Green Innovation and Synthesis of Honeybee Products-Mediated Nanoparticles: Potential Approaches and Wide Applications
Abstract
:1. Introduction
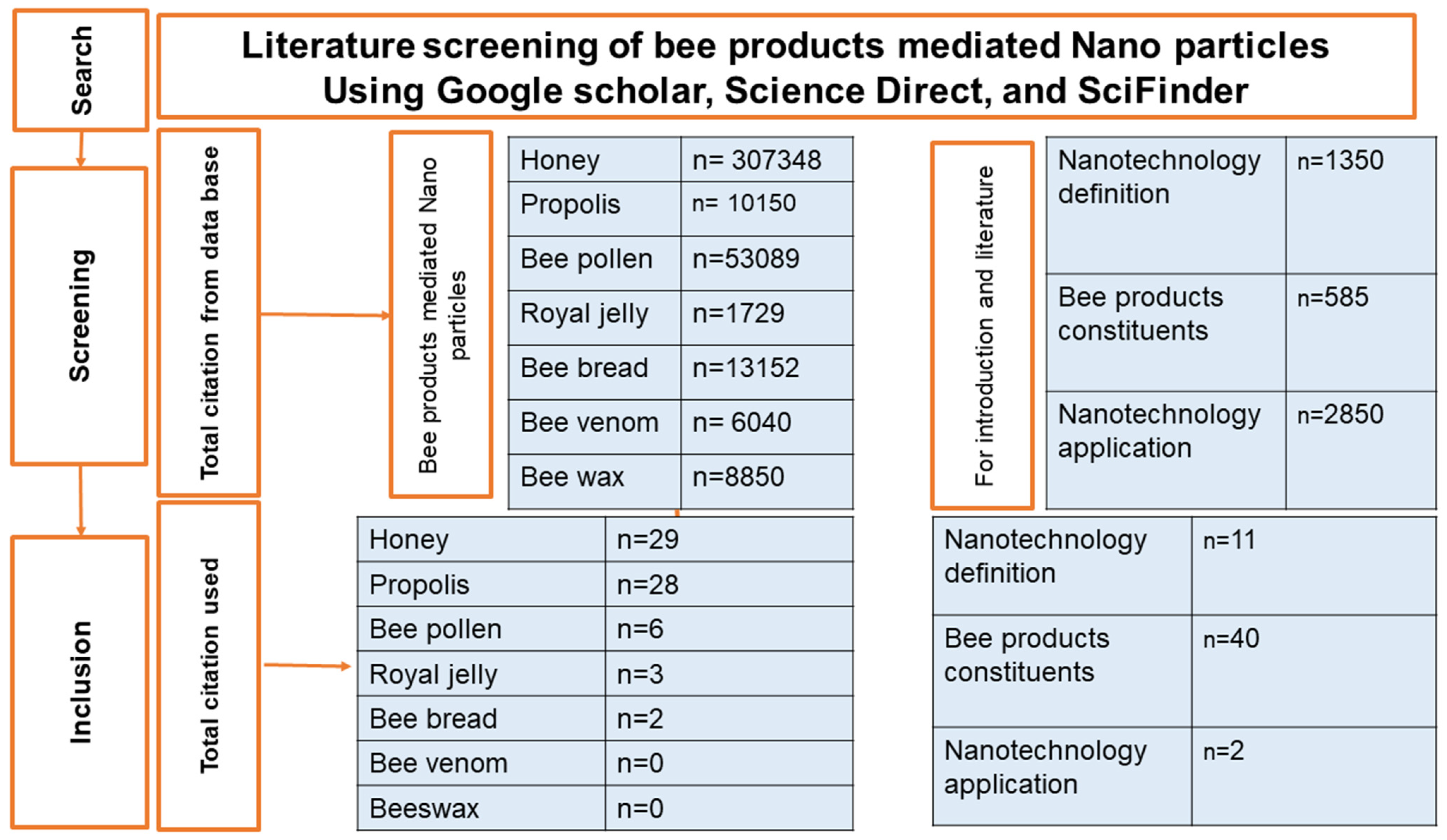
2. Method of Search
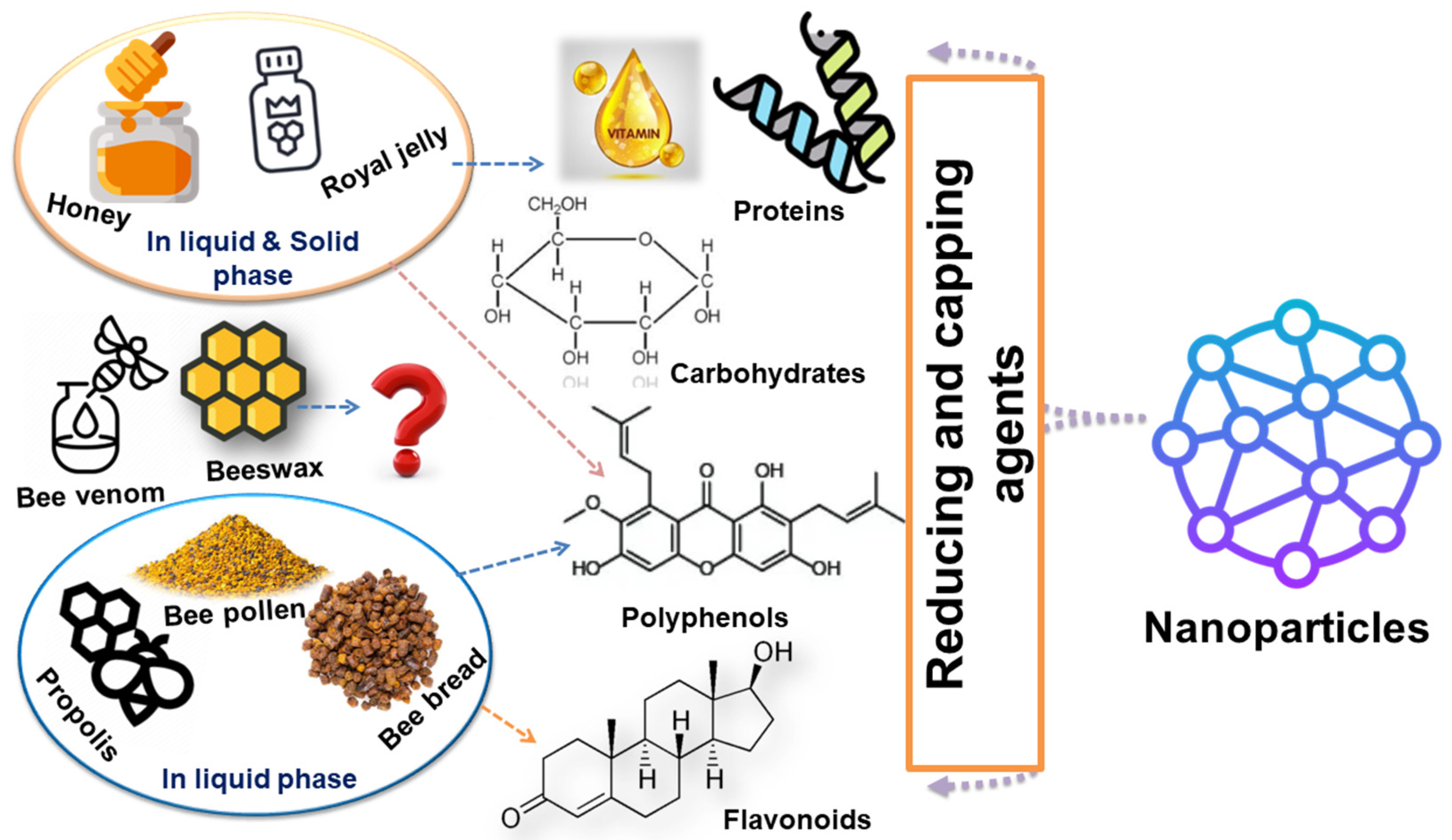
3. Bee Products and Their Metabolites
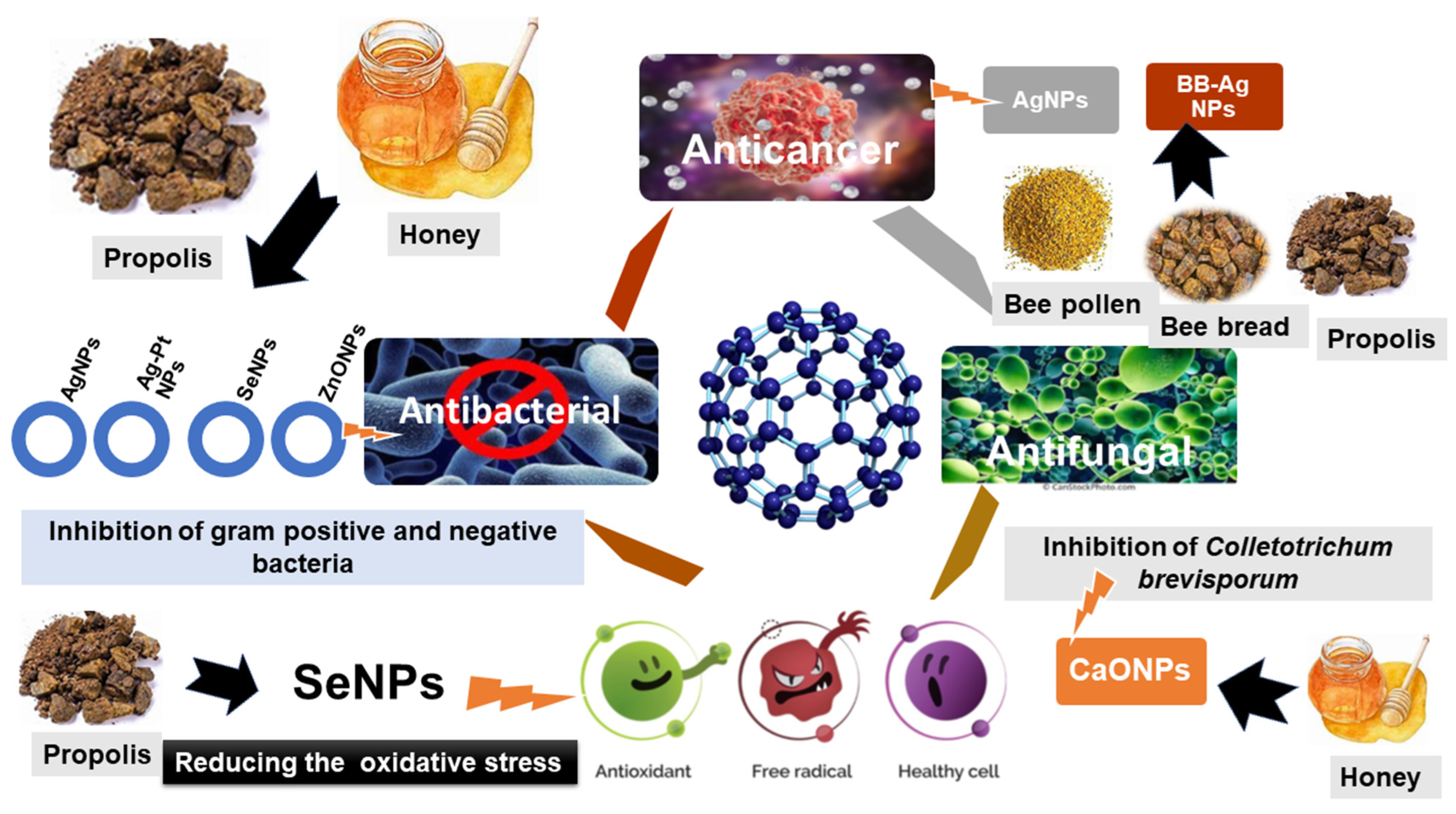
3.1. Honey
3.2. Propolis
3.3. Bee Pollen
3.4. Bee Bread
3.5. Royal Jelly
3.6. Beeswax
3.7. Bee Venom
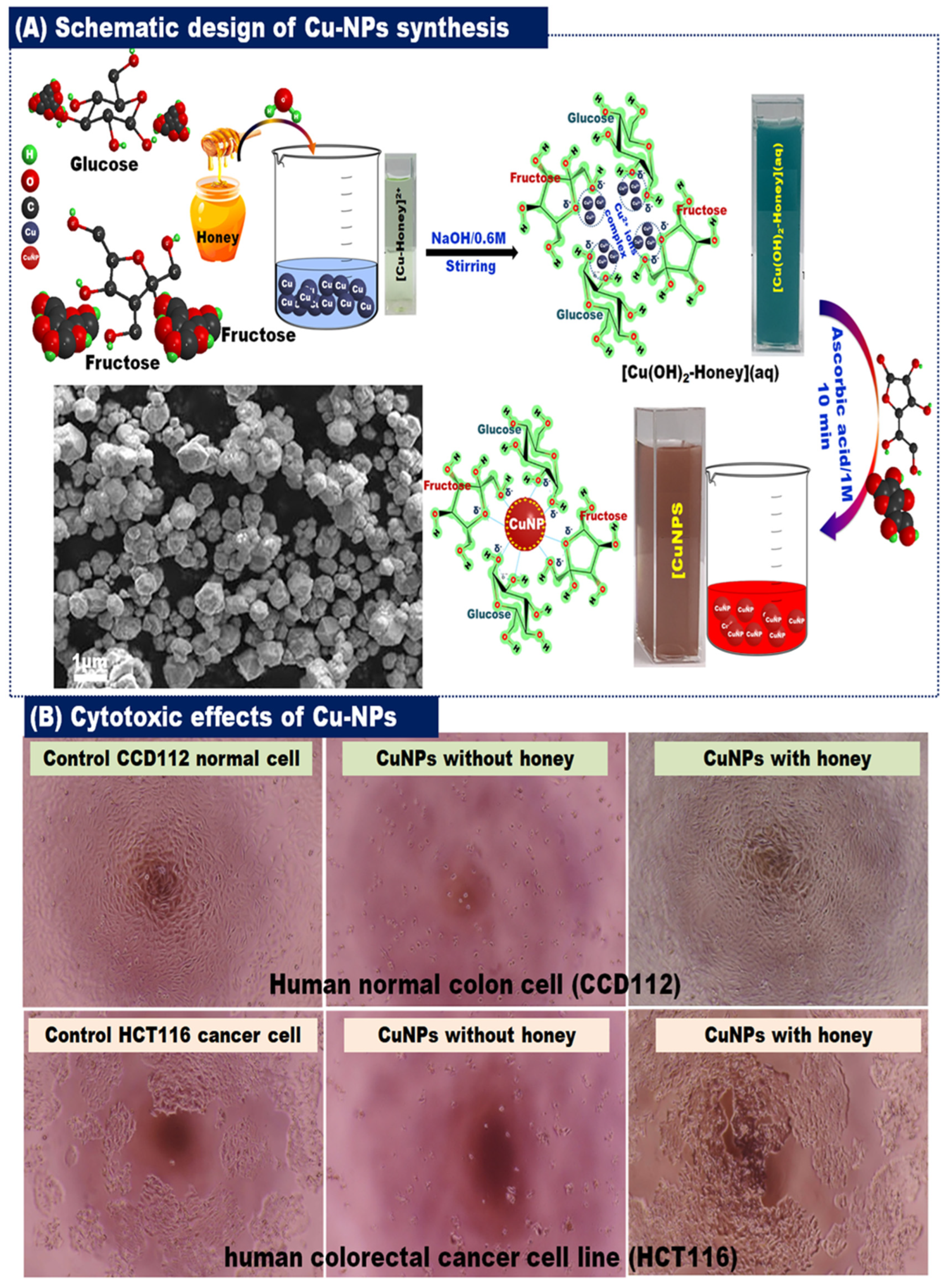
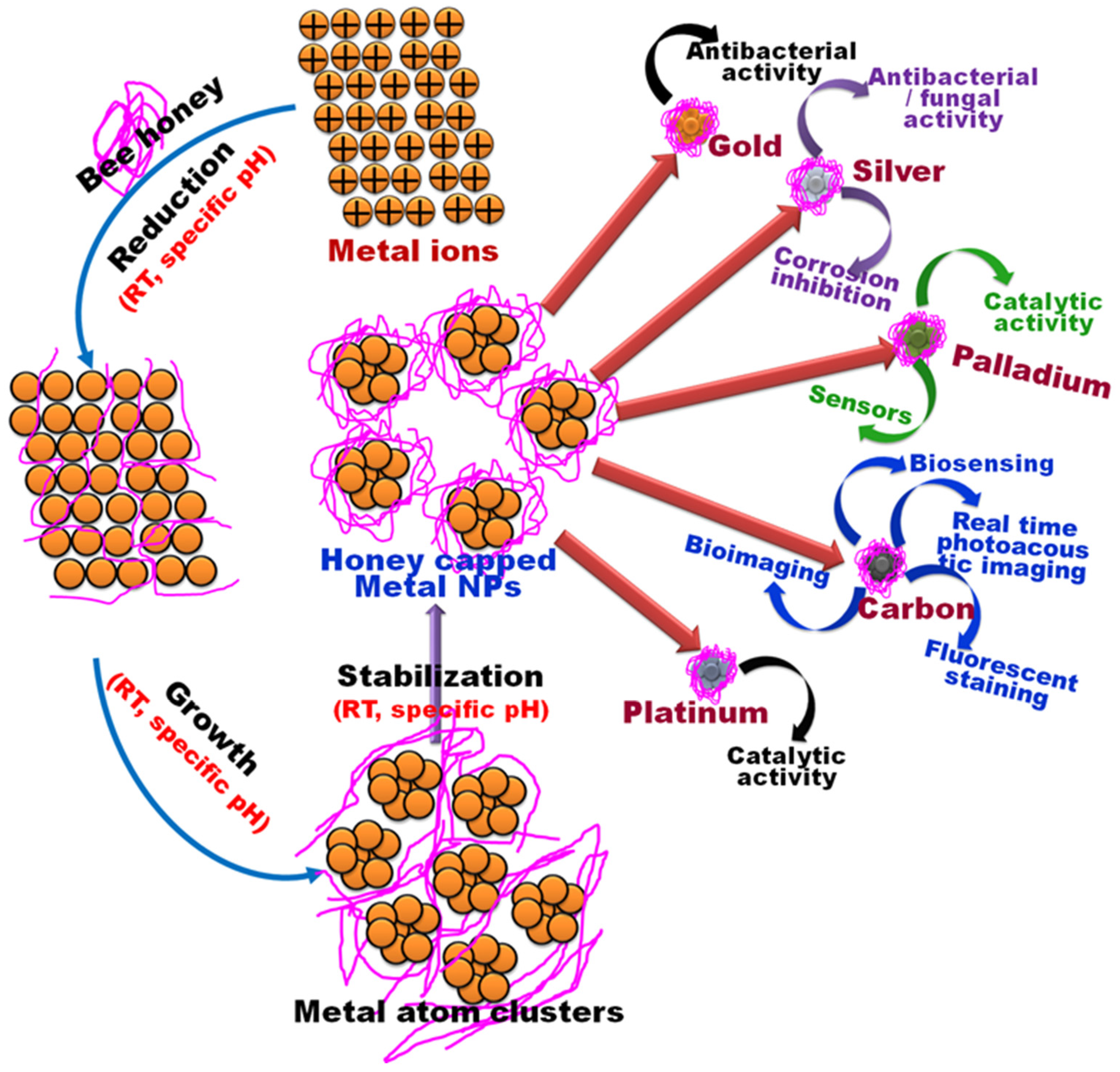
4. Green Synthesis of NPs Utilizing Bee Products
4.1. Sliver Nanoparticles (AgNPs)
4.1.1. Utilizing Honey for AgNP Synthesis
4.1.2. Utilizing Propolis for AgNPs’ Synthesis
4.1.3. Utilizing BP for AgNPs’ Synthesis
4.1.4. Utilizing Bee Bread for AgNPs’ Synthesis
4.1.5. Utilizing Royal Jelly for AgNPs Synthesis
4.2. Gold Nanoparticles (AuNPs)
4.2.1. Utilizing Honey for Gold Nanoparticles’ (AuNPs) Synthesis
4.2.2. Utilizing Propolis for Gold Nanoparticles’ (AuNPs) Synthesis
| Nanoparticles | Bee Products/Reducing and Capping Agents | Morphology | NPs Size (nm) | Biological Activities and Application | References |
|---|---|---|---|---|---|
| Gold nanoparticles (AuNPs) | Propolis/Carboxyl groups in the propolis component | Spherical | 7.8 | Anticancer activity | [99] |
| (Au@AgNPs) | Propolis/Polyphenolic molecules | Spherical | 108 | Antibacterial and anticancer activity | [44] |
| AuNPs | Propolis/Polyphenols, proteins, vitamins, and sugars | Spherical, hexagonal, and triangular | 20–60 | Catalytic and anti-tumor activity | [42] |
| AuNPs@ZnO | Propolis | Spherical | 22–73 | Antibacterial activity and cytotoxicity properties against human breast cancer cell line (MCF-7) and liver carcinoma cell line (HepG2). | [48] |
| Palladium nanoparticles (PdNPs) | Propolis/Flavonoid | Crystalline phase and face-centered cubic structure | 3.14–4.62 | Anticancer and antimicrobial | [41] |
| Selenium nanoparticles (SeNPs) | Propolis/Phenolic content | Spherical | 279 | Antibacterial activity | [49] |
| SeNPs | Propolis/Flavones, steroids, phosphoric acid, charcones, acetic acid, butanol, butyl ester, butanoic acid, hydroxyl and keto waxes, ketones, terpenoids, and sugars | Spherical | 50–60 | Antioxidant activity | [102] |
| SeNPs | Propolis/Alcohol and polyphenols | Crystalline, oval, and with smooth surface | 52.9–118 | Antioxidant and antimicrobial potential | [103] |
| Copper nanoparticles (CuNPs) | Propolis/Proteins, sugars, and polyphenols | Spherical | 15–23 | N.D. | [43] |
| CuO NPs | Propolis | Crystallite | 75–145 and 120–155 | Antimicrobial, and cytotoxic effects | [47] |
| Iron oxide nanoparticles (IONPs) | Propolis | Spherical | Around 87 and 194 | Antibacterial activity and degradation of dyes | [45] |
| Titanium oxide nanoparticles (TiO2NPs) | flavonoids and phenolic compounds | Quasi-spherical | 21 | Antimicrobial activity | [46] |
| calcium oxide nanoparticles (CaONPs) | Honey/Hydroxyl and carboxylic groups, amines, and amides | Spherical | >100 | Antifungal activity and cytotoxicity properties | [104] |
| Decahedral cinnamon nanoparticles | Honey/Vitamins, proteins, monosaccharide, and fructose | Crystalline | 17.08 ± 4.71 | Antibacterial activity | [105] |
| Cerium oxide anoparticles (CeO2NPs) | Honey/Proteins and carbohydrates | Spherical | 1.23 | Antioxidant and photocatalytic dye degradation | [106] |
| CeO2NPs | Honey/Proteins and carbohydrates | Spherical | 2.61 | Antioxidant and photocatalytic dye degradation | [106] |
| CeO2NPs | Honey/Proteins and carbohydrates | Spherical | 3.02 | Antioxidant and photocatalytic dye degradation | [106] |
| Graphene basedNiO2/Cu2O nanocomposite (Gr@NiO2/Cu2O NCs) | Honey/Reducing sugar | Crystalline | 15 | Catalyst in synthesizing the functionalized Schiff-base derivatives | [107] |
| Chromium oxide nanoparticles (Cr2O3NPs) | Honey/Proteins, and carbohydrates | Crystalline | 20 | Antioxidant and anti-inflammatory activity | [108] |
| Cr2O3NPs | Honey/Carbohydrates | Crystalline | 24.7205 | Antioxidant and antibacterial activity | [109] |
| CoFe2O4, Ag dopedNPs | Honey | Crystallite | 24–41 | Antibacterial activity | [110] |
| Platinum nanoparticles (PtNPs) | Honey/Protein | Crystalline | 5–15 | Catalytic application on preparation of organic dye | [8] |
| Zinc oxide nanoparticles (ZnONPs) | Honey/Fructose, glucose, sucrose, proteins, minerals, and vitamins. | Crystalline | 39 | Catalytic, antibacterial, and antifungal activities | [111] |
| ZnONPs | Propolis | Spherical | 17–70 | Antibacterial activity, cytotoxicity properties against cell lines (MCF-7 and HepG2). | [48] |
| NiFe2O4 spinel ferriteNPs | Honey/Glucose and fructose | Octahedral | 10–70 | Magnetic, dielectric, and electrical properties | [112] |
| Carbon Nanoparticles (CNPs) | Honey/Monosaccharides and the higher sugars | Spherical | Below 7 | - | [113] |
| AgNPs@ZnO | Propolis | Spherical | 45–75 | Antibacterial activity, cytotoxicity properties against cell lines (MCF-7 and HepG2). | [48] |
| BP@MgNPs | Bee pollen | Spherical | 36–40 | Antioxidant and antibacterial activity | [53] |
4.2.3. Bee Pollen for Gold Nanoparticles (AuNPs) Synthesis
4.3. Bee Pollen for Magnesium Nanoparticle (MgNPs) Synthesis
4.4. Bee Bread for Iron Nanoparticles (FeNPs) Synthesis
5. Biological Activities of Bee Products-Mediated Nanoparticles
5.1. Anticancer
5.2. Antibacterial
