Chemometric Approach Application in Modern Wine Studies
Abstract
1. Introduction
2. Materials and Methods
2.1. Wine Origin
2.1.1. Slovakia
2.1.2. Austria
2.1.3. Hungary
2.2. Methods
2.2.1. Chemicals and Laboratory Equipment
2.2.2. FTIR Analyses
2.2.3. Method of Total Phenolic Content (TPC) Determination
2.2.4. Method of Antioxidant Activity (DPPH Method) Determination
2.2.5. Statistical Evaluation
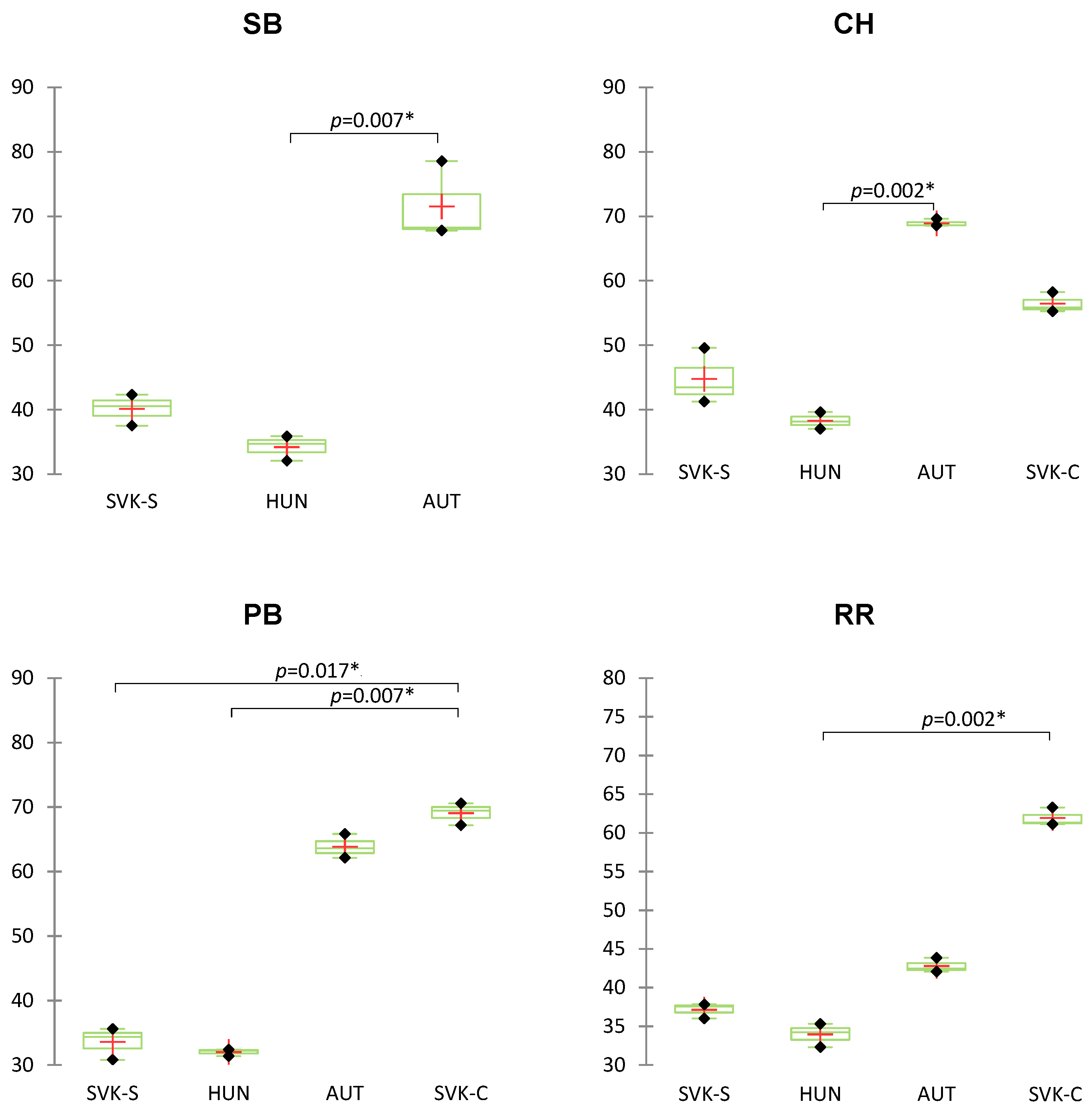
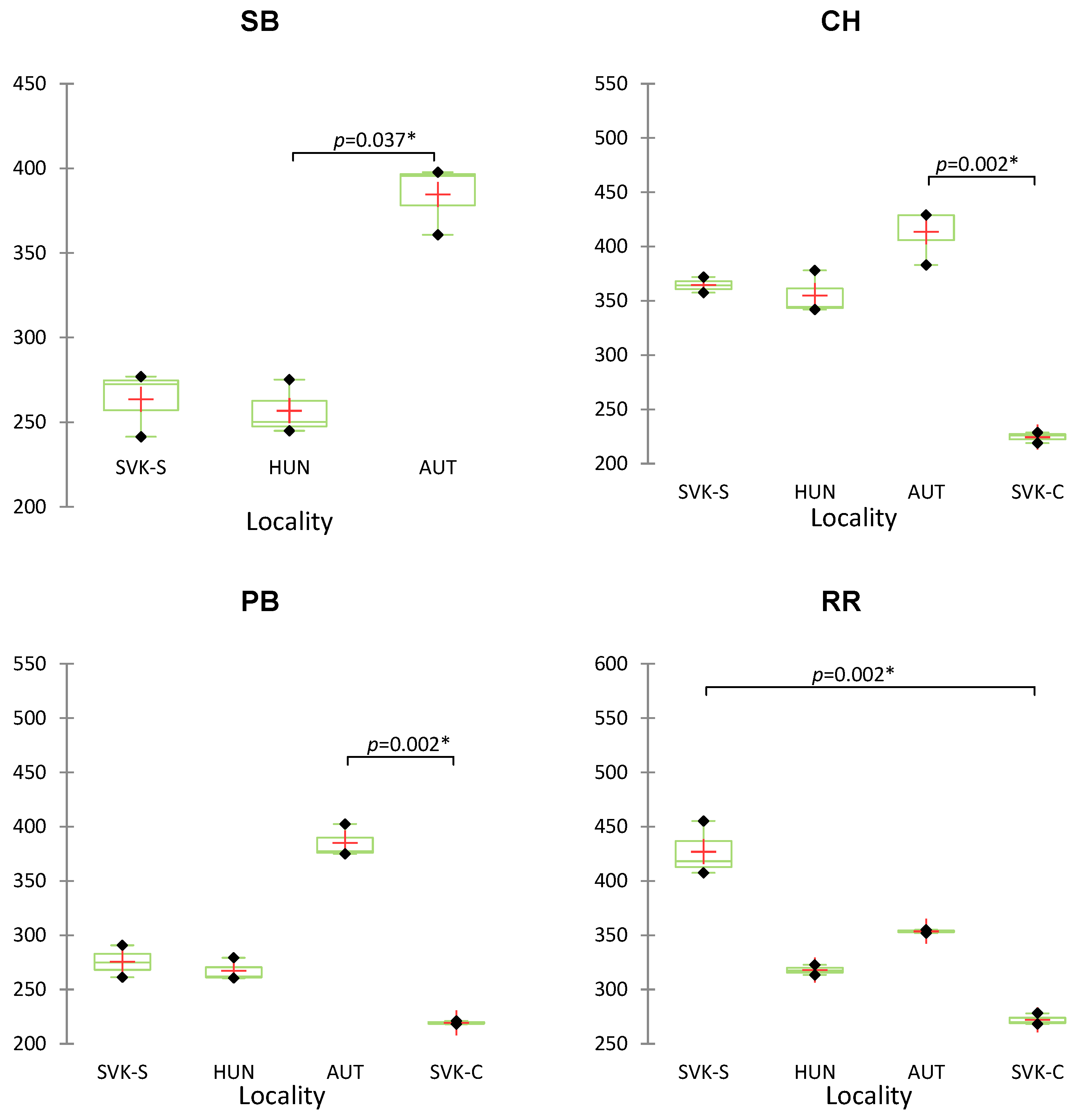
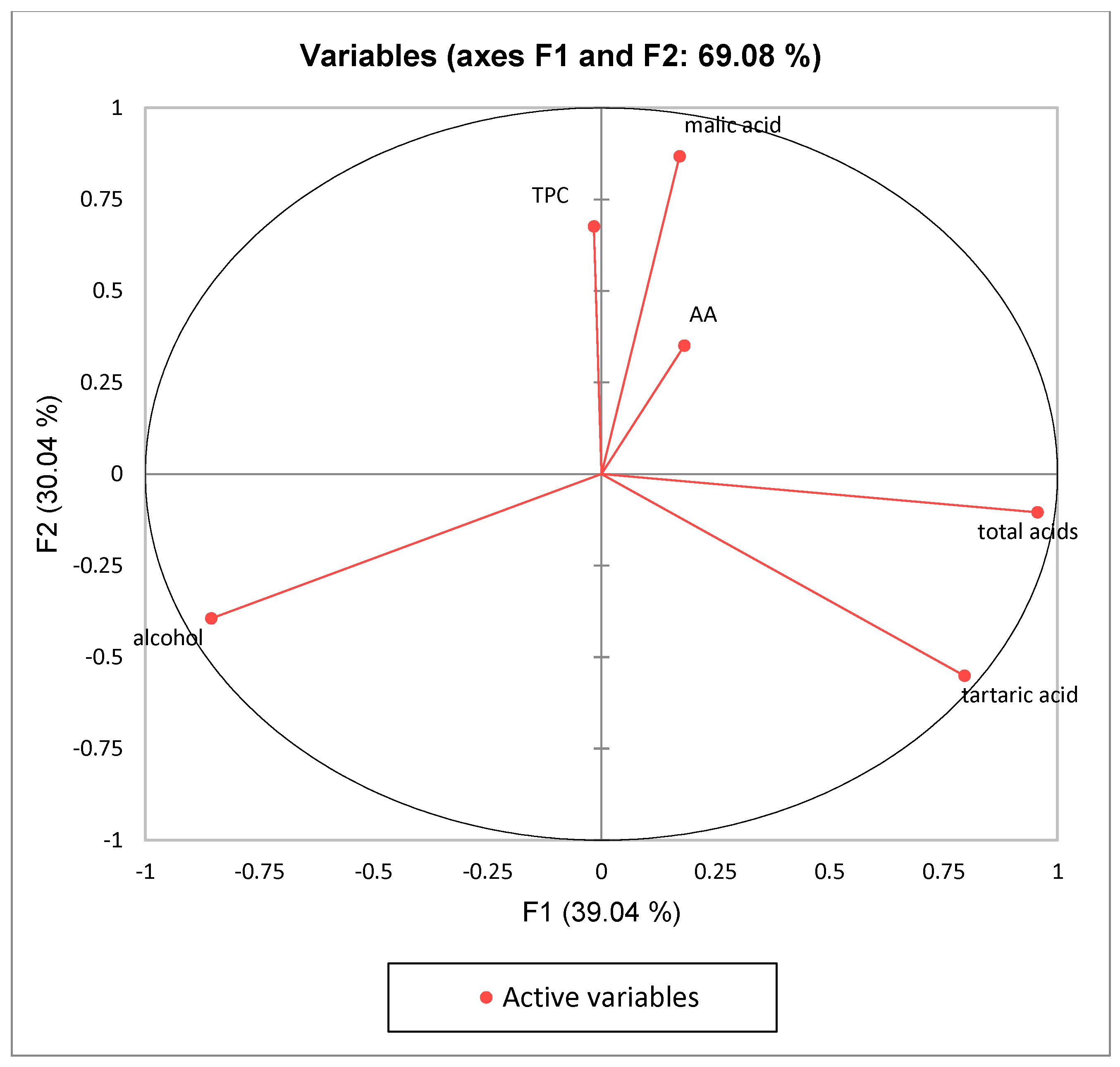
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Butnariu, M.; Butu, A. 11—Qualitative and Quantitative Chemical Composition of Wine. In Quality Control in the Beverage Industry; Grumezescu, A.M., Holban, A.M., Eds.; Academic Press: Cambridge, MA, USA, 2019; pp. 385–417. ISBN 978-0-12-816681-9. [Google Scholar]
- Rodrigues, S.M.; Otero, M.; Alves, A.A.; Coimbra, J.; Coimbra, M.A.; Pereira, E.; Duarte, A.C. Elemental Analysis for Categorization of Wines and Authentication of Their Certified Brand of Origin. J. Food Compos. Anal. 2011, 24, 548–562. [Google Scholar] [CrossRef]
- Pereira, L.; Gomes, S.; Barrias, S.; Fernandes, J.R.; Martins-Lopes, P. Applying High-Resolution Melting (HRM) Technology to Olive Oil and Wine Authenticity. Food Res. Int. 2018, 103, 170–181. [Google Scholar] [CrossRef] [PubMed]
