A Self-Polymerizing Mesh of Nano-Tethers for the Mechanical Constraint of Degraded Intervertebral Discs—A Review of 25 Years of Pre-Clinical and Early Clinical Research
Abstract
:1. Introduction
2. Methods
3. Results
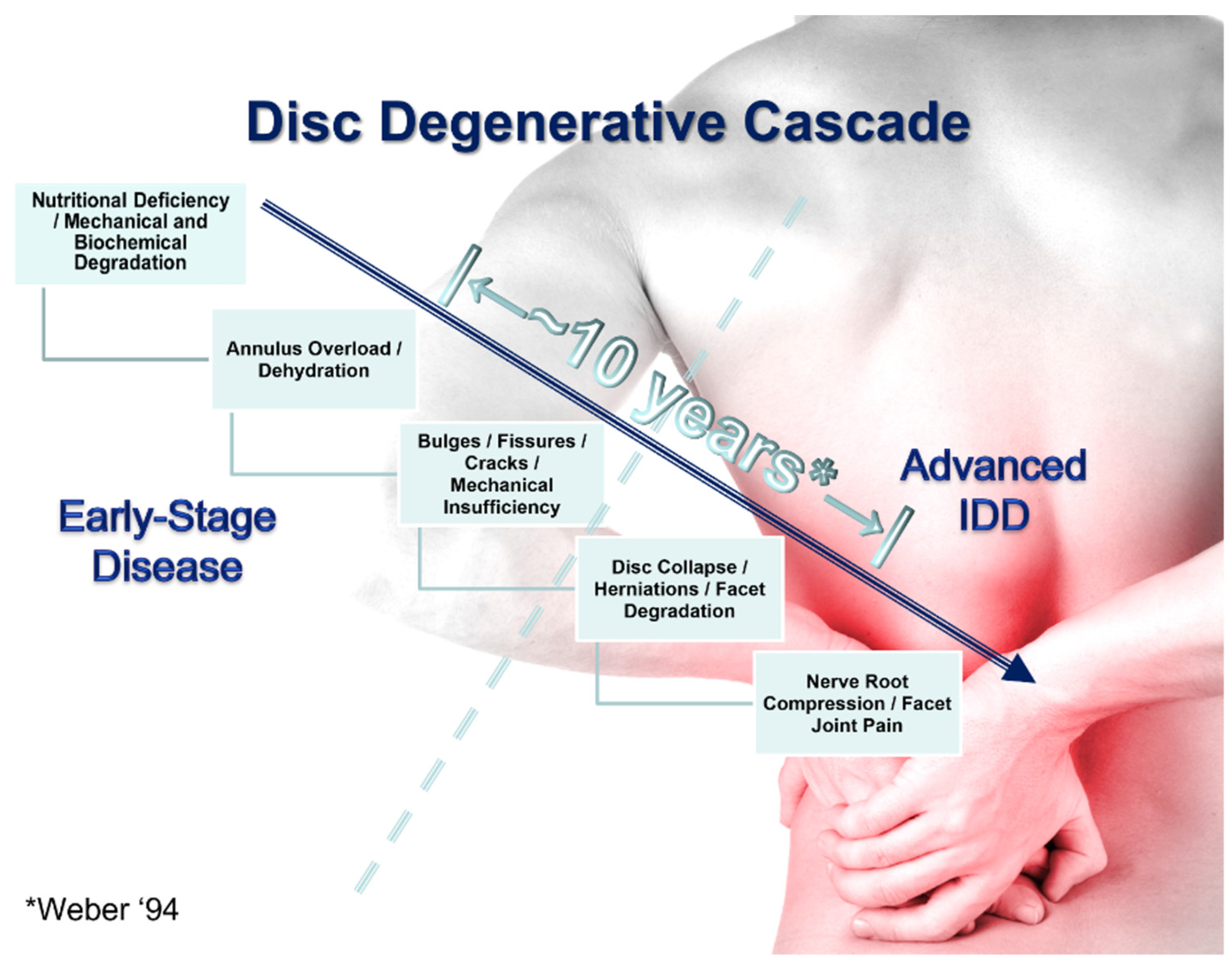
3.1. The Role of Mechanical Strength Deterioration in the Pathogenesis of IDD
3.2. The Reaction Kinetics of Genipin in Collagenous Tissues
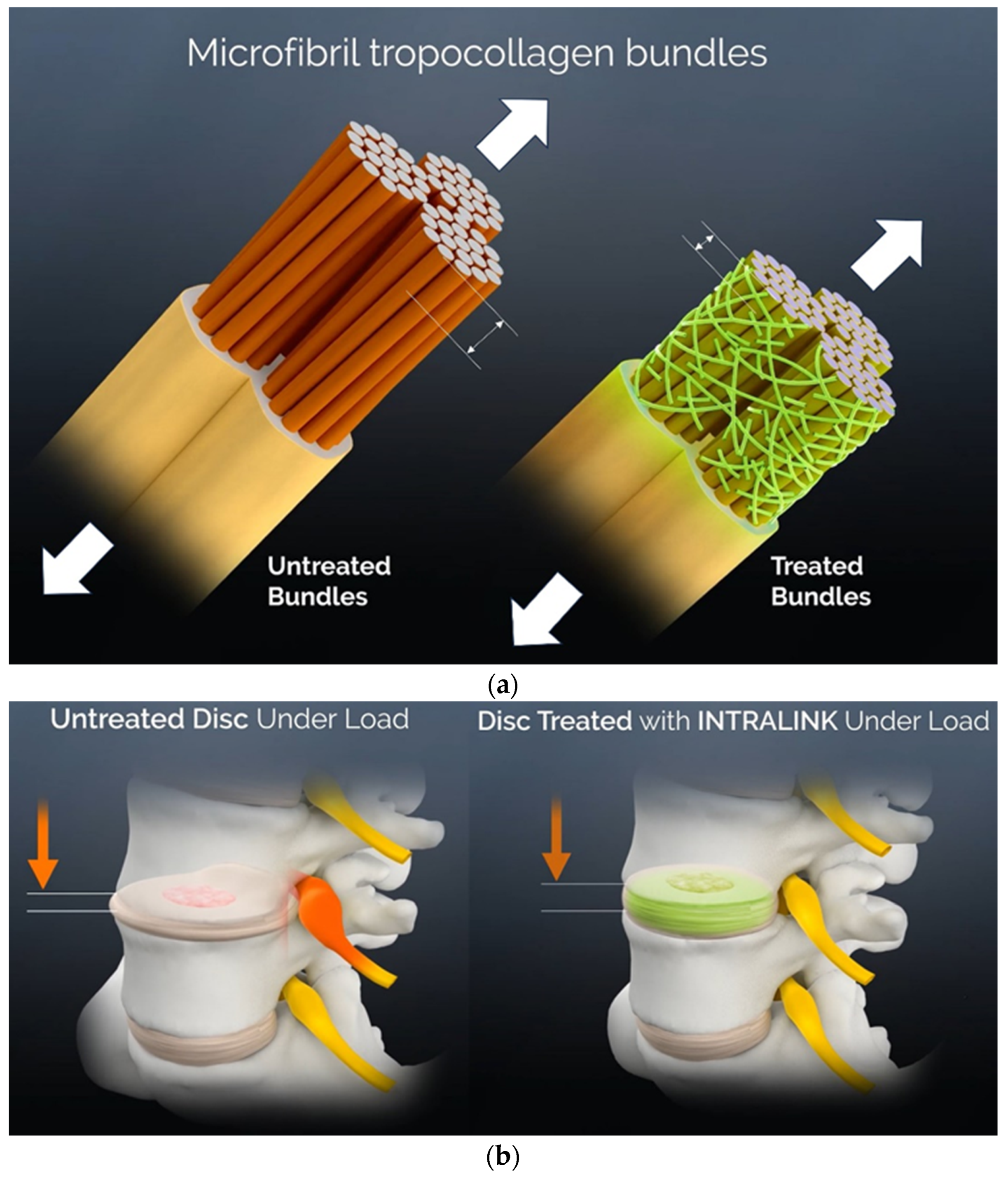
3.3. A Mechanical Effects Review
| Pain or Degeneration-Related Mechanical Factor | Effect Size |
|---|---|
| Resistance to tissue degradation from repetitive loading [33,40] | 3-fold increase |
| Joint stability, neutral zone reductions [33,41,42,43,44] † | Up to 4-fold increase |
| Annulus mechanical properties such as tensile strength, yield strength, resilience and toughness [32,36,37] | 50% or more improvement |
| Disc bulging under a load [32,38] | 38% reduction |
| Resistance to tear propagation, delamination [39,40] | Up to 70% increase |
| Tensile stress levels in annulus [47] | 3- to 8-fold lower |
| Annular sealing, disc pressure restoration [49,50] | 5- to 7-fold increase |
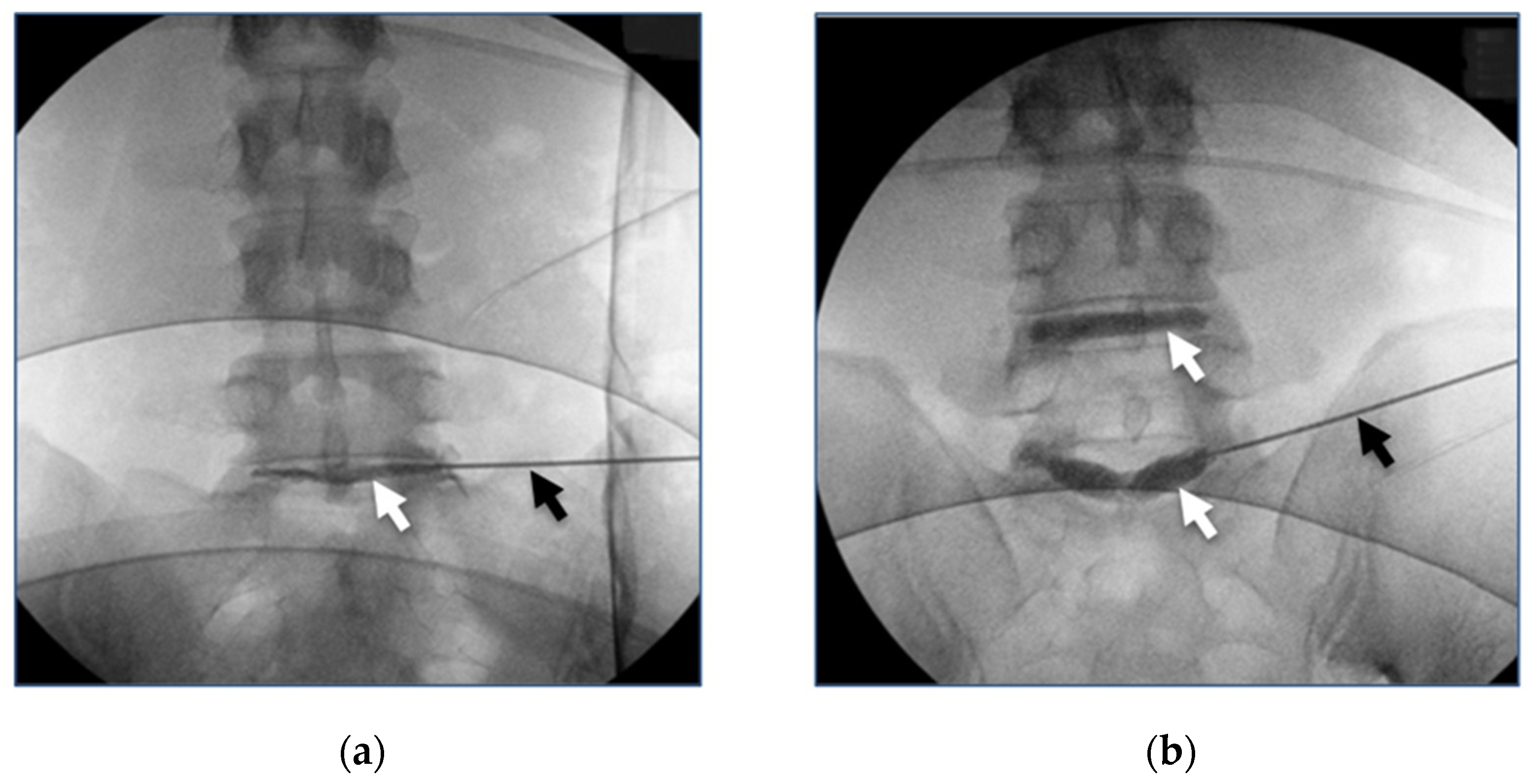
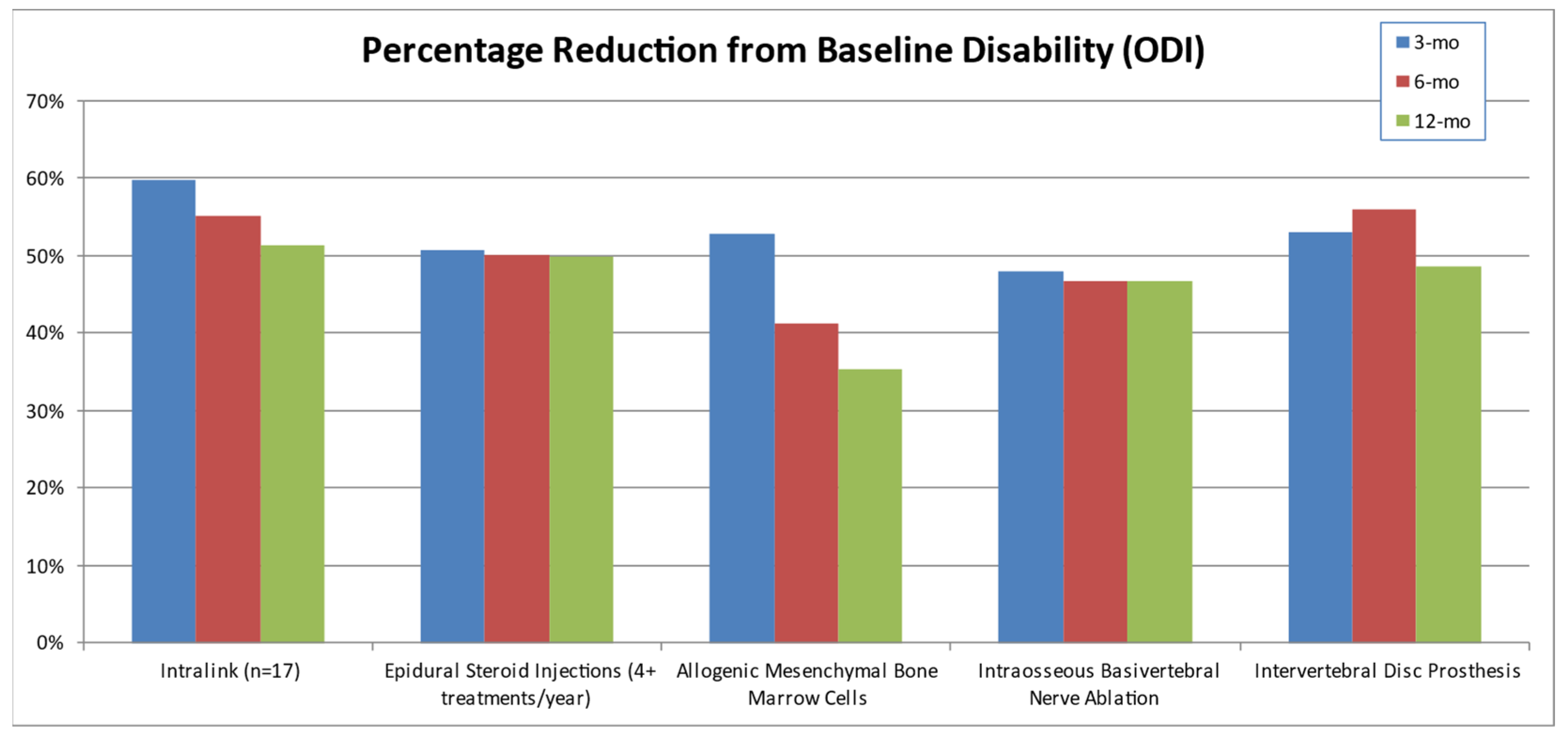
3.4. Clinical Studies Review
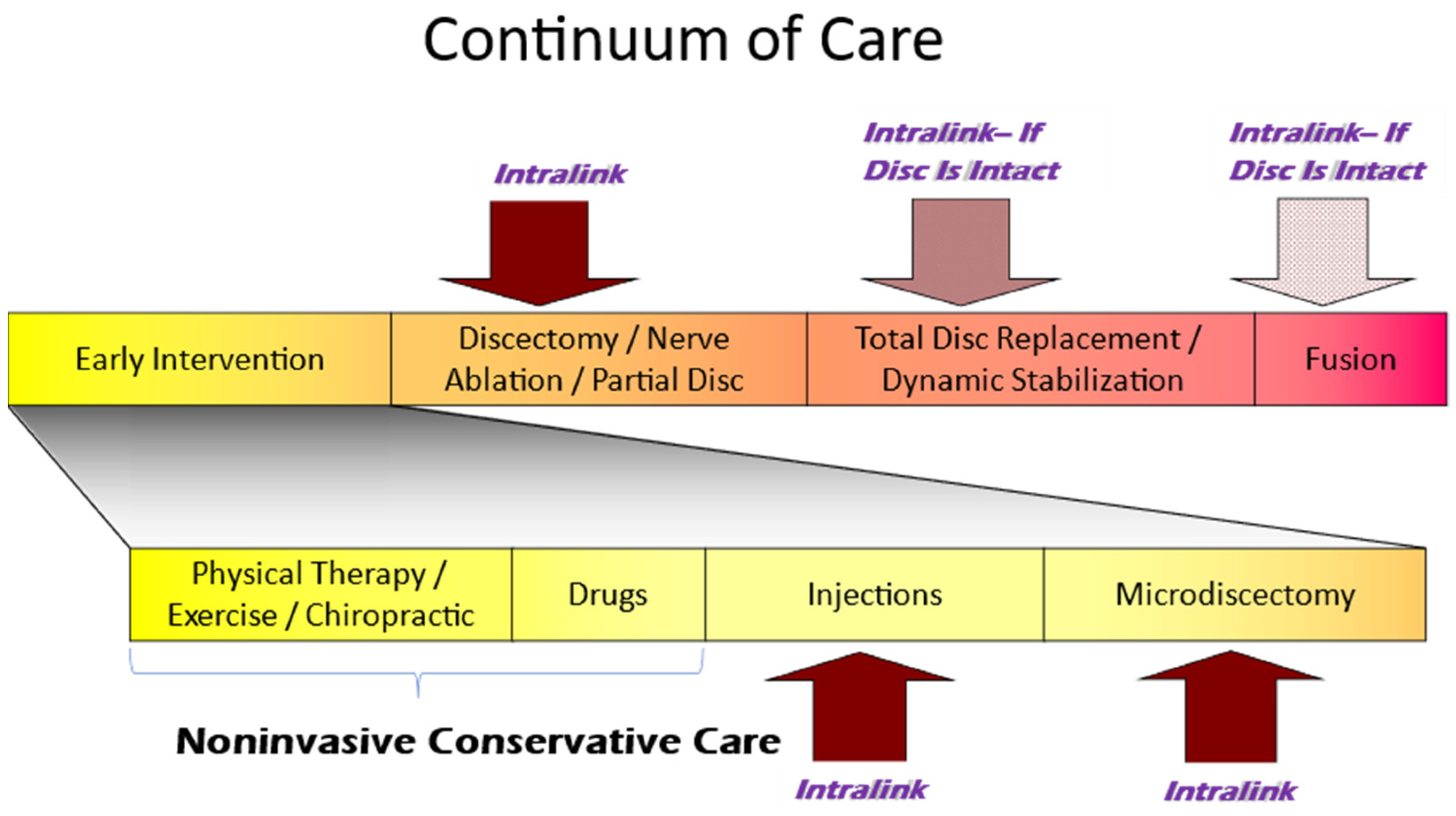
3.5. The Current Continuum of Care for Discogenic LBP
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Adams, M.A.; Roughley, P.J. What is intervertebral disc degeneration, and what causes it? Spine 2006, 31, 2151–2161. [Google Scholar] [CrossRef] [PubMed]
- Urban, J.P.; Smith, S.; Fairbank, J.C. Nutrition of the intervertebral disc. Spine 2004, 29, 2700–2709. [Google Scholar] [CrossRef]
- Urban, J.P.G.; Roberts, S. Degeneration of the intervertebral disc. Arthritis Res. Ther. 2003, 5, 120–130. [Google Scholar] [CrossRef] [PubMed]
- Adams, M.; Bogduk, N.; Burton, K.; Dolan, P. The Biomechanics of Back Pain; Churchill Livingstone: London, UK, 2002; ISBN 0-443-06207-2. [Google Scholar]
- Buckwalter, J.A. Aging and degeneration of the human intervertebral disc. Spine 1995, 20, 1307–1314. [Google Scholar] [CrossRef] [PubMed]
