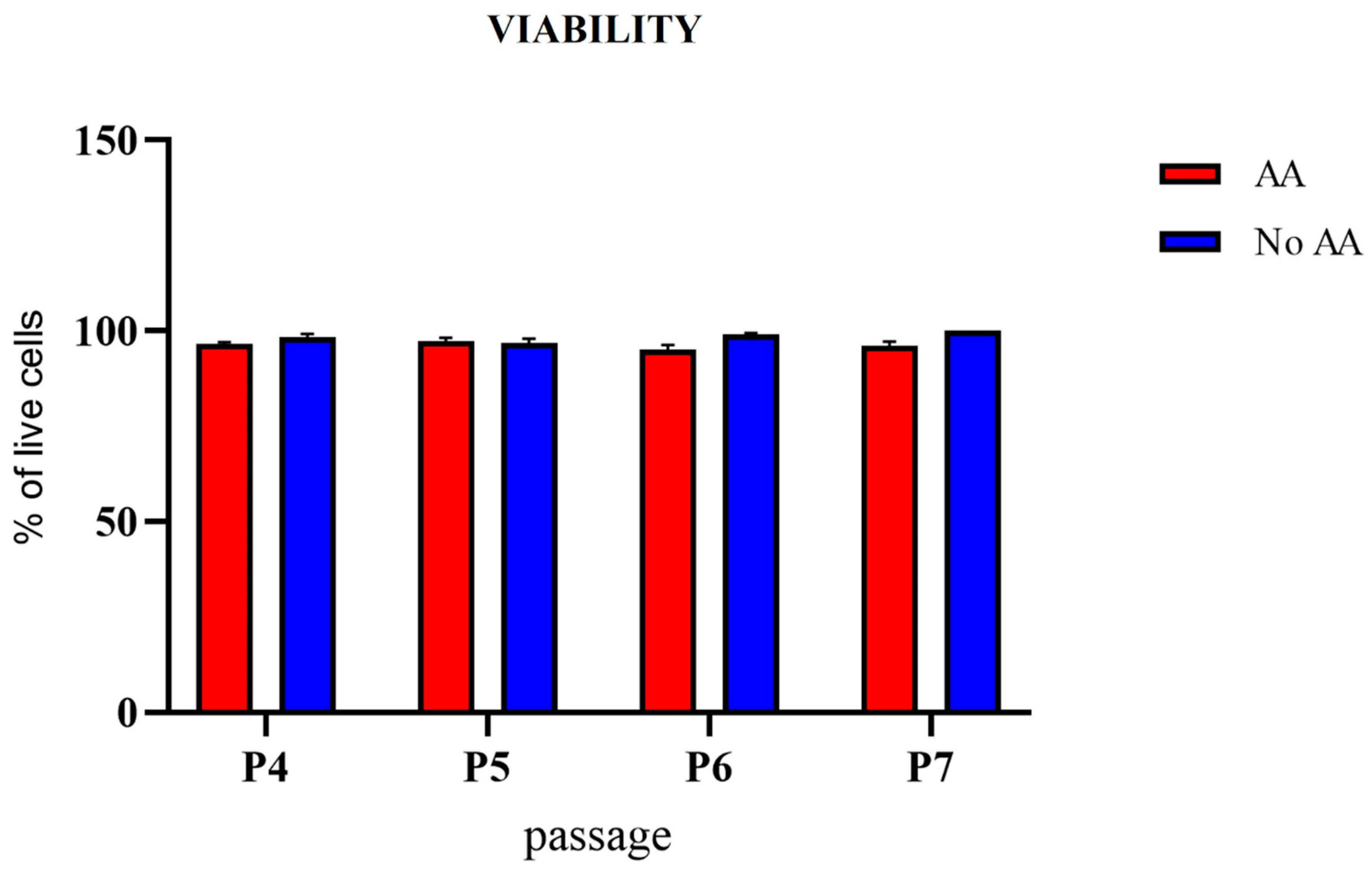
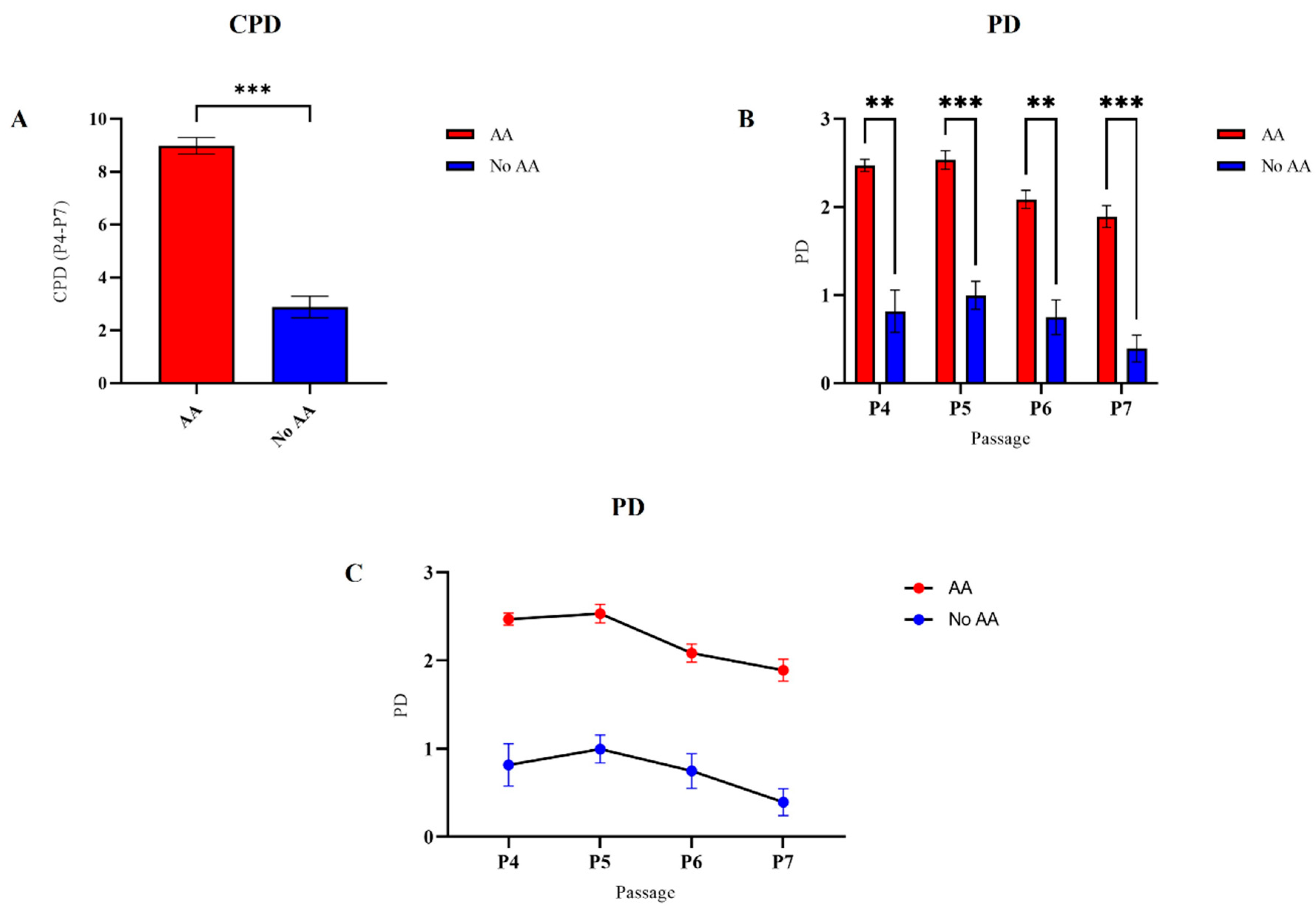
3.4. Cell Viability and Proliferation Potential
3.5. Alcian Blue Staining
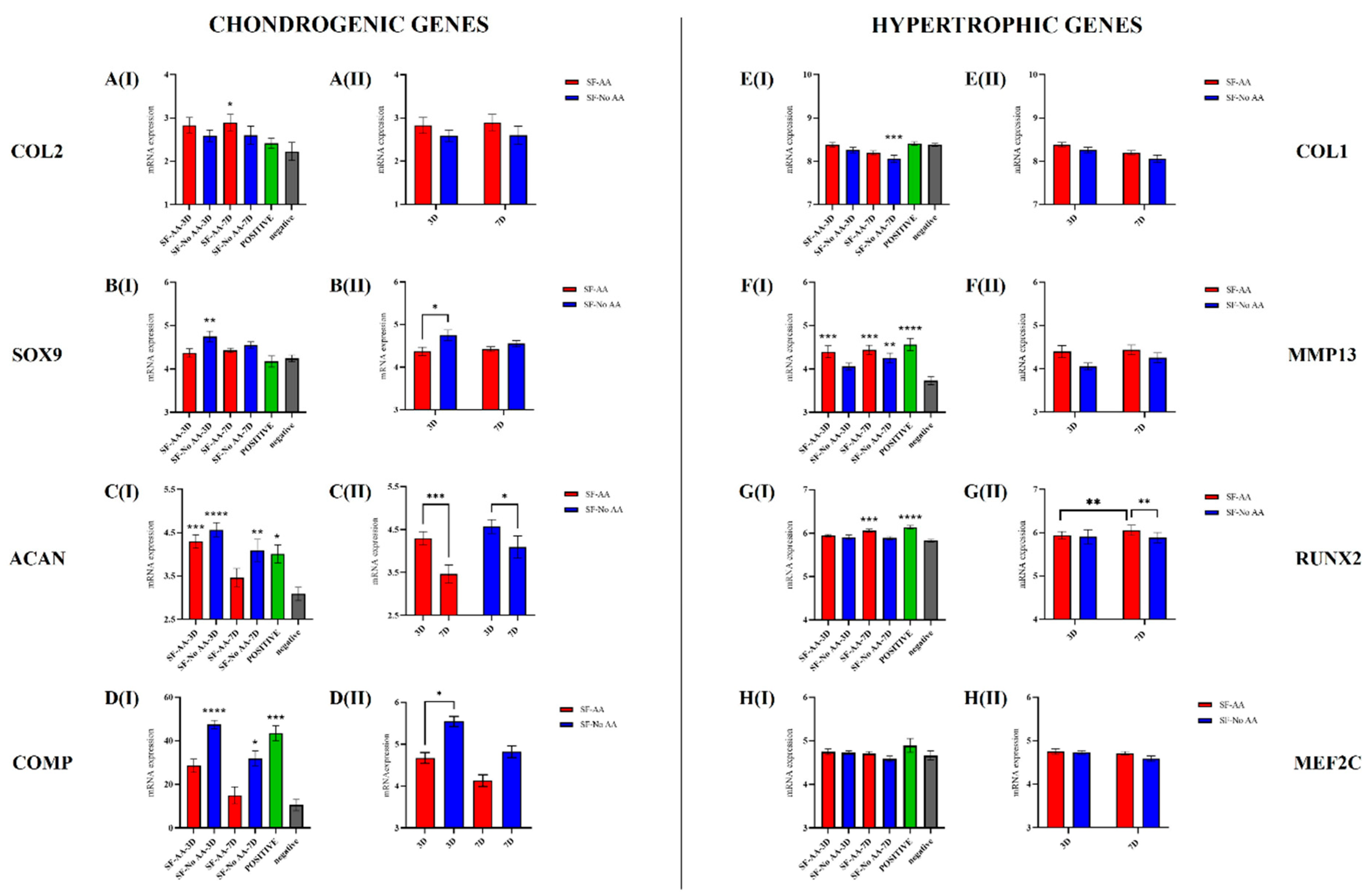
3.6. Gene Expression of Chondrogenic and Hypertrophic Marker Genes
- Chondrogenic genes: COL2: Compared with the negative control, the expression of COL2 was significantly higher in SF-AA-7D (p < 0.05; Figure 8(AI)). When comparing only SF cells, there were no differences between SF cells with respect to the different media or times of cultivation (Figure 8(AII)). SOX9: Compared with the negative control, the expression of SOX9 was significantly higher in SF-NoAA-3D (p = 0.001; Figure 8(BI)). When comparing only SF cells, the expression of SOX9 on day 3 of cell culture was significantly higher in SF-NoAA than in SF-AA (p < 0.05; Figure 8(BII)). ACAN: Compared to the negative control, the expression of ACAN was higher in SF-AA-3D (p < 0.001), SF-NoAA-3D (p < 0.0001), SF-NoAA-7D (p < 0.01), and the positive control (p < 0.05) but similar in SF-AA-7D (Figure 8(CI)). When comparing only SF cells, expression of ACAN was higher on day 3 than on day 7 of cell culture in both SF-AA and SF-NoAA (p < 0.001 and p < 0.05, respectively; Figure 8(CII)). COMP: Compared to the negative control, the expression of COMP was significantly higher in SF-NoAA-3D (p < 0.0001), SF-NoAA-7D (p < 0.05), and the positive control (p < 0.001) (Figure 8(DI)). When comparing only SF cells, the expression of COMP was significantly higher in SF-NoAA than in SF-AA on day 3 of cell culture (p < 0.05; Figure 8(DII)).
- Hypertrophic genes: COL1: Compared with the negative control, COL1 expression was significantly lower in SF-NoAA-7D (p < 0.001; Figure 8(EI)). There were no differences between SF cells with respect to the different media or times of culturing when comparing SF cells only (Figure 8(EII)). MMP13: Expression of MMP13 in SF-NoAA-3D was similar to that in the negative control. In all other cell groups, expression was higher and significantly different from the negative control (p < 0.001 for SF-AA-3D, p = 0.0001 for SF-AA-7D, p < 0.01 for SF-NoAA-7D, and p < 0.0001 for the positive control; Figure 8(FI)). There were no differences between SF cells with respect to the different media or times of culturing when comparing SF cells only (Figure 8(FII)). RUNX2: Compared with the negative control, the expression of RUNX2 was significantly higher in SF-AA-7D (p < 0.001) and the positive control (p < 0.0001), while the expression of RUNX2 in the other cell groups was similar to that in the negative control (Figure 8(GI)). When comparing only SF cells, the expression of RUNX2 was significantly higher in SF-AA on day 7 than on day 3 (p < 0.01), and on day 7, the expression was higher in SF-AA than in SF-NoAA (p < 0.01) (Figure 8(GII)). MEF2C: There were no differences in MEF2C expression between cell groups (Figure 8H).
