Pomegranate Pomace Extract with Antioxidant, Anticancer, Antimicrobial, and Antiviral Activity Enhances the Quality of Strawberry-Yogurt Smoothie
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Pomegranate Pomace Aqueous Extract (PP)
2.2. Proximate Analysis of Pomegranate Pomace Powder
2.3. Phenolic Profile in PP Extract
2.3.1. Total Phenols and Total Flavonoids Content
2.3.2. High-Resolution LC/MS Profile of Phenolic Content of PP Extract
2.4. Pomegranate Pomace Extract Activity
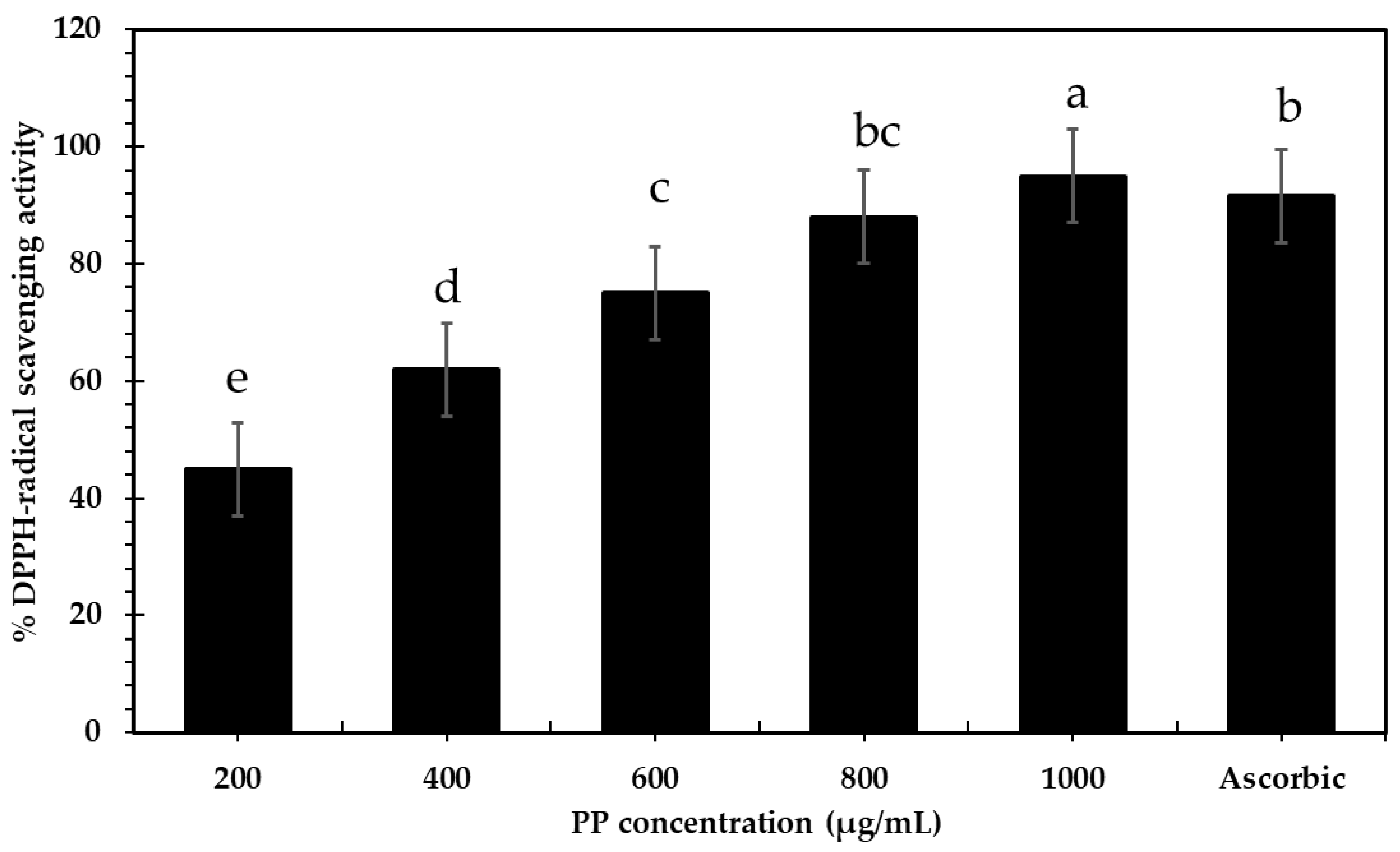
2.4.1. DPPH-Radical-Scavenging Activity
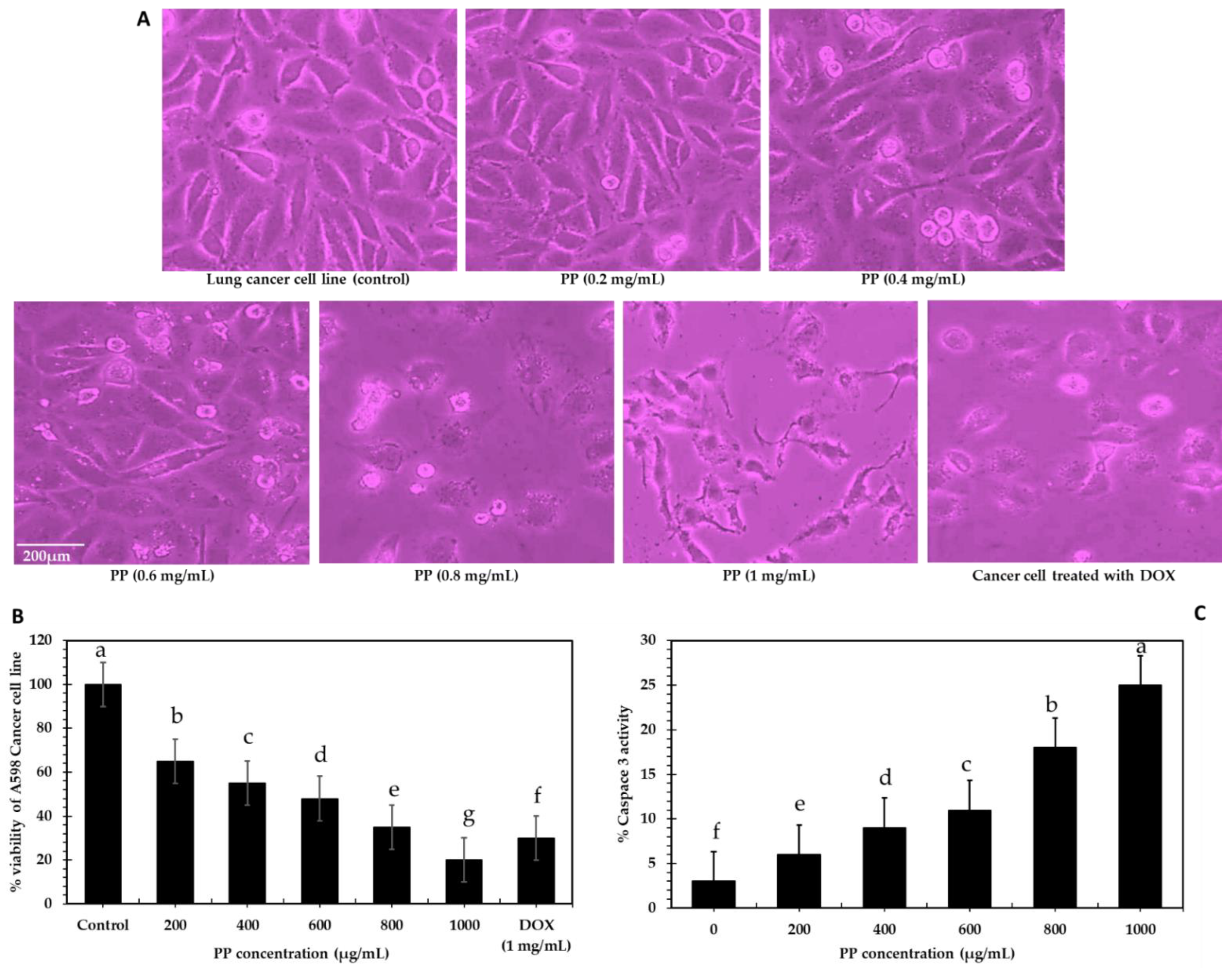
2.4.2. Anticancer
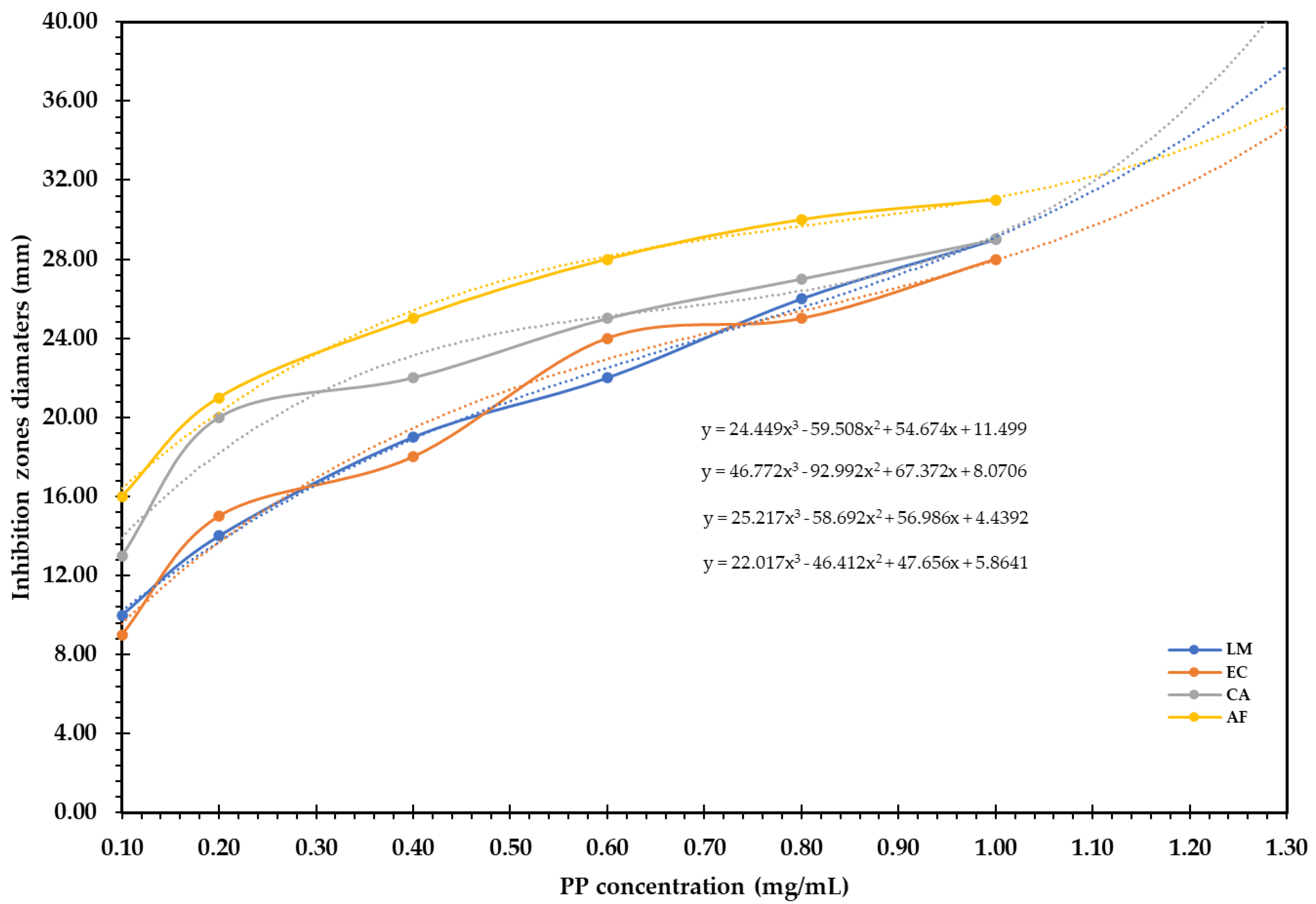
2.4.3. Antimicrobial
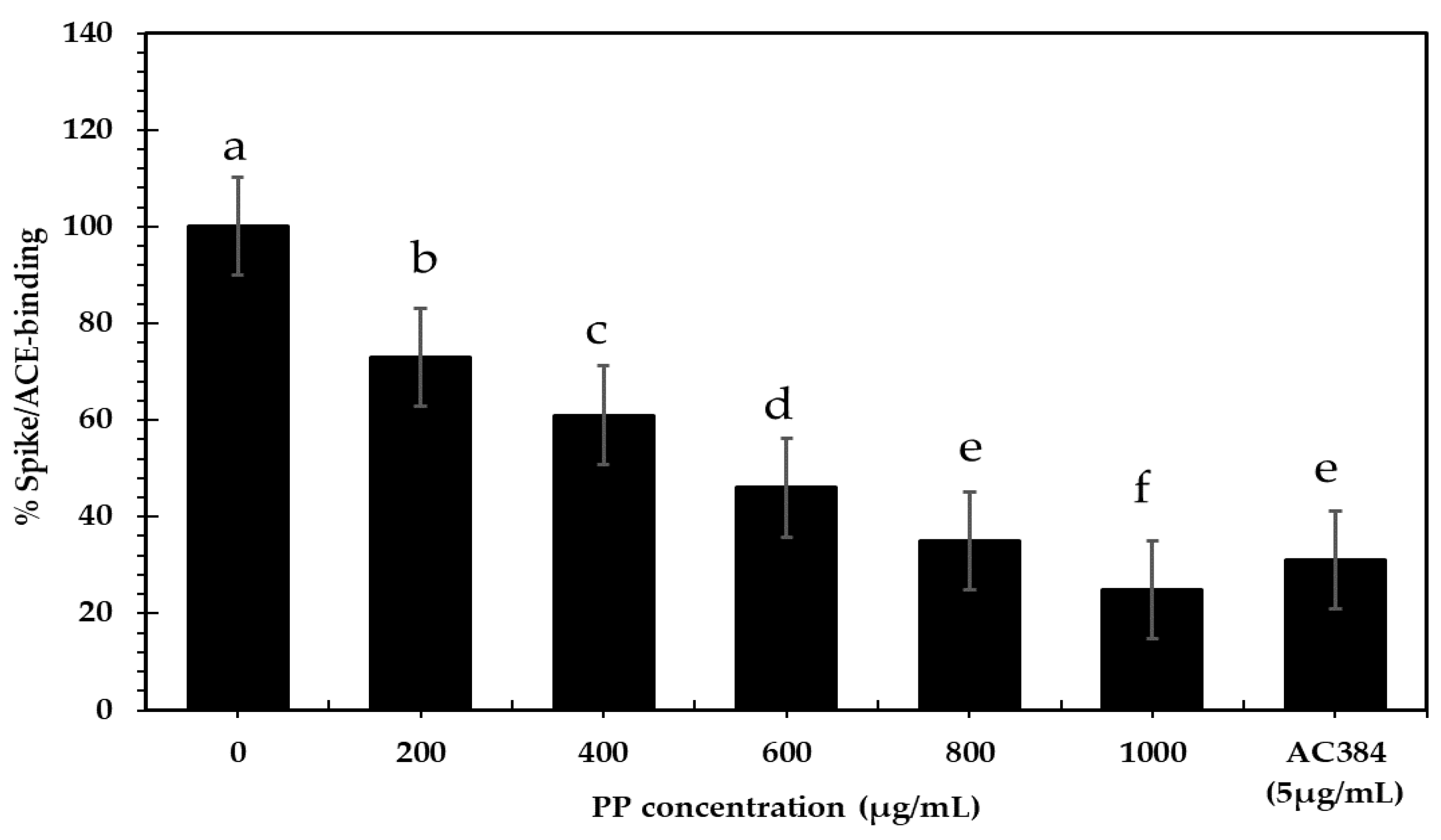
2.4.4. Antiviral
2.5. PP-Strawberry-Yogurt Smoothie Processing and Preservation
2.5.1. Preparation of Strawberry Smoothie
2.5.2. Physiochemical Properties
2.5.3. Color Differences and Sensory Properties
2.5.4. Microbial Load
2.6. Statistical Analysis
3. Results and Discussion
3.1. Chemical Composition
3.2. Phenolic Compounds in Pomegranate Pomace Aqueous Extract (PP)