- Iatridis, J.C.; Laible, J.P.; Krag, M.H. Influence of fixed charge density magnitude and distribution on the intervertebral disc: Applications of a poroelastic and chemical electric (PEACE) model. J. Biomech. Eng. 2003, 125, 12–24. [Google Scholar] [CrossRef] [PubMed]
- Adams, M.A.; Freeman, B.J.C.; Morrison, H.P.; Nelson, I.W.; Dolan, P. Mechanical initiation of intervertebral disc degeneration. Spine 2000, 25, 1625–1636. [Google Scholar] [CrossRef]
- Zhao, F.; Pollintine, P.; Hole, B.D.; Dolan, P.; Adams, M.A. Discogenic origins of spinal instability. Spine 2005, 30, 2621–2630. [Google Scholar] [CrossRef]
- Butler, D.; Trafimow, J.H.; Andersson, G.B.; McNeill, T.W.; Huckman, M.S. Discs degenerate before facets. Spine 1990, 15, 111–113. [Google Scholar] [CrossRef]
- Buckwalter, J.A.; Woo, S.L.; Goldberg, V.M.; Hadley, E.C.; Booth, F.; Oegema, T.R.; Eyre, D.R. Soft-tissue aging and musculoskeletal function. J. Bone Jt. Surg. Am. 1993, 75, 1533–1548. [Google Scholar] [CrossRef]
- Koes, B.W.; van Tulder, M.W.; Thomas, S. Diagnosis and treatment of low back pain. BMJ 2006, 332, 1430–1434. [Google Scholar] [CrossRef]
- Maher, C.; Underwood, M.; Buchbinder, R. Non-specific low back pain. Lancet 2017, 389, 736–747. [Google Scholar] [CrossRef] [PubMed]
- Bartynski, W.S.; Dejohn, L.M.; Rothfus, W.E.; Gerszten, P.C. ‘Progressive-Onset’ versus injury-associated discogenic low back pain: Features of disc internal derangement in patients studied with provocation lumbar discography. Interv. Neuroradiol. 2013, 19, 110–120. [Google Scholar] [CrossRef] [PubMed]
- Panjabi, M.M. Clinical spina instability and low back pain. J. Electromyogr. Kinesiol. 2003, 13, 371–379. [Google Scholar] [CrossRef] [PubMed]
- Von Korff, M.; Deyo, R.A.; Cherkin, D.; Barlow, W. Back pain in primary care—Outcomes at 1 year. Spine 1993, 18, 855–862. [Google Scholar] [CrossRef] [PubMed]
- Von Korff, M. Studying the natural history of back pain. Spine 1994, 19, 2041S–2046S. [Google Scholar] [CrossRef] [PubMed]
- Maniadakis, N.; Gray, A. The economic burden of back pain in the UK. Pain 2000, 84, 95–103. [Google Scholar] [CrossRef] [PubMed]
- Balague, F.; Mannion, A.F.; Pellise, F.; Cedraschi, C. Non-specific low back pain. Lancet 2012, 379, 482–491. [Google Scholar] [CrossRef] [PubMed]
