Pre-Clinical Cell Therapeutic Approaches for Repair of Volumetric Muscle Loss
Abstract
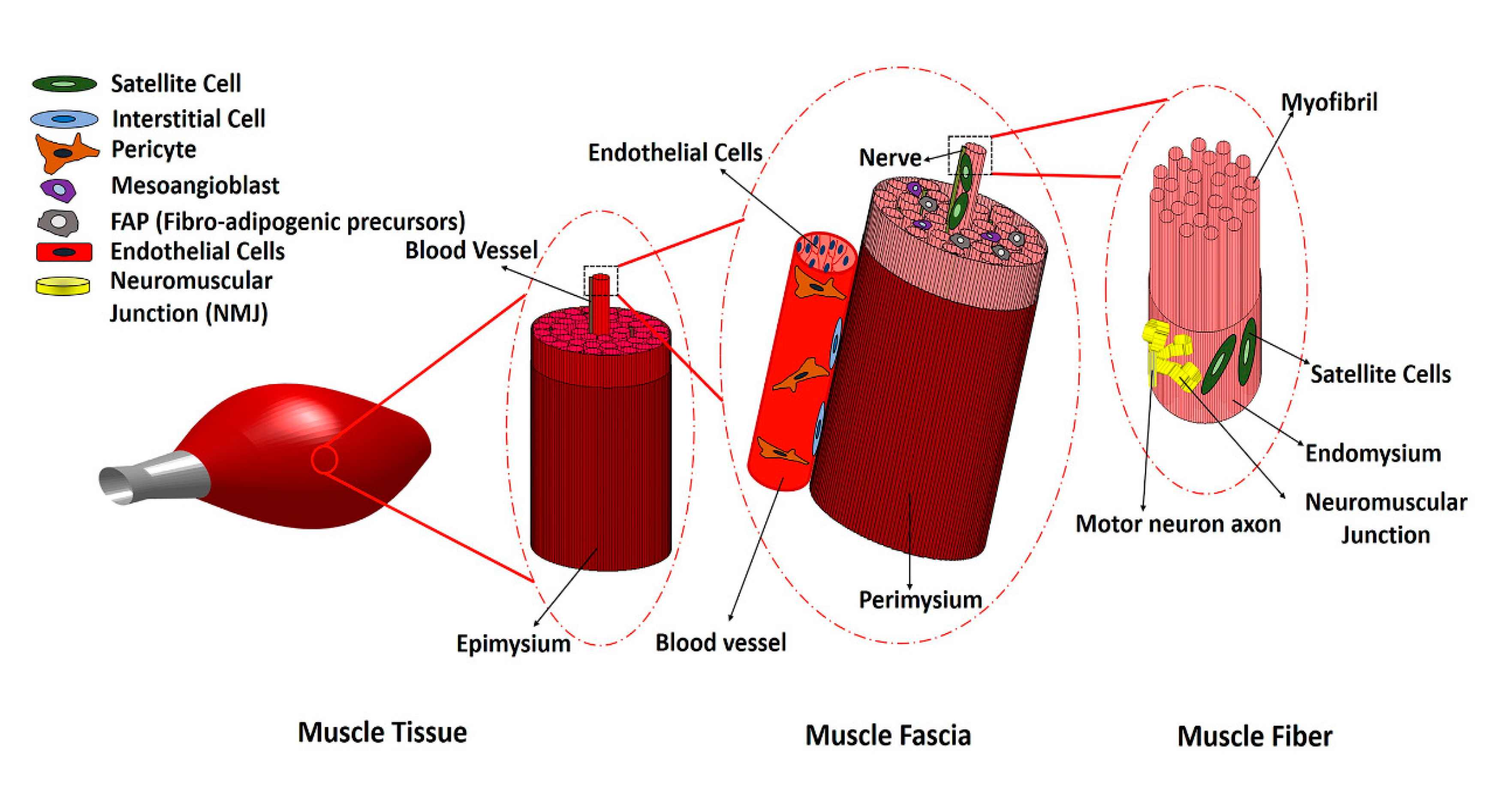
:1. Introduction
2. Main Myogenic Cell Sources
3. Non-Myogenic Cells
4. Preclinical VML Treatment Studies
4.1. Animal Models of VML
4.2. Cell-Seeded Scaffolds for Preclinical Treatment of VML
4.3. MuSC-Based Therapies for Preclinical Treatment of VML
4.4. MSC-Based Therapies for Preclinical Treatment of VML
5. Conclusions and Future Perspectives
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Corona, B.T.; Rivera, J.C.; Owens, J.G.; Wenke, J.C.; Rathbone, C.R. Volumetric muscle loss leads to permanent disability following extremity trauma. J. Rehabil. Res. Dev. 2015, 52, 785–792. [Google Scholar] [CrossRef] [PubMed]
- Garg, K.; Ward, C.L.; Hurtgen, B.J.; Wilken, J.M.; Stinner, D.J.; Wenke, J.C.; Owens, J.G.; Corona, B.T. Volumetric muscle loss: Persistent functional deficits beyond frank loss of tissue. J. Orthop. Res. 2014, 33, 40–46. [Google Scholar] [CrossRef] [PubMed]
- Corona, B.T.; Wenke, J.C.; Ward, C.L. Pathophysiology of volumetric muscle loss injury. Cells Tissues Organs 2016, 202, 180–188. [Google Scholar] [CrossRef]
- Lin, C.-H.; Lin, Y.-T.; Yeh, J.-T.; Chen, C.-T. Free Functioning muscle transfer for lower extremity posttraumatic composite structure and functional defect. Plast. Reconstr. Surg. 2007, 119, 2118–2126. [Google Scholar] [CrossRef]
- Dziki, J.; Badylak, S.; Yabroudi, M.; Sicari, B.; Ambrosio, F.; Stearns, K.; Turner, N.; Wyse, A.; Boninger, M.L.; Brown, E.H.P.; et al. An acellular biologic scaffold treatment for volumetric muscle loss: Results of a 13-patient cohort study. NPJ Regen. Med. 2016, 1, 16008. [Google Scholar] [CrossRef] [PubMed]
- Ding, S.; Wang, F.; Liu, Y.; Li, S.; Zhou, G.; Hu, P. Characterization and isolation of highly purified porcine satellite cells. Cell Death Discov. 2017, 3, 17003. [Google Scholar] [CrossRef] [Green Version]
- Pantelic, M.N.; Larkin, L.M.; Pantellic, M.N. Stem cells for skeletal muscle tissue engineering. Tissue Eng. Part B Rev. 2018, 24, 373–391. [Google Scholar] [CrossRef]
- Garcia, J.M.S.; Panitch, A.; Calve, S. Functionalization of hyaluronic acid hydrogels with ECM-derived peptides to control myoblast behavior. Acta Biomater. 2019, 84, 169–179. [Google Scholar] [CrossRef]
- Patel, K.H.; Dunn, A.J.; Talovic, M.; Haas, G.J.; Marcinczyk, M.; Elmashhady, H.; Kalaf, E.A.G.; Sell, S.A.; Garg, K.; Growney, E. Aligned nanofibers of decellularized muscle ECM support myogenic activity in primary satellite cells in vitro. Biomed. Mater. 2019, 14, 035010. [Google Scholar] [CrossRef]
- Prueller, J.; Mannhardt, I.; Eschenhagen, T.; Zammit, P.S.; Figeac, N. Satellite cells delivered in their niche efficiently generate functional myotubes in three-dimensional cell culture. PLoS ONE 2018, 13, e0202574. [Google Scholar] [CrossRef] [Green Version]
- Gilbert, P.M.; Havenstrite, K.L.; Magnusson, K.E.G.; Sacco, A.; Leonardi, N.A.; Kraft, P.; Nguyen, N.K.; Thrun, S.; Lutolf, M.P.; Blau, H.M. Substrate elasticity regulates skeletal muscle stem sell self-renewal in culture. Science 2010, 329, 1078–1081. [Google Scholar] [CrossRef] [Green Version]