6. Application of Bee Products-Mediated Nanoparticles
6.1. Catalysis Application
6.2. Food Industries
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Gour, A.; Jain, N.K. Advances in green synthesis of nanoparticles. Artif. Cells Nanomed. Biotechnol. 2019, 47, 844–851. [Google Scholar] [CrossRef] [PubMed]
- Khan, I.; Saeed, K.; Khan, I. Nanoparticles: Properties, applications and toxicities. Arab. J. Chem. 2019, 12, 908–931. [Google Scholar] [CrossRef]
- Yokoyama, T.; Masuda, H.; Suzuki, M.; Ehara, K.; Nogi, K.; Fuji, M.; Fukui, T.; Suzuki, H.; Tatami, J.; Toda, K.; et al. Basic properties and measuring methods of nanoparticles. In Nanoparticle Technology Handbook; Elsevier: Amsterdam, The Netherlands, 2008; pp. 3–48. [Google Scholar]
- Yadav, R.K.; Jangeer, S.; Parashar, R.; Sharma, G.; Meena, P.; Meena, M.K.; Patel, D.D. A critical review on nanoparticles synthesis: Physical, chemical and biological perspectives. IJCRT 2023, 11, c838–c848. [Google Scholar]
- Hussain, I.; Singh, N.B.; Singh, A.; Singh, H.; Singh, S.C. Green synthesis of nanoparticles and its potential application. Biotechnol. Lett. 2015, 38, 545–560. [Google Scholar] [CrossRef] [PubMed]
- Thomas, B.; Arumugam, R.; Veerasamy, A.; Ramamoorthy, S. Ethnomedicinal plants used for the treatment of cuts and wounds by kuruma tribes, wayanadu districts of Kerala, India. Asian Pac. J. Trop. Biomed. 2014, 4 (Suppl. S1), S488–S491. [Google Scholar] [CrossRef]
- Velusamy, P.; Kumar, G.V.; Jeyanthi, V.; Das, J.; Pachaiappan, R. Bio-inspired green nanoparticles: Synthesis, mechanism, and antibacterial application. Toxicol. Res. 2016, 32, 95–102. [Google Scholar] [CrossRef]
- Venu, R.; Ramulu, T.S.; Anandakumar, S.; Rani, V.S.; Kim, C.G. Bio-directed synthesis of platinum nanoparticles using aqueous honey solutions and their catalytic applications. Colloids Surf. A Physicochem. Eng. Asp. 2011, 384, 733–738. [Google Scholar] [CrossRef]
- Maksimović, M.; Omanovic-Miklicanin, E. Towards green nanotechnology: Maximizing benefits and minimizing harm; Springer: Singapore, 2017; pp. 164–170. [Google Scholar] [CrossRef]
- Parveen, K.; Banse, V.; Ledwani, L. Green synthesis of nanoparticles: Their advantages and disadvantages. Proc. AIP Conf. Proc. 2016, 1724, 020048. [Google Scholar]
- Sharma, V.K.; Yngard, R.A.; Lin, Y. Silver nanoparticles: Green synthesis and ttheir antimicrobial activities. Adv. Colloid. Interface Sci. 2009, 145, 83–96. [Google Scholar] [CrossRef]
- Kocot, J.; Kielczykowska, M.; Luchowska-Kocot, D.; Kurzepa, J.; Musik, I. Antioxidant potential of propolis, bee pollen, and royal jelly: Possible medical application. Oxid. Med. Cell Longev. 2018, 2018, 7074209. [Google Scholar] [CrossRef]
- El-Seedi, H.R.; Eid, N.; Abd El-Wahed, A.A.; Rateb, M.E.; Afifi, H.S.; Algethami, A.F.; Zhao, C.; Al Naggar, Y.; Alsharif, S.M.; Tahir, H.E.; et al. Honey bee products: Preclinical and clinical studies of their anti-inflammatory and immunomodulatory properties. Front. Nutr. 2022, 8, 761267. [Google Scholar] [CrossRef] [PubMed]
- Radwan, A.; Mohamed, S.O.; Khalil, M.M.; El-Sewify, I.M. Effective adsorption of fluorescent congo red azo dye from aqueous solution by green synthesized nanosphere zno/cuo composite using propolis as bee byproduct extract. Sci. Repor. 2024, 14, 9061. [Google Scholar] [CrossRef] [PubMed]
- Hanafy, N.A.; Salim, E.I.; Mahfouz, M.E.; Eltonouby, E.A.; Hamed, I.H. Fabrication and characterization of bee pollen extract nanoparticles: Their potential in combination therapy against human a549 lung cancer cells. Food Hydrocoll. Health 2023, 3, 100110. [Google Scholar] [CrossRef]
- Yosri, N.; Abd El-Wahed, A.A.; Ghonaim, R.; Khattab, O.M.; Sabry, A.; Ibrahim, M.A.; Moustafa, M.F.; Guo, Z.; Zou, X.; Algethami, A.F.; et al. Anti-viral and immunomodulatory properties of propolis: Chemical diversity, pharmacological properties, preclinical and clinical applications, and In Silico Potential against SARS-CoV-2. Foods 2021, 10, 1776. [Google Scholar] [CrossRef] [PubMed]
- Eid, N.; Yosri, N.; El-Seedi, H.R.; Awad, H.M.; Emam, H.E. Ag@Sidr honey nanocomposite: Chemical profiles, antioxidant and microbicide procurator. Biocatal. Agric. Biotechnol. 2023, 51, 102788. [Google Scholar] [CrossRef]
- Yosri, N.; Alsharif, S.M.; Xiao, J.; Musharraf, S.G.; Zhao, C.; Saeed, A.; Gao, R.; Said, N.S.; Di Minno, A.; Daglia, M.; et al. Arctium lappa (Burdock): Insights from ethnopharmacology potential, chemical constituents, clinical studies, pharmacological utility. Biomed. Pharmacother. 2023, 158, 114104. [Google Scholar] [CrossRef] [PubMed]
- Salama, S.; Shou, Q.; Abd El-Wahed, A.A.; Elias, N.; Xiao, J.; Swillam, A.; Umair, M.; Guo, Z.; Daglia, M.; Wang, K. Royal jelly: Beneficial properties and synergistic effects with chemotherapeutic drugs with particular emphasis in anticancer strategies. Nutrients 2022, 14, 4166. [Google Scholar] [CrossRef] [PubMed]
- Nikhat, S.; Fazil, M. History, phytochemistry, experimental pharmacology and clinical uses of honey: A comprehensive review with special reference to unani medicine. J. Ethnopharmacol. 2022, 282, 114614. [Google Scholar] [CrossRef]
- Alvarez-Suarez, J.M.; Gasparrini, M.; Forbes-Hernandez, T.Y.; Mazzoni, L.; Giampieri, F. The composition and biological activity of honey: A focus on manuka honey. Foods 2014, 3, 420–432. [Google Scholar] [CrossRef]