- Luykx, D.M.; Van Ruth, S.M. An Overview of Analytical Methods for Determining the Geographical Origin of Food Products. Food Chem. 2008, 107, 897–911. [Google Scholar] [CrossRef]
- Cozzolino, D.; Smyth, H.E.; Gishen, M. Feasibility Study on the Use of Visible and Near-Infrared Spectroscopy Together with Chemometrics to Discriminate between Commercial White Wines of Different Varietal Origins. J. Agric. Food Chem. 2003, 51, 7703–7708. [Google Scholar] [CrossRef]
- Tregear, A.; Kuznesof, S.; Moxey, A. Policy Initiatives for Regional Foods: Some Insights from Consumer Research. Food Policy 1998, 23, 383–394. [Google Scholar] [CrossRef]
- Cozzolino, D.; Cynkar, W.U.; Shah, N.; Smith, P.A. Can Spectroscopy Geographically Classify Sauvignon Blanc Wines from Australia and New Zealand? Food Chem. 2011, 126, 673–678. [Google Scholar] [CrossRef]
- Gajek, M.; Pawlaczyk, A.; Szynkowska-Jozwik, M.I. Multi-Elemental Analysis of Wine Samples in Relation to Their Type, Origin, and Grape Variety. Molecules 2021, 26, 214. [Google Scholar] [CrossRef]
- Cordella, C.; Moussa, I.; Martel, A.-C.; Sbirrazzuoli, N.; Lizzani-Cuvelier, L. Recent Developments in Food Characterization and Adulteration Detection: Technique-Oriented Perspectives. J. Agric. Food Chem. 2002, 50, 1751–1764. [Google Scholar] [CrossRef]
