Unleashing the Potential of Tannic Acid in Dentistry: A Scoping Review of Applications
Abstract
:1. Introduction
2. Materials and Methods
2.1. Search Strategy
2.2. Study Selection
2.2.1. Inclusion Criteria
- Original in vitro studies, in vivo studies, or clinical studies;
- Articles on the application of TA in the field of dentistry;
- Full text in English.
2.2.2. Exclusion Criteria
- Review articles;
- Studies applying other types of polyphenols, not TA.
2.3. Data Extraction and Synthesis
2.4. Quality Assessment
3. Results
3.1. Characteristics of Included Studies
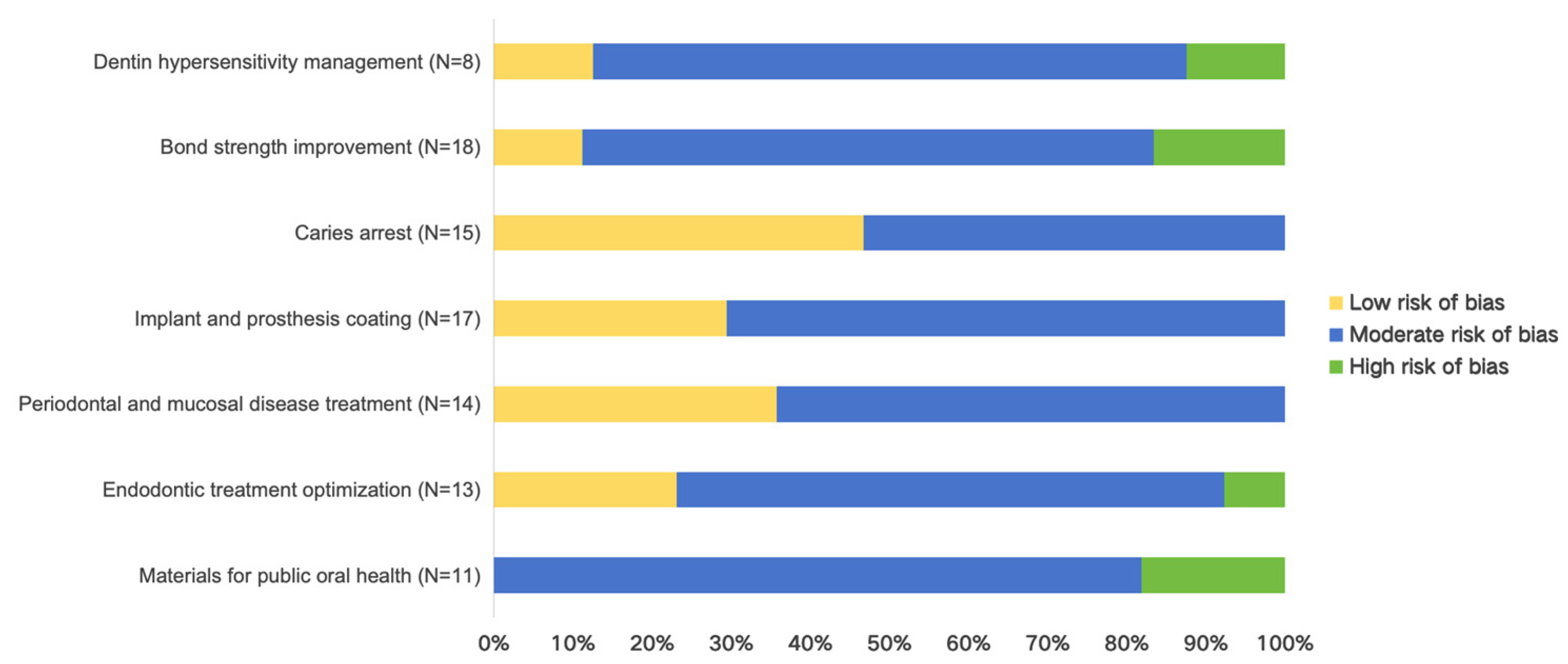
3.2. Quality Outcomes
3.3. Dentin Hypersensitivity Management
3.4. Bond Strength Improvement
3.5. Caries Arrest
3.6. Implant and Prosthesis Coating
3.7. Periodontal and Mucosal Disease Treatment
3.8. Endodontic Treatment Optimization
3.9. Materials for Public Oral Health
4. Discussion
5. Conclusions
- Management of dentin hypersensitivity
- Improvement of bond strength
- Caries arrest
- Coating for prostheses and implants
- Treatment of periodontal and mucosal diseases
- Optimization of endodontic treatment
- Materials for public oral health
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| Abbreviation | Meaning |
| TA | Tannic acid |
| IL | Interleukin |
| TNF | Tumor necrosis factor |
| DH | Dentin hypersensitivity |
| HY | Tannin–fluoride |
| FTLA | Fluoride–tannic acid–lanthanum–apatite |
| SF | Silk fibroin |
| GIC | Glass ionomer cement |
| ALP | Alkaline phosphatase |
| PA | Phosphoric acid |
| SDF | Silver diamine fluoride |
| ROS | Reactive oxygen species |
| LPS | Lipopolysaccharide |
| GGs | Gallic acid-derived pyruvate glucosides |
| RCT | Randomized controlled trial |
References
- Aelenei, N.; Popa, M.I.; Novac, O.; Lisa, G.; Balaita, L. Tannic Acid Incorporation in Chitosan-Based Microparticles and in Vitro Controlled Release. J. Mater. Sci Mater. Med. 2009, 20, 1095–1102. [Google Scholar] [CrossRef] [PubMed]
- Ejima, H.; Richardson, J.J.; Liang, K.; Best, J.P.; van Koeverden, M.P.; Such, G.K.; Cui, J.; Caruso, F. One-Step Assembly of Coordination Complexes for Versatile Film and Particle Engineering. Science 2013, 341, 154–157. [Google Scholar] [CrossRef] [PubMed]
- Bigham, A.; Rahimkhoei, V.; Abasian, P.; Delfi, M.; Naderi, J.; Ghomi, M.; Dabbagh Moghaddam, F.; Waqar, T.; Nuri Ertas, Y.; Sharifi, S.; et al. Advances in Tannic Acid-Incorporated Biomaterials: Infection Treatment, Regenerative Medicine, Cancer Therapy, and Biosensing. Chem. Eng. J. 2022, 432, 134146. [Google Scholar] [CrossRef]
- Nikkerdar, N.; Seyedi, H.; Mirzaeei, S.; Safari-Faramani, R.; Golshah, A. Comparative Effects of Three Mucoadhesive Gels Containing Lidocaine, Zinc Acetate, and Tannic Acid on the Gag Reflex of Dental Patients: A Randomized Double-Blind Clinical Trial. BMC Oral Health 2024, 24, 1442. [Google Scholar] [CrossRef]
- Li, R.; Zeng, Z.; Fu, G.; Wan, Y.; Liu, C.; McClements, D.J. Formation and Characterization of Tannic Acid/Beta-Glucan Complexes: Influence of pH, Ionic Strength, and Temperature. Food Res. Int. 2019, 120, 748–755. [Google Scholar] [CrossRef]
- Bae, S.B.; Kim, E.; Chathuranga, K.; Lee, J.S.; Park, W.H. Gelation and the Antioxidant and Antibacterial Properties of Silk Fibroin/Tannic Acid/Zn2+ Mixtures. Polymer 2021, 230, 124090. [Google Scholar] [CrossRef]
- Li, X.; Sun, S.; Feng, X.; Chen, Y.; Chen, S.; Ma, J.; Zhou, F. Tannic Acid-Crosslinked O-Carboxymethyl Chitosan Hydrogels for Enhanced Antibacterial Activity and Rapid Hemostasis. J. Biomater. Sci. Polym. Ed. 2023, 34, 184–199. [Google Scholar] [CrossRef]
- Chen, X.; Zhang, Q.; Wang, Y.; Meng, J.; Wu, M.; Xu, H.; Du, L.; Yang, X. Fabrication and Characterization of Electrospun Poly(Caprolactone)/Tannic Acid Scaffold as an Antibacterial Wound Dressing. Polymers 2023, 15, 593. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Xu, P.; Liu, C.; Chen, J.; Ren, B.; Du, E.; Guo, S.; Li, P.; Li, L.; Ding, B. Effect of Tannic Acid on Antioxidant Function, Immunity, and Intestinal Barrier of Broilers Co-Infected with Coccidia and Clostridium Perfringens. Animals 2024, 14, 955. [Google Scholar] [CrossRef]
- Dong, G.; Liu, H.; Yu, X.; Zhang, X.; Lu, H.; Zhou, T.; Cao, J. Antimicrobial and Anti-Biofilm Activity of Tannic Acid against Staphylococcus Aureus. Nat. Prod. Res. 2018, 32, 2225–2228. [Google Scholar] [CrossRef]
- Salman, M.; Tabassum, H.; Parvez, S. Tannic Acid Provides Neuroprotective Effects Against Traumatic Brain Injury Through the PGC-1α/Nrf2/HO-1 Pathway. Mol. Neurobiol. 2020, 57, 2870–2885. [Google Scholar] [CrossRef]
- Liu, W.; Guo, K. Tannic Acid Alleviates ETEC K88-induced Intestinal Damage through Regulating the p62-keap1-Nrf2 and TLR4-NF-κB-NLRP3 Pathway in IPEC-J2 Cells. J. Sci. Food Agric. 2024, 104, 5186–5196. [Google Scholar] [CrossRef]
- Jin, W.; Xue, Y.; Xue, Y.; Han, X.; Song, Q.; Zhang, J.; Li, Z.; Cheng, J.; Guan, S.; Sun, S.; et al. Tannic Acid Ameliorates Arsenic Trioxide-Induced Nephrotoxicity, Contribution of NF-κB and Nrf2 Pathways. Biomed. Pharmacother. 2020, 126, 110047. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Liu, P.; Xue, Y.; Liang, Y.; Shi, J.; Han, X.; Zhang, J.; Chu, X.; Chu, L. Tannic Acid Attenuates Hepatic Oxidative Stress, Apoptosis and Inflammation by Activating the Keap1-Nrf2/ARE Signaling Pathway in Arsenic Trioxide-toxicated Rats. Oncol. Rep. 2020, 44, 2306–2316. [Google Scholar] [CrossRef]
- Sabbak, S.A.; Hassanin, M.B. A Scanning Electron Microscopic Study of Tooth Surface Changes Induced by Tannic Acid. J. Prosthet. Dent. 1998, 79, 169–174. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Chen, J.; Duan, Y.; Zhou, Z. Sealing Effects of Different Chinese Herbal Medicines on Dentinal Tubules: A Scanning Electron Microscopic Observation. Ultrastruct. Pathol. 2020, 44, 255–261. [Google Scholar] [CrossRef] [PubMed]
- Hertel, S.; Pötschke, S.; Basche, S.; Delius, J.; Hoth-Hannig, W.; Hannig, M.; Hannig, C. Effect of Tannic Acid on the Protective Properties of the in Situ Formed Pellicle. Caries Res. 2017, 51, 34–45. [Google Scholar] [CrossRef]