- Yücel, Y.; Sultanoğlu, P. Characterization of honeys from hatay region by their physicochemical properties combined with chemometric. Food Biosci. 2013, 1, 16–25. [Google Scholar] [CrossRef]
- Pehlivan, T. Comparison study on honey in the islamic nutrition culture; the status of prophet muhammad’s (pbuh) and avicenna’s applications according to current scientific studies. J. Tour. Gastron. Stud. 2023, 11, 279–297. [Google Scholar] [CrossRef]
- Ranneh, Y.; Akim, A.M.; Hamid, H.A.; Khazaai, H.; Fadel, A.; Zakaria, Z.A.; Albujja, M.; Bakar, M.F.A. Hand its nutritional and anti-inflammatory value. BMC Complement. Med. Ther. 2021, 21, 30. [Google Scholar] [CrossRef] [PubMed]
- Dżugan, M.; Grabek-Lejko, D.; Swacha, S.; Tomczyk, M.; Bednarska, S.; Kapusta, I. Physicochemical quality parameters, antibacterial properties and cellular antioxidant activity of polish buckwheat honey. Food Biosci. 2020, 34, 100538. [Google Scholar] [CrossRef]
- Matar, G.; Akyuz, G.; Kaymazlar, E.; Andaç, M. An investigation of green synthesis of silver nanoparticles using Turkish honey against pathogenic bacterial. Biointerface Res. Appl. Chem. 2023, 13, 195–205. [Google Scholar]
- Al-Zaban, M.I.; Mahmoud, M.A.; AlHarbi, M.A. Catalytic degradation of methylene blue using silver nanoparticles synthesized by honey. Saudi J. Biol. Sci. 2021, 28, 2007–2013. [Google Scholar] [CrossRef]
- Khorrami, S.; Jafari Najafabadi, F.; Zarepour, A.; Zarrabi, A. Is Astragalus gossypinus honey a natural antibacterial and cytotoxic agent? an Investigation on a. Gossypinus honey biological activity and its green synthesized silver nanoparticles. BioNanoScience 2019, 9, 603–610. [Google Scholar] [CrossRef]
- Rasouli, E.; Basirun, W.J.; Rezayi, M.; Shameli, K.; Nourmohammadi, E.; Khandanlou, R.; Izadiyan, Z.; Khoshdel Sarkarizi, H. Ultrasmall superparamagnetic Fe3O4 Nanoparticles: Honey-based green and facile synthesis and in vitro viability assay. Int. J. Nanomed. 2018, 13, 6903–6911. [Google Scholar] [CrossRef]
- Bonsignore, G.; Patrone, M.; Martinotti, S.; Ranzato, E. “Green” biomaterials: The promising role of honey. J. Funct. Biomater. 2021, 12, 72. [Google Scholar] [CrossRef]
- Balasooriya, E.R.; Jayasinghe, C.D.; Jayawardena, U.A.; Ruwanthika, R.W.D.; Mendis de Silva, R.; Udagama, P.V. Honey mediated green synthesis of nanoparticles: New era of safe nanotechnology. J. Nanomater. 2017, 2017, 5919836. [Google Scholar] [CrossRef]
- Rufatto, L.C.; Luchtenberg, P.; Garcia, C.; Thomassigny, C.; Bouttier, S.; Henriques, J.A.P.; Roesch-Ely, M.; Dumas, F.; Moura, S. Brazilian red propolis: Chemical composition and antibacterial activity determined using bioguided fractionation. Microbiol. Res. 2018, 214, 74–82. [Google Scholar] [CrossRef]
- Boisard, S.; Shahali, Y.; Aumond, M.-C.; Derbré, S.; Blanchard, P.; Dadar, M.; Le Ray, A.-M.; Richomme, P. Anti-AGE activity of poplar-type propolis: Mechanism of action of main phenolic compounds. Int. J. Food Sci. Technol. 2020, 55, 453–460. [Google Scholar] [CrossRef]
- Katarzyna, P. Application of propolis in antimicrobial and antioxidative protection of food quality—A review. Trends Food Sci. Technol. 2019, 83, 53–62. [Google Scholar] [CrossRef]
- Ueda, T.; Inden, M.; Shirai, K.; Sekine, S.I.; Masaki, Y.; Kurita, H.; Ichihara, K.; Inuzuka, T.; Hozumi, I. The effects of brazilian green propolis that contains flavonols against mutant copper-zinc superoxide dismutase-mediated toxicity. Sci. Rep. 2017, 7, 2882. [Google Scholar] [CrossRef] [PubMed]
- Silva-Beltrán, N.P.; Galvéz-Ruíz, J.C.; Ikner, L.A.; Umsza-Guez, M.A.; de Paula Castro, T.L.; Gerba, C.P. In vitro antiviral effect of Mexican and Brazilian propolis and phenolic compounds against human coronavirus 229E. Int. J. Environ. Health Res. 2022, 33, 1591–1603. [Google Scholar] [CrossRef] [PubMed]
- Barsola, B.; Kumari, P. Green synthesis of nano-propolis and nanoparticles (Se and Ag) from ethanolic extract of propolis, their biochemical characterization: A review. Green Process. Synth. 2022, 11, 659–673. [Google Scholar] [CrossRef]
- Roy, N.; Mondal, S.; Laskar, R.A.; Basu, S.; Mandal, D.; Begum, N.A. Biogenic synthesis of Au and Ag nanoparticles by Indian propolis and its constituents. Colloids Surf. B Biointerfaces 2010, 76, 317–325. [Google Scholar] [CrossRef] [PubMed]
- Youssef, D.M.; Alshubaily, F.A.; Tayel, A.A.; Alghuthaymi, M.A.; Al-Saman, M.A. Application of nanocomposites from bees products and nano-selenium in edible coating for catfish fillets biopreservation. Polymers 2022, 14, 2378. [Google Scholar] [CrossRef]
- Jayanthi, B.; Kothai, S. Anti cancer activity of silver nano particles bio- synthesized using stingless bee propolis (Tetragonula iridipennis) of Tamilnadu. Asian J. Biomed. Pharm. Sci. 2015, 4, 30–37. [Google Scholar] [CrossRef]
- Al-Fakeh, M.S.; Osman, S.O.M.; Gassoumi, M.; Rabhi, M.; Omer, M. Characterization, antimicrobial and anticancer properties of palladium nanoparticles biosynthesized optimally using saudi propolis. Nanomaterials 2021, 11, 2666. [Google Scholar] [CrossRef]
- Gatea, F.; Teodor, E.D.; Seciu, A.-M.; Covaci, O.I.; Mănoiu, S.; Lazăr, V.; Radu, G.L. Antitumour, antimicrobial and catalytic activity of gold nanoparticles synthesized by different pH propolis extracts. J. Nanoparticle Res. 2015, 17, 320. [Google Scholar] [CrossRef]