- Mori, R.; Kamei, N.; Okawa, S.; Nakabayashi, A.; Yokota, K.; Higashi, Y.; Ochi, M. Promotion of skeletal muscle repair in a rat skeletal muscle injury model by local injection of human adipose tissue-derived regenerative cells. J. Tissue Eng. Regen. Med. 2012, 9, 1150–1160. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Berebichez-Fridman, R.; Montero-Olvera, P.R. Sources and Clinical Applications of Mesenchymal Stem Cells. Sultan Qaboos Univ. Med. J. 2018, 18, 264–277. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jacobs, S.A.; Roobrouck, V.D.; Verfaillie, C.M.; Van Gool, S.W. Immunological characteristics of human mesenchymal stem cells and multipotent adult progenitor cells. Immunol. Cell Biol. 2013, 91, 32–39. [Google Scholar] [CrossRef] [PubMed]
- Kodaka, Y.; Rabu, G.; Asakura, A. Skeletal Muscle Cell Induction from Pluripotent Stem Cells. Stem Cells Int. 2017, 2017, 1–16. [Google Scholar] [CrossRef] [PubMed]
- del Carmen Ortuño-Costela, M.; García-López, M.; Cerrada, V.; Gallardo, M.E. iPSC s: A powerful tool for skeletal muscle tissue engineering. J. Cell. Mol. Med. 2019, 23, 3784–3794. [Google Scholar] [CrossRef] [PubMed]
- Rao, L.; Qian, Y.; Khodabukus, A.; Ribar, T.; Bursac, N. Engineering human pluripotent stem cells into a functional skeletal muscle tissue. Nat. Commun. 2018, 9, 126. [Google Scholar] [CrossRef] [PubMed]
- De Micheli, A.J.; Laurilliard, E.J.; Heinke, C.L.; Ravichandran, H.; Fraczek, P.; Soueid-Baumgarten, S.; De Vlaminck, I.; Elemento, O.; Cosgrove, B.D. Single-Cell Analysis of the Muscle Stem Cell Hierarchy Identifies Heterotypic Communication Signals Involved in Skeletal Muscle Regeneration. Cell Rep. 2020, 30, 3583–3595.e5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dunn, A.; Talovic, M.; Patel, K.; Patel, A.; Marcinczyk, M.; Garg, K. Biomaterial and stem cell-based strategies for skeletal muscle regeneration. J. Orthop. Res. 2019, 37, 1246–1262. [Google Scholar] [CrossRef] [PubMed]
- Yin, H.; Price, F.; Rudnicki, M.A. Satellite Cells and the Muscle Stem Cell Niche. Physiol. Rev. 2013, 93, 23–67. [Google Scholar] [CrossRef] [Green Version]
- Zheng, B.; Cao, B.; Crisan, M.; Sun, B.; Li, G.; Logar, A.; Yap, S.; Pollett, J.B.; Drowley, L.; Cassino, T.; et al. Prospective identification of myogenic endothelial cells in human skeletal muscle. Nat. Biotechnol. 2007, 25, 1025–1034. [Google Scholar] [CrossRef] [PubMed]
- Sirabella, D.; De Angelis, L.; Berghella, L. Sources for skeletal muscle repair: From satellite cells to reprogramming. J. Cachexia Sarcopenia Muscle 2013, 4, 125–136. [Google Scholar] [CrossRef]
- Torrente, Y.; Belicchi, M.; Marchesi, C.; D’Antona, G.; Cogiamanian, F.; Pisati, F.; Gavina, M.; Giordano, R.; Tonlorenzi, R.; Fagiolari, G.; et al. Autologous transplantation of muscle-derived CD133+ stem cells in Duchenne muscle patients. Cell Transplant. 2007, 16, 563–577. [Google Scholar] [CrossRef]