4. Discussion
5. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Voga, M.; Adamic, N.; Vengust, M.; Majdic, G. Stem Cells in Veterinary Medicine-Current State and Treatment Options. Front. Vet. Sci. 2020, 7, 278. [Google Scholar] [CrossRef] [PubMed]
- Caplan, A.I. Mesenchymal Stem Cells: Time to Change the Name! Stem Cells Transl. Med. 2017, 6, 1445–1451. [Google Scholar] [CrossRef] [PubMed]
- Guimaraes-Camboa, N.; Cattaneo, P.; Sun, Y.; Moore-Morris, T.; Gu, Y.; Dalton, N.D.; Rockenstein, E.; Masliah, E.; Peterson, K.L.; Stallcup, W.B.; et al. Pericytes of Multiple Organs Do Not Behave as Mesenchymal Stem Cells In Vivo. Cell Stem Cell 2017, 20, 345–359.e1–e5. [Google Scholar] [CrossRef] [PubMed]
- Meirelles Lda, S.; Fontes, A.M.; Covas, D.T.; Caplan, A.I. Mechanisms involved in the therapeutic properties of mesenchymal stem cells. Cytokine Growth Factor Rev. 2009, 20, 419–427. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.R.; Grodzinsky, A.J.; Hsu, H.P.; Martin, S.D.; Spector, M. Effects of harvest and selected cartilage repair procedures on the physical and biochemical properties of articular cartilage in the canine knee. J. Orthop. Res. 2000, 18, 790–799. [Google Scholar] [CrossRef] [PubMed]
- Hallett, S.A.; Ono, W.; Ono, N. The hypertrophic chondrocyte: To be or not to be. Histol. Histopathol. 2021, 36, 1021–1036. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Tsang, K.Y.; Tang, H.C.; Chan, D.; Cheah, K.S. Hypertrophic chondrocytes can become osteoblasts and osteocytes in endochondral bone formation. Proc. Natl. Acad. Sci. USA 2014, 111, 12097–12102. [Google Scholar] [CrossRef]
- Park, S.; Bello, A.; Arai, Y.; Ahn, J.; Kim, D.; Cha, K.-Y.; Baek, I.; Park, H.; Lee, S.-H. Functional Duality of Chondrocyte Hypertrophy and Biomedical Application Trends in Osteoarthritis. Pharmaceutics 2021, 13, 1139. [Google Scholar] [CrossRef]
- Brigelius-Flohe, R.; Flohe, L. Ascorbic acid, cell proliferation, and cell differentiation in culture. Subcell. Biochem. 1996, 25, 83–107. [Google Scholar] [CrossRef]
- Fujisawa, K.; Hara, K.; Takami, T.; Okada, S.; Matsumoto, T.; Yamamoto, N.; Sakaida, I. Evaluation of the effects of ascorbic acid on metabolism of human mesenchymal stem cells. Stem Cell Res. Ther. 2018, 9, 93. [Google Scholar] [CrossRef]
- Zhitkovich, A. Ascorbate: Antioxidant and biochemical activities and their importance for in vitro models. Arch. Toxicol. 2021, 95, 3623–3631. [Google Scholar] [CrossRef] [PubMed]
- Choi, K.-M.; Seo, Y.-K.; Yoon, H.-H.; Song, K.-Y.; Kwon, S.-Y.; Lee, H.-S.; Park, J.-K. Effect of ascorbic acid on bone marrow-derived mesenchymal stem cell proliferation and differentiation. J. Biosci. Bioeng. 2008, 105, 586–594. [Google Scholar] [CrossRef] [PubMed]
- Potdar, P.D.; D’Souza, S.B. Ascorbic acid induces in vitro proliferation of human subcutaneous adipose tissue derived mesenchymal stem cells with upregulation of embryonic stem cell pluripotency markers Oct4 and SOX 2. Hum. Cell 2010, 23, 152–155. [Google Scholar] [CrossRef] [PubMed]
- Wahyuningsih, K.A.; Karina, K.; Rosadi, I.; Rosliana, I.; Subroto, W.R. Effect of ascorbic acid on morphology of post-thawed human adipose-derived stem cells. Stem Cell Investig. 2020, 7, 16. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Pei, M. A Protocol to Prepare Decellularized Stem Cell Matrix for Rejuvenation of Cell Expansion and Cartilage Regeneration. Methods Mol. Biol. 2018, 1577, 147–154. [Google Scholar] [CrossRef] [PubMed]
- Solchaga, L.A.; Penick, K.J.; Welter, J.F. Chondrogenic differentiation of bone marrow-derived mesenchymal stem cells: Tips and tricks. Methods Mol. Biol. 2011, 698, 253–278. [Google Scholar] [CrossRef]
- Langenbach, F.; Handschel, J. Effects of dexamethasone, ascorbic acid and beta-glycerophosphate on the osteogenic differentiation of stem cells in vitro. Stem Cell Res. Ther. 2013, 4, 117. [Google Scholar] [CrossRef] [PubMed]