- Huang, X.; Deng, M.; Liu, M.; Cheng, L.; Exterkate, R.A.M.; Li, J.; Zhou, X.; Ten Cate, J.M. Comparison of Composition and Anticaries Effect of Galla Chinensis Extracts with Different Isolation Methods. Open Dent. J. 2017, 11, 447–459. [Google Scholar] [CrossRef]
- Steffi, C.; Shi, Z.; Kong, C.H.; Wang, W. Bioinspired Polydopamine and Polyphenol Tannic Acid Functionalized Titanium Suppress Osteoclast Differentiation: A Facile and Efficient Strategy to Regulate Osteoclast Activity at Bone-Implant Interface. J. R. Soc. Interface 2019, 16, 20180799. [Google Scholar] [CrossRef]
- Iqbal, M.H.; Schroder, A.; Kerdjoudj, H.; Njel, C.; Senger, B.; Ball, V.; Meyer, F.; Boulmedais, F. Effect of the Buffer on the Buildup and Stability of Tannic Acid/Collagen Multilayer Films Applied as Antibacterial Coatings. ACS Appl. Mater. Interfaces 2020, 12, 22601–22612. [Google Scholar] [CrossRef]
- Prati, C.; Montanari, G.; Biagini, G.; Fava, F.; Pashley, D.H. Effects of Dentin Surface Treatments on the Shear Bond Strength of Vitrabond. Dent. Mater. 1992, 8, 21–26. [Google Scholar] [CrossRef] [PubMed]
- Bedran-Russo, A.K.B.; Yoo, K.J.; Ema, K.C.; Pashley, D.H. Mechanical Properties of Tannic-Acid-Treated Dentin Matrix. J. Dent. Res. 2009, 88, 807–811. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Zhong, K.; Zong, Y.; Wang, S.; Yang, H.; Zhen, L.; Tao, S.; Sun, L.; Yang, J.; Li, J. A Mussel-Inspired Wet-Adhesion Hydrogel with Hemostasis and Local Anti-Inflammation for Managing the Development of Acute Wounds. Mater. Des. 2022, 213, 110347. [Google Scholar] [CrossRef]
- Liu, J.; Liu, H.; Jia, Y.; Tan, Z.; Hou, R.; Lu, J.; Luo, D.; Fu, X.; Wang, L.; Wang, X. Glucose-Sensitive Delivery of Tannic Acid by a Photo-Crosslinked Chitosan Hydrogel Film for Antibacterial and Anti-Inflammatory Therapy. J. Biomater. Sci. Polym. Ed. 2022, 33, 1644–1663. [Google Scholar] [CrossRef]
- Bitter, N.C. A 25% Tannic Acid Solution as a Root Canal Irrigant Cleanser: A Scanning Electron Microscope Study. Oral Surg. Oral Med. Oral Pathol. 1989, 67, 333–337. [Google Scholar] [CrossRef]
- Nordbö, H.; Attramadal, A.; Eriksen, H.M. Iron Discoloration of Acrylic Resin Exposed to Chlorhexidine or Tannic Acid: A Model Study. J. Prosthet. Dent. 1983, 49, 126–129. [Google Scholar] [CrossRef]
- Anil, A.; Sekhar, A.; Thomas, M.S.; Ginjupalli, K. Haemostatic Agents on the Shear Bond Strength of Self-Adhesive Resin. J. Clin. Exp. Dent. 2015, 7, e356–e360. [Google Scholar] [CrossRef]
- Geißler, S.; Gomez-Florit, M.; Wiedmer, D.; Barrantes, A.; Petersen, F.C.; Tiainen, H. In Vitro Performance of Bioinspired Phenolic Nanocoatings for Endosseous Implant Applications. ACS Biomater. Sci. Eng. 2019, 5, 3340–3351. [Google Scholar] [CrossRef]
- Tran, L.; Tam, D.N.H.; Elshafay, A.; Dang, T.; Hirayama, K.; Huy, N.T. Quality Assessment Tools Used in Systematic Reviews of in Vitro Studies: A Systematic Review. BMC Med. Res. Methodol. 2021, 21, 101. [Google Scholar] [CrossRef]
- Addy, M.; Absi, E.G.; Adams, D. Dentine Hypersensitivity. The Effects in Vitro of Acids and Dietary Substances on Root-Planed and Burred Dentine. J. Clin. Periodontol. 1987, 14, 274–279. [Google Scholar] [CrossRef]
- Yamaga, M.; Koide, T.; Hieda, T.; Daito, M. Obturation of Dentinal Tubules with Tannin-Fluoride Preparation (HY Agent) Incorporated into Glass Ionomer Cement. J. Osaka Dent. Univ. 1993, 27, 77–87. [Google Scholar] [PubMed]
- Mukai, Y.; Tomiyama, K.; Okada, S.; Mukai, K.; Negishi, H.; Fujihara, T. Dentinal Tubule Occlusion with Lanthanum Fluoride and Powdered Apatite Glass Ceramics In Vitro. Dent. Mater. J. 1998, 17, 253–263. [Google Scholar] [CrossRef] [PubMed]
- Tomiyama, K.; Mukai, Y.; Okada, S.; Negishi, H.; Fujihara, T.; Kawase, T.; Ueda, M.; Nakagawa, S.; Teranaka, T. Durability of FTLA Treatment as a Medicament for Dentin Hypersensitivity: Abrasion Resistance and Profiles of Fluoride Release. Dent. Mater. J. 2004, 23, 585–592. [Google Scholar] [CrossRef] [PubMed]
- Oh, D.X.; Prajatelistia, E.; Ju, S.-W.; Jeong Kim, H.; Baek, S.-J.; Joon Cha, H.; Ho Jun, S.; Ahn, J.-S.; Soo Hwang, D. A Rapid, Efficient and Facile Solution for Dental Hypersensitivity: The Tannin–Iron Complex. Sci. Rep. 2015, 5, 10884. [Google Scholar] [CrossRef]
- Gao, W.; Liu, Y.; Li, M.; Ding, M.; Cheng, L.; Ding, C.; Yang, J.; Li, J.; Luo, J.; Qiu, R. A Drop-By-Drop Self-Assembled All-Natural Hydrogel as a Desensitizer for Rapid and Enduring Management of Dentin Hypersensitivity. Adv. Healthc. Mater. 2024, 13, e2303153. [Google Scholar] [CrossRef]
- Powis, D.R.; Follerås, T.; Merson, S.A.; Wilson, A.D. Materials Science: Improved Adhesion of a Glass Ionomer Cement to Dentin and Enamel. J. Dent. Res. 1982, 61, 1416–1422. [Google Scholar] [CrossRef]
- Prati, C.; Nucci, C.; Montanari, G. Effects of Acid and Cleansing Agents on Shear Bond Strength and Marginal Microleakage of Glass-Ionomer Cements. Dent. Mater. 1989, 5, 260–265. [Google Scholar] [CrossRef]
- Bitter, N.C. Tannic Acid for Smear Layer Removal: Pilot Study with Scanning Electron Microscope. J. Prosthet. Dent. 1989, 61, 503–507. [Google Scholar] [CrossRef]
- Bitter, N.C. The Effect of 25% Tannic Acid on Prepared Dentin: A Scanning Electron Microscope-Methylene Blue Dye Study. J. Prosthet. Dent. 1990, 64, 12–16. [Google Scholar] [CrossRef]
- Okamoto, Y.; Shintani, H.; Yamaki, M. A Medicated Polycarboxylate Cement to Prevent Complications in Composite Resin Therapy. J. Prosthet. Dent. 1990, 63, 37–40. [Google Scholar] [CrossRef]
- Okamoto, Y.; Heeley, J.D.; Dogon, I.L.; Shintani, H. Effects of Phosphoric Acid and Tannic Acid on Dentine Collagen. J. Oral Rehabil. 1991, 18, 507–512. [Google Scholar] [CrossRef] [PubMed]
- Natsir, N.; Wakasa, K.; Yoshida, Y.; Satou, N.; Shintani, H. Effect of Tannic Acid Solution on Collagen Structures for Dental Restoration. J. Mater. Sci. Mater. Med. 1999, 10, 489–492. [Google Scholar] [CrossRef] [PubMed]