- Asakura, A.; Seale, P.; Girgis-Gabardo, A.; Rudnicki, M.A. Myogenic specification of side population cells in skeletal muscle. J. Cell Boil. 2002, 159, 123–134. [Google Scholar] [CrossRef] [PubMed]
- Joe, A.W.B.; Yi, L.; Natarajan, A.; Le Grand, F.; So, L.; Wang, J.; Rudnicki, M.A.; Rossi, F.M. Muscle injury activates resident fibro/adipogenic progenitors that facilitate myogenesis. Nature 2010, 12, 153–163. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wosczyna, M.N.; Rando, T.A. A Muscle Stem Cell Support Group: Coordinated Cellular Responses in Muscle Regeneration. Dev. Cell 2018, 46, 135–143. [Google Scholar] [CrossRef] [Green Version]
- Ostrovidov, S.; Ahadian, S.; Ramon-Azcon, J.; Hosseini, V.; Fujie, T.; Parthiban, S.P.; Shiku, H.; Matsue, T.; Kaji, H.; Ramalingam, M. Three-dimensional co-culture of C2C12/PC12 cells improves skeletal muscle tissue formation and function. J. Tissue Eng. Regen. Med. 2017, 11, 582–595. [Google Scholar] [CrossRef]
- Biferali, B.; Proietti, D.; Mozzetta, C.; Madaro, L. Fibro–Adipogenic Progenitors Cross-Talk in Skeletal Muscle: The Social Network. Front. Physiol. 2019, 10, 10. [Google Scholar] [CrossRef]
- Gao, F.; Chiu, S.M.; Motan, D.A.L.; Zhang, Z.; Chen, L.; Ji, H.-L.; Tse, H.-F.; Fu, Q.-L.; Lian, Q. Mesenchymal stem cells and immunomodulation: Current status and future prospects. Cell Death Dis. 2016, 7, 2062. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.H.; Kim, I.; Seol, Y.-J.; Ko, I.K.; Yoo, J.J.; Atala, A.; Lee, S.J. Neural cell integration into 3D bioprinted skeletal muscle constructs accelerates restoration of muscle function. Nat. Commun. 2020, 11, 1–12. [Google Scholar] [CrossRef]
- Laternser, S.; Keller, H.; Leupin, O.; Rausch, M.; Graf-Hausner, U.; Rimann, M. A Novel Microplate 3D Bioprinting Platform for the Engineering of Muscle and Tendon Tissues. SLAS Technol. Transl. Life Sci. Innov. 2018, 23, 599–613. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Latroche, C.; Weiss-Gayet, M.; Muller, L.; Gitiaux, C.; Leblanc, P.; Liot, S.; Ben-Larbi, S.; Abou-Khalil, R.; Verger, N.; Bardot, P.; et al. Coupling between Myogenesis and Angiogenesis during Skeletal Muscle Regeneration is Stimulated by Restorative Macrophages. Stem Cell Rep. 2017, 9, 2018–2033. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sarrafian, T.L.; Bodine, S.C.; Murphy, B.; Grayson, J.K.; Stover, S.M. Extracellular matrix scaffolds for treatment of large volume muscle injuries: A review. Veter. Surg. 2018, 47, 524–535. [Google Scholar] [CrossRef] [PubMed]
- Anderson, S.E.; Han, W.M.; Srinivasa, V.; Mohiuddin, M.; Ruehle, M.A.; Moon, J.Y.; Shin, E.; Emeterio, C.L.S.; Ogle, M.E.; Botchwey, E.A.; et al. Determination of a Critical Size Threshold for Volumetric Muscle Loss in the Mouse Quadriceps. Tissue Eng. Part C Methods 2019, 25, 59–70. [Google Scholar] [CrossRef]
- Skuk, D.; Caron, N.J.; Goulet, M.; Roy, B.; Tremblay, J.P. Resetting the Problem of Cell Death Following Muscle-Derived Cell Transplantation: Detection, Dynamics and Mechanisms. J. Neuropathol. Exp. Neurol. 2003, 62, 951–967. [Google Scholar] [CrossRef] [Green Version]