- Bhandi, S.; Alkahtani, A.; Mashyakhy, M.; Abumelha, A.S.; Albar, N.H.M.; Renugalakshmi, A.; Alkahtany, M.F.; Robaian, A.; Almeslet, A.S.; Patil, V.R.; et al. Effect of Ascorbic Acid on Differentiation, Secretome and Stemness of Stem Cells from Human Exfoliated Deciduous Tooth (SHEDs). J. Pers. Med. 2021, 11, 589. [Google Scholar] [CrossRef] [PubMed]
- Kundu, B.; Rajkhowa, R.; Kundu, S.C.; Wang, X. Silk fibroin biomaterials for tissue regenerations. Adv. Drug Deliv. Rev. 2013, 65, 457–470. [Google Scholar] [CrossRef]
- Rockwood, D.N.; Preda, R.C.; Yucel, T.; Wang, X.; Lovett, M.L.; Kaplan, D.L. Materials fabrication from Bombyx mori silk fibroin. Nat. Protoc. 2011, 6, 1612–1631. [Google Scholar] [CrossRef]
- Voga, M.; Drnovsek, N.; Novak, S.; Majdic, G. Silk fibroin induces chondrogenic differentiation of canine adipose-derived multipotent mesenchymal stromal cells/mesenchymal stem cells. J. Tissue Eng. 2019, 10, 2041731419835056. [Google Scholar] [CrossRef] [PubMed]
- Sartore, L.; Manferdini, C.; Saleh, Y.; Dey, K.; Gabusi, E.; Ramorino, G.; Zini, N.; Almici, C.; Re, F.; Russo, D.; et al. Polysaccharides on gelatin-based hydrogels differently affect chondrogenic differentiation of human mesenchymal stromal cells. Mater. Sci. Eng. C 2021, 126, 112175. [Google Scholar] [CrossRef] [PubMed]
- Feng, Q.; Lin, S.; Zhang, K.; Dong, C.; Wu, T.; Huang, H.; Yan, X.; Zhang, L.; Li, G.; Bian, L. Sulfated hyaluronic acid hydrogels with retarded degradation and enhanced growth factor retention promote hMSC chondrogenesis and articular cartilage integrity with reduced hypertrophy. Acta Biomater. 2017, 53, 329–342. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Huang, Z.; Liang, Y.; Yuan, W.; Bian, L.; Duan, L.; Rong, Z.; Xiong, J.; Wang, D.; Xia, J. 3D printed gelatin/hydroxyapatite scaffolds for stem cell chondrogenic differentiation and articular cartilage repair. Biomater. Sci. 2021, 9, 2620–2630. [Google Scholar] [CrossRef]
- Cristofalo, V.J.; Allen, R.G.; Pignolo, R.J.; Martin, B.G.; Beck, J.C. Relationship between donor age and the replicative lifespan of human cells in culture: A reevaluation. Proc. Natl. Acad. Sci. USA 1998, 95, 10614–10619. [Google Scholar] [CrossRef] [PubMed]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef] [PubMed]
- Ruifrok, A.C.; Johnston, D.A. Quantification of histochemical staining by color deconvolution. Anal. Quant. Cytol. Histol. 2001, 23, 291–299. [Google Scholar] [PubMed]
- Landini, G.; Martinelli, G.; Piccinini, F. Colour deconvolution: Stain unmixing in histological imaging. Bioinformatics 2020, 37, 1485–1487. [Google Scholar] [CrossRef]
- Carrasco, G.C. Dynamic Threshold. Available online: https://www.gcsca.net/IJ/Dynamic.html (accessed on 20 August 2023).
- Bi, W.; Deng, J.M.; Zhang, Z.; Behringer, R.R.; de Crombrugghe, B. Sox9 is required for cartilage formation. Nat. Genet. 1999, 22, 85–89. [Google Scholar] [CrossRef]
- Goldring, M.B. Chondrogenesis, chondrocyte differentiation, and articular cartilage metabolism in health and osteoarthritis. Ther. Adv. Musculoskelet. Dis. 2012, 4, 269–285. [Google Scholar] [CrossRef]
- Chen, S.; Xu, Z.; Shao, J.; Fu, P.; Wu, H. MicroRNA-218 promotes early chondrogenesis of mesenchymal stem cells and inhibits later chondrocyte maturation. BMC Biotechnol. 2019, 19, 6. [Google Scholar] [CrossRef] [PubMed]
- Branly, T.; Contentin, R.; Desance, M.; Jacquel, T.; Bertoni, L.; Jacquet, S.; Mallein-Gerin, F.; Denoix, J.M.; Audigie, F.; Demoor, M.; et al. Improvement of the Chondrocyte-Specific Phenotype upon Equine Bone Marrow Mesenchymal Stem Cell Differentiation: Influence of Culture Time, Transforming Growth Factors and Type I Collagen siRNAs on the Differentiation Index. Int. J. Mol. Sci. 2018, 19, 435. [Google Scholar] [CrossRef]
- Armiento, A.; Alini, M.; Stoddart, M. Articular fibrocartilage—Why does hyaline cartilage fail to repair? Adv. Drug Deliv. Rev. 2018, 146, 289–305. [Google Scholar] [CrossRef]
- Yasui, N.; Ono, K.; Konomi, H.; Nagai, Y. Transitions in collagen types during endochondral ossification in human growth cartilage. Clin. Orthop. Relat. Res. 1984, 215–218. [Google Scholar]