- Haldemann, S.; Dagenais, S. A supermarket approach to the evidence-informed management of chronic low back pain. Spine J. 2008, 8, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Deyo, R.A.; Mirza, S.K.; Martin, B.I. Back pain prevalence and visit rates—Estimates from US National Surveys—2002. Spine 2006, 31, 2724–2727. [Google Scholar] [CrossRef]
- Dagenais, S.; Caro, J.; Haldemann, S. A systematic review of low back pain cost of illness studies in the United States and international. Spine J. 2008, 8, 8–20. [Google Scholar] [CrossRef]
- Mi, F.L.; Shyu, S.S.; Peng, C.K. Characterization of ring-opening polymerization of genipin and pH-dependent cross-linking reactions between chitosan and genipin. J. Polym. Sci. Part A Polym. Chem. 2005, 43, 1985–2000. [Google Scholar] [CrossRef]
- Butler, M.F.; Ng, Y.F.; Pudney, P.D.A. Mechanism and Kinetics of the Crosslinking Reaction between Biopolymers Containing Primary Amine Groups and Genipin. J. Polym. Sci. Part A Polym. Chem. 2003, 41, 3941–3953. [Google Scholar] [CrossRef]
- Slusarewicz, P.; Zhu, K.; Hedman, T. Kinetic analysis of genipin degradation in aqueous solution. Nat. Prod. Commun. 2010, 5, 1853–1858. [Google Scholar] [CrossRef] [PubMed]
- Zhu, K.; Slusarewicz, P.; Hedman, T. Thermal Analysis Reveals Differential Effects of Various Crosslinkers on Bovine Annulus Fibrosis. J. Orthop. Res. 2011, 29, 8–13. [Google Scholar] [CrossRef] [PubMed]
- Battie, M.C.; Videman, T.; Parent, E. Lumbar disc degeneration: Epidemiology and genetic influences. Spine 2004, 29, 2679–2690. [Google Scholar] [CrossRef] [PubMed]
- Roughley, P.J. Biology of intervertebral disc aging and degeneration: Involvement of the extracellular matrix. Spine 2004, 29, 2691–2699. [Google Scholar] [CrossRef] [PubMed]
- Stokes, I.A.; Iatridis, J.C. Mechanical conditions that accelerate intervertebral disc degeneration: Overload versus immobilization. Spine 2004, 29, 2724–2732. [Google Scholar] [CrossRef] [PubMed]
- Lotz, J.C.; Ulrich, J.A. Innervation, inflammation, and hypermobility may characterize pathologic disc degeneration: Review of animal model data. J. Bone Jt. Surg. Am. 2006, 88 (Suppl. S2), 76–82. [Google Scholar] [CrossRef]
