The Anti-Inflammatory and Antithrombotic Properties of Bioactives from Orange, Sanguine and Clementine Juices and from Their Remaining By-Products
Abstract
:1. Introduction
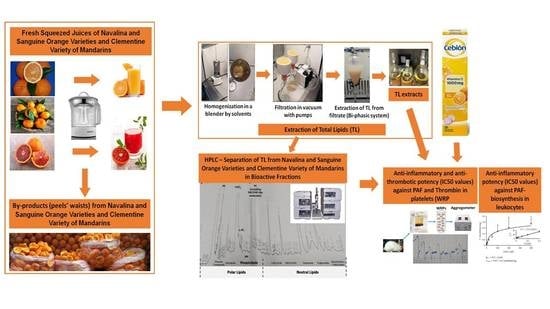
2. Materials and Methods
2.1. Samples Preparation and Lipids’ Extraction
2.2. Determination of Vitamin C
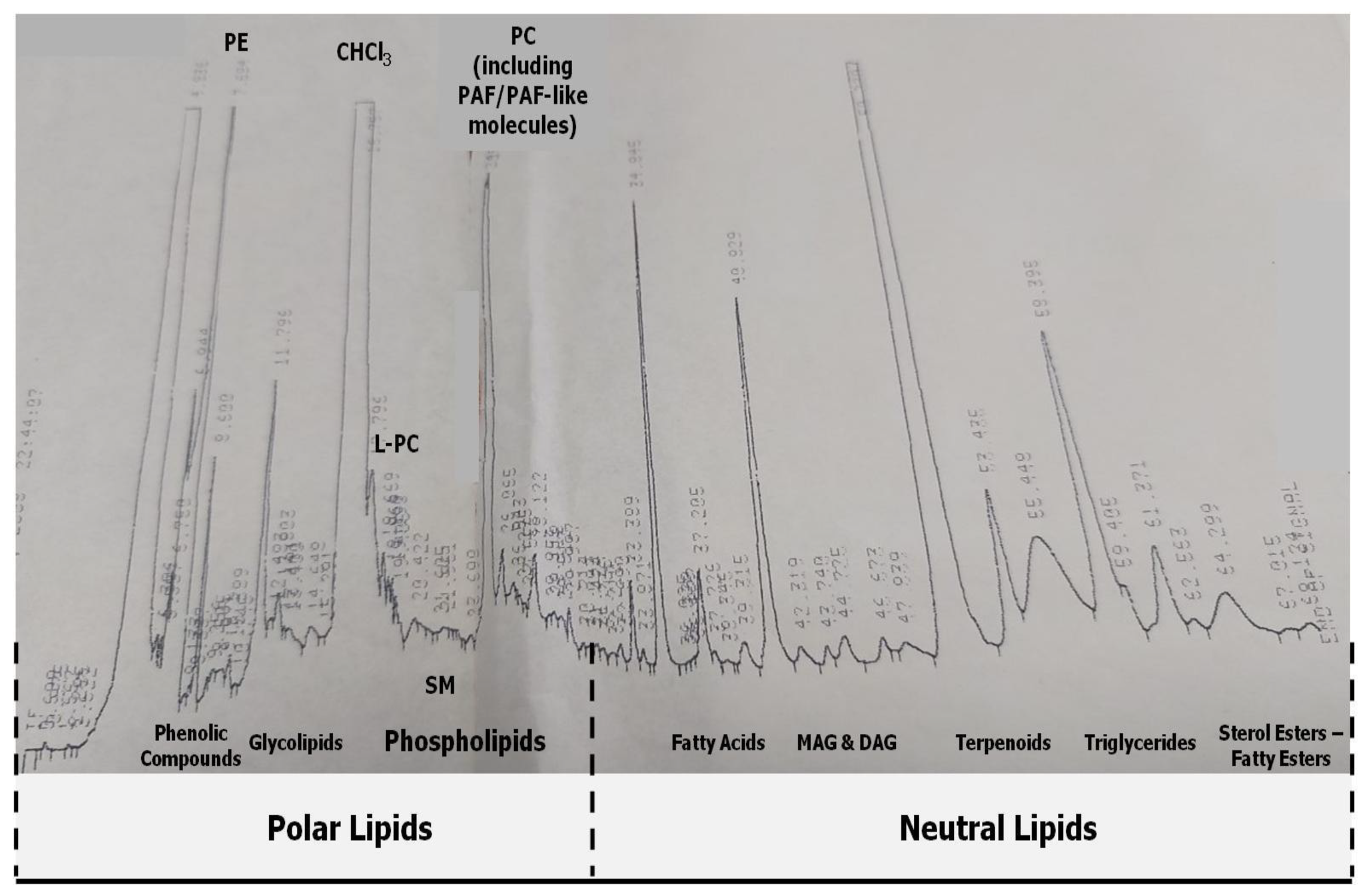
2.3. HPLC Analysis and Separation of Bioactive Lipid Molecules from Orange, Sanguine and Clementine Juices and Peels
2.4. Biological Assays on Washed Rabbit Platelets
2.5. Enzymatic Assays of PAF-CPT and Lyso-PAF-AT in Rabbit Leukocytes
2.5.1. Isolation of Rabbit Leukocytes
2.5.2. DTT-Insensitive PAF-Cholinephosphotransferase (PAF-CPT) Activity Assays
2.5.3. Lyso-PAF-AT Activity Assays
2.6. Statistical Analysis
3. Results and Discussion
3.1. Anti-Inflammatory and Antithrombotic Potency of TLs from Juices of Oranges and Mandarines and from Their by-Products in Platelets
3.2. Anti-Inflammatory and Antithrombotic Potency of HPLC-Derived Bioactive Fractions from TLs of Juices of Oranges and Mandarines and from Their by-Products in Platelets
3.3. Anti-Inflammatory Effects of TLs and HPLC-Derived Bioactive Fractions from Juices of Oranges and Mandarines and from Their by-Products against PAF-Synthesis in Leukocytes
4. Conclusions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tsoupras, A.; Lordan, R.; Zabetakis, I. Inflammation and cardiovascular diseases. In The Impact of Nutrition and Statins on Cardiovascular Diseases; Zabetakis, I., Lordan, R., Tsoupras, A., Eds.; Academic Press: Cambridge, MA, USA, 2019; Chapter 3; pp. 53–117. [Google Scholar]
- Tsoupras, A.; Lordan, R.; Zabetakis, I. Inflammation, not Cholesterol, Is a Cause of Chronic Disease. Nutrients 2018, 10, 604. [Google Scholar] [CrossRef] [Green Version]
- Tsoupras, A.B.; Iatrou, C.; Frangia, C.; Demopoulos, C.A. The implication of platelet activating factor in cancer growth and metastasis: Potent beneficial role of PAF-inhibitors and antioxidants. Infect. Disord.-Drug Targets 2009, 4, 390–399. [Google Scholar] [CrossRef] [PubMed]
- De Lorgeril, M.; Salen, P.; Martin, J.L.; Monjaud, I.; Delaye, J.; Mamelle, N. Mediterranean Diet, Traditional Risk Factors and the Rate of Cardiovascular Complications after Myocardial Infarction: Final report of the Lyon Diet Heart Study. Circulation 1999, 99, 779–785. [Google Scholar] [CrossRef] [PubMed]
- Hoevenaar-Blom, M.P.; Nooyens, A.C.; Kromhout, D.; Spijkerman, A.M.; Beulens, J.W.; Van Der Schouw, Y.T.; Bueno-De-Mesquita, B.; Verschuren, W.M. Mediterranean style diet and 12-year incidence of cardiovascular diseases: The EPIC-NL cohort study. PLoS ONE 2012, 7, e45458. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fung, T.T.; Willett, W.C.; Stampfer, M.J.; Manson, J.E.; Hu, F.B. Dietary patterns and the risk of coronary heart disease in women. Arch. Intern. Med. 2001, 161, 1857–1862. [Google Scholar] [CrossRef] [Green Version]