- Bertram, H.; Boeuf, S.; Wachters, J.; Boehmer, S.; Heisel, C.; Hofmann, M.W.; Piecha, D.; Richter, W. Matrix metalloprotease inhibitors suppress initiation and progression of chondrogenic differentiation of mesenchymal stromal cells in vitro. Stem Cells Dev. 2009, 18, 881–892. [Google Scholar] [CrossRef]
- Bedingfield, S.; Yu, F.; Liu, D.; Jackson, M.; Himmel, L.; Cho, H.; Colazo, J.; Crofford, L.; Hasty, K.; Duvall, C. Matrix-Targeted Nanoparticles for MMP13 RNA Interference Blocks Post-Traumatic Osteoarthritis. bioRxiv 2020. [Google Scholar] [CrossRef]
- Zheng, Q.; Zhou, G.; Morello, R.; Chen, Y.; Garcia-Rojas, X.; Lee, B. Type X collagen gene regulation by Runx2 contributes directly to its hypertrophic chondrocyte-specific expression in vivo. J. Cell Biol. 2003, 162, 833–842. [Google Scholar] [CrossRef] [PubMed]
- Dreher, S.I.; Fischer, J.; Walker, T.; Diederichs, S.; Richter, W. Significance of MEF2C and RUNX3 Regulation for Endochondral Differentiation of Human Mesenchymal Progenitor Cells. Front. Cell Dev. Biol. 2020, 8, 81. [Google Scholar] [CrossRef]
- Ding, M.; Lu, Y.; Abbassi, S.; Li, F.; Li, X.; Song, Y.; Geoffroy, V.; Im, H.J.; Zheng, Q. Targeting Runx2 expression in hypertrophic chondrocytes impairs endochondral ossification during early skeletal development. J. Cell. Physiol. 2012, 227, 3446–3456. [Google Scholar] [CrossRef]
- Diederichs, S.; Tonnier, V.; Marz, M.; Dreher, S.I.; Geisbusch, A.; Richter, W. Regulation of WNT5A and WNT11 during MSC in vitro chondrogenesis: WNT inhibition lowers BMP and hedgehog activity, and reduces hypertrophy. Cell. Mol. Life Sci. 2019, 76, 3875–3889. [Google Scholar] [CrossRef]
- Pfaffl, M.W. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001, 29, e45. [Google Scholar] [CrossRef]
- Bearden, R.N.; Huggins, S.S.; Cummings, K.J.; Smith, R.; Gregory, C.A.; Saunders, W.B. In-vitro characterization of canine multipotent stromal cells isolated from synovium, bone marrow, and adipose tissue: A donor-matched comparative study. Stem Cell Res. Ther. 2017, 8, 218. [Google Scholar] [CrossRef] [PubMed]
- Trindade, A.B.; Therrien, J.; Garcia, J.M.; Smith, L.C. Mesenchymal-like stem cells in canine ovary show high differentiation potential. Cell Prolif. 2017, 50. [Google Scholar] [CrossRef] [PubMed]
- Takemitsu, H.; Zhao, D.; Yamamoto, I.; Harada, Y.; Michishita, M.; Arai, T. Comparison of bone marrow and adipose tissue-derived canine mesenchymal stem cells. BMC Vet. Res. 2012, 8, 150. [Google Scholar] [CrossRef] [PubMed]
- Ronziere, M.C.; Farjanel, J.; Reyria, A.; Hartmann, D.J.; Herbage, D. Analysis of types I, II, III, IX and XI collagens synthesized by fetal bovine chondrocytes in high-density culture. Osteoarthr. Cartil. 1997, 5, 205–214. [Google Scholar] [CrossRef] [PubMed]
- Pizzute, T.; Zhang, Y.; He, F.; Pei, M. Ascorbate-dependent impact on cell-derived matrix in modulation of stiffness and rejuvenation of infrapatellar fat derived stem cells toward chondrogenesis. Biomed. Mater. 2016, 11, 045009. [Google Scholar] [CrossRef] [PubMed]
- Guo, P.; Zeng, J.-j.; Zhou, N. A novel experimental study on the fabrication and biological characteristics of canine bone marrow mesenchymal stem cells sheet using vitamin C. Scanning 2015, 37, 42–48. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.K.; Tu, Y.K.; Yu, J.; Cheng, N.C. The Influence of Cell Culture Density on the Cytotoxicity of Adipose-Derived Stem Cells Induced by L-Ascorbic Acid-2-Phosphate. Sci. Rep. 2020, 10, 104. [Google Scholar] [CrossRef] [PubMed]
- Pinnell, S.R. Regulation of collagen biosynthesis by ascorbic acid: A review. Yale J. Biol. Med. 1985, 58, 553–559. [Google Scholar]
- Franceschi, R.T.; Iyer, B.S. Relationship between collagen synthesis and expression of the osteoblast phenotype in MC3T3-E1 cells. J. Bone Min. Res. 1992, 7, 235–246. [Google Scholar] [CrossRef]
- Rosadi, I.; Karina, K.; Rosliana, I.; Sobariah, S.; Afini, I.; Widyastuti, T.; Barlian, A. In vitro study of cartilage tissue engineering using human adipose-derived stem cells induced by platelet-rich plasma and cultured on silk fibroin scaffold. Stem Cell Res. Ther. 2019, 10, 369. [Google Scholar] [CrossRef] [PubMed]