- Slusarewicz, P.; Zhu, K.; Hedman, T. Kinetic characterization and comparison of various protein crosslinking reagents for matrix modification. J. Mater. Sci. Mater. Med. 2010, 21, 1175–1181. [Google Scholar] [CrossRef]
- Weber, H. The natural history of disc herniation and the influence of intervention. Spine 1994, 19, 2234–2238. [Google Scholar] [CrossRef]
- Slusarewicz, P.; Zhu, K.; Kirking, B.; Toungate, J.; Hedman, T. Optimization of protein crosslinking formulations for the treatment of degenerative disc disease. Spine 2011, 36, E7–E13. [Google Scholar] [CrossRef] [PubMed]
- Hedman, T.P.; Chuang, S.Y.; Syed, B.G.D. Biomechanical benefits of crosslink augmentation in spinal discs. In Proceedings of the IMECE, Washington, DC, USA, 16–21 November 2003; Volume 43256. [Google Scholar]
- Brown, M.; Racadio, J.; Sundararaj, S.; Hedman, T. Long Term Biocompatibility of Intradiscal Injections with Genipin in Ovine Model. Orthop. Trans. 2016, 41, 0871. [Google Scholar]
- Hedman, T.; Yu, J.; Singh, H.; Deer, T. Early clinical results of intervertebral joint stabilization by injectable load-sharing polymers. J. Pain Res. 2023, 16, 2777–2789. [Google Scholar] [CrossRef] [PubMed]
- Chuang, S.Y.; Odono, R.M.; Hedman, T.P. Effects of exogenous crosslinking on in vitro tensile and compressive moduli of lumbar intervertebral discs. Clin. Biomech. 2007, 22, 14–20. [Google Scholar] [CrossRef] [PubMed]
- Kirking, B.; Toungate, J.; Slusarewicz, P.; Zhu, K.; Hedman, T. The effects of different crosslinking agents on the tensile properties of the annulus fibrosus. Orthop. Trans. 2009, 34, 1676. [Google Scholar]
- Kirking, B.; Toungate, J.; Slusarewicz, P.; Zhu, K.; Hedman, T. The effect of chemical crosslinking agent injection on intervertebral disc bulge. Orthop. Trans. 2010, 35, 1560. [Google Scholar]
- Kirking, B.; Hedman, T.; Criscione, J. Changes in the interfacial shear resistance of disc annulus fibrosus from genipin crosslinking. J. Biomech. 2014, 47, 293–296. [Google Scholar] [CrossRef] [PubMed]
- Fessel, G.; Wernli, J.; Li, Y.; Gerber, C.; Snedeker, J.G. Exogenous collagen cross-linking recovers tendon functional integrity in an experimental model of partial tear. J. Orthop. Res. 2012, 30, 973–981. [Google Scholar] [CrossRef]