- Hajizadeh, Y.S.; Harzandi, N.; Babapour, E.; Yazdanian, M.; Ranjbar, R. Green synthesize and characterization of copper nanoparticles using Iranian propolis extracts. Adv. Mater. Sci. Eng. 2022, 2022, 8100440. [Google Scholar] [CrossRef]
- Rezk, N.; Abdelsattar, A.S.; Makky, S.; Hussein, A.H.; Kamel, A.G.; El-Shibiny, A. New formula of the green synthesised Au@Ag core@shell nanoparticles using propolis extract presented high antibacterial and anticancer activity. AMB Express 2022, 12, 108. [Google Scholar] [CrossRef] [PubMed]
- Matar, G.H.; Andac, M. Green synthesis of iron oxide nanoparticles using brown Egyptian propolis extract for evaluation of their antibacterial activity and degradation of dyes. Inorg. Chem. Commun. 2023, 153, 110889. [Google Scholar] [CrossRef]
- Tahmasebi, E.; Mohammadi, M.; Yazdanian, M.; Alam, M.; Abbasi, K.; Hosseini, H.M.; Tavakolizadeh, M.; Khayatan, D.; Hassani, Z.; Tebyaniyan, H. Antimicrobial properties of green synthesized novel TiO2 nanoparticles using Iranian propolis extracts. J. Basic Microbiol. 2023, 63, 1030–1048. [Google Scholar] [CrossRef] [PubMed]
- Seyyed Hajizadeh, Y.; Babapour, E.; Harzandi, N.; Yazdanian, M.; Ranjbar, R. The effect of cytotoxicity and antimicrobial of synthesized CuONPs from propolis on HEK-293 cells and Lactobacillus acidophilus. Evid. Based Complement. Altern. Med. 2023, 2023, 1430839. [Google Scholar] [CrossRef] [PubMed]
- Abdelsattar, A.S.; Kamel, A.G.; Hussein, A.H.; Azzam, M.; Makky, S.; Rezk, N.; Essam, K.; Agwa, M.M.; El-Shibiny, A. The promising antibacterial and anticancer activity of green synthesized zinc nanoparticles in combination with silver and gold nanoparticles. J. Inorg. Organomet. Polym. Mater. 2023, 33, 1868–1881. [Google Scholar] [CrossRef]
- Długosz, O.; Chmielowiec-Korzeniowska, A.; Drabik, A.; Tymczyna, L.; Banach, M. Bioactive selenium nanoparticles synthesized from propolis extract and quercetin based on natural deep eutectic solvents (NDES). J. Clust. Sci. 2023, 34, 1401–1412. [Google Scholar] [CrossRef]
- Pernal, S.F.; Currie, R.W. The influence of pollen quality on foraging behavior in honeybees (Apis mellifera L.). Behav. Ecol. Sociobiol. 2001, 51, 53–68. [Google Scholar] [CrossRef]
- Khalifa, S.A.M.; Elashal, M.H.; Yosri, N.; Du, M.; Musharraf, S.G.; Nahar, L.; Sarker, S.D.; Guo, Z.; Cao, W.; Zou, X. Bee pollen: Current status and therapeutic potential. Nutrients 2021, 13, 1876. [Google Scholar] [CrossRef]
- Komosinska-Vassev, K.; Olczyk, P.; Kazmierczak, J.; Mencner, L.; Olczyk, K. Bee pollen: Chemical composition and therapeutic application. Evid. Based Complement. Altern. Med. 2015, 2015, 297425. [Google Scholar] [CrossRef]
- Bıldır, B.; Nur Çobanoğlu, D.; Kaya, B. Antioxidant, Antimicrobial, and Neurotoxicity (in Sh-Sy5y Cell Lines) properties of Mg nanoparticle system (BP@ MgNPs) prepared by green synthesis with bee pollen. Chem. Biodivers. 2023, 20, e202201093. [Google Scholar] [CrossRef] [PubMed]
- Kumar, B.; Smita, K.; Angulo, Y.; Debut, A.; Cumbal, L. Honeybee pollen assisted biosynthesis of nanogold and its application as catalyst in reduction of 4-nitrophenol. Heliyon 2022, 8, e10191. [Google Scholar] [CrossRef] [PubMed]
- Turunc, E.; Kahraman, O.; Binzet, R. Green synthesis of silver nanoparticles using pollen extract: Characterization, assessment of their electrochemical and antioxidant activities. Anal. Biochem. 2021, 621, 114123. [Google Scholar] [CrossRef] [PubMed]
- Khalifa, S.A.; Elashal, M.; Kieliszek, M.; Ghazala, N.E.; Farag, M.A.; Saeed, A.; Xiao, J.; Zou, X.; Khatib, A.; Göransson, U.; et al. Recent insights into chemical and pharmacological studies of bee bread. Trends Food Sci. Technol. 2020, 97, 300–316. [Google Scholar] [CrossRef]
- Bayram, N.E.; Gercek, Y.C.; Çelik, S.; Mayda, N.; Kostić, A.Ž.; Dramićanin, A.M.; Özkök, A. Phenolic and free amino acid profiles of bee bread and bee pollen with the same botanical origin—Similarities and differences. Arab. J. Chem. 2021, 14, 103004. [Google Scholar] [CrossRef]
- Ahmad, S.; Campos, M.G.; Fratini, F.; Altaye, S.Z.; Li, J. New insights into the biological and pharmaceutical properties of royal jelly. Int. J. Mol. Sci. 2020, 21, 382. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Wang, Z.; Chen, Y.; Cao, J.; Tian, W.; Ma, B.; Dong, Y. Active components and biological functions of royal jelly. J. Funct. Foods 2021, 82, 104514. [Google Scholar] [CrossRef]
- Mendoza-Reséndez, R.; Gómez-Treviño, A.; Barriga-Castro, E.D.; Núñez, N.O.; Luna, C. Synthesis of antibacterial silver-based nanodisks and dendritic structures mediated by royal jelly. RSC Adv. 2014, 4, 1650–1658. [Google Scholar] [CrossRef]
- Gevorgyan, S.; Schubert, R.; Yeranosyan, M.; Gabrielyan, L.; Trchounian, A.; Lorenzen, K.; Trchounian, K. Antibacterial activity of royal jelly-mediated green synthesized silver nanoparticles. AMB Express 2021, 11, 51. [Google Scholar] [CrossRef]
- Fratini, F.; Cilia, G.; Turchi, B.; Felicioli, A. Beeswax: A minireview of its antimicrobial activity and its application in medicine. Asian Pac. J. Trop. Med. 2016, 9, 839–843. [Google Scholar] [CrossRef]
- Maia, M.; Nunes, F.M. Authentication of beeswax (Apis mellifera) by high-temperature gas chromatography and chemometric analysis. Food Chem. 2013, 136, 961–968. [Google Scholar] [CrossRef] [PubMed]