3.3. Activity of Pomegranate Pomace Aqueous Extract
3.3.1. Antioxidant
3.3.2. Anticancer
3.3.3. Antimicrobial
3.3.4. Antiviral
3.4. Changes in Physiochemical Properties of PP-Strawberry-Yogurt Smoothie
3.4.1. Physiochemical Properties
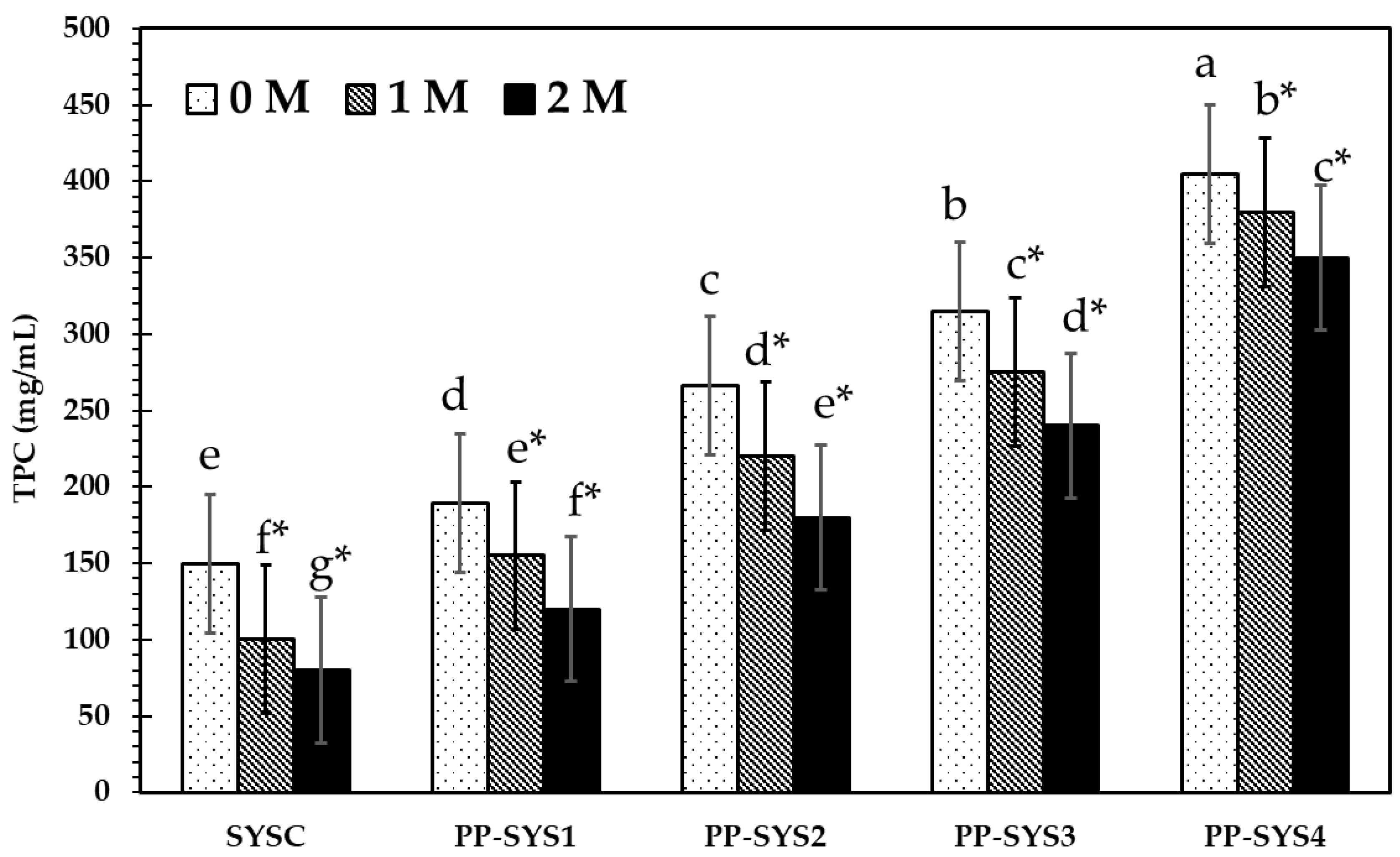
3.4.2. Total Phenolic Content
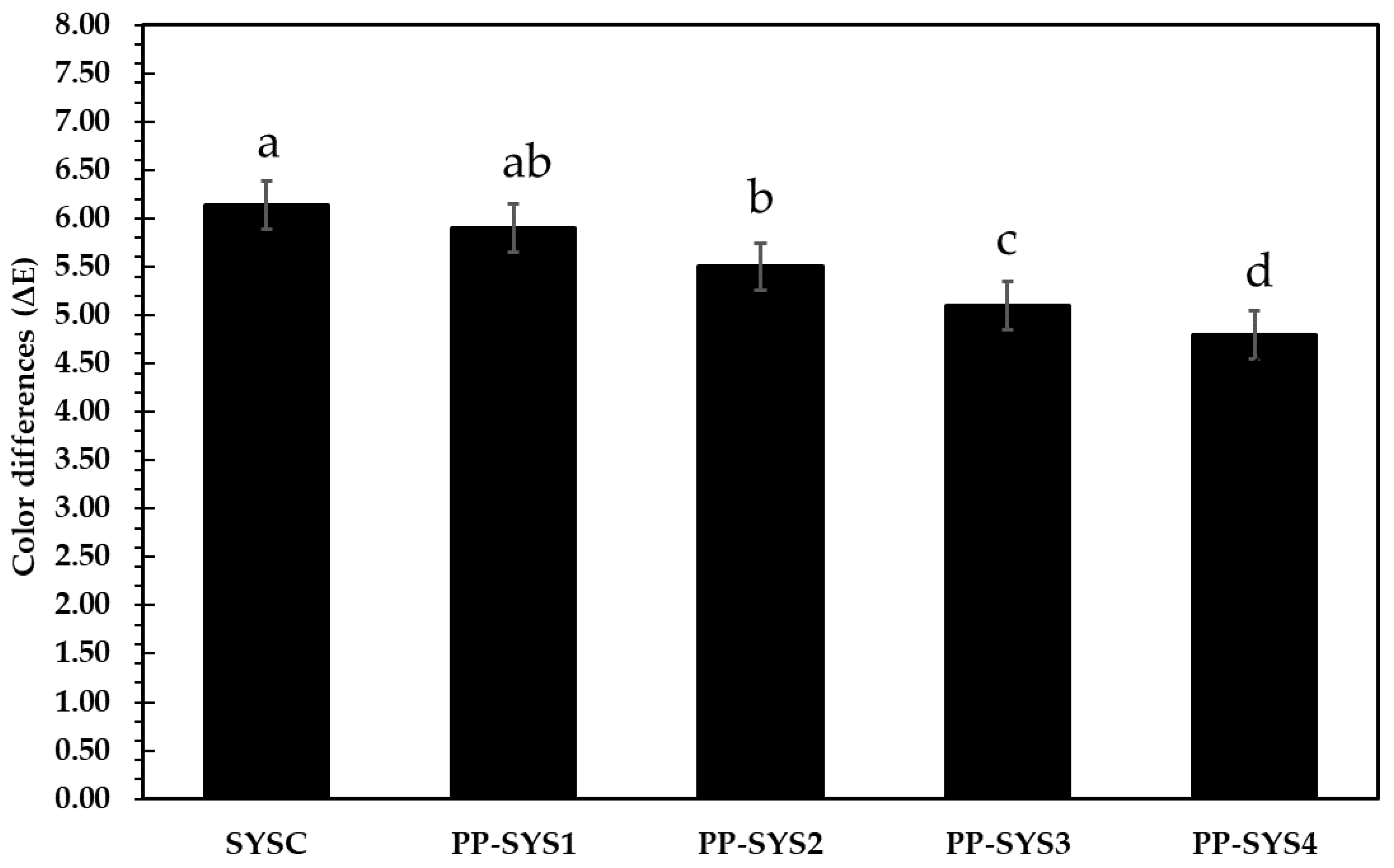
3.4.3. Color Difference and Sensorial Properties
3.5. Microbial Load Changes during Storage of Smoothie
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Abou-Kassem, D.E.; Mahrose, K.M.; El-Samahy, R.A.; Shafi, M.E.; El-Saadony, M.T.; Abd El-Hack, M.E.; Emam, M.; El-Sharnouby, M.; Taha, A.E.; Ashour, E.A. Influences of dietary herbal blend and feed restriction on growth, carcass characteristics and gut microbiota of growing rabbits. Ital. J. Anim. Sci. 2021, 20, 896–910. [Google Scholar] [CrossRef]
- El-Saadony, M.T.; Abd El-Hack, M.E.; Swelum, A.A.; Al-Sultan, S.I.; El-Ghareeb, W.R.; Hussein, E.O.; Ba-Awadh, H.A.; Akl, B.A.; Nader, M.M. Enhancing quality and safety of raw buffalo meat using the bioactive peptides of pea and red kidney bean under refrigeration conditions. Ital. J. Anim. Sci. 2021, 20, 762–776. [Google Scholar] [CrossRef]
- El-Saadony, M.T.; Sitohy, M.Z.; Ramadan, M.F.; Saad, A.M. Green nanotechnology for preserving and enriching yogurt with biologically available iron (II). Innov. Food Sci. Emerg. Technol. 2021, 69, 102645. [Google Scholar] [CrossRef]
- Lansky, E.P.; Newman, R.A. Punica granatum (pomegranate) and its potential for prevention and treatment of inflammation and cancer. J. Ethnopharmacol. 2007, 109, 177–206. [Google Scholar] [CrossRef] [PubMed]
- Saad, A.M.; El-Saadony, M.T.; Mohamed, A.S.; Ahmed, A.I.; Sitohy, M.Z. Impact of cucumber pomace fortification on the nutritional, sensorial and technological quality of soft wheat flour-based noodles. Int. J. Food Sci. Technol. 2021, 56, 3255–3268. [Google Scholar] [CrossRef]
- FAO. Statistical Database. Food and Agriculture Organization of the United Nations, Codex Alimentarius Commission: Tunis, Tunisia. Available online: http://www.fao.org (accessed on 23 May 2012).