- Wolf, M.T.; Dearth, C.L.; Sonnenberg, S.B.; Loboa, E.G.; Badylak, S.F. Naturally derived and synthetic scaffolds for skeletal muscle reconstruction. Adv. Drug Deliv. Rev. 2015, 84, 208–221. [Google Scholar] [CrossRef] [Green Version]
- Corona, B.T.; Ward, C.L.; Baker, H.B.; Walters, J.T.R.; Christ, G.J. Implantation ofIn VitroTissue Engineered Muscle Repair Constructs and Bladder Acellular Matrices Partially RestoreIn VivoSkeletal Muscle Function in a Rat Model of Volumetric Muscle Loss Injury. Tissue Eng. Part A 2013, 20, 705–715. [Google Scholar] [CrossRef] [Green Version]
- Nakayama, K.H.; Quarta, M.; Paine, P.; Alcazar, C.; Karakikes, I.; Garcia, V.; Abilez, O.; Calvo, N.S.; Simmons, C.S.; Rando, T.A.; et al. Treatment of volumetric muscle loss in mice using nanofibrillar scaffolds enhances vascular organization and integration. Commun. Biol. 2019, 2, 170. [Google Scholar] [CrossRef]
- Borselli, C.; Cezar, C.A.; Shvartsman, D.; VanDenburgh, H.H.; Mooney, D.J. The role of multifunctional delivery scaffold in the ability of cultured myoblasts to promote muscle regeneration. Biomaterials 2011, 32, 8905–8914. [Google Scholar] [CrossRef] [Green Version]
- Grasman, J.; Zayas, M.J.; Page, R.L.; Pins, G.D. Biomimetic scaffolds for regeneration of volumetric muscle loss in skeletal muscle injuries. Acta Biomater. 2015, 25, 2–15. [Google Scholar] [CrossRef] [Green Version]
- Matthias, N.; Hunt, S.D.; Wu, J.; Lo, J.; Callahan, L.A.S.; Li, Y.; Huard, J.; Darabi, R. Volumetric muscle loss injury repair using in situ fibrin gel cast seeded with muscle-derived stem cells (MDSCs). Stem Cell Res. 2018, 27, 65–73. [Google Scholar] [CrossRef]
- Quarta, M.; Cromie, M.; Chacon, R.; Blonigan, J.; Garcia, V.; Akimenko, I.; Hamer, M.; Paine, P.; Stok, M.; Shrager, J.B.; et al. Bioengineered constructs combined with exercise enhance stem cell-mediated treatment of volumetric muscle loss. Nat. Commun. 2017, 8, 15613. [Google Scholar] [CrossRef]
- Passipieri, J.A.; Baker, H.; Siriwardane, M.; Ellenburg, M.D.; Vadhavkar, M.; Saul, J.M.; Tomblyn, S.; Burnett, L.; Christ, G.J. Keratin Hydrogel Enhances In Vivo Skeletal Muscle Function in a Rat Model of Volumetric Muscle Loss. Tissue Eng. Part A 2017, 23, 556–571. [Google Scholar] [CrossRef] [PubMed]
- Li, M.-T.; Ruehle, M.A.; Stevens, H.Y.; Servies, N.; Willett, N.J.; Karthikeyakannan, S.; Warren, G.L.; Guldberg, R.E.; Krishnan, L. Skeletal Myoblast-Seeded Vascularized Tissue Scaffolds in the Treatment of a Large Volumetric Muscle Defect in the Rat Biceps Femoris Muscle. Tissue Eng. Part A 2017, 23, 989–1000. [Google Scholar] [CrossRef] [PubMed]
- Kesireddy, V. Evaluation of adipose-derived stem cells for tissue-engineered muscle repair construct-mediated repair of a murine model of volumetric muscle loss injury. Int. J. Nanomed. 2016, 11, 1461–1473. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qiu, X.; Liu, S.; Zhang, H.; Zhu, B.; Su, Y.; Zheng, C.; Tian, R.; Wang, M.; Kuang, H.; Zhao, X.; et al. Mesenchymal stem cells and extracellular matrix scaffold promote muscle regeneration by synergistically regulating macrophage polarization toward the M2 phenotype. Stem Cell Res. Ther. 2018, 9, 88. [Google Scholar] [CrossRef]