- Kapoor, D.N.; Sharma, V.P.; Tandon, P.; Pandey, K. Comparative Evaluation of Tannic Acid, Citric Acid and Phosphoric Acid as Etching Agents for Direct Bonding. J. Indian. Orthod. Soc. 2002, 36, 54–62. [Google Scholar] [CrossRef]
- Buchalla, W.; Lennon, Á.; Becker, K.; Lucke, T.; Attin, T. Smear Layer and Surface State Affect Dentin Fluoride Uptake. Arch. Oral Biol. 2007, 52, 932–937. [Google Scholar] [CrossRef]
- Pavan, S.; Dos Santos, P.H.; Berger, S.; Bedran-Russo, A.K.B. The Effect of Dentin Pretreatment on the Microtensile Bond Strength of Self-Adhesive Resin Cements. J. Prosthet. Dent. 2010, 104, 258–264. [Google Scholar] [CrossRef]
- Boruziniat, A.; Babazadeh, M.; Gifani, M.; Nasirzadeh, M. Effect of Tannic Acid Application on Durability of Bond of Etch and Rinse Adhesive Resins. J. Dent. Mater. Tech. 2017, 6, 125–130. [Google Scholar] [CrossRef]
- Abdollahi, M.; Ebrahimi, M.; Shirazi, A.S.; Abdolhoseinpour, F. Effect of Tannic Acid on Bond Strength of Etch and Rinse and Self-Etch Adhesive Systems in Dentin of Primary Teeth. J. Contemp. Dent. Pract. 2017, 18, 34–38. [Google Scholar] [CrossRef]
- Cecchin, D.; Farina, A.P.; Bedran-Russo, A.K. Efficacy of Natural Collagen Crosslinkers on the Compromised Adhesive Bond Strength to NaOCl-Treated Pulp Chamber Dentin. J. Adhes. Dent. 2018, 20, 365–369. [Google Scholar] [CrossRef]
- Fattah, Z.; Shafiei, F.; Rajabi, F. Effect of Tannic Acid and Quercetin Antioxidants on Bond Strength of Resin Cement to Dentin after Internal Bleaching. Eur. J. Prosthodont. Restor. Dent. 2022, 30, 126–133. [Google Scholar] [CrossRef]
- Zheng, X.; Liu, Y.; Li, M.; Li, Y.; Gao, W.; Qiu, R.; Xing, J.; Yang, J.; Chen, Y.; Xu, X.; et al. Bioinspired Mineral-in-Shell Nanoarchitectonics: Functional Empowerment of Mineral Precursors for Guiding Intradentinal Mineralization. Nano Res. 2023, 17, 4338–4349. [Google Scholar] [CrossRef]
- Yu, H.; Lx, X.; Oho, T.; Morioka, T. The Effect of a Tannin-Fluoride Mixture on Human Dental Enamel. Caries Res. 1993, 27, 161–168. [Google Scholar] [CrossRef]
- Yu, H.; Oho, T.; Xu, L.X. Effects of Several Tea Components on Acid Resistance of Human Tooth Enamel. J. Dent. 1995, 23, 101–105. [Google Scholar] [CrossRef] [PubMed]
- Yamaga, T.; Nokubi, T. Clinical Observations of Noncoping Overdenture Abutments Protected by Tannin-Fluoride Preparation. J. Prosthet. Dent. 1997, 78, 315–319. [Google Scholar] [CrossRef]
- Koide, T.; Daito, M. Effects of Various Collagen Crosslinking Techniques on Mechanical Properties of Collagen Film. Dent. Mater. J. 1997, 16, 1–9+109. [Google Scholar] [CrossRef]
- Yee, R.; Holmgren, C.; Mulder, J.; Lama, D.; Walker, D.; van Palenstein Helderman, W. Efficacy of Silver Diamine Fluoride for Arresting Caries Treatment. J. Dent. Res. 2009, 88, 644–647. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Yang, B.; He, L.; Li, R.; Liao, Y.; Zhang, S.; Yang, Y.; Xu, X.; Zhang, D.; Tan, H.; et al. Bioinspired Peptide-Decorated Tannic Acid for in Situ Remineralization of Tooth Enamel: In Vitro and in Vivo Evaluation. ACS Biomater. Sci. Eng. 2017, 3, 3553–3562. [Google Scholar] [CrossRef] [PubMed]
- Xi, Q.; Hoth-Hannig, W.; Deng, S.; Jin, X.; Fu, B.; Hannig, M. The Effect of Polyphenol-Containing Solutions on in Situ Biofilm Formation on Enamel and Dentin. J. Dent. 2020, 102, 103482. [Google Scholar] [CrossRef]
- Schestakow, A.; Hannig, M. Effects of Experimental Agents Containing Tannic Acid or Chitosan on the Bacterial Biofilm Formation in Situ. Biomolecules 2020, 10, 1315. [Google Scholar] [CrossRef]
- Schestakow, A.; Guth, M.S.; Eisenmenger, T.A.; Hannig, M. Evaluation of Anti-Biofilm Activity of Mouthrinses Containing Tannic Acid or Chitosan on Dentin In Situ. Molecules 2021, 26, 1351. [Google Scholar] [CrossRef]
- Schestakow, A.; Nekrashevych, Y.; Hoth-Hannig, W.; Hannig, M. Influence of Periodic Polyphenol Treatment on the Anti-Erosive Potential of the Acquired Enamel Pellicle—A Qualitative Exploratory Study. J. Dent. 2022, 124, 104236. [Google Scholar] [CrossRef]
- Zhen, L.; Liang, K.; Luo, J.; Ke, X.; Tao, S.; Zhang, M.; Yuan, H.; He, L.; Bidlack, F.B.; Yang, J.; et al. Mussel-Inspired Hydrogels for Fluoride Delivery and Caries Prevention. J. Dent. Res. 2022, 101, 1597–1605. [Google Scholar] [CrossRef] [PubMed]
- Kong, W.; Du, Q.; Qu, Y.; Shao, C.; Chen, C.; Sun, J.; Mao, C.; Tang, R.; Gu, X. Tannic Acid Induces Dentin Biomineralization by Crosslinking and Surface Modification. RSC Adv. 2022, 12, 3454–3464. [Google Scholar] [CrossRef]
- Selvaraj, K.; Venkatesan, L.S.; Ganapathy, D.; Sathishkumar, P. Treatment of Dental Biofilm-Forming Bacterium Streptococcus Mutans Using Tannic Acid-Mediated Gold Nanoparticles. Microb. Pathog. 2024, 189, 106568. [Google Scholar] [CrossRef]
- Yang, X.; Huang, P.; Wang, H.; Cai, S.; Liao, Y.; Mo, Z.; Xu, X.; Ding, C.; Zhao, C.; Li, J. Antibacterial and Anti-Biofouling Coating on Hydroxyapatite Surface Based on Peptide-Modified Tannic Acid. Colloids Surf. B Biointerfaces 2017, 160, 136–143. [Google Scholar] [CrossRef]
- Weber, F.; Liao, W.; Barrantes, A.; Edén, M.; Tiainen, H. Silicate-Phenolic Networks: Coordination-Mediated Deposition of Bioinspired Tannic Acid Coatings. Chem. A Eur. J. 2019, 25, 9870–9874. [Google Scholar] [CrossRef] [PubMed]
- Weber, F.; Barrantes, A.; Tiainen, H. Silicic Acid-Mediated Formation of Tannic Acid Nanocoatings. Langmuir 2019, 35, 3327–3336. [Google Scholar] [CrossRef]
- Li, H.; Gao, C.; Tang, L.; Wang, C.; Chen, Q.; Zheng, Q.; Yang, S.; Sheng, S.; Zan, X. Lysozyme (Lys), Tannic Acid (TA), and Graphene Oxide (GO) Thin Coating for Antibacterial and Enhanced Osteogenesis. ACS Appl. Bio. Mater. 2020, 3, 673–684. [Google Scholar] [CrossRef]
- Dong, Z.; Ke, X.; Tang, S.; Wu, S.; Wu, W.; Chen, X.; Yang, J.; Xie, J.; Luo, J.; Li, J. A Stable Cell Membrane-Based Coating with Antibiofouling and Macrophage Immunoregulatory Properties for Implants at the Macroscopic Level. Chem. Mater. 2021, 33, 7994–8006. [Google Scholar] [CrossRef]