- Jiang, B.; Xi, Z.; Luo, M.; Zhang, Z. Comparison on Aroma Compounds in Cabernet Sauvignon and Merlot Wines from Four Wine Grape-Growing Regions in China. Food Res. Int. 2013, 51, 482–489. [Google Scholar] [CrossRef]
- Liu, L.; Cozzolino, D.; Cynkar, W.U.; Gishen, M.; Colby, C.B. Geographic Classification of Spanish and Australian Tempranillo Red Wines by Visible and Near-Infrared Spectroscopy Combined with Multivariate Analysis. J. Agric. Food Chem. 2006, 54, 6754–6759. [Google Scholar] [CrossRef]
- Mandrile, L.; Zeppa, G.; Giovannozzi, A.M.; Rossi, A.M. Controlling Protected Designation of Origin of Wine by Raman Spectroscopy. Food Chem. 2016, 211, 260–267. [Google Scholar] [CrossRef]
- Liu, S.; Wei, W.; Bai, Z.; Wang, X.; Li, X.; Wang, C.; Liu, X.; Liu, Y.; Xu, C. Rapid Identification of Pearl Powder from Hyriopsis Cumingii by Tri-Step Infrared Spectroscopy Combined with Computer Vision Technology. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2018, 189, 265–274. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.-H.; Liu, S.-L.; Zhao, S.-N.; Li, S.-Z.; Sun, S.-Q. Unveiling Ontogenesis of Herbal Medicine in Plant Chemical Profiles by Infrared Macro-Fingerprinting. Planta Med. 2013, 79, 1068–1076. [Google Scholar] [CrossRef] [PubMed]
- Hu, W.; Guo, X.-X.; Wang, X.-C.; Zhao, Y.; Sun, S.-Q.; Xu, C.-H.; Liu, Y. Rapid Discrimination of Different Grades of White Croaker Surimi by Tri-Step Infrared Spectroscopy Combined with Soft Independent Modeling of Class Analogy (SIMCA). Food Anal. Methods 2016, 9, 831–839. [Google Scholar] [CrossRef]