- Shaltouki, P.; Mohamadi, E.; Moghaddasi, M.A.; Farahbakhsh, A.; Bahmanpour, H. Synthesis and characterization of nanoparticles propolis using beeswax. Iran. J. Chem. Chem. Eng. (IJCCE) 2019, 38, 9–19. [Google Scholar] [CrossRef]
- Tanugur-Samanc, A.E.; Kekecoglu, M. An Evaluation of the chemical content and microbiological contamination of anatolian bee venom. PLoS ONE 2021, 16, e0255161. [Google Scholar] [CrossRef]
- Abd El-Wahed, A.; Yosri, N.; Sakr, H.H.; Du, M.; Algethami, A.F.; Zhao, C.; Abdelazeem, A.H.; Tahir, H.E.; Masry, S.H.; Abdel-Daim, M.M.; et al. Wasp venom biochemical components and their potential in biological applications and nanotechnological interventions. Toxins 2021, 13, 206. [Google Scholar] [CrossRef] [PubMed]
- Alalawy, A.I.; El Rabey, H.A.; Almutairi, F.M.; Tayel, A.A.; Al-Duais, M.A.; Zidan, N.S.; Sakran, M.I. Effectual anticancer potentiality of loaded bee venom onto fungal chitosan nanoparticles. Int. J. Polym. Sci. 2020, 2020, 2785304. [Google Scholar] [CrossRef]
- Walaa, A.M.; Samia, E.; Fatma, A.T.; Aly, F.M.; Karima, M. Evaluation of anticancer potentials of bee free venom and chitosan nano-conjugated one: In vitro study. Int. J. Sci. Res. Manag. (IJSRM) 2017, 5, 5253–5262. [Google Scholar]
- Tarek A El-Desouky, H.A.M.A. Honey mediated silver nanoparticles and their inhibitory effect on aflatoxins and ochratoxin A. J. App. Pharm. Sci. 2016, 6, 83–90. [Google Scholar] [CrossRef]
- Sreelakshmi, C.; Datta, K.K.; Yadav, J.S.; Reddy, B.V. Honey derivatized Au and Ag nanoparticles and evaluation of its antimicrobial activity. J. Nanosci. Nanotechnol. 2011, 11, 6995–7000. [Google Scholar] [CrossRef] [PubMed]
- Javed, S.; Mangla, B.; Ahsan, W. From propolis to nanopropolis: An exemplary journey and a paradigm shift of a resinous substance produced by bees. Phytother. Res. 2022, 36, 2016–2041. [Google Scholar] [CrossRef] [PubMed]
- Al-Khedhairy, A.A.; Wahab, R. Silver nanoparticles: An instantaneous solution for anticancer activity against human liver (HepG2) and breast (MCF-7) cancer cells. Metals 2022, 12, 148. [Google Scholar] [CrossRef]
- Khan, S.; Zahoor, M.; Sher Khan, R.; Ikram, M.; Islam, N.U. The impact of silver nanoparticles on the growth of plants: The agriculture applications. Heliyon 2023, 9, e16928. [Google Scholar] [CrossRef]
- Moradi, F.; Sedaghat, S.; Moradi, O.; Arab Salmanabadi, S. Review on green nano-biosynthesis of silver nanoparticles and their biological activities: With an emphasis on medicinal plants. Inorg. Nano-Met. Chem. 2021, 51, 133–142. [Google Scholar] [CrossRef]
- González Fá, A.J.; Juan, A.; Di Nezio, M.S. Synthesis and characterization of silver nanoparticles prepared with honey: The role of carbohydrates. Anal. Lett. 2017, 50, 877–888. [Google Scholar] [CrossRef]
- Al-Brahim, J.S.; Mohammed, A.E. Antioxidant, cytotoxic and antibacterial potential of biosynthesized nanoparticles using bee honey from two different floral sources in Saudi Arabia. Saudi J. Biol. Sci. 2020, 27, 363–373. [Google Scholar] [CrossRef] [PubMed]
- Youssef, G.A.; El-Boraey, A.M.; Abdel-Tawab, M.M. Eco-Friendly green synthesis of silver nanoparticles from Egyptian honey: Evaluating its antibacterial activities. Egypt. J. Bot. 2019, 59, 709–721. [Google Scholar] [CrossRef]
- Ghramh, H.A.; Ibrahim, E.H.; Kilany, M. Study of anticancer, antimicrobial, immunomodulatory, and silver nanoparticles production by Sidr honey from three different sources. Food Sci. Nutr. 2020, 8, 445–455. [Google Scholar] [CrossRef] [PubMed]
- Ghramh, H.A.; Ibrahim, E.H.; Ahmad, Z. Antimicrobial, immunomodulatory and cytotoxic activities of green synthesized nanoparticles from Acacia honey and Calotropis procera. Saudi J. Biol. Sci. 2021, 28, 3367–3373. [Google Scholar] [CrossRef] [PubMed]
- Obot, I.B.; Umoren, S.A.; Johnson, A.S. Sunlight-mediated synthesis of silver nanoparticles using honey and its promising anticorrosion potentials for mild steel in acidic environments. J. Mater. Environ. Sci. 2013, 4, 1013–1018. [Google Scholar]
- Marsudi, M.A.; Sari, F.F.; Wicaksono, P.M.; Asmoro, A.; Basuki, A.; Wibowo, A. Optimization of green synthesis approach of silver nanoparticles using indonesian wild honey. Key Eng. Mater. 2021, 891, 111–115. [Google Scholar] [CrossRef]
- Mendez-Pfeiffer, P.; Ballesteros-Monrreal, M.G.; Gaona-Ochoa, J.; Juarez, J.; Gastelum-Cabrera, M.; Montano-Leyva, B.; Arenas-Hernandez, M.; Caporal-Hernandez, L.; Ortega-Garcia, J.; Barrios-Villa, E.; et al. Biosynthesis of silver nanoparticles using seasonal samples of sonoran desert propolis: Evaluation of its antibacterial activity against clinical isolates of multi-drug resistant bacteria. Pharmaceutics 2022, 14, 1853. [Google Scholar] [CrossRef]
- Tosun, N.G.; Kaplan, Ö. Biosynthesis of silver nanoparticles using white propolis extract as a reduction agent and optimized by box-behnken design. Kahramanmaraş Sütçü İmam Üniversitesi Tarım Ve Doğa Derg. 2022, 25, 933–945. [Google Scholar] [CrossRef]
- Al-saggaf, M.S. Formulation of insect chitosan stabilized silver nanoparticles with propolis extract as potent antimicrobial and wound healing composites. Int. J. Polym. Sci. 2021, 2021, 5578032. [Google Scholar] [CrossRef]
- Kischkel, B.; Castilho, P.F.D.; de Oliveira, K.M.; Rezende, P.S.; Bruschi, M.L.; Svidzinski, T.I.; Negri, M. Silver nanoparticles stabilized with propolis show reduced toxicity and potential activity against fungal infections. Future Microbiol. 2020, 15, 521–539. [Google Scholar] [CrossRef]