- Hedman, T.P.; Saito, H.; Vo, C.; Chuong, S.Y. Exogenous cross-linking increases the stability of spinal motion segments. Spine 2006, 31, 480–485. [Google Scholar] [CrossRef]
- Yerramalli, C.S.; Chou, A.I.; Miller, G.J.; Nicoll, S.B.; Chin, K.R.; Elliott, D.M. The effect of nucleus pulposus crosslinking and glycosaminoglycan degradation on disc mechanical function. Biomech. Model. Mechanobiol. 2007, 6, 13–20. [Google Scholar] [CrossRef]
- Barbir, A.; Michalek, A.J.; Abbott, R.D.; Iatridis, J.C. Effects of enzymatic digestion on compressive properties of rat intervertebral discs. J. Biomech. 2010, 43, 1067–1073. [Google Scholar] [CrossRef]
- Kirking, B.C.; Toungate, J.K.; Hedman, T.P. The dose response relationship between intervertebral disc flexion-extension neutral zone metrics and injected genipin concentration. J. Appl. Biomater. Funct. Mater. 2013, 11, e73–e79. [Google Scholar] [CrossRef]
- Chuang, S.Y.; Lin, L.C.; Hedman, T.P. The influence of exogenous cross-linking and compressive creep loading on intradiscal pressure. Biomech. Model. Mechanobiol. 2010, 9, 533–538. [Google Scholar] [CrossRef] [PubMed]
- Hedman, T.P.; Chen, W.P.; Lin, L.C.; Lin, H.J.; Chuang, S.Y. Effects of Collagen Crosslink Augmentation on Mechanism of Compressive Load Sharing in Intervertebral Discs. J. Med. Biol. Eng. 2017, 37, 94–101. [Google Scholar] [CrossRef] [PubMed]
- Chuang, S.Y.; Popovich, J.M.; Lin, S.Y.; Hedman, T.P. The Effects of Exogenous Crosslinking on Hydration and Fluid Flow in the Intervertebral Disc Subjected to Compressive Creep Loading and Unloading. Spine 2010, 35, E1362–E1366. [Google Scholar] [CrossRef]
- Popovich, J.M.; Yau, D.; Chuang, S.Y.; Hedman, T.P. Exogenous Collagen Crosslinking of the Intervertebral Disc Restores Joint Stability following Lumbar Posterior Decompression Surgery. Spine 2011, 36, 939–944. [Google Scholar] [CrossRef]
- Chuang, S.Y.; Lin, L.C.; Tsai, Y.C.; Wang, J.L. Exogenous crosslinking recovers the functional integrity of intervertebral disc secondary to stab injury. J. Biomed. Mater. Res. A 2010, 92, 297–302. [Google Scholar] [CrossRef]