- Tierney, A.; Lordan, R.; Tsoupras, A.; Zabetakis, I. Diet and cardiovascular disease: The Mediterranean diet. In The Impact of Nutrition and Statins on Cardiovascular Diseases; Zabetakis, I., Lordan, R., Tsoupras, A., Eds.; Academic Press: Cambridge, MA, USA, 2019; Chapter 8; pp. 267–288. [Google Scholar]
- Lehr, H.A.; Weyrich, A.S.; Saetzler, R.K.; Jurek, A.; Arfors, K.E.; Zimmerman, G.A.; Prescott, S.M.; McIntyre, T.M. Vitamin C blocks inflammatory platelet-activating factor mimetics created by cigarette smoking. J. Clin. Investig. 1997, 99, 2358–2364. [Google Scholar] [CrossRef]
- Olsson, M.E.; Gustavsson, K.E.; Andersson, S.; Nilsson, A.; Duan, R.D. Inhibition of cancer cell proliferation in vitro by fruit and berry extracts and correlations with antioxidant levels. J. Agric. Food Chem. 2004, 52, 7264–7271. [Google Scholar] [CrossRef]
- Guthrie, N.; Carroll, K.K. Inhibition of mammary cancer by citrus flavonoids. Adv. Exp. Med. Biol. 1998, 439, 227–236. [Google Scholar] [CrossRef]
- Lloberas, N.; Torras, J.; Herrero-Fresneda, I.; Cruzado, J.M.; Riera, M.; Hurtado, I.; Grinyó, J.M. Postischemic renal oxidative stress induces inflammatory response through PAF and oxidized phospholipids. Prevention by antioxidant treatment. FASEB J. 2002, 16, 908–910. [Google Scholar] [CrossRef]
- Yao, Y.; Harrison, K.A.; Al-Hassani, M.; Murphy, R.C.; Rezania, S.; Konger, R.L.; Travers, J.B. Platelet-Activating Factor Receptor Agonists Mediate Xeroderma Pigmentosum A Photosensitivity. J. Biol. Chem. 2012, 287, 9311–9321. [Google Scholar] [CrossRef] [Green Version]
- Jin, Y.R.; Han, X.H.; Zhang, Y.H.; Lee, J.J.; Lim, Y.; Chung, J.H.; Yun, Y.P. Antiplatelet activity of hesperetin, a bioflavonoid, is mainly mediated by inhibition of PLC-gamma2 phosphorylation and cyclooxygenase-1 activity. Atherosclerosis 2007, 194, 144–152. [Google Scholar] [CrossRef]
- Kim, T.H.; Kim, H.M.; Park, S.W.; Jung, Y.S. Inhibitory effects of yuzu and its components on human platelet aggregation. Biomol. Ther. 2015, 23, 149–155. [Google Scholar] [CrossRef] [Green Version]
- Assefa, A.D.; Ko, E.Y.; Moon, S.H.; Keum, Y.-S. Antioxidant and antiplatelet activities of flavonoid-rich fractions of three citrus fruits from Korea. Biotech 2016, 6, 109. [Google Scholar] [CrossRef] [Green Version]
- Osman, H.E.; Maalej, N.; Shanmuganayagam, D.; Folts, J.D. Grape Juice but Not Orange or Grapefruit Juice Inhibits Platelet Activity in Dogs and Monkeys (Macaca fasciularis). J. Nutr. 1998, 128, 2307–2312. [Google Scholar] [CrossRef] [Green Version]