- Lalegül-Ülker, Ö.; Şeker, Ş.; Elçin, A.E.; Elçin, Y.M. Encapsulation of bone marrow-MSCs in PRP-derived fibrin microbeads and preliminary evaluation in a volumetric muscle loss injury rat model: Modular muscle tissue engineering. Artif. Cells Nanomed. Biotechnol. 2018, 47, 10–21. [Google Scholar] [CrossRef] [Green Version]
- Huang, H.; Liu, J.; Hao, H.; Chen, D.; Zhizhong, L.; Li, M.; Song, H.; Xiang, R.; Jiang, C.; Fu, X.; et al. Preferred M2 Polarization by ASC-Based Hydrogel Accelerated Angiogenesis and Myogenesis in Volumetric Muscle Loss Rats. Stem Cells Int. 2017, 2017, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Gilbert-Honick, J.; Ginn, B.; Zhang, Y.; Salehi, S.; Wagner, K.R.; Mao, H.-Q.; Grayson, W.L. Adipose-derived Stem/Stromal Cells on Electrospun Fibrin Microfiber Bundles Enable Moderate Muscle Reconstruction in a Volumetric Muscle Loss Model. Cell Transplant. 2018, 27, 1644–1656. [Google Scholar] [CrossRef] [Green Version]
- Zhang, D.; Yan, K.; Zhou, J.; Xu, T.; Xu, M.; Lin, J.; Bai, J.; Ge, G.; Hu, D.; Si, W.; et al. Myogenic differentiation of human amniotic mesenchymal cells and its tissue repair capacity on volumetric muscle loss. J. Tissue Eng. 2019, 10, 2041731419887100. [Google Scholar] [CrossRef] [Green Version]
- Choi, Y.-J.; Jun, Y.-J.; Kim, D.Y.; Yi, H.-G.; Chae, S.-H.; Kang, J.; Lee, J.; Gao, G.; Kong, J.-S.; Jang, J.; et al. A 3D cell printed muscle construct with tissue-derived bioink for the treatment of volumetric muscle loss. Biomaterials 2019, 206, 160–169. [Google Scholar] [CrossRef]
- Boldrin, L.; Malerba, A.; Vitiello, L.; Cimetta, E.; Piccoli, M.; Messina, C.; Gamba, P.; Elvassore, N.; De Coppi, P. Efficient delivery of human single fiber-derived muscle precursor cells via biocompatible scaffold. Cell Transplant. 2008, 17, 577–584. [Google Scholar] [CrossRef]
- Baker, H.; Passipieri, J.A.; Siriwardane, M.; Ellenburg, M.D.; Vadhavkar, M.; Bergman, C.R.; Saul, J.M.; Tomblyn, S.; Burnett, L.; Christ, G.J. Cell and Growth Factor-Loaded Keratin Hydrogels for Treatment of Volumetric Muscle Loss in a Mouse Model. Tissue Eng. Part A 2017, 23, 572–584. [Google Scholar] [CrossRef]
- Rodriques, S.G.; Stickels, R.R.; Goeva, A.; Martin, C.A.; Murray, E.; Vanderburg, C.R.; Welch, J.; Chen, L.M.; Chen, F.; Macosko, E.Z. Slide-seq: A scalable technology for measuring genome-wide expression at high spatial resolution. Science 2019, 363, 1463–1467. [Google Scholar] [CrossRef]
- Wang, J.; Khodabukus, A.; Rao, L.; VanDusen, K.; Abutaleb, N.; Bursac, N. Engineered skeletal muscles for disease modeling and drug discovery. Biomaterials 2019, 221, 119416. [Google Scholar] [CrossRef]
| Cell Types | Markers | Location | Advantages | Disadvantages | Reference |
|---|---|---|---|---|---|