- Selvaraju, G.D.; Umapathy, V.R.; SumathiJones, C.; Cheema, M.S.; Jayamani, D.R.; Dharani, R.; Sneha, S.; Yamuna, M.; Gayathiri, E.; Yadav, S. Fabrication and characterization of surgical sutures with propolis silver nano particles and analysis of its antimicrobial properties. J. King Saud. Univ.-Sci. 2022, 34, 102082. [Google Scholar] [CrossRef]
- Taqi, Z.J.; Abdul-Wahed, H.E.; Al-Saadi, H.K.; Jabir, M.S. Potential activity of silver nanoparticles synthesized by Iraqi propolis on phagocytosis. AIP Conf. Proc. 2020, 2213, 020104. [Google Scholar] [CrossRef]
- Tiri, R.N.E.; Gulbagca, F.; Aygun, A.; Cherif, A.; Sen, F. Biosynthesis of Ag–Pt bimetallic nanoparticles using propolis extract: Antibacterial effects and catalytic activity on NaBH4 hydrolysis. Environ. Res. 2022, 206, 112622. [Google Scholar] [CrossRef] [PubMed]
- Lee, I.K.; Wang, C.C.; Lin, M.C.; Kung, C.T.; Lan, K.C.; Lee, C.T. Effective strategies to prevent coronavirus disease-2019 (COVID-19) outbreak in hospital. J. Hosp. Infect. 2020, 105, 102–103. [Google Scholar] [CrossRef] [PubMed]
- Attia, W.Y.; El-Naggar, R.E.; Bawadekji, A.; Al Ali, M. Evaluation of some in vitro anti-carcinogenic activities of polysaccharides extracted from Ascomata of the desert truffle Terfezia claveryi Chatin. J. Appl. Environ. Biol. Sci. 2018, 8, 152–159. [Google Scholar]
- Urcan, A.C.; Criste, A.D.; Szanto, K.I.; Stefan, R.; Zahan, M.; Musca, A.S.; Focsan, M.; Burtescu, R.F.; Olah, N.K. Antimicrobial and antiproliferative activity of green synthesized silver nanoparticles using bee bread extracts. Pharmaceutics 2023, 15, 1797. [Google Scholar] [CrossRef]
- Philip, D. Honey mediated green synthesis of silver nanoparticles. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2010, 75, 1078–1081. [Google Scholar] [CrossRef]
- Mohamed, S.O.; El-Naggar, K.; Khalil, M.M.H. Green synthesis of silver nanoparticles using Egyptian propolis extract and its antimicrobial activity. Egypt. J. Chem. 2022, 65, 453–464. [Google Scholar] [CrossRef]
- Keskin, M. Synthesis, characterization and antidiabetic potential of bee pollen based silver nanoparticles. El-Cezeri 2022, 9, 266–275. [Google Scholar] [CrossRef]
- Tavares, L.; Smaoui, S.; Lima, P.S.; de Oliveira, M.M.; Santos, L. Propolis: Encapsulation and application in the food and pharmaceutical industries. Trends Food Sci. Technol. 2022, 127, 169–180. [Google Scholar] [CrossRef]
- Kumar, B.; Smita, K.; Angulo, Y.; Debut, A.; Cumbal, L. Single-step biogenic synthesis of silver nanoparticles using honeybee-collected pollen. Inorg. Nano-Met. Chem. 2022, 6, 322. [Google Scholar] [CrossRef]
- Gevorgyan, S.; Schubert, R.; Falke, S.; Lorenzen, K.; Trchounian, K.; Betzel, C. Structural characterization and antibacterial activity of silver nanoparticles synthesized using a low-molecular-weight Royal Jelly extract. Sci. Rep. 2022, 12, 14077. [Google Scholar] [CrossRef]
- Al-darwesh, M.Y.; Ibrahim, S.S.; Faiad Naief, M.; Mishaal Mohammed, A.; Chebbi, H. Synthesis and characterizations of zinc oxide nanoparticles and its ability to detect O2 and NH3 gases. Results Chem. 2023, 6, 101064. [Google Scholar] [CrossRef]
- Aljohani, F.S.; Hamed, M.T.; Bakr, B.A.; Shahin, Y.H.; Abu-Serie, M.M.; Awaad, A.K.; El-Kady, H.; Elwakil, B.H. In vivo bio-distribution and acute toxicity evaluation of greenly synthesized ultra-small gold nanoparticles with different biological activities. Sci. Rep. 2022, 12, 6269. [Google Scholar] [CrossRef] [PubMed]
- Botteon, C.E.A.; Silva, L.B.; Ccana-Ccapatinta, G.V.; Silva, T.S.; Ambrosio, S.R.; Veneziani, R.C.S.; Bastos, J.K.; Marcato, P.D. Biosynthesis and characterization of gold nanoparticles using Brazilian red propolis and evaluation of its antimicrobial and anticancer activities. Sci. Rep. 2021, 11, 1974. [Google Scholar] [CrossRef] [PubMed]
- Haiza, H.; Azizan, A.; Mohidin, A.H.; Halin, D.S.C. Green synthesis of silver nanoparticles using local honey. Nano Hybrids 2013, 4, 87–98. [Google Scholar] [CrossRef]
- Hatami, R.; Javadi, A.; Jafarizadeh-Malmiri, H. Effectiveness of six different methods in green synthesis of selenium nanoparticles using propolis extract: Screening and characterization. Green Process. Synth. 2020, 9, 685–692. [Google Scholar] [CrossRef]
- Shubharani, R.; Mahesh, M.; Yogan, V.N.; Murthy, A. Biosynthesis and characterization, antioxidant and antimicrobial activities of selenium nanoparticles from ethanol extract of bee propolis. J. Nanomed. Nanotechnol. 2019, 10, 1–7. [Google Scholar]
- Maringgal, B.; Hashim, N.; Tawakkal, I.S.M.A.; Hamzah, M.H.; Mohamed, M.T.M. Biosynthesis of CaO nanoparticles using Trigona sp. Honey: Physicochemical characterization, antifungal activity, and cytotoxicity properties. J. Mater. Res. Technol. 2020, 9, 11756–11768. [Google Scholar] [CrossRef]
- Salim, A.A.; Bakhtiar, H.; Bidin, N.; Ghoshal, S.K. Antibacterial activity of decahedral cinnamon nanoparticles prepared in honey using PLAL technique. Mater. Lett. 2018, 232, 183–186. [Google Scholar] [CrossRef]
- Kalaycıoğlu, Z.; Geçim, B.; Erim, F.B. Green synthesis of cerium oxide nanoparticles from turmeric and kinds of honey: Characterisations, antioxidant and photocatalytic dye degradation activities. Adv. Nat. Sci. Nanosci. Nanotechnol. 2022, 13, 015016. [Google Scholar] [CrossRef]