- Wang, G.; Zhu, Y.; Zan, X.; Li, M. Endowing Orthopedic Implants’ Antibacterial, Antioxidation, and Osteogenesis Properties Through a Composite Coating of Nano-Hydroxyapatite, Tannic Acid, and Lysozyme. Front. Bioeng. Biotechnol. 2021, 9, 718255. [Google Scholar] [CrossRef]
- Weber, F.; Quach, H.Q.; Reiersen, M.; Sarraj, S.Y.; Bakir, D.N.; Jankowski, V.A.; Nilsson, P.H.; Tiainen, H. Characterization of the Foreign Body Response of Titanium Implants Modified with Polyphenolic Coatings. J. Biomed. Mater. Res. Part. A 2022, 110, 1341–1355. [Google Scholar] [CrossRef]
- Weber, F.; Dornelas-Figueira, L.M.; Hafiane, N.; Zaytseva-Zotova, D.; Barrantes, A.; Petersen, F.C.; Tiainen, H. Can Polyphenolic Surface Modifications Prevent Fungal Colonization of Titanium Dental Implants? Colloids Surf. B Biointerfaces 2022, 219, 112813. [Google Scholar] [CrossRef]
- Kim, K.-H.; Mai, H.-N.; Hyun, D.-C.; Lee, D.-H. New Autonomous Water-Enabled Self-Healing Coating Material with Antibacterial-Agent-Releasing Properties. Pharmaceutics 2022, 14, 1005. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; He, L.; Li, J.; Luo, J.; Liang, K.; Yin, D.; Tao, S.; Yang, J.; Li, J. Mussel-Inspired Organic–Inorganic Implant Coating Based on a Layer-by-Layer Method for Anti-Infection and Osteogenesis. Ind. Eng. Chem. Res. 2022, 61, 13040–13051. [Google Scholar] [CrossRef]
- Ren, L.; Gong, P.; Gao, X.; Wang, Q.; Xie, L.; Tang, W.; Long, J.; Liu, C.; Tian, W.; He, M. Metal–Phenolic Networks Acted as a Novel Bio-Filler of a Barrier Membrane to Improve Guided Bone Regeneration via Manipulating Osteoimmunomodulation. J. Mater. Chem. B 2022, 10, 10128–10138. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Tan, X.; Zheng, L.; Tang, H.; Hu, S.; Zhai, Q.; Jing, X.; Liang, P.; Zhang, Y.; He, Q.; et al. A Dual-Antioxidative Coating on Transmucosal Component of Implant to Repair Connective Tissue Barrier for Treatment of Peri-Implantitis. Adv. Healthc. Mater. 2023, 12, 2301733. [Google Scholar] [CrossRef]
- Shen, L.; Hu, J.; Yuan, Y.; Wang, X.; Jiang, Q. Photothermal-Promoted Multi-Functional Gallic Acid Grafted Chitosan Hydrogel Containing Tannic Acid Miniaturized Particles for Peri-Implantitis. Int. J. Biol. Macromol. 2023, 253, 127366. [Google Scholar] [CrossRef]
- Zhao, B.; Dong, Y.; Shen, X.; He, W.; Jin, H.; Yao, L.; Zheng, S.; Zan, X.; Liu, J. Construction of Multifunctional Coating with Cationic Amino Acid-Coupled Peptides for Osseointegration of Implants. Mater. Today Bio 2023, 23, 100848. [Google Scholar] [CrossRef]
- Homer, K.A.; Manji, F.; Beighton, D. Inhibition of Protease Activities of Periodontopathic Bacteria by Extracts of Plants Used in Kenya as Chewing Sticks (Mswaki). Arch. Oral Biol. 1990, 35, 421–424. [Google Scholar] [CrossRef]
- Darvin, P.; Baeg, S.J.; Joung, Y.H.; Sp, N.; Kang, D.Y.; Byun, H.J.; Park, J.U.; Yang, Y.M. Tannic Acid Inhibits the Jak2/STAT3 Pathway and Induces G1/S Arrest and Mitochondrial Apoptosis in YD-38 Gingival Cancer Cells. Int. J. Oncol. 2015, 47, 1111–1120. [Google Scholar] [CrossRef]
- Sheng, H.; Ogawa, T.; Niwano, Y.; Sasaki, K.; Tachibana, K. Effects of Polyphenols on Doxorubicin-Induced Oral Keratinocyte Cytotoxicity and Anticancer Potency against Oral Cancer Cells. J. Oral Pathol. Med. 2018, 47, 368–374. [Google Scholar] [CrossRef]
- Shahbazi, M.-A.; Shrestha, N.; Pierchala, M.K.; Kadumudi, F.B.; Mehrali, M.; Hasany, M.; Préat, V.; Leeuwenburgh, S.; Dolatshahi-Pirouz, A. A Self-Healable, Moldable and Bioactive Biomaterial Gum for Personalised and Wearable Drug Delivery. J. Mater. Chem. B 2020, 8, 4340–4356. [Google Scholar] [CrossRef] [PubMed]
- Lengert, E.V.; Savkina, A.A.; Ermakov, A.V.; Saveleva, M.S.; Lagutina, D.D.; Stepanova, T.V.; Ivanov, A.N. Influence of the New Formulation Based on Silver Alginate Microcapsules Loaded with Tannic Acid on the Microcirculation of the Experimental Periodontitis in Rats. Mater. Sci. Eng. C 2021, 126, 112144. [Google Scholar] [CrossRef] [PubMed]
- Ding, T.; Zou, J.; Qi, J.; Dan, H.; Tang, F.; Zhao, H.; Chen, Q. Mucoadhesive Nucleoside-Based Hydrogel Delays Oral Leukoplakia Canceration. J. Dent. Res. 2022, 101, 921–930. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Shi, Y.; Yang, H.; Liu, M.; Shen, L.; Zhang, S.; Liu, Y.; Zhu, J.; Lan, J.; Li, J.; et al. Stem Cell Microencapsulation Maintains Stemness in Inflammatory Microenvironment. Int. J. Oral Sci. 2022, 14, 48. [Google Scholar] [CrossRef]
- Zhu, J.; Li, Y.; Xie, W.; Yang, L.; Li, R.; Wang, Y.; Wan, Q.; Pei, X.; Chen, J.; Wang, J. Low-Swelling Adhesive Hydrogel with Rapid Hemostasis and Potent Anti-Inflammatory Capability for Full-Thickness Oral Mucosal Defect Repair. ACS Appl. Mater. Interfaces 2022, 14, 53575–53592. [Google Scholar] [CrossRef]
- Shi, Y.; Su, C.; Ding, T.; Zhao, H.; Wang, Y.; Ren, Y.; Wu, L.; Zhang, Q.; Liang, J.; Sun, S.; et al. Manganese Suppresses the Development of Oral Leukoplakia by Activating the Immune Response. Oral Dis. 2022, 30, 462–476. [Google Scholar] [CrossRef]
- Cheng, X.; Yang, Y.; Liao, Z.; Yi, Q.; Zhou, Y.; Dai, X.; Liu, Y.; Liu, O. Drug-Loaded Mucoadhesive Microneedle Patch for the Treatment of Oral Submucous Fibrosis. Front. Bioeng. Biotechnol. 2023, 11, 1251583. [Google Scholar] [CrossRef]
- He, S.; Bai, J.; Liu, Y.; Zeng, Y.; Wang, L.; Chen, X.; Wang, J.; Weng, J.; Zhao, Y.; Peng, W.; et al. A Polyglutamic Acid/Tannic Acid-Based Nano Drug Delivery System: Antibacterial, Immunoregulation and Sustained Therapeutic Strategies for Oral Ulcers. Int. J. Pharm. 2023, 648, 123607. [Google Scholar] [CrossRef]
- Liu, H.; Li, Q.; Xu, Y.; Sun, Y.; Fan, X.; Fang, H.; Hu, B.; Huang, L.; Liao, L.; Wang, X. Dual-Light Defined in Situ Oral Mucosal Lesion Therapy through a Mode Switchable Anti-Bacterial and Anti-Inflammatory Mucoadhesive Hydrogel. Biomater. Sci. 2023, 11, 3180–3196. [Google Scholar] [CrossRef]
- Raiden, G.; Olguín, A.; Peralta, G.; Posleman, I.; Lagarrigue, G. Apical Leakage in Canals Filled with Glass Ionomer Sealer and Gutta-Percha after Dentin Conditioning. Endod. Dent. Traumatol. 1997, 13, 289–291. [Google Scholar] [CrossRef]