| MuSCs | Pax7+, CD56+, MyoD+ | Under basal lamina of muscle fibers. | Critical to native skeletal muscle regeneration. High myogenic potential. | Isolation is invasive and low yield. Loss of self-renewal potential during in vitro expansion. Loss of differentiation potential after in vivo transplantation. | [7,19,20] |
| Mesenchymal stem cells (MSCs) | CD90+, CD44+, CD29+, CD105+, CD13+, CD73+, CD166+, CD45−, CD34−, CD14− | Adipose tissue, bone marrow (BM), umbilical cord (UC). | Abundance of adipose tissue. Ease of isolation from adipose tissue. Low expression of MHC-I and MHC-II Immunomodulatory effect. | Invasive isolation for BM-MSCs. Poor myogenic differentiation capacity. | [7,19] |
| Myo-endothelial cells | CD34+, CD144+, CD56+, CD31+, CD45− | Periphery of myofibers close to blood vessels. | Have both angiogenic and myogenic capacity. | Laborious isolation and purification process. Limited literature on their role in skeletal muscle regeneration. | [21] |
| Mesoangioblasts | CD34+, Sca-1+, CD31+, c-Kit+, CD45− | Walls of microvessels. | High proliferative capacity in vitro. Multipotent cells with potential to differentiate into skeletal muscle | Invasive isolation procedure. Lack of studies for VML treatment. | [22] |
| Pericytes | CD146+, NG2+, ALP+, PDGFR-β+ | Periphery of capillaries and microvessels. | Pericyte myogenesis naturally occurs during development and regeneration of muscle. High muscle differentiation potential. Lack of MHCII expression. | Limited literature on their potential in skeletal muscle regeneration and VML. | [7,19] |
| CD133+ progenitor cells | CD133+, CD34+/−, CD90+/−, CD146+ | Periphery of myofibers close to blood vessels. | Availability and ease of purification from peripheral blood Myogenic and angiogenic capacity. | Reduction of myogenic potential following in vitro culture. | [19,23] |
| Induced pluripotent stem cells (iPSCs) | Oct4+, Sox2+, KLF4+, and c-Myc+ | All tissues, mainly skin. | Unlimited self-renewal in vitro. Patient-derived autologous cells. Myogenic differentiation capacity. | Risk of tumorigenicity and genetic instability. | [7,19] |
| Embryonic stem cells (ESCs) | Oct4+, Sox2+, KLF4+, and c-Myc+ | Inner cell mass of blastocyst. | Unlimited self-renewal in vitro. Myogenic differentiation capacity | Ethical concerns Inefficient isolation process. Risk of tumorigenicity. Risk associated with immune response. | [7] |
| Muscle side population cells (SPs) | CD45−, c-Kit−, Sca1+, ABCG2+, Pax7−, Myf5−, Desmin− | Interstitial space of skeletal muscle. | Myogenic differentiation capacity in vivo. | Low availability Lack of specific phenotypic markers. Poor myogenic differentiation in vitro. Limited literature on their potential for skeletal muscle regeneration and VML. | [20,24] |
| Cell Type | In Vitro Findings | Animal Model | Delivery Technique | In Vivo Findings | Reference |
|---|---|---|---|---|---|
| MPCs ASCs | - | Murine LD muscle (50% defect). | Cells seeded in Bladder acellular matrix (BAM) scaffolds. | Histological and immunohistochemical analysis shows ADSCs could create regenerated muscle comparable to MPCs seeded scaffolds, but mainly through participation in vascularization. | [45] |