- Ansari, M.Z.; Shoeb, M.; Nayab, P.S.; Mobin, M.; Khan, I.; Siddiqi, W.A. Honey mediated green synthesis of graphene based NiO2/Cu2O nanocomposite (Gr@ NiO2/Cu2O NCs): Catalyst for the synthesis of functionalized Schiff-base derivatives. J. Alloys Compd. 2018, 738, 56–71. [Google Scholar] [CrossRef]
- Shahid, H.; Arooj, I.; Zafar, S. Honey-mediated synthesis of Cr2O3 nanoparticles and their potent anti-bacterial, anti-oxidant and anti-inflammatory activities. Arab. J. Chem. 2023, 16, 104544. [Google Scholar] [CrossRef]
- Nivethitha, P.R.; Rachel, D.C.J. A study of antioxidant and antibacterial activity using honey mediated Chromium oxide nanoparticles and its characterization. Mater. Today Proc. 2022, 48, 276–281. [Google Scholar] [CrossRef]
- Satheeshkumar, M.K.; Kumar, E.R.; Srinivas, C.; Suriyanarayanan, N.; Deepty, M.; Prajapat, C.L.; Rao, T.V.C.; Sastry, D.L. Study of structural, morphological and magnetic properties of Ag substituted cobalt ferrite nanoparticles prepared by honey assisted combustion method and evaluation of their antibacterial activity. J. Magn. Magn. Mater. 2019, 469, 691–697. [Google Scholar] [CrossRef]
- Sharmila, M.; Jothi Mani, R.; Kader, A.; Ahmad, A.; Eldesoky, G.; Yahya, A.; Bahajjaj, A. Photocatalytic and biological activity of zno nanoparticles using honey. Coatings 2021, 11, 1046. [Google Scholar] [CrossRef]
- Yadav, R.S.; Kuřitka, I.; Vilcakova, J.; Havlica, J.; Masilko, J.; Kalina, L.; Tkacz, J.; Enev, V.; Hajdúchová, M. Structural, magnetic, dielectric, and electrical properties of NiFe2O4 spinel ferrite nanoparticles prepared by honey-mediated sol-gel combustion. J. Phys. Chem. Solids 2017, 107, 150–161. [Google Scholar] [CrossRef]
- Wu, L.; Cai, X.; Nelson, K.; Xing, W.; Xia, J.; Zhang, R.; Stacy, A.J.; Luderer, M.; Lanza, G.M.; Wang, L.V.; et al. A Green synthesis of carbon nanoparticle from honey for real-time photoacoustic imaging. Nano Res. 2013, 6, 312–325. [Google Scholar] [CrossRef] [PubMed]
- Ismail, N.A.; Shameli, K.; Wong, M.M.-T.; Teow, S.-Y.; Chew, J.; Sukri, S.N.A.M. Antibacterial and cytotoxic effect of honey mediated copper nanoparticles synthesized using ultrasonic assistance. Mater. Sci. Eng. C 2019, 104, 109899. [Google Scholar] [CrossRef] [PubMed]
- Bildir, B.; DemİRkan, Z.; Kaya, B. Biosynthesis, characterization and determination of sun protection factor (Spf) of iron nanoparticles with bee bread. Türk Doğa Ve Fen. Derg. 2022, 11, 110–117. [Google Scholar] [CrossRef]
- Neupane, B.P.; Chaudhary, D.; Paudel, S.; Timsina, S.; Chapagain, B.; Jamarkattel, N.; Tiwari, B.R. Himalayan honey loaded iron oxide nanoparticles: Synthesis, characterization and study of antioxidant and antimicrobial activities. Int. J. Nanomed. 2019, 14, 3533–3541. [Google Scholar] [CrossRef] [PubMed]
- Corciovă, A.M.C.; Burlec Af Cioancă, O.; Tuchiluş, C.; Fifere, A.; Lungoci Al Marangoci, N.; Hăncianu, M. Antioxidant, antimicrobial and photocatalytic activities of silver nanoparticles obtained by bee propolis extract assisted biosynthesis. Farmacia. 2019, 67, 482–489. [Google Scholar] [CrossRef]
- Siegel, R.L.; Miller, K.D.; Wagle, N.S.; Jemal, A. Cancer statistics, 2023. CA Cancer J. Clin. 2023, 73, 17–48. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Huang, W.; Huang, S.; Zhu Ge, Q.; Jin, K.; Zhao, Y. Magnetically active Fe3O4 nanorods loaded with tissue plasminogen activator for enhanced thrombolysis. Nano Res. 2016, 9, 2652–2661. [Google Scholar] [CrossRef]
- Al-Yousef, H.M.; Amina, M.; Alqahtani, A.S.; Alqahtani, M.S.; Malik, A.; Hatshan, M.R.; Siddiqui, M.R.H.; Khan, M.; Shaik, M.R.; Ola, M.S.; et al. Pollen bee aqueous extract-based synthesis of silver nanoparticles and evaluation of their anti-cancer and anti-bacterial activities. Processes 2020, 8, 524. [Google Scholar] [CrossRef]
- Azarshinfam, N.; Tanomand, A.; Soltanzadeh, H.; Arjomandirad, F. Evaluation of anticancer effects of propolis extract with or without combination with layered double hydroxide nanoparticles on Bcl-2 and Bax genes expression in HT-29 cell lines. Gene Rep. 2021, 23, 101031. [Google Scholar] [CrossRef]
- Geng, Z.; Cao, Z.; Liu, J. Recent advances in targeted antibacterial therapy basing on nanomaterials. Exploration 2023, 3, 20210117. [Google Scholar] [CrossRef]
- Malik, S.; Muhammad, K.; Waheed, Y. Nanotechnology: A revolution in modern industry. Molecules 2023, 28, 661. [Google Scholar] [CrossRef]
- Vijayakumar, M.D.; Surendhar, G.J.; Natrayan, L.; Patil, P.P.; Ram, P.M.B.; Paramasivam, P.R.L. Evolution and recent scenario of nanotechnology in agriculture and food industries. J. Nanomater. 2022, 2022, 1280411. [Google Scholar] [CrossRef]
- Maringgal, B.; Hashim, N.; Mohamed Amin Tawakkal, I.S.; Mohamed, M.T.M.; Hamzah, M.H.; Mohd Ali, M. Effect of Kelulut honey nanoparticles coating on the changes of respiration rate, ascorbic acid, and total phenolic content of Papaya (Carica papaya L.) during cold storage. Foods 2021, 10, 432. [Google Scholar] [CrossRef]
- Perinelli, D.R.; Fagioli, L.; Campana, R.; Lam, J.K.W.; Baffone, W.; Palmieri, G.F.; Casettari, L.; Bonacucina, G. Chitosan-based nanosystems and their exploited antimicrobial activity. Eur. J. Pharm. Sci. 2018, 117, 8–20. [Google Scholar] [CrossRef]
- Alsaggaf, M.S.; Moussa, S.H.; Tayel, A.A. Application of fungal chitosan incorporated with pomegranate peel extract as edible coating for microbiological, chemical and sensorial quality enhancement of Nile tilapia fillets. Int. J. Biol. Macromol. 2017, 99, 499–505. [Google Scholar] [CrossRef] [PubMed]