- Krebiehl, A. Get to Know Austria’s Wine Regions. Wine Enthusiast. Available online: https://www.winemag.com/2018/05/15/austrian-wine-regions/ (accessed on 29 April 2024).
- Austrian Wine Marketing Board. Austrian Wine in Depth. Training Manual. Available online: https://www.oesterreichwein.at/fileadmin/user_upload/PDF/Broschueren/SU_EN_2022_04_web.pdf (accessed on 29 April 2024).
- Gilby, C. Exploring Hungary: Around the Wine Regions. Decanter. Available online: https://www.decanter.com/sponsored/exploring-hungary-around-the-wine-regions-456060/ (accessed on 29 April 2024).
- Kielmayer, K.; Herzeg, Á. Pinot Blanc. Wines of Hungary Personally. Available online: https://winesofhungary.hu/grape-varieties/white-grape-varieties-white-wine-styles/pinot-blanc (accessed on 29 April 2024).
- Jakabová, S.; Fikselová, M.; Mendelová, A.; Ševčík, M.; Jakab, I.; Aláčová, Z.; Kolačkovská, J.; Ivanova-Petropulos, V. Chemical Composition of White Wines Produced from Different Grape Varieties and Wine Regions in Slovakia. Appl. Sci. 2021, 11, 11059. [Google Scholar] [CrossRef]
- Lachman, J.; Pronek, D.; Hejtmánková, A.; Dudjak, J.; Pivec, V.; Faitová, K. Total Polyphenol and Main Flavonoid Antioxidants in Different Onion (Allium cepa L.) Varieties. Hortic. Sci. 2003, 30, 142–147. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.-E.; Berset, C. Use of a Free Radical Method to Evaluate Antioxidant Activity. LWT Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Han, S.; Yang, J.; Choi, K.; Kim, J.; Adhikari, K.; Lee, J. Chemical Analysis of Commercial White Wines and Its Relationship with Consumer Acceptability. Foods 2022, 11, 603. [Google Scholar] [CrossRef]
- Vidal, N.P.; Manful, C.F.; Pham, T.H.; Stewart, P.; Keough, D.; Thomas, R. The Use of XLSTAT in Conducting Principal Component Analysis (PCA) When Evaluating the Relationships between Sensory and Quality Attributes in Grilled Foods. MethodsX 2020, 7, 100835. [Google Scholar] [CrossRef]
- Fikselová, M.; Mendelová, A.; Gažo, J. Wine Quality as a Part of Cultural Heritage Affected by Its Different Geographical Origins. In Cultural Heritage—Possibilities for Land-Centered Societal Development; Hernik, J., Walczycka, M., Sankowski, E., Harris, B.J., Eds.; Environmental History; Springer International Publishing: Cham, Switzerland, 2022; Volume 13, pp. 69–78. ISBN 978-3-030-58091-9. [Google Scholar]
- Boulton, R. The Relationships between Total Acidity, Titratable Acidity and pH in Grape Tissue. Vitis 1980, 19, 113–120. [Google Scholar]
- Regmi, U.; Palma, M.; Barroso, C.G. Direct Determination of Organic Acids in Wine and Wine-Derived Products by Fourier Transform Infrared (FT-IR) Spectroscopy and Chemometric Techniques. Anal. Chim. Acta 2012, 732, 137–144. [Google Scholar] [CrossRef]