- Akhtar, S.; Ismail, T.; Fraternale, D.; Sestili, P. Pomegranate peel and peel extracts: Chemistry and food features. Food Chem. 2015, 174, 417–425. [Google Scholar] [CrossRef] [PubMed]
- Belkacem, N.; Djaziri, R.; Lahfa, F.; El-Haci, I.; Boucherit, Z. Phytochemical screening and in vitro antioxidant activity of various Punica granatum l. Peel extracts from Algeria: A comparative study. Phytothérapie 2014, 12, 372–379. [Google Scholar] [CrossRef]
- Gözlekçi, Ş.; Saraçoğlu, O.; Onursal, E.; Özgen, M. Total phenolic distribution of juice, peel, and seed extracts of four pomegranate cultivars. Pharmacogn. Mag. 2011, 7, 161. [Google Scholar] [CrossRef] [PubMed]
- Sorrenti, V.; Randazzo, C.L.; Caggia, C.; Ballistreri, G.; Romeo, F.V.; Fabroni, S.; Timpanaro, N.; Raffaele, M.; Vanella, L. Beneficial effects of pomegranate peel extract and probiotics on pre-adipocyte differentiation. Front. Microbiol. 2019, 10, 660. [Google Scholar] [CrossRef] [PubMed]
- Khaleel, A.; Sijam, K.; Rashid, T. Determination of antibacterial compounds of punica Granatum peel extract by tlc direct bio-autography and GCMS analysis. Biochem. Cell. Arch. 2018, 18, 379–384. [Google Scholar]
- Kesur, P.; Gahlout, M.; Chauhan, P.B.; Prajapati, H. Evaluation of antimicrobial properties of peels and juice extract of Punica granatum (Pomegranate). Int. J. Res. Sci. Innov. 2016, 3, 11–20. [Google Scholar]
- Chaudhary, A.; Rahul, S.N. Antibacterial activity of Punica granatum (pomegranate) fruit peel extract against pathogenic and drug resistance bacterial strains. Int. J. Curr. Microbiol. Appl. Sci. 2017, 6, 3802–3807. [Google Scholar] [CrossRef]
- Li, Y.; Yang, F.; Zheng, W.; Hu, M.; Wang, J.; Ma, S.; Deng, Y.; Luo, Y.; Ye, T.; Yin, W. Punica granatum (pomegranate) leaves extract induces apoptosis through mitochondrial intrinsic pathway and inhibits migration and invasion in non-small cell lung cancer in vitro. Biomed. Pharmacother. 2016, 80, 227–235. [Google Scholar] [CrossRef]
- Zam, W.; Khaddour, A. Anti-virulence effects of aqueous pomegranate peel extract on E. coli urinary tract infection. Progr. Nutr. 2017, 19, 98–104. [Google Scholar]
- Abou El-Nour, M.M. Functional properties and medical benefits of pomegranate (Punica granatum L.) peels as agro-industrial wastes. Egypt. J. Exp. Biol. 2019, 15, 377–392. [Google Scholar] [CrossRef]
- Mekni, M.; Kharroubi, W.; Flamimi, G.; Garrab, M.; Mastouri, M.; Hammami, M. Comparative study between extracts of different pomegranate parts issued from five tunisian cultivars (Punica granatum L.): Phytochemical content, volatile composition and biological activity. Int. J. Curr. Microbiol. Appl. Sci. 2018, 7, 1663–1682. [Google Scholar] [CrossRef]
- Ohshima, H.; Tazawa, H.; Sylla, B.S.; Sawa, T. Prevention of human cancer by modulation of chronic inflammatory processes. Mutat. Res. Fundam. Mol. Mech. Mutagen. 2005, 591, 110–122. [Google Scholar] [CrossRef]
- Rocha, A.; Wang, L.; Penichet, M.; Martins-Green, M. Pomegranate juice and specific components inhibit cell and molecular processes critical for metastasis of breast cancer. Breast Cancer Res. Treat. 2012, 136, 647–658. [Google Scholar] [CrossRef]
- Mahmoudi, R.; Servatkhah, M.; Fallahzadeh, A.R.; Abidi, H.; Shirazi, H.R.G.; Delaviz, H.; Nikseresht, M. Pomegranate seed oil shows inhibitory effect on invasion of human breast cancer cell lines. J. Clin. Diagn. Res. 2017, 11, 5–10. [Google Scholar] [CrossRef]
- Mastrodi Salgado, J.; Baroni Ferreira, T.R.; de Oliveira Biazotto, F.; dos Santos Dias, C.T. Increased antioxidant content in juice enriched with dried extract of pomegranate (Punica granatum) peel. Plant Foods Hum. Nutr. 2012, 67, 39–43. [Google Scholar] [CrossRef] [PubMed]
- Barros, Z.M.P.; Salgado, J.M.; Melo, P.S.; de Oliveira Biazotto, F. Enrichment of commercially-prepared juice with pomegranate (Punica granatum L.) peel extract as a source of antioxidants. J. Food Res. 2014, 3, 179. [Google Scholar] [CrossRef]
- Altunkaya, A.; Hedegaard, R.V.; Harholt, J.; Brimer, L.; Gökmen, V.; Skibsted, L.H. Palatability and chemical safety of apple juice fortified with pomegranate peel extract. Food Funct. 2013, 4, 1468–1473. [Google Scholar] [CrossRef] [PubMed]
- AOAC. Official Methods of Analysis of AOAC International, 19th ed.; AOAC, International: Gaithersburg, MA, USA, 2012. [Google Scholar]
- Saad, A.M.; Sitohy, M.Z.; Ahmed, A.I.; Rabie, N.A.; Amin, S.A.; Aboelenin, S.M.; Soliman, M.M.; El-Saadony, M.T. Biochemical and functional characterization of kidney bean protein alcalase-hydrolysates and their preservative action on stored chicken meat. Molecules 2021, 26, 4690. [Google Scholar] [CrossRef] [PubMed]
- Saad, A.M.; Mohamed, A.S.; El-Saadony, M.T.; Sitohy, M.Z. Palatable functional cucumber juices supplemented with polyphenols-rich herbal extracts. LWT Food Sci. Technol. 2021, 148, 111668. [Google Scholar] [CrossRef]
- Namir, M.; Iskander, A.; Alyamani, A.; Sayed-Ahmed, E.T.A.; Saad, A.M.; Elsahy, K.; El-Tarabily, K.A.; Conte-Junior, C.A. Upgrading common wheat pasta by fiber-rich fraction of potato peel byproduct at different particle sizes: Effects on physicochemical, thermal, and sensory properties. Molecules 2022, 27, 2868. [Google Scholar] [CrossRef]
- Colantuono, A.; Vitaglione, P.; Ferracane, R.; Campanella, O.H.; Hamaker, B.R. Development and functional characterization of new antioxidant dietary fibers from pomegranate, olive and artichoke byproducts. Food Res. Int. 2017, 101, 155–164. [Google Scholar] [CrossRef]