- Correa-Pacheco, Z.; Bautista-Baños, S.; Ramos-García, M.; Martínez-González, M.; Hernández-Romano, J. Physicochemical characterization and antimicrobial activity of edible propolis-chitosan nanoparticle films. Prog. Org. Coat. 2019, 137, 105326. [Google Scholar] [CrossRef]


| Bee products Used/Reducing and Capping Agents | Morphology | NPs Size (nm) | Biological Activities and Application | References |
|---|---|---|---|---|
| Utilizing honey for silver nanoparticles (AgNPs) synthesis | ||||
| Honey/Honey | Spherical | 9–22 | Inhibitory effect on aflatoxins and ochratoxin | [69] |
| Honey/Glucose | Spherical | >20 | [75] | |
| Proteins, minerals, and polyphenols | Spherical with smooth edges | 50–98 | Antioxidant, cytotoxic, and antibacterial agent | [76] |
| Honey/Glucose, fructose, organic acids, vitamins, and minerals | Spherical | 14.3 and 14.7 | Antibacterial properties with inhibition zone of 14 mm against Staphylococcus aureus | [26] |
| Honey/Phenolic compounds, fructose, glucose, vitamins, and proteins | Spherical | 42.7 | Cytotoxic effect against L-929 cell line and antibacterial activity | [28] |
| Honey/Honey (black seed) | Spherical | 25–70 | Antibacterial activity | [77] |
| Honey/Fructose, glucose, and vitamin C | Spherical and monodispersed | 5–25 | Catalytic degradation of methylene blue | [27] |
| Honey/Alkane, ketone, alkene, nitro compounds, vinyl ether, and alkyl halide | Spherical | 50–90 | Anticancer, antimicrobial, immunomodulatory | [78] |
| Honey/Vinyl ether, alkene, and bromo compounds | Spherical | 70–80 | Antimicrobial, immunomodulatory, and cytotoxic activities | [79] |
| Honey/Fructose and proteins | Spherical | N.D. | Anticorrosion potentials for mild steel in acidic environments | [80] |
| Honey | Crystalline | 11.1 | - | [81] |
| Utilizing propolis for AgNPs’ synthesis | ||||
| Propolis/Polyphenols and flavonoids | Crystalline | 91 | Anticancer | [40] |
| Propolis/Quercetin and galangin | Spherical | 16.5 ± 5.3 | Antimicrobial activity | [82] |
| Propolis/Aliphatic –CH, –CH2 groups and carboxylate compounds in the propolis extract | N.D. | 108.2 | Antibacterial activity | [83] |
| Propolis/Amino acids, flavonoids, and phenolics | Spherical | 8.7 | Potent antimicrobial and wound-healing composites | [84] |
| Propolis/Polyol | N.D. | 2–40 | Antifungal, antibiofilm, and non-mutagenic properties | [85] |
| Propolis | Spherical | 20 | Antimicrobial activity | [86] |
| Propolis | Spherical | 13–45 | Immunomodulatory effect | [87] |
| Propolis | N.D. | 5–15 | Antibacterial effects and catalytic activity | [88] |
| Utilizing bee pollen (BP) for AgNPs’ synthesis | ||||
| BP | Spherical | 11.5 | Antioxidant activity | [55] |
| BP | Spherical | 10–30 | Anticancer activity and antioxidant activity | [89] |
| BP | Spherical | 40–60 nm | Antidiabetic activity | [90] |
| Utilizing bee bread (Bb) for AgNPs’ synthesis | ||||
| Bb | Spherical | 48.3–150.1 | Antioxidant and antimicrobial activity | [91] |
| Utilizing royal jelly (RJ) for AgNPs’ synthesis | ||||
| RJ | Disk-like morphologies | 4 | Antimicrobial activity | [60] |
| RJ | Spherical | 7.8–192.7 and 8.6–61.8 | Antimicrobial activity | [61] |
| Parameters | Z. spina-christi Honey-Mediated AgNPs | A. gerrardii Honey-Mediated AgNPs | Amoxicillin | Cefuroxime | Ciprofloxacin |
|---|---|---|---|---|---|
| Average size (nm) | 50.5 ± 0.7 | 98.2 ± 0.9 | |||
| Inhibition zone of Staphylococcus aureus (mm) | 19.4 ± 0.6 | 17.0 ± 0.1 | 20 ± 0.6 | 24 ± 0.9 | 22 ± 1.7 |
| Inhibition zone of Escherichia coli (mm) | 22.8 ± 1.2 | 19.0 ± 1.3 | 18 ± 1.4 | 25 ± 1.9 | 34 ± 1.3 |
| Inhibition zone of Pseudomonas aeruginosa (mm) | 21.0 ± 0.9 | 18.6 ± 0.8 | - | - | 35 ± 1.8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khalifa, S.A.M.; Shetaia, A.A.; Eid, N.; Abd El-Wahed, A.A.; Abolibda, T.Z.; El Omri, A.; Yu, Q.; Shenashen, M.A.; Hussain, H.; Salem, M.F.; et al. Green Innovation and Synthesis of Honeybee Products-Mediated Nanoparticles: Potential Approaches and Wide Applications. Bioengineering 2024, 11, 829. https://doi.org/10.3390/bioengineering11080829
Khalifa SAM, Shetaia AA, Eid N, Abd El-Wahed AA, Abolibda TZ, El Omri A, Yu Q, Shenashen MA, Hussain H, Salem MF, et al. Green Innovation and Synthesis of Honeybee Products-Mediated Nanoparticles: Potential Approaches and Wide Applications. Bioengineering. 2024; 11(8):829. https://doi.org/10.3390/bioengineering11080829
Chicago/Turabian StyleKhalifa, Shaden A. M., Aya A. Shetaia, Nehal Eid, Aida A. Abd El-Wahed, Tariq Z. Abolibda, Abdelfatteh El Omri, Qiang Yu, Mohamed A. Shenashen, Hidayat Hussain, Mohamed F. Salem, and et al. 2024. "Green Innovation and Synthesis of Honeybee Products-Mediated Nanoparticles: Potential Approaches and Wide Applications" Bioengineering 11, no. 8: 829. https://doi.org/10.3390/bioengineering11080829
APA StyleKhalifa, S. A. M., Shetaia, A. A., Eid, N., Abd El-Wahed, A. A., Abolibda, T. Z., El Omri, A., Yu, Q., Shenashen, M. A., Hussain, H., Salem, M. F., Guo, Z., Alanazi, A. M., & El-Seedi, H. R. (2024). Green Innovation and Synthesis of Honeybee Products-Mediated Nanoparticles: Potential Approaches and Wide Applications. Bioengineering, 11(8), 829. https://doi.org/10.3390/bioengineering11080829