- Barlian, A.; Judawisastra, H.; Alfarafisa, N.M.; Wibowo, U.A.; Rosadi, I. Chondrogenic differentiation of adipose-derived mesenchymal stem cells induced by L-ascorbic acid and platelet rich plasma on silk fibroin scaffold. PeerJ 2018, 6, e5809. [Google Scholar] [CrossRef] [PubMed]
- Fan, L.-P.; Wang, H.; Zhang, K.; Cai, Z.; He, C.; Sheng, X.; Mo, X. Vitamin C-reinforcing silk fibroin nanofibrous matrices for skin care application. RSC Adv. 2012, 2, 4110–4119. [Google Scholar] [CrossRef]
- Gandhimathi, C. Controlled Release of Dexamethasone in PCL/Silk Fibroin/Ascorbic Acid Nanoparticles for the Initiation of Adipose Derived Stem Cells into Osteogenesis. J. Drug Metab. Toxicol. 2015, 06, 2. [Google Scholar] [CrossRef]
- Aulthouse, A.L. Prolonged exposure of human chondrocytes to ascorbic acid modifies cellular behavior in an agarose gel. Anat. Rec. 1994, 238, 31–37. [Google Scholar] [CrossRef] [PubMed]
- Altaf, F.M.; Hering, T.M.; Kazmi, N.H.; Yoo, J.U.; Johnstone, B. Ascorbate-enhanced chondrogenesis of ATDC5 cells. Eur. Cell Mater. 2006, 12, 64–69. [Google Scholar] [CrossRef]
- Clark, A.G.; Rohrbaugh, A.L.; Otterness, I.; Kraus, V.B. The effects of ascorbic acid on cartilage metabolism in guinea pig articular cartilage explants. Matrix Biol. 2002, 21, 175–184. [Google Scholar] [CrossRef] [PubMed]
- Ibold, Y.; Lübke, C.; Pelz, S.; Augst, H.; Kaps, C.; Ringe, J.; Sittinger, M. Effect of different ascorbate supplementations on in vitro cartilage formation in porcine high-density pellet cultures. Tissue Cell 2009, 41, 249–256. [Google Scholar] [CrossRef]
- Kim, G.; Okumura, M.; Bosnakovski, D.; Ishiguro, T.; Park, C.H.; Kadosawa, T.; Fujinaga, T. Effects of ascorbic acid on proliferation and biological properties of bovine chondrocytes in alginate beads. Jpn. J. Vet. Res. 2003, 51, 83–94. [Google Scholar]
- de Crombrugghe, B.; Lefebvre, V.; Behringer, R.R.; Bi, W.; Murakami, S.; Huang, W. Transcriptional mechanisms of chondrocyte differentiation. Matrix Biol. 2000, 19, 389–394. [Google Scholar] [CrossRef]
- Liu, C.J.; Zhang, Y.; Xu, K.; Parsons, D.; Alfonso, D.; Di Cesare, P.E. Transcriptional activation of cartilage oligomeric matrix protein by Sox9, Sox5, and Sox6 transcription factors and CBP/p300 coactivators. Front. Biosci. 2007, 12, 3899–3910. [Google Scholar] [CrossRef]
- Hering, T.M.; Kollar, J.; Huynh, T.D.; Varelas, J.B.; Sandell, L.J. Modulation of extracellular matrix gene expression in bovine high-density chondrocyte cultures by ascorbic acid and enzymatic resuspension. Arch. Biochem. Biophys. 1994, 314, 90–98. [Google Scholar] [CrossRef] [PubMed]
- Shukunami, C.; Ishizeki, K.; Atsumi, T.; Ohta, Y.; Suzuki, F.; Hiraki, Y. Cellular hypertrophy and calcification of embryonal carcinoma-derived chondrogenic cell line ATDC5 in vitro. J. Bone Min. Res. 1997, 12, 1174–1188. [Google Scholar] [CrossRef] [PubMed]
- Leboy, P.S.; Vaias, L.; Uschmann, B.; Golub, E.; Adams, S.L.; Pacifici, M. Ascorbic acid induces alkaline phosphatase, type X collagen, and calcium deposition in cultured chick chondrocytes. J. Biol. Chem. 1989, 264, 17281–17286. [Google Scholar] [CrossRef] [PubMed]
- Chan, D.; Lamande, S.R.; Cole, W.G.; Bateman, J.F. Regulation of procollagen synthesis and processing during ascorbate-induced extracellular matrix accumulation in vitro. Biochem. J. 1990, 269, 175–181. [Google Scholar] [CrossRef]
- Chojkier, M.; Houglum, K.; Solis-Herruzo, J.; Brenner, D.A. Stimulation of collagen gene expression by ascorbic acid in cultured human fibroblasts. A role for lipid peroxidation? J. Biol. Chem. 1989, 264, 16957–16962. [Google Scholar] [CrossRef]
- Geesin, J.C.; Darr, D.; Kaufman, R.; Murad, S.; Pinnell, S.R. Ascorbic acid specifically increases type I and type III procollagen messenger RNA levels in human skin fibroblast. J. Investig. Dermatol. 1988, 90, 420–424. [Google Scholar] [CrossRef]