- Abdel-Moneim, A.-M.E.; El-Saadony, M.T.; Shehata, A.M.; Saad, A.M.; Aldhumri, S.A.; Ouda, S.M.; Mesalam, N.M. Antioxidant and antimicrobial activities of Spirulina platensis extracts and biogenic selenium nanoparticles against selected pathogenic bacteria and fungi. Saudi J. Biol. Sci. 2022, 29, 1197–1209. [Google Scholar] [CrossRef]
- El-Saadony, M.T.; Saad, A.M.; Elakkad, H.A.; El-Tahan, A.M.; Alshahrani, O.A.; Alshilawi, M.S.; El-Sayed, H.; Amin, S.A.; Ahmed, A.I. Flavoring and extending the shelf life of cucumber juice with aroma compounds-rich herbal extracts at 4℃ through controlling chemical and microbial fluctuations. Saudi J. Biol. Sci. 2022, 29, 346–354. [Google Scholar] [CrossRef]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Gomha, S.M.; Riyadh, S.M.; Mahmmoud, E.A. Synthesis and anticancer activities of thiazoles, 1, 3-thiazines, and thiazolidine using chitosan-grafted-poly (vinylpyridine) as basic catalyst. Heterocycles: An Int. J. Rev. Comm. Heterocycl.Chem. 2015, 91, 1227–1243. [Google Scholar]
- Dai, Z.; Nair, V.; Khan, M.; Ciolino, H.P. Pomegranate extract inhibits the proliferation and viability of MMTV-Wnt-1 mouse mammary cancer stem cells in vitro. Oncol. Rep. 2010, 24, 1087–1091. [Google Scholar] [PubMed]
- Dahham, S.S.; Ali, M.N.; Tabassum, H.; Khan, M. Studies on antibacterial and antifungal activity of pomegranate (Punica granatum L.). Am. Eurasian J. Agric. Environ. Sci. 2010, 9, 273–281. [Google Scholar]
- El-Saadony, M.T.; Saad, A.M.; Najjar, A.A.; Alzahrani, S.O.; Alkhatib, F.M.; Shafi, M.E.; Selem, E.; Desoky, E.-S.M.; Fouda, S.E.; El-Tahan, A.M. The use of biological selenium nanoparticles to suppress Triticum aestivum L. crown and root rot diseases induced by Fusarium species and improve yield under drought and heat stress. Saudi J. Biol. Sci. 2021, 28, 4461–4471. [Google Scholar] [CrossRef] [PubMed]
- El-Saadony, M.T.; El-Wafai, N.A.; El-Fattah, H.I.A.; Mahgoub, S.A. Biosynthesis, optimization and characterization of silver nanoparticles using a soil isolate of Bacillus pseudomycoides MT32 and their antifungal activity against some pathogenic fungi. Adv. Anim. Vet. Sci 2019, 7, 238–249. [Google Scholar] [CrossRef]
- El-Saadony, M.T.; Alkhatib, F.M.; Alzahrani, S.O.; Shafi, M.E.; Abdel-Hamid, S.E.; Taha, T.F.; Aboelenin, S.M.; Soliman, M.M.; Ahmed, N.H. Impact of mycogenic zinc nanoparticles on performance, behavior, immune response, and microbial load in Oreochromis niloticus. Saudi J. Biol. Sci. 2021, 28, 4592–4604. [Google Scholar] [CrossRef] [PubMed]
- El-Saadony, M.T.; Elsadek, M.F.; Mohamed, A.S.; Taha, A.E.; Ahmed, B.M.; Saad, A.M. Effects of chemical and natural additives on cucumber juice’s quality, shelf life, and safety. Foods 2020, 9, 639. [Google Scholar] [CrossRef] [PubMed]
- Namir, M.; Suleiman, A.R.; Hassanien, M.F.R. Characterization and functionality of alcohol insoluble solids from tomato pomace as fat substitute in low fat cake. J. Food Meas. Charact. 2015, 9, 557–563. [Google Scholar] [CrossRef]
- El-Saadony, M.T.; Osama, S.F.; Osman, A.; Mashaeal, S.; Ayman, E.T.; Aboelenin, S.M.; Shukry, M.; Saad, A.M. Bioactive peptides supplemented raw buffalo milk: Biological activity, shelf life and quality properties during cold preservation. Saudi J. Biol. Sci. 2021, 28, 4581–4591. [Google Scholar] [CrossRef] [PubMed]
- Ranjitha, J.; Bhuvaneshwari, G.; Terdal, D.; Kavya, K. Nutritional composition of fresh pomegranate peel powder. Int. J. Chem. Stud. 2018, 6, 692–696. [Google Scholar]
- El-Hadary, A.E.; Taha, M. Pomegranate peel methanolic-extract improves the shelf-life of edible-oils under accelerated oxidation conditions. Food Sci. Nutr. 2020, 8, 1798–1811. [Google Scholar] [CrossRef]
- Bchir, B.; Rabetafika, H.N.; Paquot, M.; Blecker, C. Effect of pear, apple and date fibres from cooked fruit byproducts on dough performance and bread quality. J. Food Bioprocess Eng. 2014, 7, 1114–1127. [Google Scholar] [CrossRef]
- Dey, D.; Richter, J.K.; Ek, P.; Gu, B.-J.; Ganjyal, G.M. Utilization of food processing byproducts in extrusion processing: A review. Front. Sustain. Food Syst. 2021, 4, 603751. [Google Scholar] [CrossRef]
- Viuda-Martos, M.; Ruiz-Navajas, Y.; Martin-Sánchez, A.; Sánchez-Zapata, E.; Fernández-López, J.; Sendra, E.; Sayas-Barberá, E.; Navarro, C.; Pérez-Álvarez, J. Chemical, physico-chemical and functional properties of pomegranate (Punica granatum L.) bagasses powder co-product. J. Food Eng. 2012, 110, 220–224. [Google Scholar] [CrossRef]
- Jalal, H.; Pal, M.A.; Ahmad, S.R.; Rather, M.; Andrabi, M.; Hamdani, S. Physico-chemical and functional properties of pomegranate peel and seed powder. J. Pharm. Innov. 2018, 7, 1127–1131. [Google Scholar]
- Ruan, J.-H.; Li, J.; Adili, G.; Sun, G.-Y.; Abuduaini, M.; Abdulla, R.; Maiwulanjiang, M.; Aisa, H.A. Phenolic Compounds and Bioactivities from Pomegranate (Punica granatum L.) Peels. J.Agric. Food Chem. 2022, 70, 3678–3686. [Google Scholar] [CrossRef]
- Yassin, M.T.; Mostafa, A.A.-F.; Al Askar, A.A. In Vitro evaluation of biological activities and phytochemical analysis of different solvent extracts of Punica granatum L.(Pomegranate) Peels. Plants 2021, 10, 2742. [Google Scholar] [CrossRef] [PubMed]
- Živković, I.; Šavikin, K.; Živković, J.; Zdunić, G.; Janković, T.; Lazić, D.; Radin, D. Antiviral effects of pomegranate peel extracts on human Norovirus in food models and simulated gastrointestinal fluids. Plant Foods Hum. Nutr. 2021, 76, 203–209. [Google Scholar] [CrossRef]
- Coronado-Reyes, J.A.; Cortes-penagos, C.d.J.; Gonzalez-hernandez, J.C. Chemical composition and great applications to the fruit of the pomegranate (Punica granatum): A review. Food Sci. Technol. 2021, 42, 1–7. [Google Scholar] [CrossRef]