- Lin, H.J.; Lin, L.C.; Hedman, T.P.; Chen, W.P.; Chuang, S.Y. Exogenous Crosslinking Restores Intradiscal Pressure of Injured Porcine Intervertebral Discs: An In Vivo Examination Using Quantitative Discomanometry. Spine 2015, 40, 1572–1577. [Google Scholar] [CrossRef] [PubMed]
- Beall, D.P.; Amirdelfan, K.; Nunley, P.D.; Phillips, T.R.; Navarro, L.C.I.; Spath, A. Hydrogel Augmentation of the Lumbar Intervertebral Disc: An Early Feasibility Study of a Treatment for Discogenic Low Back Pain. J. Vasc. Interv. Radiol. 2024, 35, 51–58. [Google Scholar] [CrossRef]
- Michalek, A.J.; Buckley, M.R.; Bonassar, L.J.; Cohen, I.; Iatridis, J.C. The effects of needle puncture injury on microscale shear strain in the intervertebral disc annulus fibrosus. Spine J. 2010, 10, 1098–1105. [Google Scholar] [CrossRef]
- Carragee, E.J.; Don, A.S.; Hurwitz, E.L.; Cuellar, J.M.; Carrino, J.; Herzog, R. Does Discography Cause Accelerated Progression of Degeneration Changes in the Lumbar Disc: A Ten-Year Cohort Study. Spine 2009, 34, 2338–2345. [Google Scholar] [CrossRef] [PubMed]
- Manchikanti, L.; Pampati, V.; Benyamin, R.M.; Boswell, M.V. Analysis of efficacy differences between caudal and lumbar interlaminar epidural injections in chronic lumbar axial discogenic pain: Local anesthetic alone vs. local combined with steroids. Int. J. Med. Sci. 2015, 12, 214–222. [Google Scholar] [CrossRef] [PubMed]
- Noriega, D.C.; Ardura, F.; Hernandez-Ramajo, R.; Martin-Ferrero, M.A.; Sanches-Lite, I.; Toribio, B.; Alberca, M.; Garcia, V.; Moraleda, J.M.; Sanches, A.; et al. Intervertebral Disc Repair by Allogeneic Mesenchymal Bone Marrow Cells: A Randomized Controlled Trial. Transplantation 2017, 101, 1945–1951. [Google Scholar] [CrossRef] [PubMed]
- Fischgrund, J.S.; Rhyne, A.; Franke, J.; Sasso, R.; Kitchel, S.; Bae, H.; Yeung, C.; Truumees, E.; Shaufele, M.; Yuan, P.; et al. Intraosseous basivertebral nerve ablation for the treatment of chronic low back pain: A prospective randomized double-blind sham-controlled multi-center study. Eur. Spine J. 2018, 27, 1146–1156. [Google Scholar] [CrossRef] [PubMed]