- Mizutani, A.; Sugiyama, I.; Kuno, E.; Matsunaga, S.; Tsukagoshi, N. Expression of matrix metalloproteinases during ascorbate-induced differentiation of osteoblastic MC3T3-E1 cells. J. Bone Min. Res. 2001, 16, 2043–2049. [Google Scholar] [CrossRef]



| Surface Marker | Conjugation | Antibody Clone | Isotype | Target Species | Catalogue Number | Source | Antibody Dilution per 1 × 106 Cells |
|---|---|---|---|---|---|---|---|
| CD44 | APC | IM7 | Rat IgG2b | Mouse, human | 103012 | Biolegend, USA | 1:67 |
| CD90 | PE | YKIX337.217 | Rat IgG2b | Dog | 12-5900-42 | eBioscience, USA | 1:20 |
| CD29 | FITC | MEM-101A | Mouse IgG1 | Dog, Human, Pig | MA1-19566 | ThermoFisher Scientific, USA | 1:5 |
| CD34 | FITC | 581 | Mouse IgG1 | Human | 60013FI | Stemcell technologies, Vancouver, BC, Canada | 1:20 |
| Cell Culture Name | Cell Seeding Surface | Culture Medium | Culture Time | Cell Seeding Density | Culture Conditions |
|---|---|---|---|---|---|
| SF-AA-3D | SF film (12.5 mg/mL) | Cell culture medium with ascorbic acid | 3 days | 104 per cm2 | 37 °C, 5% CO2 |
| SF-AA-7D | SF film (12.5 mg/mL) | Cell culture medium with ascorbic acid | 7 days | 104 per cm2 | 37 °C, 5% CO2 |
| SF-NoAA-3D | SF film (12.5 mg/mL) | Cell culture medium without ascorbic acid | 3 days | 104 per cm2 | 37 °C, 5% CO2 |
| SF-NoAA-7D | SF film (12.5 mg/mL) | Cell culture medium without ascorbic acid | 7 days | 104 per cm2 | 37 °C, 5% CO2 |
| POZ K | Standard polystyrene | Chondrogenic medium | P4 | 5 µL droplets of 4 × 104 cells | 37 °C, 5% CO2, high humidity |
| NEG K | Standard polystyrene | Cell culture medium | P4 | 104 per cm2 | 37 °C, 5% CO2 |
| Gene Symbol | Gene Name | Assay ID | Gene Role in Chondrogenesis |
|---|---|---|---|
| COL2A1 | Collagen type II, alpha 1 | Cf02622868_m1 | Cartilage-specific marker gene [30] |
| SOX9 | SRY (sex determining region Y)-box9 | cf02625134_g1 | The first transcription factor, essential for chondrocyte differentiation and cartilage formation [30] |
| ACAN | Aggrecan | Cf02674826_m1 | Cartilage-specific marker gene [31] |
| COMP | Cartilage oligomeric matrix protein | Cf02690298_g1 | Cartilage-specific marker gene [32] |
| COL1A1 | Collagen type I, alpha 1 | Cf01076765_m1 | A main component of fibrocartilage characteristic of OA [33,34] and distinctive of endochondral ossification [35] |
| MMP13 | Matrix Metalloproteinase 13 (Collagenase 3) | Cf02741638_m1 | Hypertrophic marker gene known to break down collagen type 2 [36,37]. |
| RUNX2 | Runt-related transcription factor 2 | Cf02694692_m1 | Direct transcriptional factor of COL10A1 during chondrogenesis [38] and hypertrophic marker gene [32,39,40] |
| MEF2C | Myocyte enhancer factor 2C | Cf02696950_m1 | Hypertrophic marker gene [39,41] |
| TBP | TATA box binding protein | Cf02637231_m1 | Reference gene |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Voga, M. Modulation of Canine Adipose-Derived Mesenchymal Stem/Medicinal Signalling Cells with Ascorbic Acid: Effect on Proliferation and Chondrogenic Differentiation on Standard Plastic and Silk Fibroin Surfaces. Bioengineering 2024, 11, 513. https://doi.org/10.3390/bioengineering11050513
Voga M. Modulation of Canine Adipose-Derived Mesenchymal Stem/Medicinal Signalling Cells with Ascorbic Acid: Effect on Proliferation and Chondrogenic Differentiation on Standard Plastic and Silk Fibroin Surfaces. Bioengineering. 2024; 11(5):513. https://doi.org/10.3390/bioengineering11050513
Chicago/Turabian StyleVoga, Metka. 2024. "Modulation of Canine Adipose-Derived Mesenchymal Stem/Medicinal Signalling Cells with Ascorbic Acid: Effect on Proliferation and Chondrogenic Differentiation on Standard Plastic and Silk Fibroin Surfaces" Bioengineering 11, no. 5: 513. https://doi.org/10.3390/bioengineering11050513
APA StyleVoga, M. (2024). Modulation of Canine Adipose-Derived Mesenchymal Stem/Medicinal Signalling Cells with Ascorbic Acid: Effect on Proliferation and Chondrogenic Differentiation on Standard Plastic and Silk Fibroin Surfaces. Bioengineering, 11(5), 513. https://doi.org/10.3390/bioengineering11050513