- Raiden, G.; Posleman, I.; Peralta, G.; Olguin, A.; Lagarrigue, G. Dowel Space Preparation in Root Canals Filled with Glass Ionomer Cement. J. Endod. 1998, 24, 197–198. [Google Scholar] [CrossRef] [PubMed]
- Yoshikawa, M.; Terada, Y.; Toda, T. Setting Time and Sealing Ability of Alpha-Tricalcium Phosphate Cement Containing Titanic Oxide. J. Osaka Dent. Univ. 1998, 32, 67–70. [Google Scholar] [PubMed]
- Yoshikawa, M.; Toda, T. Reconstruction of Alveolar Bone Defect by Calcium Phosphate Compounds. J. Biomed. Mater. Res. 2000, 53, 430–437. [Google Scholar] [CrossRef]
- Yoshikawa, M.; Toda, T.; Mandai, Y. Pulpal and Periapical Tissue Responses to a Calcium Phosphate Cement Containing Barium Sulfate. Key Eng. Mater. 2003, 240–242, 345–348. [Google Scholar] [CrossRef]
- Yoshikawa, M.; Hayami, S.; Toda, T.; Mandai, Y. Effects of Tannic Acid in α-Tricalcium Phophate Cement for the Physical Properties and Tissue Responses. Key Eng. Mater. 2001, 218–220, 353–356. [Google Scholar] [CrossRef]
- Nakamura, K.; Deyama, Y.; Yoshimura, Y.; Hashimoto, M.; Kaga, M.; Suzuki, K.; Yawaka, Y. Tannin-Fluoride Preparation Attenuates Prostaglandin E2 Production by Dental Pulp Cells. Mol. Med. Rep. 2011, 4, 641–644. [Google Scholar] [CrossRef] [PubMed]
- Christopher, S.R.; Mathai, V.; Nair, R.S.; Angelo, J.M.C. The Effect of Three Different Antioxidants on the Dentinal Tubular Penetration of Resilon and Real Seal SE on Sodium Hypochlorite-Treated Root Canal Dentin: An In Vitro Study. J. Conserv. Dent. 2016, 19, 161–165. [Google Scholar] [CrossRef]
- Kharouf, N.; Zghal, J.; Addiego, F.; Gabelout, M.; Jmal, H.; Haikel, Y.; Bahlouli, N.; Ball, V. Tannic Acid Speeds up the Setting of Mineral Trioxide Aggregate Cements and Improves Its Surface and Bulk Properties. J. Colloid. Interface Sci. 2021, 589, 318–326. [Google Scholar] [CrossRef]
- Wu, I.-T.; Chu, Y.-H.; Huang, Y.-R.; Chen, C.-C.; Ding, S.-J. Antibacterial Ability and Osteogenic Activity of Polyphenol-Tailored Calcium Silicate Bone Cement. J. Mater. Chem. B 2022, 10, 4640–4649. [Google Scholar] [CrossRef]
- Louvrier, A.; Kroemer, M.; Terranova, L.; Meyer, F.; Tissot, M.; Euvrard, E.; Gindraux, F.; Meyer, C.; Rolin, G. Development of a Biomimetic Bioreactor for Regenerative Endodontics Research. J. Tissue Eng. Regen. Med. 2022, 16, 998–1007. [Google Scholar] [CrossRef]
- Zhou, L.; Shi, W.; Zhang, X.; Liu, M.; Zhang, L.; Jiang, X.; Chen, Z. Injectable Tannin-Containing Hydroxypropyl Chitin Hydrogel as Novel Bioactive Pulp Capping Material Accelerates Repair of Inflamed Dental Pulp. Biomolecules 2024, 14, 1129. [Google Scholar] [CrossRef] [PubMed]
- Kageyama, M.; Kado, H.; Kajiyama, N.; Kado, K.; Nagai, K.; Fujita, M.; Kashiwaya, Y. Ultrastructural Visualization of Complex Carbohydrates in Odontoblasts, Predentin, and Dentin Matrix by the Tannic Acid-Uranyl Acetate Method. J. Nihon Univ. Sch. Dent. 1985, 27, 211–217. [Google Scholar] [CrossRef] [PubMed]
- Takagi, M.; Kazama, T.; Shimada, K.; Hosokawa, Y.; Hishikawa, H. Differential Distribution and Ultrastructural Staining of Oxytalan and Elastic Fibers in the Periodontal Ligament of Alligator Mississippiensis. Anat. Rec. 1989, 225, 279–287. [Google Scholar] [CrossRef]
- Ishizeki, K.; Nagano, H.; Nawa, T. Features of the Aperiodic Microfibrils Associated with Mouse Dental Basement Membrane Demonstrated by Ultrastructural Histochemistry. J. Anat. 1990, 173, 139–150. [Google Scholar]
- Kim, S.; Inoue, S.; Akisaka, T. Ultrastructure of Quick-Frozen Secretory Ameloblasts of the Rat Molar Tooth. Tissue Cell 1994, 26, 29–41. [Google Scholar] [CrossRef] [PubMed]
- Joiner, A.; Muller, D.; Elofsson, U.M.; Arnebrant, T. Ellipsometry Analysis of the in Vitro Adsorption of Tea Polyphenols onto Salivary Pellicles. Eur. J. Oral Sci. 2004, 112, 510–515. [Google Scholar] [CrossRef]
- Haruyama, A.; Kameyama, A.; Ono, T.; Baba, Y.; Sugiyama, T.; Sugiyama, S.; Takahashi, T. Combined Effects of Electric Toothbrushing and Dentifrice on Artificial Stain Removal: An in Vitro Study. J. Clin. Exp. Dent. 2018, 10, e200–e205. [Google Scholar] [CrossRef]
- Haruyama, A.; Kojima, M.; Kameyama, A.; Muramatsu, T. Combined Use of Baking Soda and Electric Toothbrushing for Removal of Artificial Extrinsic Stain on Enamel Surface: An In Vitro Study. J. Clin. Exp. Dent. 2022, 14, e9–e15. [Google Scholar] [CrossRef]
- Marquillas, C.B.; Procaccini, R.; Malmagro, M.V.; Sánchez-Martín, M.-J. Breaking the Rules: Tooth Whitening by Means of a Reducing Agent. Clin. Oral Investig. 2020, 24, 2773–2779. [Google Scholar] [CrossRef]
- Asghar, M.; Yahya, R.; Yap, A.U.J.; Azzahari, A.D.; Omar, R.A. Incorporation of Green Capping Agents to Reduce Silver-Mediated Dentine Staining. Caries Res. 2022, 56, 149–160. [Google Scholar] [CrossRef]
- Cen, X.; Pan, X.; Wang, R.; Huang, X.; Zhao, Z. The Complex of Tannic Acid and Cetylpyridinium Chloride: An Antibacterial and Staining-Removal Cleaner for the Aligners. Am. J. Orthod. Dentofac. Orthop. 2023, 165, 173–185. [Google Scholar] [CrossRef] [PubMed]
- Vinod, K.S.; Sunil, K.S.; Sethi, P.; Bandla, R.C.; Singh, S.; Patel, D. A Novel Herbal Formulation versus Chlorhexidine Mouthwash in Efficacy against Oral Microflora. J. Int. Soc. Prev. Community Dent. 2018, 8, 184–190. [Google Scholar] [CrossRef]
- Yudaev, P.A.; Chistyakov, E.M. Progress in Dental Materials: Application of Natural Ingredients. Russ. Chem. Rev. 2024, 93, RCR5108. [Google Scholar] [CrossRef]
- Contaldo, M.; Di Stasio, D.; Romano, A.; Fiori, F.; Della Vella, F.; Rupe, C.; Lajolo, C.; Petruzzi, M.; Serpico, R.; Lucchese, A. Oral Candidiasis and Novel Therapeutic Strategies: Antifungals, Phytotherapy, Probiotics, and Photodynamic Therapy. Curr. Drug Deliv. 2023, 20, 441–456. [Google Scholar] [CrossRef]
- Valenti, C.; Pagano, S.; Bozza, S.; Ciurnella, E.; Lomurno, G.; Capobianco, B.; Coniglio, M.; Cianetti, S.; Marinucci, L. Use of the Er:YAG Laser in Conservative Dentistry: Evaluation of the Microbial Population in Carious Lesions. Materials 2021, 14, 2387. [Google Scholar] [CrossRef]