- Siepe, C.J.; Heider, F.; Haas, E.; Hitzl, W.; Szeimies, U.; Stabler, A.; Weiler, C.; Nerlich, A.G.; Mayer, M.H. Influence of lumbar intervertebral disc degeneration on the outcome of total lumbar disc replacement: A prospective clinical, histological, X-ray and MRI investigation. Eur. Spine J. 2012, 21, 2287–2299. [Google Scholar] [CrossRef] [PubMed]
- Proietti, L.; Scaramuzzo, L.; Schiro, G.R.; Sessa, S.; Logroscino, C.A. Complications in lumbar spine surgery: A retrospective analysis. Indian J. Orthop. 2013, 47, 340–345. [Google Scholar] [CrossRef] [PubMed]
- Greenwood, J.; McGregor, A.; Jones, F.; Mullane, J.; Hurley, M. Rehabilitation Following Lumbar Fusion Surgery: A Systematic Review and Meta-Analysis. Spine 2016, 41, E28–E36. [Google Scholar] [CrossRef] [PubMed]
- Sanford, Z.; Broda, A.; Taylor, H.; Turcotte, J.; Patton, C.M. Predictive Risk Factors Associated with Increased Opioid Use among Patients Undergoing Elective Spine Surgery. Int. J. Spine Surg. 2020, 14, 189–194. [Google Scholar] [CrossRef]
- Karhade, A.V.; Cha, T.D.; Fogel, H.A.; Hershman, S.H.; Tobert, D.G.; Schoenfeld, A.J.; Bono, C.M.; Schwab, J.H. Predicting prolonged opioid prescriptions in opioid-naive lumbar spine surgery patients. Spine J. 2020, 20, 888–895. [Google Scholar] [CrossRef]
- Sharma, M.; Ugiliweneza, B.; Aljuboori, Z.; Boakkye, M. Health care utilization and overall costs based on opioid dependence in patients undergoing surgery for degenerative spondylolisthesis. Neurosurg. Focus 2018, 44, E14. [Google Scholar] [CrossRef]
- Chou, R. Pharmacological management of low back pain. Drugs 2010, 70, 387–402. [Google Scholar] [CrossRef] [PubMed]
- Lorio, M.P.; Beall, D.P.; Calodney, A.K.; Lewandrowski, K.-U.; Block, J.E.; Mekhail, N. Defining the Patient with Lumbar Discogenic Pain: Real-World Implications for Diagnosis and Effective Clinical Management. J. Pers. Med. 2023, 13, 821. [Google Scholar] [CrossRef] [PubMed]
- Lorio, M.P.; Tate, J.L.; Myers, T.J.; Block, J.E.; Beall, D.P. Perspective on Intradiscal Therapies for Lumbar Discogenic Pain: State of the Science, Knowledge Gaps, and Imperatives for Clinical Adoption. J. Pain Res. 2024, 17, 1171–1182. [Google Scholar] [CrossRef] [PubMed]