- Singh, M.; Jha, A.; Kumar, A.; Hettiarachchy, N.; Rai, A.K.; Sharma, D. Influence of the solvents on the extraction of major phenolic compounds (punicalagin, ellagic acid and gallic acid) and their antioxidant activities in pomegranate aril. J. Food Sci. Technol. 2014, 51, 2070–2077. [Google Scholar] [CrossRef] [PubMed]
- Ko, K.; Dadmohammadi, Y.; Abbaspourrad, A. Nutritional and bioactive components of pomegranate waste used in food and cosmetic applications: A review. Foods 2021, 10, 657. [Google Scholar] [CrossRef] [PubMed]
- Gaber, N.B.; El-Dahy, S.I.; Shalaby, E.A. Comparison of ABTS, DPPH, permanganate, and methylene blue assays for determining antioxidant potential of successive extracts from pomegranate and guava residues. Biomass Convers. Biorefinery 2021, 1–10. [Google Scholar] [CrossRef]
- Khalil, A.A.; Khan, M.R.; Shabbir, M.A. In vitro antioxidant activity and punicalagin content quantification of pomegranate peel obtained as agro-waste after juice extraction. Pak. J. Agric. Sci. 2018, 55, 197–201. [Google Scholar]
- Bopitiya, D.; Madhujith, T. Efficacy of pomegranate (Punica granatum L.) peel extracts in suppressing oxidation of white coconut oil used for deep frying. Trop. Agric. Res. 2014, 25, 298–306. [Google Scholar] [CrossRef]
- Lobo, V.; Patil, A.; Phatak, A.; Chandra, N. Free radicals, antioxidants and functional foods: Impact on human health. Pharmacogn Rev. 2010, 4, 118. [Google Scholar] [CrossRef]
- Tzulker, R.; Glazer, I.; Bar-Ilan, I.; Holland, D.; Aviram, M.; Amir, R. Antioxidant activity, polyphenol content, and related compounds in different fruit juices and homogenates prepared from 29 different pomegranate accessions. J. Agric. Food Chem. 2007, 55, 9559–9570. [Google Scholar] [CrossRef] [PubMed]
- Jesse Joel, T.; Suluvoy, J.K.; Varghese, J. Evaluation of the secondary metabolites of the waste pomegranate rind and its cytotoxicity against oral cancer (KB 3-1). J Pure Appl Microbiol. 2019, 13, 1667–1672. [Google Scholar] [CrossRef]
- An, J.; Guo, Y.; Wang, T.; Pantuck, A.J.; Rettig, M.B. Pomegranate extract inhibits EMT in clear cell renal cell carcinoma in a NF-κB and JNK dependent manner. Asian J. Urol. 2015, 2, 38–45. [Google Scholar] [CrossRef]
- Aqil, F.; Munagala, R.; Vadhanam, M.V.; Kausar, H.; Jeyabalan, J.; Schultz, D.J.; Gupta, R.C. Anti-proliferative activity and protection against oxidative DNA damage by punicalagin isolated from pomegranate husk. Food Res. Int. 2012, 49, 345–353. [Google Scholar] [CrossRef] [PubMed]
- Berköz, M.; Krosniak, M. Punicalagin induces apoptosis in A549 cell line through mitochondria-mediated pathway. Gen. Physiol. Biophys. 2020, 39, 557–567. [Google Scholar] [CrossRef] [PubMed]
- Yadav, P.; Yadav, R.; Jain, S.; Vaidya, A. Caspase-3: A primary target for natural and synthetic compounds for cancer therapy. Chem. Biol. Drug Des. 2021, 98, 144–165. [Google Scholar] [CrossRef]
- Jain, A.S.; Prasad, A.; Pradeep, S.; Dharmashekar, C.; Achar, R.R.; Silina, E.; Stupin, V.; Amachawadi, R.G.; Prasad, S.K.; Pruthvish, R. Everything old is new again: Drug repurposing approach for non-small cell lung cancer targeting MAPK signaling pathway. Front.Oncol. 2021, 11, 741326. [Google Scholar] [CrossRef] [PubMed]
- Venkatachalapathy, D.; Shivamallu, C.; Prasad, S.K.; Thangaraj Saradha, G.; Rudrapathy, P.; Amachawadi, R.G.; Patil, S.S.; Syed, A.; Elgorban, A.M.; Bahkali, A.H. Assessment of chemopreventive potential of the plant extracts against liver cancer using HepG2 cell line. Molecules 2021, 26, 4593. [Google Scholar] [CrossRef]
- Saad, A.M.; El-Saadony, M.T.; El-Tahan, A.M.; Sayed, S.; Moustafa, M.A.; Taha, A.E.; Taha, T.F.; Ramadan, M.M. Polyphenolic extracts from pomegranate and watermelon wastes as substrate to fabricate sustainable silver nanoparticles with larvicidal effect against Spodoptera littoralis. Saudi J. Biol. Sci. 2021, 28, 5674–5683. [Google Scholar] [CrossRef]
- Oliveira, D.A.; Salvador, A.A.; Smânia Jr, A.; Smânia, E.F.; Maraschin, M.; Ferreira, S.R. Antimicrobial activity and composition profile of grape (Vitis vinifera) pomace extracts obtained by supercritical fluids. J. Biotechnol. 2013, 164, 423–432. [Google Scholar] [CrossRef] [PubMed]
- Ali Redha, A.; Hasan, A.; Mandeel, Q. Phytochemical determinations of Pomegranate (Punica granatum) Rind and Aril extracts and their antioxidant, antidiabetic and antibacterial activity. Nat. Prod. Chem. Res. 2018, 6, 1–9. [Google Scholar]
- Naziri, Z.; Rajaian, H.; Firouzi, R. Antibacterial effects of Iranian native sour and sweet pomegranate (Punica granatum) peel extracts against various pathogenic bacteria. 2012, 13, 282–288. Iran Vet. Res. J. 2012, 13, 282–288. [Google Scholar]
- Rosas-Burgos, E.C.; Burgos-Hernández, A.; Noguera-Artiaga, L.; Kačániová, M.; Hernández-García, F.; Cárdenas-López, J.L.; Carbonell-Barrachina, Á.A. Antimicrobial activity of pomegranate peel extracts as affected by cultivar. J. Sci. Food Agric. 2017, 97, 802–810. [Google Scholar] [CrossRef] [PubMed]
- Vasconcelos, L.C.d.S.; Sampaio, F.C.; Sampaio, M.C.C.; Pereira, M.d.S.V.; Higino, J.S.; Peixoto, M.H.P. Minimum inhibitory concentration of adherence of Punica granatum Linn (pomegranate) gel against S. mutans, S. mitis and C. albicans. Braz. Dent. J. 2006, 17, 223–227. [Google Scholar] [CrossRef] [PubMed]
- Devatkal, S.K.; Jaiswal, P.; Jha, S.N.; Bharadwaj, R.; Viswas, K. Antibacterial activity of aqueous extract of pomegranate peel against Pseudomonas stutzeri isolated from poultry meat. J. Food Sci. Technol. 2013, 50, 555–560. [Google Scholar] [CrossRef] [PubMed]