- Gholami, L.; Shahabi, S.; Jazaeri, M.; Hadilou, M.; Fekrazad, R. Clinical Applications of Antimicrobial Photodynamic Therapy in Dentistry. Front. Microbiol. 2022, 13, 1020995. [Google Scholar] [CrossRef] [PubMed]
- Yan, R.; Liu, J.; Dong, Z.; Peng, Q. Nanomaterials-Mediated Photodynamic Therapy and Its Applications in Treating Oral Diseases. Biomater. Adv. 2023, 144, 213218. [Google Scholar] [CrossRef] [PubMed]
- Mirzaie, M.; Yassini, E.; Ashnagar, S.; Hadadi, A.; Chiniforush, N. Evaluation of Temperature Change during Antimicrobial Photodynamic Therapy with Two Different Photosensitizers in Dental Caries. Photodiagn. Photodyn. Ther. 2016, 14, 115–118. [Google Scholar] [CrossRef]
- Li, H.; Liu, Y.; Li, X.; Du, J.; Guo, L.; Liu, Y. A Histological Evaluation of the Mice Oral Mucosal Tissue Wounds Excised with Diode Laser, Er:YAG Laser, and Cold Scalpel. Lasers Med. Sci. 2022, 37, 2707–2715. [Google Scholar] [CrossRef]
- Hu, N.; Qin, H.; Chen, X.; Huang, Y.; Xu, J.; He, H. Tannic Acid Assisted Metal-Chelate Interphase toward Highly Stable Zn Metal Anodes in Rechargeable Aqueous Zinc-Ion Batteries. Front. Chem. 2022, 10, 981623. [Google Scholar] [CrossRef]
- Li, H.; Yuan, M.; Li, P.-J.; Yang, J.-Y.; Chao, C.-Y. Tannic Acid as a Pioneering Chelating Agent for Nickel–Cobalt Supercapacitor Electrodes. Ionics 2024, 30, 7525–7535. [Google Scholar] [CrossRef]
- Wang, P.; Liu, J.; Luo, X.; Xiong, P.; Gao, S.; Yan, J.; Li, Y.; Cheng, Y.; Xi, T. A Tannic Acid-Modified Fluoride Pre-Treated Mg-Zn-Y-Nd Alloy with Antioxidant and Platelet-Repellent Functionalities for Vascular Stent Application. J. Mater. Chem. B Mater. Biol. Med. 2019, 7, 7314–7325. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Liang, J.; Li, Z.; Xiao, F.; Wu, Y.; Zhang, C. Preparation of Forward Osmosis Composite Membrane with Chitosan/Tannic Acid Co-Deposition Interlayer and Its Fluoride Removal Performance. Sep. Purif. Technol. 2024, 330, 125300. [Google Scholar] [CrossRef]
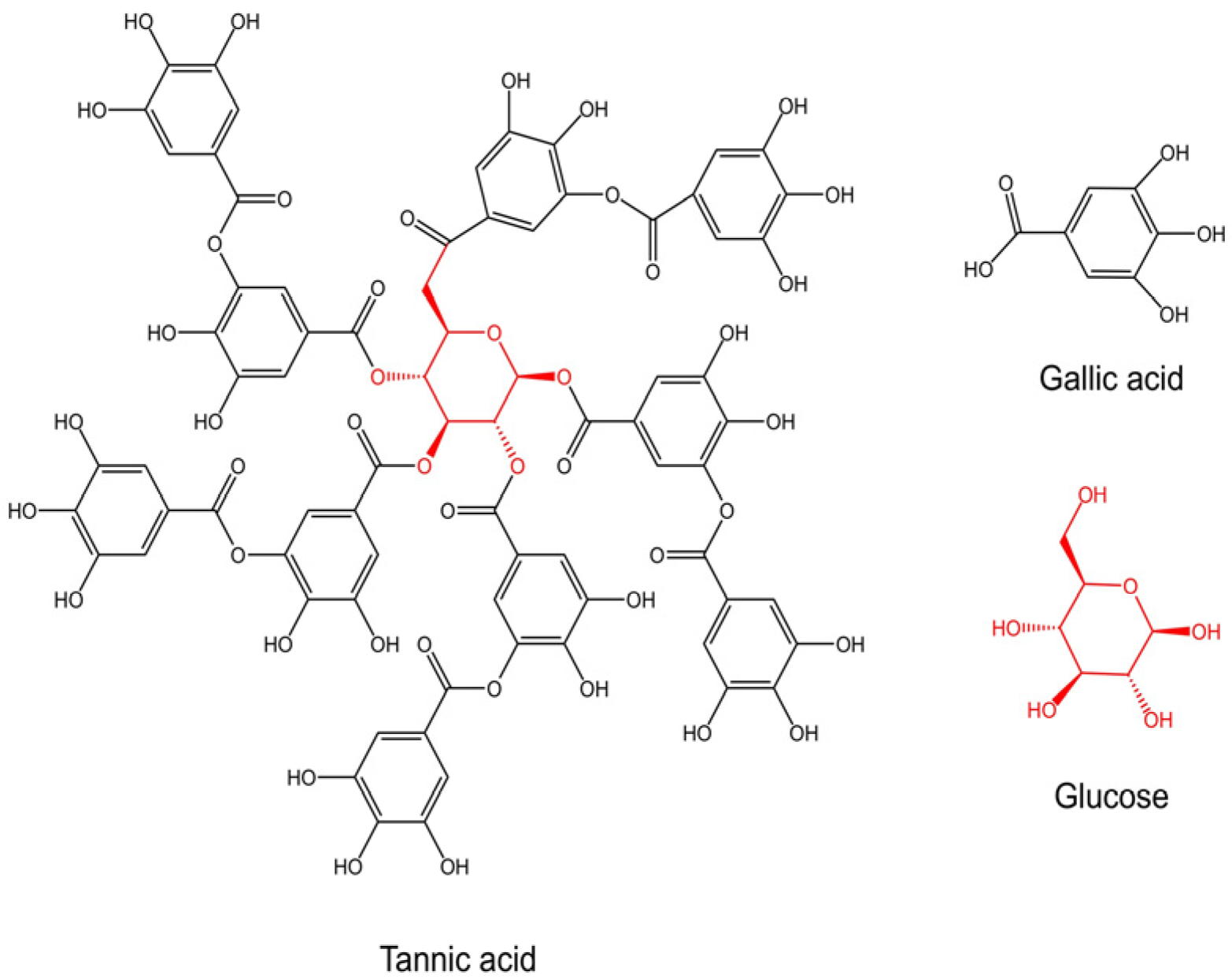

| Studies | Treatment | Study Type | Subject | Assessment | Key Outcomes |
|---|---|---|---|---|---|
| Addy et al. (1987) [30] | TA (pH 3.3) | In vitro | Human dentin | TA application for 5 min |
|
| Yamaga et al. (1993) [31] | HY (20% TA) | In vitro | Bovine dentin | HY application with GIC |
|
| Sabbak et al. (1998) [15] | 15%, 20%, and 25% TA | In vitro | Human dentin | TA application for 5–15 min |
|
| Mukai et al. (1998) [32] | FTLA (5% TA) | In vitro | Bovine dentin | FLTA application for 6 weeks |
|
| Tomiyama et al. (2004) [33] | FTLA (5% TA) | In vitro | Bovine dentin | FLTA treatment and toothbrush abrasion test (6000 cycles) |
|
| Oh et al. (2015) [34] | TA/Fe3+ (0.04% TA) | In vitro | Human dentin | TA/Fe3+ treatment and toothbrush abrasion test (1000 cycles) |
|
| Li et al. (2020) [16] | Commercial TA (≥98%) | In vitro | Bovine dentin | Triple TA application (5 min each) |
|
| Gao et al. (2024) [35] | SF-TA-DTs (10% TA) | In vitro and in vivo | Human dentin, rat model, rabbit model | SF-TA-DTs application and in vivo evaluation in rat and rabbit models |
|
| Studies | Treatment | Study Type | Subject | Assessment | Key Outcomes |
|---|---|---|---|---|---|
| Powis et al. (1982) [36] | 25% TA | In vitro | Human enamel and dentin | TA application for 60 s, bonded with GIC |
|
| Prati et al. (1989) [37] | 25% TA | In vitro | Human dentin | TA application for 30 s, bonded with GIC |
|
| Bitter (1989) [38] | 25% TA | In vitro | Human dentin | TA application for 15, 30, and 60 s |
|
| Bitter (1990) [39] | 25% TA | In vitro | Human dentin | TA application for 15 s |
|
| Okamoto et al. (1990) [40] | HY (20% TA) | In vitro | Human dentin | HY application with GIC intraoral and teeth extraction performed after 2–9 months |
|
| Okamoto et al. (1991) [41] | 1% TA | In vitro | Bovine dentin | PA application followed by TA treatment for 5, 10, 30, 60, and 120 min |
|
| Prati et al. (1992) [21] | 25% TA | In vitro | Human dentin | TA application for 60 s, bonded with GIC |
|
| Natsir et al. (1999) [42] | 1%, 3%, 5%, and 10% TA | In vitro | Bovine dentin | TA application for 1, 3, 6, 12, or 24 h, followed by PA treatment with trypsin |
|
| Kapoor et al. (2002) [43] | 50% TA | In vitro | Human enamel | TA application for 60 s and 90 s, bonded with brackets |
|
| Buchalla et al. (2007) [44] | 5% TA | In vitro | Bovine dentin | TA application for 20 s |
|
| Bedran-Russo et al. (2009) [22] | 1%, 10%, and 20% TA | In vitro | Human dentin | TA application for 10 min, 30 min, 1 h, 2 h, 24 h, and 48 h, bonded with resin |
|
| Pavan et al. (2010) [45] | 20% TA | In vitro | Human dentin | TA application for 10 min, bonded with resin |
|
| Anil et al. (2015) [27] | 25% TA | In vitro | Human dentin | TA application for 5 min, bonded with resin |
|
| Alireza et al. (2017) [46] | 20% and 30% TA | In vitro | Human dentin | TA application for 30 s, 1 min, 3 min, and 5 min, bonded with resin |
|
| Abdollahi et al. (2017) [47] | 20% TA | In vitro | Human dentin | TA application for 2 min, bonded with resin |
|
| Cecchin et al. (2018) [48] | 10% TA | In vitro | Human dentin and root canal | NaOCl application followed by TA treatment for 5 min, bonded with resin |
|
| Shafeie et al. (2022) [49] | 20% TA | In vitro | Human dentin | Internal bleaching followed by TA application for 10 min, bonded with resin |
|
| Zheng et al. (2023) [50] | ACP@TS (0.8% TA) | In vitro and in vivo | Human dentin, rat model | ACP@TS treatment and in vivo evaluation in rats |
|
| Studies | Treatment | Study Type | Subject | Assessment | Key Outcomes |
|---|---|---|---|---|---|
| Yu et al. (1993) [51] | TA-F (0.5% TA) | In vitro | Human enamel | Rinsed after treatment with TA-F |
|
| Yu et al. (1995) [52] | TA-F (1% TA) | In vitro | Human enamel | Rinsed after treatment with TA-F |
|
| Yamaga et al. (1997) [53] | HY (20% TA) | Clinical | Exposed dentin of overdenture abutments | Denture base mixed with HY |
|