- Gornet, M.G.; Peacock, J.; Ryken, T.; Schranck, F.W.; Eastlack, R.K.; Lotz, J.C. Establishing a Gold Standard for Noninvasive Identification of Painful Lumbar Discs: Prospective Comparison of Magnetic Resonance Spectroscopy vs Low-Pressure Provocation Discography. Int. J. Spine Surg. 2024, 18, 91–100. [Google Scholar] [CrossRef] [PubMed]
- Braunstein, J.; Hipp, J.A.; Browning, R.; Grieco, T.F.; Reitman, C.A. Analysis of translation and angular motion in loaded and unloaded positions in the lumbar spine. N. Am. Spine Soc. J. 2020, 4, 100038. [Google Scholar] [CrossRef] [PubMed]
- Chatprem, T.; Puntumetakul, R.; Kanpittaya, J.; Selfe, J.; Yeowell, G. A diagnostic tool for people with lumbar instability: A criterion-related validity study. BMC Musculoskelet. Disord. 2021, 22, 976. [Google Scholar] [CrossRef] [PubMed]
- Kasai, Y.; Morishita, K.; Kawakita, E.; Kondo, T.; Uchida, A. A New Evaluation Method for Lumbar Spinal Instability: Passive Lumbar Extension Test. Phys. Ther. 2006, 86, 1661–1667. [Google Scholar] [CrossRef]
- Sayed, D.; Grider, J.; Strand, N.; Hagedorn, J.M.; Falowski, S.; Lam, C.M.; Francio, V.T.; Beall, D.P.; Tomycz, N.D.; Davanzo, J.R.; et al. The American Society of Pain and Neuroscience (ASPN) Evidence-Based Clinical Guideline of Interventional Treatments for Low Back Pain. J. Pain Res. 2022, 15, 3729–3832. [Google Scholar]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hedman, T.; Rogers, A.; Beall, D. A Self-Polymerizing Mesh of Nano-Tethers for the Mechanical Constraint of Degraded Intervertebral Discs—A Review of 25 Years of Pre-Clinical and Early Clinical Research. Bioengineering 2024, 11, 535. https://doi.org/10.3390/bioengineering11060535
Hedman T, Rogers A, Beall D. A Self-Polymerizing Mesh of Nano-Tethers for the Mechanical Constraint of Degraded Intervertebral Discs—A Review of 25 Years of Pre-Clinical and Early Clinical Research. Bioengineering. 2024; 11(6):535. https://doi.org/10.3390/bioengineering11060535
Chicago/Turabian StyleHedman, Thomas, Adam Rogers, and Douglas Beall. 2024. "A Self-Polymerizing Mesh of Nano-Tethers for the Mechanical Constraint of Degraded Intervertebral Discs—A Review of 25 Years of Pre-Clinical and Early Clinical Research" Bioengineering 11, no. 6: 535. https://doi.org/10.3390/bioengineering11060535