- Suručić, R.; Travar, M.; Petković, M.; Tubić, B.; Stojiljković, M.P.; Grabež, M.; Šavikin, K.; Zdunić, G.; Škrbić, R. Pomegranate peel extract polyphenols attenuate the SARS-CoV-2 S-glycoprotein binding ability to ACE2 Receptor: In silico and in vitro studies. Bioorg. Chem. 2021, 114, 105145. [Google Scholar] [CrossRef] [PubMed]
- Hikal, W.M.; Said-Al Ahl, H.A.; Tkachenko, K.G.; Mahmoud, A.A.; Bratovcic, A.; Hodžić, S.; Atanassova, M. An Overview of Pomegranate Peel: A Waste Treasure for Antiviral Activity. Trop. J. Nat. Prod. Res. (TJNPR) 2022, 6, 15–19. [Google Scholar] [CrossRef]
- Al-Hindi, R.R.; Abd El Ghani, S. Production of functional fermented milk beverages supplemented with pomegranate peel extract and probiotic lactic acid bacteria. J. Food Qual. 2020, 2020, 1–9. [Google Scholar] [CrossRef]
- Kaderides, K.; Kyriakoudi, A.; Mourtzinos, I.; Goula, A.M. Potential of pomegranate peel extract as a natural additive in foods. Trends Food Sci Technol. 2021, 115, 380–390. [Google Scholar] [CrossRef]
- Cao, H.; Saroglu, O.; Karadag, A.; Diaconeasa, Z.; Zoccatelli, G.; Conte-Junior, C.A.; Gonzalez-Aguilar, G.A.; Ou, J.; Bai, W.; Zamarioli, C.M. Available technologies on improving the stability of polyphenols in food processing. Food Front. 2021, 2, 109–139. [Google Scholar] [CrossRef]
- Ahmed, M.; Ali, A.; Sarfraz, A.; Hong, Q.; Boran, H. Effect of freeze-drying on apple pomace and pomegranate peel powders used as a source of bioactive ingredients for the development of functional yogurt. J. Food Qual. 2022, 2022, 1–9. [Google Scholar] [CrossRef]
- Reda, F.M.; El-Saadony, M.T.; El-Rayes, T.K.; Farahat, M.; Attia, G.; Alagawany, M. Dietary effect of licorice (Glycyrrhiza glabra) on quail performance, carcass, blood metabolites and intestinal microbiota. Poult. Sci. 2021, 100, 101266. [Google Scholar] [CrossRef] [PubMed]
- Yaqoob, M.; Abd El-Hack, M.; Hassan, F.; El-Saadony, M.; Khafaga, A.; Batiha, G.; Yehia, N.; Elnesr, S.; Alagawany, M.; El-Tarabily, K. The potential mechanistic insights and future implications for the effect of prebiotics on poultry performance, gut microbiome, and intestinal morphology. Poult. Sci. 2021, 100, 101143. [Google Scholar] [CrossRef]
- Aadil, R.M.; Madni, G.M.; Roobab, U.; ur Rahman, U.; Zeng, X.-A. Quality control in beverage production: An overview. Q. Control Bever. Indust. 2019, 1–38. [Google Scholar] [CrossRef]
- Trigo, J.P.; Alexandre, E.M.; Silva, S.; Costa, E.; Saraiva, J.A.; Pintado, M. Study of viability of high pressure extract from pomegranate peel to improve carrot juice characteristics. Food Funct. 2020, 11, 3410–3419. [Google Scholar] [CrossRef] [PubMed]
- Pathare, P.B.; Opara, U.L.; Al-Said, F.A.-J. Colour measurement and analysis in fresh and processed foods: A review. Food Bioproc. Tech. 2013, 6, 36–60. [Google Scholar] [CrossRef]
- Ebrahimian, M.; Mehdizadeh, T.; Aliakbarlu, J. Chemical and microbiological stability and sensorial properties of traditional Iranian butter incorporated with pomegranate peel extract. Int. J. Dairy Technol. 2022, 21, 1–9. [Google Scholar] [CrossRef]
- Al-Huqail, A.A.; Elgaaly, G.A.; Ibrahim, M.M. Identification of bioactive phytochemical from two Punica species using GC–MS and estimation of antioxidant activity of seed extracts. Saudi J. Biol. Sci. 2018, 25, 1420–1428. [Google Scholar] [CrossRef] [PubMed]
- Karaman, M.; Jovin, E.; Malbaša, R.; Matavuly, M.; Popović, M. Medicinal and edible lignicolous fungi as natural sources of antioxidative and antibacterial agents. Phytother. Res. 2010, 24, 1473–1481. [Google Scholar] [CrossRef] [PubMed]


| Ingredients | SYSC | PP-SYS1 | PP-SYS2 | PP-SYS3 | PP-SYS4 |
|---|---|---|---|---|---|
| Strawberry Juice (mL) | 100 | 100 | 100 | 100 | 100 |
| PP-yogurt (g) | 40 | 40 | 40 | 40 | 40 |
| Sugar (g) | 10 | 10 | 10 | 10 | 10 |
| PP (mL) | ˗ | 50 | 50 | 50 | 50 |
| Water (mL) | 50 | ˗ | ˗ | ˗ | ˗ |
| PP Composition | Content (g/100 g) |
|---|---|
| Chemical | |
| Moisture | 6.98 ± 0.2 |
| Protein | 9.11 ± 0.1 |
| Fat | 0.61 ± 0.01 |
| Ash | 4.12 ± 0.3 |
| Fiber | 20.66 ± 0.8 |
| Soluble carbohydrates | 58.52 ± 0.6 |
| Physical | |
| WAC (mL/g) | 8.21 ± 0.1 |
| OAC (mL/g) | 7.77 ± 0.1 |
| L* | 61.22 ± 0.5 |
| a* | 25.52 ± 0.8 |
| b* | 15.66 ± 0.3 |
| RT | Compound | Equivalent | MW | PP (g/L) |
|---|---|---|---|---|
| 6.3 | Gallic acid | - | 169.014 | 1.20 ± 0.2 j |
| 6.5 | Punicalin | - | 781.053 | 65.35 ± 0.9 b |
| 7.8 | Punicalagin | - | 1083.059 | 199.85 ± 1.2 a |
| 8.2 | Pedunculagin A bis-Hexahydroxy diphenic acid (HHDP) | Punicalagin | 783.069 | 52.98 ± 0.8 c |
| 8.2 | Causarinin | Punicalagin | 935.080 | 22.96 ± 0.8 f |
| 8.5 | Galloyl-HHDP-hexoside | Punicalagin | 633.073 | 48.15 ± 0.7 d |
| 8.7 | Ellagic acid hexoside | Ellagic acid | 463.052 | 4.50 ± 0.6 h |
| 8.8 | Pedunculagin B (digalloyl- HHDP) | Punicalagin | 785.084 | 31.66 ± 0.9 e |
| 8.8 | Ellagic acid dihexoside | Ellagic acid | 625.105 | 1.21 ± 0.1 ij |
| 9.0 | Lagerstannin B | Punicalagin | 949.059 | 1.93 ± 0.2 i |
| 9.3 | Granatin B | Punicalagin | 951.075 | 62.42 ± 0.8 b |
| 9.5 | Ellagic acid pentoside | Ellagic acid | 433.041 | 3.12 ± 0.1 h |
| 9.6 | Ellagic acid deoxyhexoside | Ellagic acid | 447.057 | 3.89 ± 0.1 h |
| 10.5 | Ellagic acid | - | 300.999 | 12.55 ± 0.3 g |
| Total punicalagin derivatives | 479.35 A | |||
| Total ellagic acid derivatives | 24.29 B | |||
| Gallic acid | 1.20 C | |||
| Total phenolic compounds | 504.84 |
| Pathogenic Bacteria | PP Concentration (µg/mL) | ||||||
|---|---|---|---|---|---|---|---|
| Inhibition Zone Diameters (mm) | |||||||
| 100 | 200 | 400 | 600 | 800 | 1000 | St * (1 mg/mL) | |