- Bhattacharya, S. Cultivation of essential oils. In Essential Oils in Food Preservation, Flavor and Safety; Preedy, V.R., Ed.; Academic Press: Cambridge, MA, USA, 2016; Chapter 3; pp. 19–29. ISBN 9780124166417. [Google Scholar] [CrossRef]
- Sharmila, V.G.; Kavitha, S.; Obulisamy, P.K.; Rajesh Banu, J. Production of fine chemicals from food wastes. In Food Waste to Valuable Resources; Rajesh Banu, J., Kumar, G., Gunasekaran, M., Kavitha, S., Eds.; Academic Press: Cambridge, MA, USA, 2020; Chapter 8; pp. 163–188. ISBN 9780128183533. [Google Scholar] [CrossRef]
- Williams, C.A. Specialized dietary supplements. In Equine Applied and Clinical Nutrition; Raymond, J., Geor, P., Harris, A., Coenen, M., Saunders, W.B., Eds.; Elsevier: Edinburgh, UK, 2013; Chapter 19; pp. 351–366. ISBN 9780702034220. [Google Scholar] [CrossRef]
- Ladaniya, M.S. (Ed.) Fruit biochemistry. In Citrus Fruit; Academic Press: Cambridge, MA, USA, 2008; Chapter 6; pp. 125–190. ISBN 9780123741301. [Google Scholar] [CrossRef]
- Khan, N.; Monagas, M.; Urpi-sarda, M.; Llorach, R.; Andres-Lacueva, C. Contribution of bioactive foods and their emerging role in immunomodulation, inflammation, and arthritis. In Preedy, Bioactive Food as Dietary Interventions for Arthritis and Related Inflammatory Diseases; Watson, R.R., Victor, R., Eds.; Academic Press: Cambridge, MA, USA, 2013; Chapter 4; pp. 43–65. ISBN 9780123971562. [Google Scholar] [CrossRef]
- Moharam, B.A.; Jantan, I.; Ahmad, F.B.; Jalil, J. Antiplatelet Aggregation and Platelet Activating Factor (PAF) Receptor Antagonistic Activities of the Essential Oils of Five Goniothalamus Species. Molecules 2010, 15, 5124–5138. [Google Scholar] [CrossRef] [Green Version]
- Kuttan, G.; Pratheeshkumar, P.; Manu, K.A.; Kuttan, R. Inhibition of tumor progression by naturally occurring terpenoids. Pharm. Biol. 2011, 49, 995–1007. [Google Scholar] [CrossRef]
- Bligh, E.G.; Dyer, W.J. A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol. 1959, 37, 911–917. [Google Scholar] [CrossRef]
- Tsoupras, A.; Donal, M.; Pleskach, H.; Durkin, M.; Traas, C.; Zabetakis, I. Beneficial Anti-Platelet and Anti-Inflammatory Properties of Irish Apple Juice and Cider Bioactives. Foods 2021, 10, 412. [Google Scholar] [CrossRef]
- Tsoupras, A.; Moran, D.; Byrne, T.; Ryan, J.; Barrett, L.; Traas, C.; Zabetakis, I. Anti-Inflammatory and Anti-Platelet Properties of Lipid Bioactives from Apple Cider By-Products. Molecules 2021, 26, 2869. [Google Scholar] [CrossRef]
- Sapei, L.; Hwa, L. Study on the Kinetics of Vitamin C Degradation in Fresh Strawberry Juices. Procedia Chem. 2014, 9, 62–68. [Google Scholar] [CrossRef] [Green Version]
- Tsoupras, A.; Kouvelis, V.N.; Pappas, K.M.; Demopoulos, C.A.; Typas, M.A. Anti-inflammatory and anti-thrombotic properties of lipid bioactives from the entomopathogenic fungus Beauveria bassiana. Prostaglandins Other Lipid Mediat. 2022, 158, 106606. [Google Scholar] [CrossRef]