- Zanette, T.; Ana, D.R.S.; Giacomelli, S.R.; Datsch, V.C.; Datsch, A.C.; Demari, G.H.; Souza, V.Q. Determination of the Antioxidant Activity of Red and White Wines Produced in Rio Grande Do Sul, Brazil. Int. J. Curr. Res. 2016, 8, 36710–36713. [Google Scholar]
- Li, H.; Wang, X.; Li, Y.; Li, P.; Wang, H. Polyphenolic Compounds and Antioxidant Properties of Selected China Wines. Food Chem. 2009, 112, 454–460. [Google Scholar] [CrossRef]
- Ma, T.-T.; Sun, X.-Y.; Gao, G.-T.; Wang, X.-Y.; Liu, X.-Y.; Du, G.-R.; Zhan, J.-C. Phenolic Characterisation and Antioxidant Capacity of Young Wines Made from Different Grape Varieties Grown in Helanshan Donglu Wine Zone (China). S. Afr. J. Enol. Vitic. 2014, 35, 321–331. [Google Scholar] [CrossRef]
- Ailer, Š.; Serenčéš, R.; Kozelová, D.; Poláková, Z.; Jakabová, S. Possibilities for Depleting the Content of Undesirable Volatile Phenolic Compounds in White Wine with the Use of Low-Intervention and Economically Efficient Grape Processing Technology. Appl. Sci. 2021, 11, 6735. [Google Scholar] [CrossRef]
- Bajčan, D.; Árvay, J.; Vollmannová, A.; Bystrická, J.; Trebichalskỳ, P.; Harangozo, L.; Šimanskỳ, V. Antioxidant Properties, Total Phenolic and Total Flavonoid Content of the Slovak White Wines-Welschriesling and Chardonnay. Potravin. Slovak J. F. Sci. 2017, 11, 266–271. [Google Scholar] [CrossRef]