| Koide et al. (1997) [54] | 0.5% TA | In vitro | Type I dentinal collagen from bovines | TA application for 5 min, 1 h, and 1 day |
|
| Yee et al. (2009) [55] | TA (Mechhi Tea) | Clinical | Primary teeth | Applied to tooth surface with SDF |
|
| Yang et al. (2017) [56] | SAP-TA (1.7% TA) | In vitro and in vivo | Human enamel, rat caries model | SAP-TA added after enamel demineralization |
|
| Hertel et al. (2017) [17] | 1.7% TA | In situ and ex vivo | Enamel from bovines worn by volunteers as maxillary splints | TA application for 10 min |
|
| Huang et al. (2017) [18] | 0.4% TA | In vitro | Bovine enamel | TA application for 5 min in pH-cycling and polymicrobial biofilm model |
|
| Xi et al. (2020) [57] | 1% TA | In situ and ex vivo | Enamel from bovines worn by volunteers as maxillary splints | Rinsed with TA twice |
|
| Schestakow et al. (2020) [58] | 5% TA | In situ and ex vivo | Enamel from bovines worn by volunteers as maxillary splints | Rinsed with TA for four or five times |
|
| Schestakow et al. (2021) [59] | 5% TA | In situ and ex vivo | Dentin from bovines worn by volunteers as maxillary splints | Rinsed with TA for four or five times |
|
| Schestakow et al. (2022) [60] | 1% TA | In situ and ex vivo | Enamel from bovines worn by volunteers as maxillary splints | Rinsed with TA every 25 min |
|
| Zhen et al. (2022) [61] | TS@NaF (10% TA) | In vitro and in vivo | Bovine enamel, rodent caries model | Demineralized enamel immersed in TS@NaF, TS@NaF hydrogels applied to rodent molars for caries prevention |
|
| Kong et al. (2022) [62] | 1.7% TA | In vitro | Human dentin | Demineralized dentin sections and collagen membranes immersed in TA for 2 h |
|
| Selvaraj et al. (2024) [63] | TA-AuNP (0.5% TA) | In vitro | S. mutans | TA-AuNP added to BHI agar plate of bacterial biofilms |
|
| Studies | Treatment | Study Type | Subject | Assessment | Key Outcomes |
|---|---|---|---|---|---|
| Yang et al. (2017) [64] | SAP-TA | In vitro | HA, MG63, and S. mutans | Coated with SAP-TA for 24 h |
|
| Weber et al. (2019) [65,66] | Siaq-TA (0.1% TA) | In vitro | SiO2, Au, HA, and Ti | Coated with Siaq-TA for 24 h |
|
| Steffi et al. (2019) [19] | 0.2% TA | In vitro | Ti, RAW 264.7, and preosteoclast cells | Coated with TA overnight |
|
| Geissler et al. (2019) [28] | 0.01% TA | In vitro | Ti, human osteoblasts, and S. aureus | Coated with TA for 2 or 24 h |
|
| Iqbal et al. (2020) [20] | TA/COL (0.01% TA) | In vitro | SiO2, hGFs, and S. aureus | Coated with TA/COL using the LBL technique (5 min deposition) |
|
| Li et al. (2020) [67] | TA-GO/Lys (0.1% TA) | In vitro | Glass slides, silicon wafers, and quartz plates, DPSCs, E. coli, and S. aureus | Coated with GO-Lys-TA using the LBL technique (10 min deposition) |
|
| Dong et al. (2021) [68] | pTA (2% TA) | In vitro | Ti, hGFs, l, E. coli, and S. aureus | Coated with the solution and irradiated by UV at 285 nm for 4 h |
|
| Wang et al. (2021) [69] | TA@HA/Lys (0.5% TA) | In vitro and in vivo | Cover glass, silicon wafer, and titanium rod, DPSCs, MC3T3-E1, E. coli, S. aureus, and New Zealand rabbits | Coated with TA@HA/Lys using the LBL technique (10 min deposition), implant inserted into rabbit femoral condyle |
|
| Weber et al. (2022) [70] | 0.1% TA | In vitro | Ti, hGFs | Coated with TA for 24 h |
|
| Weber et al. (2022) [71] | 0.1% TA | In vitro | Ti, C. albicans | Coated with TA for 24 h |
|
| Kim et al. (2022) [72] | CHX-loaded TA-PEG (0.5% TA) | In vitro | PMMA, MC3T3-E1 | Coated with CHX-loaded TA-PEG for 10, 20, 40, and 90 min |
|
| Liu et al. (2022) [73] | TA-nHA-PEG (0.2% TA) | In vitro and in vivo | Ti, BMSCs, S. aureus, E. coli, and rat model | Coated with TA-nHA-PEG using the LBL technique (4 h deposition), rod implanted in rat femur |
|
| Ren et al. (2022) [74] | TA/Sr2+ (1:1) | In vitro and in vivo | BMSCs, RAW264.7, and rat model | Cells cultured and treated with TA/Sr2+ (1 h), membrane inserted into rat alveolar bone |
|
| Li et al. (2023) [75] | TA-Ce-Mino (0.4% TA) | In vitro and in vivo | Ti, PEEK, hGFs, and rat model | Immersed in TA-Ce-Mino and vortexed for 30 s, Ti disk inserted into rat model |
|
| Shen et al. (2023) [76] | CS-GA/TAMP (40% TA) | In vitro and in vivo | Ti, hGFs, P. gingivalis, F. nucleatum, and pig model | Hydrogel samples soaked in lysozyme solution for 21 days, hydrogels injected in pig mandible around implant and exposed to bacterial suspensions |
|
| Zhao et al. (2023) [77] | TA-OGP@(RGD)n (0.1% TA) | In vitro and in vivo | Ti, MC3T3-E1, and rat model | Coated with TA-OGP@(RGD)n using the LBL technique (20 min deposition), Ti implants inserted into rat model |
|
| Studies | Treatment | Study Type | Subject | Assessment | Key Outcomes |
|---|---|---|---|---|---|
| Homer et al. (1990) [78] | 0.000001%, 0.00001%, 0.0001%, 0.001%, and 0.01% TA | In vitro | B. gingivalis, B. intermedius, and T. denticola | Bacteria strains cultivated with TA |
|
| Darvin et al. (2015) [79] | 3.4–17% TA | In vitro | GSCC | TA applied to cells for 24–48 h, cell cycle and pathways examined |
|
| Sheng et al. (2018) [80] | 0.002% and 0.008% TA | In vitro | Human normal OLK and oral cancer cells | Cells treated with TA for 48 h after doxorubicin induction. |
|
| Shahbazi et al. (2020) [81] | PVA-TA, PATA (50% TA) | In vitro | Customized wearable holder, S. aureus | Hydrogel performance tested in drug delivery device |
|
| Lengert et al. (2021) [82] | AgNPs-TA (0.1% TA) | In vivo | Rat lower jaw anterior incisors region | Hydrogel capsule suspension applied to the rat model |
|
| Zhu et al. (2022) [23] | TA-SF-DP, TSD (10% TA) | In vitro and in vivo | Porcine buccal mucosa, rat tail truncation model, and coronal plane through extraction socket | Hydrogel tested in vitro and applied to animal models |
|
| Liu et al. (2022) [24] | CS-GOx-TA (0.2%, 0.4%, 0.6%, 0.8%, and 1% TA) | In vitro | MC3T3-E1, RAW264.7, and P. gingivalis | Hydrogel tested in vitro |
|
| Ding et al. (2022) [83] | isoG-TA (TA: 1/10 of isoG molar) | In vitro and in vivo | Mice anti-OLK model | Hydrogel tested in vitro and applied to the animal model |