- Koukouraki, P.; Tsoupras, A.; Sotiroudis, G.; Demopoulos, C.A.; Sotiroudis, T.G. Antithrombotic properties of Spirulina extracts against platelet-activating factor and thrombin. Food Biosci. 2020, 37, 100686. [Google Scholar] [CrossRef]
- Tsoupras, A.B.; Demopoulos, C.A.; Pappas, K.M. Platelet-activating factor detection, metabolism, and inhibitors in the ethanologenic bacterium Zymomonas mobilis. Eur. J. Lipid Sci. Technol. 2012, 114, 123–133. [Google Scholar] [CrossRef]
- Tsoupras, A.; Pappas, K.M.; Sotiroudis, T.G.; Demopoulos, C.A. One-step separation system of bio-functional lipid compounds from natural sources. MethodsX 2021, 8, 101380. [Google Scholar] [CrossRef]
- Tsoupras, A.B.; Chini, M.; Mangafas, N.; Tsogas, N.; Stamatakis, G.; Tsantila, N.; Fragopoulou, E.; Antonopoulou, S.; Gargalianos, P.; Demopoulos, C.A.; et al. Platelet-activating factor and its basic metabolic enzymes in blood of naive HIV-infected patients. Angiology 2012, 63, 343–352. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Tsoupras, A.B.; Fragopoulou, E.; Nomikos, T.; Iatrou, C.; Antonopoulou, S.; Demopoulos, C.A. Characterization of the de novo biosynthetic enzyme of platelet activating factor, DDT-insensitive cholinephosphotransferase, of human mesangial cells. Mediat. Inflamm. 2007, 2007, 27683. [Google Scholar] [CrossRef] [Green Version]
- Tsoupras, A.B.; Chini, M.; Tsogas, N.; Fragopoulou, E.; Nomikos, T.; Lioni, A.; Mangafas, N.; Demopoulos, C.A.; Antonopoulou, S.; Lazanas, M.C. Anti-platelet-activating factor effects of highly active antiretroviral therapy (HAART): A new insight in the drug therapy of HIV infection? AIDS Res. Hum. Retrovir. 2008, 24, 1079–1086. [Google Scholar] [CrossRef] [PubMed]
- Martí, N.; Mena, P.; Cánovas, J.A.; Micol, V.; Saura, D. Vitamin C and the role of citrus juices as functional food. Nat. Prod. Commun. 2009, 4, 677–700. [Google Scholar] [CrossRef] [Green Version]
- Violi, F.; Pignatelli, P.; Basili, S. Nutrition, supplements and vitamins in platelet function and bleeding. Circulation 2010, 121, 1033–1044. [Google Scholar] [CrossRef]
- Tamer, F.; Tullemans, B.M.E.; Kuijpers, M.J.E.; Claushuis, T.A.M.; Heemskerk, J.W.M. Nutrition Phytochemicals Affecting Platelet Signaling and Responsiveness: Implications for Thrombosis and Hemostasis. Thromb. Haemost. 2021. [Google Scholar] [CrossRef] [PubMed]
- Ling, S.K.; Pisar, M.M.; Man, S. Platelet-activating factor (PAF) receptor binding antagonist activity of the methanol extracts and isolated flavonoids from Chromolaena odorata (L.) King and Robinson. Biol. Pharm. Bull. 2007, 30, 1150–1152. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tsoupras, A.; Brummell, C.; Kealy, C.; Vitkaitis, K.; Redfern, S.; Zabetakis, I. Cardio-Protective Properties and Health Benefits of Fish Lipid Bioactives; The Effects of Thermal Processing. Mar. Drugs 2022, 20, 187. [Google Scholar] [CrossRef] [PubMed]