- Brad, C.C.; Muste, S.; Mudura, E.; Bobis, O. The Content of Polyphenolic Compounds and Antioxidant Activity of Three Monovarietal Wines and Their Blending, Used for Sparkling Wine Production. Bull. UASVM Serie Agric. 2012, 69, 2. [Google Scholar]
- Feher, I.; Magdas, D.A.; Dehelean, A.; Sârbu, C. Characterization and Classification of Wines According to Geographical Origin, Vintage and Specific Variety Based on Elemental Content: A New Chemometric Approach. J. Food Sci. Technol. 2019, 56, 5225–5233. [Google Scholar] [CrossRef]
- Téthal, J.; Baroň, M.; Sotolář, R.; Ailer, S.; Sochor, J. Effect of Grapevine Rootstocks on Qualitative Parameters of the Cerason Variety. Czech J. Food Sci. 2015, 33, 570–579. [Google Scholar] [CrossRef]
- Hu, X.-Z.; Liu, S.-Q.; Li, X.-H.; Wang, C.-X.; Ni, X.-L.; Liu, X.; Wang, Y.; Liu, Y.; Xu, C.-H. Geographical Origin Traceability of Cabernet Sauvignon Wines Based on Infrared Fingerprint Technology Combined with Chemometrics. Sci. Rep. 2019, 9, 8256. [Google Scholar] [CrossRef]
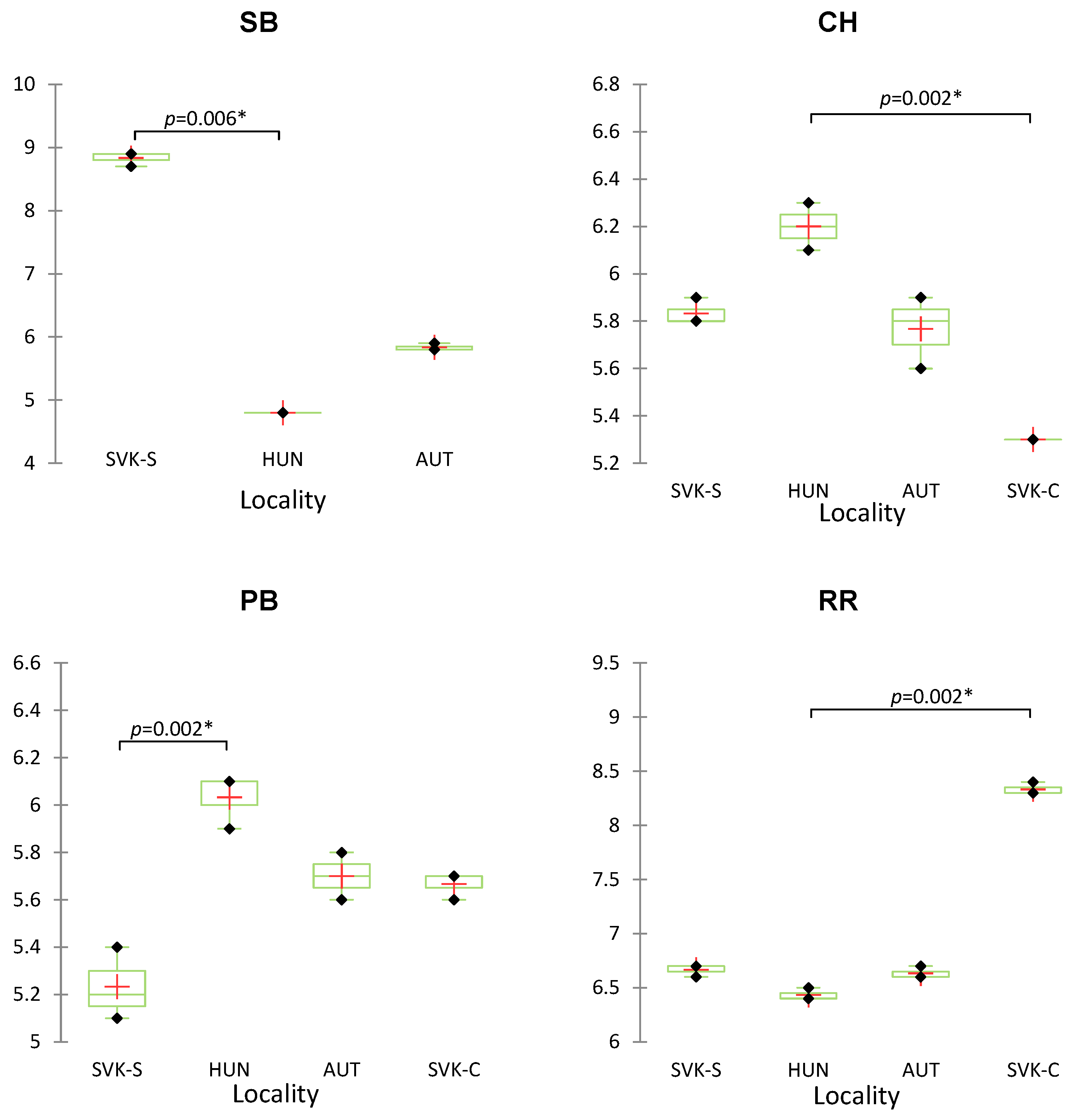
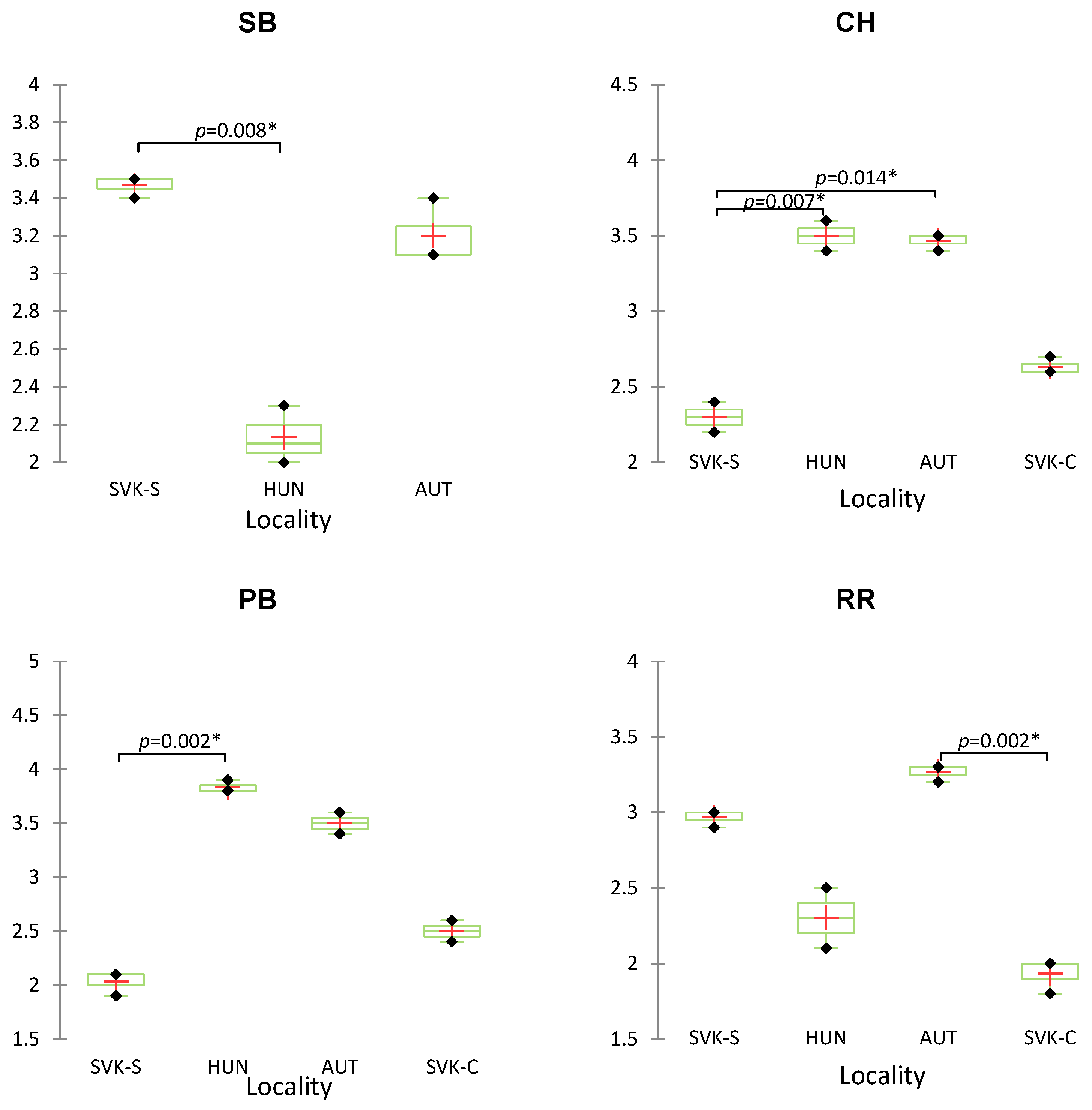
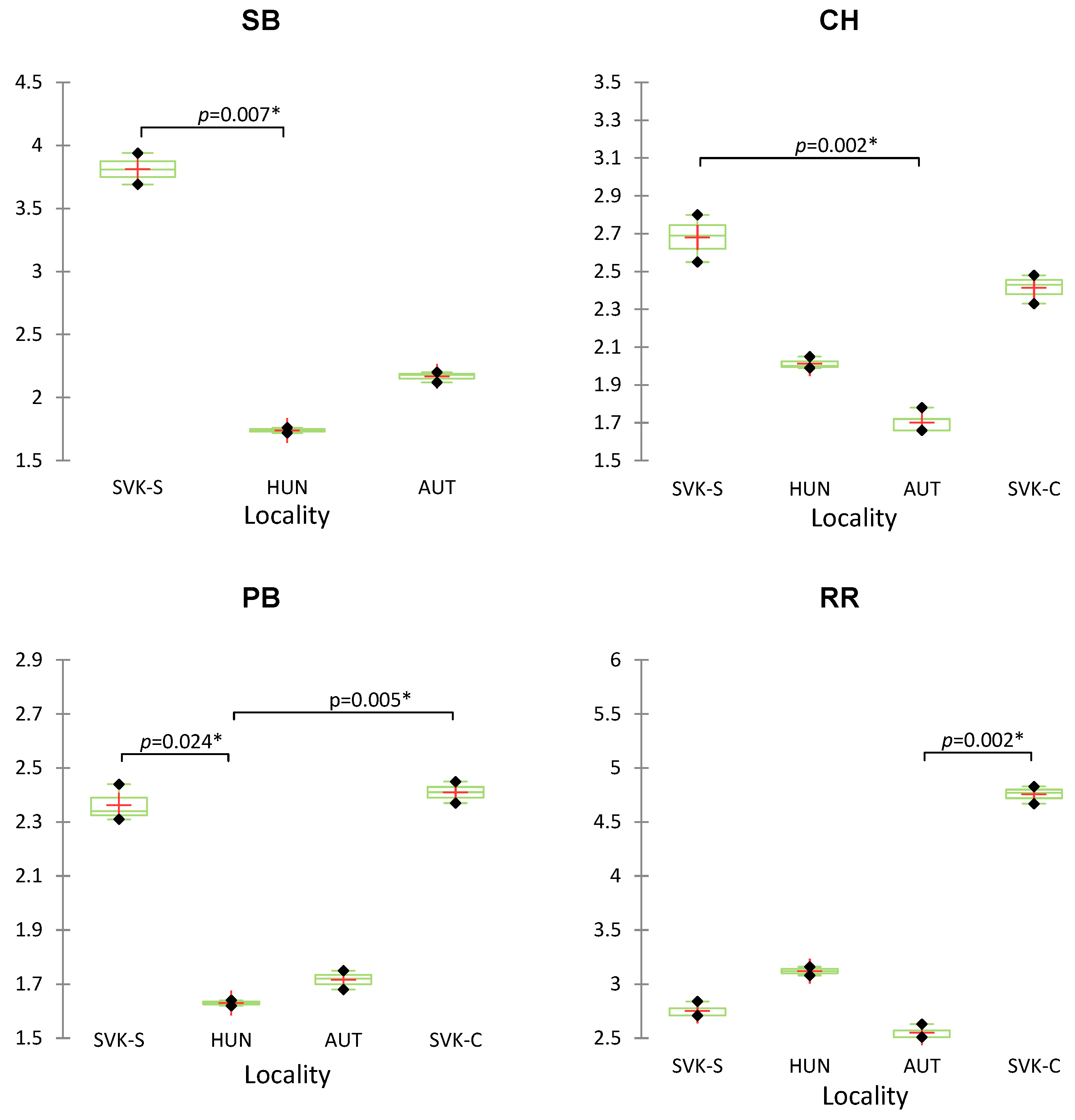
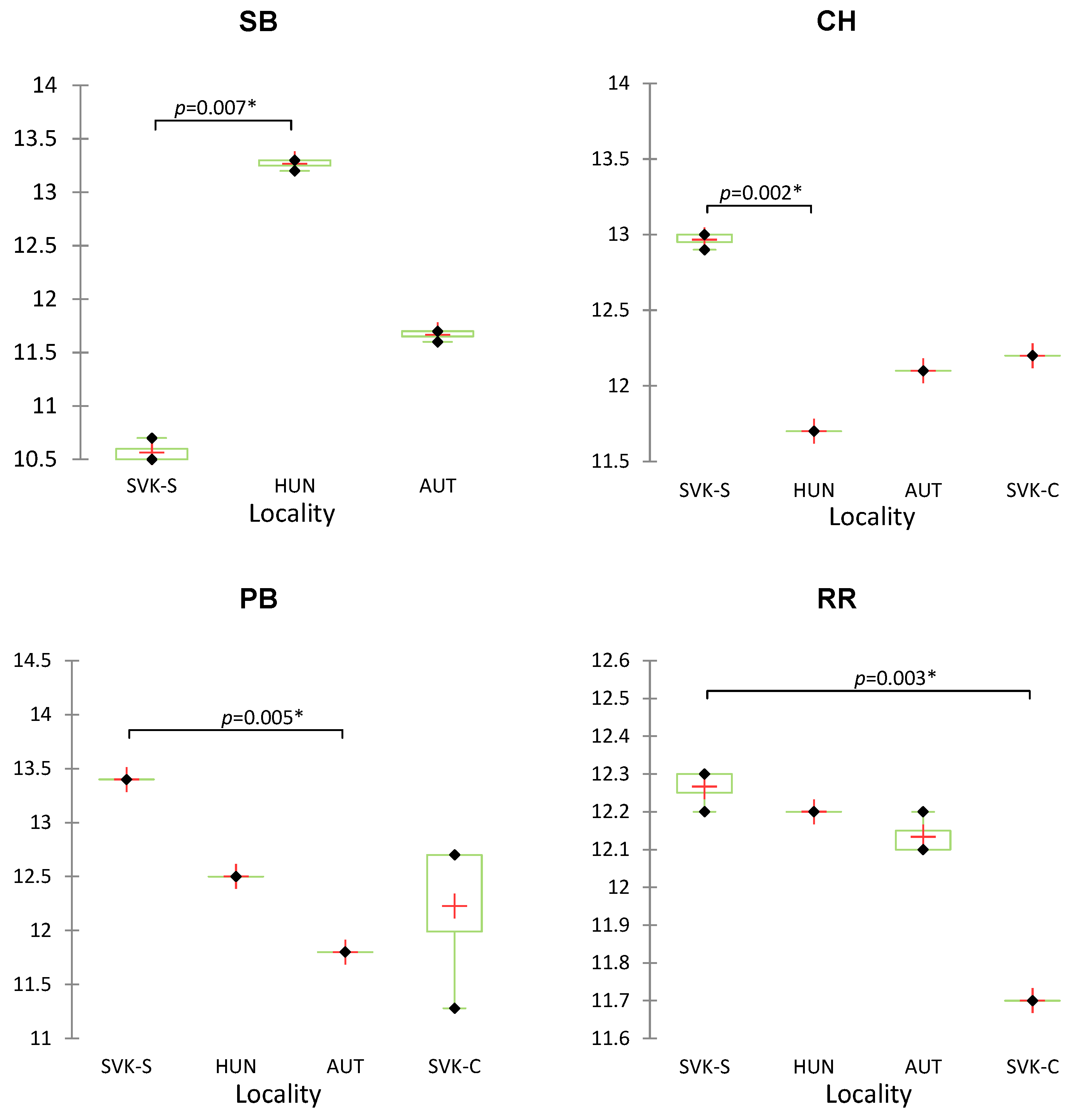

| Sample of Wine | Origin | Variety |
|---|---|---|
| 1 | SVK-S—Southern Slovak wine-growing region | SB |