| Human UC-MSCs | - | Rat TA muscle (20% defect). | Placing cells in aggregate in the muscle defect with and without decellularized porcine heart ECM powder. | Histological analysis and mechanical function evaluation show MSCs and decellularized ECM have a synergistic effect on promoting skeletal muscle regeneration. | [46] |
| Combination of MuSCs, ECs, FAPs, hematopoietic cells, fibroblast like cells | Bioluminescence imaging (BLI) measurements demonstrated viability was significantly enhanced in the presence of support cells. Ex vivo force measurement shows force recovery reaches up to 90% of the uninjured muscle. | Murine TA muscle (40% defect). | Decellularized murine TA ECM-based hydrogel. | The combination of cells with scaffolds could generate functional vascularized muscle tissue in VML models; however, innervation and muscle force are not sufficient, yet could be enhanced by exercise. | [42] |
| Human skeletal muscle cells (hSKMCs) | Printed 3D cell constructs demonstrate high cell viability (>90%), differentiation, myotube formation and contractility. | Rat TA muscle (40% defect) | Cell-laden muscle decellularized ECM (mdECM) bioink. | Pre-vascularized 3D cell printed muscle constructs improve muscle regeneration, vascularization and innervation, as well as 85% of functional recovery. | [51] |
| ASCs | ASCs proliferate and align on fibers with acceptable cell viability, but do not fully express myotube characterization and myogenesis fails after 2 months in vitro. | Murine TA and extensor digitorum longus (EDL) removal. | Cells-seeded electrospun fibrin scaffold. | ASCs combined with electrospun fibrin microfibers demonstrate more tissue regeneration in vivo compared with acellular fibers, but limited expression of myogenic markers in ASCs is observed. | [49] |
| Human MPCs | - | Murine TA muscle. | Poly-lactic-glycolic acid (PLGA) 3D scaffold. | Scaffolds increase the viability of cells in vivo and regeneration of muscle is enhanced following 1 and 4 week implantation compared to direct cell injection. | [52] |
| Rat Bone-marrow MSCs | - | Rat biceps femoris resection size: 8 × 4 × 4 mm3. | Fibrin-based microbeads. | Fibrin microbeads with and without MSCs accelerate muscle regeneration and prevent scar formation; MSCs shorten the regeneration period. Sham group has in incomplete repair and fibrotic scar formation. | [47] |
| Rat ASCs | - | Rat TA muscle resection size: 10 × 5 × 3 mm3. | Type I hydrogel. | ASCs encapsulated in hydrogel reduced inflammation and collagen deposition and accelerated muscle regeneration and angiogenesis compared with the hydrogel group. | [48] |
| Human ASCs | Viability and growth of ASCs on electrospun fibers were assessed by Live/Dead and PicoGreen assays for up to 21 days. After 2 months in culture, both induced and uninduced ASCs formed elongated and aligned fibers on electrospun fibers and expressed high levels of desmin, but they expressed low and non-nuclear Myogenin and could not fully recapitulate myotube formation. | Removal of TA and EDL muscles from the anterior tibial compartment in immunodeficient mice. | Electrospun fibrin hydrogel microfiber bundles. | ASC-seeded fibers exhibited up to four times higher volume retention than acellular fibers and lower levels of fibrosis. Unlike acellular scaffolds, ASC-seeded scaffolds showed mature muscle cells. | [49] |