| S. aureus | 12 ± 0.1 a | 18 ± 0.3 a | 23 ± 0.2 a | 27 ± 0.5 a | 31 ± 0.4 a | 35 ± 0.2 a | 37 ± 0.1 a |
| L. monocytogenes | 10 ± 0.2 b | 14 ± 0.2 cd | 19 ± 0.1 c | 22 ± 0.3 c | 26 ± 0.2 c | 29 ± 0.1 bc | 31 ± 0.5 c |
| B. cereus | 11 ± 0.5 ab | 16 ± 0.5 b | 20 ± 0.2 b | 25 ± 0.4 b | 28 ± 0.5 b | 30 ± 0.3 b | 33 ± 0.3 b |
| P. aeruginosa | − | 14 ± 0.1 cd | 17 ± 0.6 d | 22 ± 0.5 c | 24 ± 0.3 d | 27 ± 0.7 cd | 29 ± 0.6 d |
| K. pneumonia | − | 12 ± 0.3 d | 16 ± 0.9 d | 21 ± 0.2 cd | 23 ± 0.3 e | 25 ± 0.5 d | 27 ± 0.6 e |
| E. coli | 9 ± 0.2 c | 15 ± 0.5 c | 18 ± 0.6 cd | 24 ± 0.1 bc | 25 ± 0.5 cd | 28 ± 0.1 c | 30 ± 0.2 cd |
| Pathogenic fungi | Inhibition Zone Diameters (mm) | ||||||
| A. niger | − | 18 ± 0.6 c | 21 ± 0.5 bc | 25 ± 0.2 bc | 26 ± 0.3 c | 28 ± 0.3 c | 30 ± 0.3 bc |
| A. flavus | 16 ± 0.2 ab | 21 ± 0.3 ab | 25 ± 0.1 a | 28 ± 0.3 ab | 30 ± 0.1 a | 31 ± 0.4 ab | 34 ± 0.1 ab |
| P. expansum | 17 ± 0.6 a | 22 ± 0.2 a | 25 ± 0.3 a | 29 ± 0.1 a | 30 ± 0.4 a | 32 ± 0.3 a | 35 ± 0.3 a |
| C. glabrata | − | 17 ± 0.5 c | 19 ± 0.2 c | 21 ± 0.9 c | 23 ± 0.6 d | 25 ± 0.6 d | 26 ± 0.2 c |
| C. albicans | 13 ± 0.1 c | 20 ± 0.7 b | 22 ± 0.5 b | 25 ± 0.5 bc | 27 ± 0.4 bc | 29 ± 0.9 bc | 31 ± 0.0 b |
| C. davenportii | 15 ± 0.3 b | 21 ± 0.1 ab | 24 ± 0.8 ab | 27 ± 0.2 b | 28 ± 0.3 b | 30 ± 0.1 b | 31 ± 0.1 b |
| Pathogenic Bacteria | MIC * | MBC * |
| S. aureus | 50 f | 90 f |
| L. monocytogenes | 70 d | 130 d |
| B. cereus | 65 e | 120 e |
| P. aeruginosa | 110 b | 190 b |
| K. pneumonia | 150 a | 250 a |
| E. coli | 90 c | 150 c |
| Pathogenic fungi | MIC | MFC * |
| A. niger | 120 b | 200 b |
| A. flavus | 55 d | 90 e |
| P. expansum | 45 e | 80 f |
| C. glabrata | 160 a | 290 a |
| C. albicans | 75 c | 130 c |
| C. davenportii | 70 cd | 120 d |
| Smoothie Samples | Storage Period (Months) | pH | Acidity (mg/10 mL) | Vit. C (mg/mL) | Total Sugars (mg/mL) | Fat (%) | TSS (%) |
|---|---|---|---|---|---|---|---|
| SYSC | 0 | 4.98 ± 0.4 a | 55.32 ± 0.1 d | 6.3 ± 0.1 a | 1.20 ± 0.01 a | 1.10 ± 0.2 g | 9.30 ± 0.2 e |
| 1 | 4.75 ± 0.2 b | 58.78 ± 0.2 d | 4.0 ± 0.2 cd | 0.91 ± 0.02 cd | 1.30 ± 0.4 f | 10.10 ± 0.1 de | |
| 2 | 4.69 ± 0.3 c | 61.22 ± 0.3 cd | 2.7 ± 0.2 e | 0.78 ± 0.01 e | 1.50 ± 0.7 e | 10.90 ± 0.3 d | |
| PP-SYS1 | 1 | 4.68 ± 0.2 c | 62.39 ± 0.7 c | 4.2 ± 0.0 c | 0.92 ± 0.09 cd | 1.53 ± 0.5 e | 10.90 ± 0.95 d |
| 2 | 4.60 ± 0.0 c | 64.72 ± 0.4 bc | 3.0 ± 0.1 de | 0.81 ± 0.03 d | 1.60 ± 0.2 de | 11.60 ± 0.1 c | |
| PP-SYS2 | 1 | 4.55 ± 0.1 d | 62.59 ± 0.9 c | 4.4 ± 0.3 c | 0.93 ± 0.05 c | 1.54 ± 0.1 e | 11.02 ± 0.7 cd |
| 2 | 4.43 ± 0.3 ef | 65.00 ± 0.1 b | 3.5 ± 0.5 d | 0.89 ± 0.06 d | 1.72 ± 0.3 c | 12.03 ± 0.0 b | |
| PP-SYS3 | 1 | 4.51 ± 0.4 d | 62.99 ± 0.3 c | 4.8 ± 0.8 c | 0.95 ± 0.04 c | 1.62 ± 0.4 de | 11.72 ± 0.0 c |
| 2 | 4.44 ± 0.6 ef | 66.05 ± 0.7 b | 3.8 ± 0.6 d | 0.92 ± 0.01 cd | 1.79 ± 0.7 b | 12.55 ± 0.2 ab | |
| PP-SYS4 | 1 | 4.49 ± 0.1 e | 63.20 ± 0.5 c | 5.2 ± 0.3 b | 1.09 ± 0.00 b | 1.69 ± 0.3 d | 11.88 ± 0.1 c |
| 2 | 4.40 ± 0.5 f | 67.02 ± 0.2 a | 4.2 ± 0.1 c | 1.00 ± 0.03 bc | 1.82 ± 0.2 a | 12.89 ± 0.2 a |
| Smoothie Samples | Storage Period (Months) | Flavor | Color | Texture | Over Acceptability |
|---|---|---|---|---|---|
| SYSC | 0 | 8.4 ± 0.1 bc | 8.3 ± 0.3 d | 8.4 ± 0.5 b | 8.3 ± 0.2 c |
| 1 | 8.1 ± 0.3 c | 8.0 ± 0.2 e | 8.0 ± 0.5 c | 8.0 ± 0.1 cd | |
| 2 | 7.5 ± 0.2 d | 7.7 ± 0.1 f | 7.5 ± 0.3 d | 7.6 ± 0.3 d | |
| PP-SYS1 | 1 | 8.6 ± 0.0 b | 8.5 ± 0.2 cd | 8.4 ± 0.4 b | 8.5 ± 0.4 bc |
| 2 | 8.2 ± 0.3 c | 8.3 ± 0.2 d | 7.9 ± 0.6 d | 8.1 ± 0.3 cd | |
| PP-SYS2 | 1 | 8.8 ± 0.1 b | 8.6 ± 0.0 c | 8.4 ± 0.7 b | 8.6 ± 0.2 b |
| 2 | 8.4 ± 0.2 bc | 8.4 ± 0.0 cd | 8.0 ± 0.3 c | 8.2 ± 0.4 c | |
| PP-SYS3 | 1 | 8.9 ± 0.3 ab | 8.8 ± 0.2 b | 8.5 ± 0.4 b | 8.7 ± 0.3 b |
| 2 | 8.5 ± 0.5 bc | 8.5 ± 0.1 cd | 8.0 ± 0.6 c | 8.3 ± 0.2 c | |
| PP-SYS4 | 1 | 9.0 ± 0.0 a | 9.0 ± 0.0 a | 8.8 ± 0.1 a | 8.9 ± 0.3 a |
| 2 | 8.6 ± 0.3 b | 8.8 ± 0.3 b | 8.5 ± 0.2 b | 8.6 ± 0.4 b |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alsubhi, N.H.; Al-Quwaie, D.A.; Alrefaei, G.I.; Alharbi, M.; Binothman, N.; Aljadani, M.; Qahl, S.H.; Jaber, F.A.; Huwaikem, M.; Sheikh, H.M.; et al. Pomegranate Pomace Extract with Antioxidant, Anticancer, Antimicrobial, and Antiviral Activity Enhances the Quality of Strawberry-Yogurt Smoothie. Bioengineering 2022, 9, 735. https://doi.org/10.3390/bioengineering9120735
Alsubhi NH, Al-Quwaie DA, Alrefaei GI, Alharbi M, Binothman N, Aljadani M, Qahl SH, Jaber FA, Huwaikem M, Sheikh HM, et al. Pomegranate Pomace Extract with Antioxidant, Anticancer, Antimicrobial, and Antiviral Activity Enhances the Quality of Strawberry-Yogurt Smoothie. Bioengineering. 2022; 9(12):735. https://doi.org/10.3390/bioengineering9120735
Chicago/Turabian StyleAlsubhi, Nouf H., Diana A. Al-Quwaie, Ghadeer I. Alrefaei, Mona Alharbi, Najat Binothman, Majidah Aljadani, Safa H. Qahl, Fatima A. Jaber, Mashael Huwaikem, Huda M. Sheikh, and et al. 2022. "Pomegranate Pomace Extract with Antioxidant, Anticancer, Antimicrobial, and Antiviral Activity Enhances the Quality of Strawberry-Yogurt Smoothie" Bioengineering 9, no. 12: 735. https://doi.org/10.3390/bioengineering9120735
APA StyleAlsubhi, N. H., Al-Quwaie, D. A., Alrefaei, G. I., Alharbi, M., Binothman, N., Aljadani, M., Qahl, S. H., Jaber, F. A., Huwaikem, M., Sheikh, H. M., Alrahimi, J., Abd Elhafez, A. N., & Saad, A. (2022). Pomegranate Pomace Extract with Antioxidant, Anticancer, Antimicrobial, and Antiviral Activity Enhances the Quality of Strawberry-Yogurt Smoothie. Bioengineering, 9(12), 735. https://doi.org/10.3390/bioengineering9120735