| 2 | SVK-S—Southern Slovak wine-growing region | RR |
| 3 | SVK-S—Southern Slovak wine-growing region | PB |
| 4 | SVK-S—Southern Slovak wine-growing region | CH |
| 5 | SVK-C—Central Slovak wine-growing region | RR |
| 6 | SVK-C—Central Slovak wine-growing region | PB |
| 7 | SVK-C—Central Slovak wine-growing region | CH |
| 8 | HUN | SB |
| 9 | HUN | RR |
| 10 | HUN | PB |
| 11 | HUN | CH |
| 12 | AUT | SB |
| 13 | AUT | RR |
| 14 | AUT | PB |
| 15 | AUT | CH |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fikselová, M.; Benešová, L.; Jakabová, S.; Mezey, J.; Čapla, J.; Golian, J. Chemometric Approach Application in Modern Wine Studies. Beverages 2024, 10, 84. https://doi.org/10.3390/beverages10030084
Fikselová M, Benešová L, Jakabová S, Mezey J, Čapla J, Golian J. Chemometric Approach Application in Modern Wine Studies. Beverages. 2024; 10(3):84. https://doi.org/10.3390/beverages10030084
Chicago/Turabian StyleFikselová, Martina, Lucia Benešová, Silvia Jakabová, Ján Mezey, Jozef Čapla, and Jozef Golian. 2024. "Chemometric Approach Application in Modern Wine Studies" Beverages 10, no. 3: 84. https://doi.org/10.3390/beverages10030084
APA StyleFikselová, M., Benešová, L., Jakabová, S., Mezey, J., Čapla, J., & Golian, J. (2024). Chemometric Approach Application in Modern Wine Studies. Beverages, 10(3), 84. https://doi.org/10.3390/beverages10030084