|
| Zhao et al. (2022) [84] | spheroid@[Fe3+-TA] (4% TA) | In vitro | PDLSCs, P. gingivalis | PDLSCs spheroid coated with Fe3+-TA coordination network |
|
| Zhu et al. (2022) [85] | GNT (7.5%, 15% and 30% TA) | In vitro and in vivo | S. aureus, E. coli, rat and rabbit full-thickness oral mucosa model | Hydrogel tested in vitro and applied to the animal model |
|
| Shi et al. (2022) [86] | G-TA@Mn2+ | In vitro and in vivo | DCs/macrophages, mice anti-OLK model | Hydrogel tested in vitro and applied to the animal model |
|
| Cheng et al. (2023) [87] | SF/TA (10% TA) | Ex vivo | NIH3T3, S. aureus, E. coli, and rat oral mucosa model | Patch applied to rat oral mucosa for 5 min |
|
| He et al. (2023) [88] | PGA/TA-NPs (0.3% TA) | In vitro and in vivo | RAW264.7, L929, S. aureus, E. coli, and rat oral ulcer model | Drug delivery system tested in vitro and applied to the rat model |
|
| Liu et al. (2023) [89] | ZPTA-G/HMA (0.02% TA) | In vitro and in vivo | RAW264.7, S. aureus, E. coli, C. albicans, and rat oral ulcer model | Hydrogel tested in vitro and applied to the animal model |
|
| Studies | Treatment | Study Type | Subject | Assessment | Key Outcomes |
|---|---|---|---|---|---|
| Bitter (1989) [25] | 25% TA | In vitro | Human teeth | Root canal preparation and irrigation with H2O2 and NaClO, followed by TA irrigation for 10–90 s |
|
| Raiden et al. (1997) [90] | 25% TA | In vitro | Human teeth | Root canal cleaning with TA for 20 s, followed by dental cement filling and immersion in Indian ink |
|
| Raiden et al. (1998) [91] | 25% TA | In vitro | Human teeth | Root canal cleaning with TA for 20 s, followed by dental cement filling and dowel space preparation |
|
| Yoshikawa et al. (1998) [92] | TA-α-TCP (5% TA) | In vitro | Human teeth | Root canal filled with TA-α-TCP |
|
| Yoshikawa et al. (2000, 2003) [93,94] | TA-α-TCP (5% TA) | In vitro and in vivo | Rat teeth | TA-α-TCP placed in periapical area of mechanical injury |
|
| Yoshikawa et al. (2001) [95] | TA-α-TCP (5% TA) | In vitro | Rat teeth | TA-α-TCP positioned over residual pulp after molar pulp cutting |
|
| Nakamura et al. (2011) [96] | HY (20% TA) | In vitro | Rat clonal dental pulp cell line RPC-C2A | Cells cultured with cement extract for 24 h |
|
| Christopher et al. (2016) [97] | 10% TA | In vitro | Human teeth | Root canal preparation followed by TA irrigation for 10 min and obturation |
|
| Kharouf et al. (2021) [98] | MTA-TA (6%, 12.5%, 18.75%, and 25% TA) | In vitro | MTA | MTA-TA preparation and performance examined |
|
| Wu et al. (2022) [99] | CSC-TA (1%, 5%, and 10% TA) | In vitro | MG63, E. coli, and S. aureus | TA mixed with bioactive calcium silicate as liquid phase |
|
| Louvrier et al. (2022) [100] | PLA/PCL-TA (particles) | In vitro | DPSCs | PLA/PCL-TA inserted into biomimetic bioreactor mimicking human tooth root canal |
|
| Zhou et al. (2024) [101] | HPCH/TA (7.5 mg/mL) | In vitro and in vivo | hDPCs, S. mutans, E. faecalis, and rat model | HPCH/TA hydrogel tested in vitro and evaluated as pulp capping material in vivo |
|
| Studies | Treatment | Study Type | Application Type | Subject | Assessment | Key Outcomes |
|---|---|---|---|---|---|---|
| Nordbö et al. (1983) [26] | 0.006%, 0.05%, 0.1%, and 0.2% TA | In vitro and in vivo | Dental staining | Acrylic resin surfaces | Plaque formation on acrylic resin, soaked in TA |
|
| Kageyama et al. (1985) [102] | TA-UA (5% TA) | In vitro | Observing ultrastructural carbohydrates | Odontoblast, predentin, and dentin matrix of rat | Observing ultrastructural carbohydrates within cells and microscopic structures |
|
| Takagi et al. (1989) [103] | TA-UA (5% TA) | In vitro | Observing ultrastructural cytochemical properties | Periodontal ligament of Alligator mississippiensis | Observing ultrastructural cytochemical properties of elastic elements in the periodontal ligament |
|
| Ishizeki et al. (1990) [104] | 2% TA | In vitro | Fixing | AMF of mouse tooth germs | Microstructures examined by fixation with a substance containing TA |
|
| Kim et al. (1994) [105] | Acetone-TA (1% TA) | In vitro | Fixing | Ameloblastsin molar tooth germs of neonatal rats | Microstructures examined by fixation and freeze-substituted with a substance containing TA |
|
| Joiner et al. (2004) [106] | 1% TA | In vitro and in situ | Dental staining | HA surface | Ellipsometry used to measure the coloring effect of TA on dental acquired film |
|
| Haruyama et al. (2018, 2022) [107,108] | 0.1% TA | In vitro | Dental staining | Bovine teeth | Enamel polished, leveled, and stained with FeCl3 and TA |
|
| Marquillas et al. (2020) [109] | 8% TA | In vitro | Dental staining | Bovine teeth | TA solution used as dyeing model |
|
| Asghar et al. (2022) [110] | 5%, 10%, and 15% TA | In vitro | Cleaning agent | Bovine teeth | TA used to modify SDF |
|
| Cen et al. (2023) [111] | TA-CPC (0.5% TA) | In vitro | Cleaning agent | Orthodontic aligners | Aligners stained by coffee and immersed in the complex |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ding, X.; Zhang, G.; Yiu, C.K.Y.; Li, X.; Shan, Z. Unleashing the Potential of Tannic Acid in Dentistry: A Scoping Review of Applications. Bioengineering 2025, 12, 438. https://doi.org/10.3390/bioengineering12050438
Ding X, Zhang G, Yiu CKY, Li X, Shan Z. Unleashing the Potential of Tannic Acid in Dentistry: A Scoping Review of Applications. Bioengineering. 2025; 12(5):438. https://doi.org/10.3390/bioengineering12050438
Chicago/Turabian StyleDing, Xiaoqian, Guanning Zhang, Cynthia Kar Yung Yiu, Xin Li, and Zhiyi Shan. 2025. "Unleashing the Potential of Tannic Acid in Dentistry: A Scoping Review of Applications" Bioengineering 12, no. 5: 438. https://doi.org/10.3390/bioengineering12050438
APA StyleDing, X., Zhang, G., Yiu, C. K. Y., Li, X., & Shan, Z. (2025). Unleashing the Potential of Tannic Acid in Dentistry: A Scoping Review of Applications. Bioengineering, 12(5), 438. https://doi.org/10.3390/bioengineering12050438