| Human amniotic MSCs | Results of Live/Dead test and immunofluorescence staining of desmin and MyoD showed that the cell viability and induction of the myogenic differentiation of hAMCs by 5-Aza was not affected by GelMA gel. | Sprague Dawley (SD) rats 5 mm diameter muscle defect in TA muscle using a hole punch. | GelMA gel. | Results showed 5-Aza induced cells in GelMA reduced the scar formation and increased the vascularization 2 weeks and 4 weeks post-implantation compared to blank and GelMA groups. | [50] |
| Microvessel fragment (MVF) construct with myoblasts (MVF + Myoblasts) | Live/Dead assay demonstrates high viability of microvessels and seeded myoblasts and immunofluorescent staining shows microvessel networks increase more in MVF-Myoblast constructs than in MVF-only constructs. | 12 mm biopsy punch in biceps femoris muscle of Sprague Dawley rats. | Collagen hydrogel. | MVF-Myoblast constructs did not show muscle regeneration at both 2 weeks and 8 weeks post-implantation. | [44] |
| Rat MPCs | Adult female Lewis rats 20% TA muscle. | Keratin hydrogel. | [43] | ||
| Mouse MPCs | Female C57/BL6 Mouse 50% LD muscle. | Keratin hydrogel. | [53] | ||
| Newborn mice MuSCs | - | Three month old immunodeficient NSG mice TA muscle 4 × 2 × 2 mm3 partial thickness wedge resection. | Fibrin hydrogel | Transplanted MuSCs in fibrin contribute to forming new fibers and new vessels and increase muscle mass as well as reduce fibrotic response. | [41] |
| Human MPCs and human microvascular endothelial cells | Human MPCs expressed Pax7 protein and were aligned along the direction of the scaffold nanofibers. | 20% TA muscle ablation in NOD SCID male mice. | Nanofibrillar collagen scaffold. | Vascular perfusion and donor-derived human myofiber density increased in endothelialized human skeletal muscle formed from aligned scaffolds compared to randomly-oriented scaffolds. | [38] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shayan, M.; Huang, N.F. Pre-Clinical Cell Therapeutic Approaches for Repair of Volumetric Muscle Loss. Bioengineering 2020, 7, 97. https://doi.org/10.3390/bioengineering7030097
Shayan M, Huang NF. Pre-Clinical Cell Therapeutic Approaches for Repair of Volumetric Muscle Loss. Bioengineering. 2020; 7(3):97. https://doi.org/10.3390/bioengineering7030097
Chicago/Turabian StyleShayan, Mahdis, and Ngan F. Huang. 2020. "Pre-Clinical Cell Therapeutic Approaches for Repair of Volumetric Muscle Loss" Bioengineering 7, no. 3: 97. https://doi.org/10.3390/bioengineering7030097
APA StyleShayan, M., & Huang, N. F. (2020). Pre-Clinical Cell Therapeutic Approaches for Repair of Volumetric Muscle Loss. Bioengineering, 7(3), 97. https://doi.org/10.3390/bioengineering7030097