- Nasopoulou, C.; Tsoupras, A.B.; Karantonis, H.C.; Demopoulos, C.A.; Zabetakis, I. Fish polar lipids retard atherosclerosis in rabbits by down-regulating PAF biosynthesis and up-regulating PAF catabolism. Lipids Health Dis. 2011, 10, 213. [Google Scholar] [CrossRef] [Green Version]
- Tsoupras, A.B.; Fragopoulou, E.; Iatrou, C.; Demopoulos, C.A. In Vitro Protective Effects of Olive Pomace Polar Lipids towards Platelet Activating Factor Metabolism in Human Renal Cells. Curr. Top. Nutraceutical Res. 2011, 9, 105–110. [Google Scholar]
| Samples | IC50-Valuesa against Platelet Aggregation Induced by PAF 1 | IC50-Valuesa against Platelet Aggregation Induced by Thrombin 1 | Platelet Aggregation—Cross Desensitization Tests (EC50 Values) 2 |
|---|---|---|---|
| Freshly dissolved Vitamin C supplement | 87 ± 15 1a | 178.5 ± 28.2 1a | ND |
| Oxidized Vitamin C supplement (left uncapped in light and room temperature for 4 h) | 160.2 ± 25.0 1a | 244.2 ± 34.2 1a | ND |
| Fresh squeezed juice of Navalina oranges | 23.2 ± 4.2 1a | 42.3 ± 3.8 1a | ND |
| Fresh squeezed juice of Sanguine oranges | 21.4 ± 3.1 1a | 36.9 ± 2.9 1a | ND |
| Fresh squeezed juice of Clementines | 28.6 ± 4.5 1a | 53.6 ± 4.4 1a | ND |
| Oxidized juice of Navalina Oranges (left uncapped in light and room temperature for 4 h) | 39 ± 8.1 1a | 122.2 ± 11.2 1a | ND |
| Oxidized juice of Sanguine Oranges (left uncapped in light and room temperature for 4 h) | 34 ± 5.3 1a | 113.9 ± 9.6 1a | ND |
| Oxidized juice of Mandarins- Clementines (left uncapped in light and room temperature for 4 h) | 49 ± 6.2 1a | 139.6 ± 14.6 1a | ND |
| TLs from Fresh squeezed juice of Navalina Oranges | 14.3 ± 3.2 1b,* | 47.2 ± 4.8 1a | ND |
| TLs from Fresh squeezed juice of Sanguine Oranges | 15.3 ± 3.2 1b,* | 41.3 ± 3.8 1a | ND |
| TLs from Fresh squeezed juice of Clementines | 17.3 ± 4.2 1b,* | 59.9 ± 4.7 1a | ND |
| TLs from peels’ wastes that remained after the production of the squeezed juice from Navalina Oranges | 1.5 ± 0.8 1b,# | 32.3 ± 2.5 1a,# | +/+ (133.6 ± 11.2) 2 |
| TLs from peels’ wastes that remained after the production of the squeezed juice from Sanguine Oranges | 1.2 ± 0.6 1b,# | 30.7 ± 3.8 1a,# | +/+ (122.3 ± 10.1) 2 |
| TLs from peels’ wastes that remained after the production of the squeezed juice from Clementines | 1.7 ± 1.1 1b,# | 43.6 ± 4.4 1a,# | +/+ (146.7 ± 12.4) 2 |
| Samples | Bioactive Amphiphilic/Lipophilic Fraction | IC50-Valuesa against Platelet Aggregation Induced by PAF 1 | SD | Platelet Aggregation—Cross Desensitization Tests (EC50 Values) 2 |
|---|---|---|---|---|
| TLs from Fresh squeezed juice of Navalina Oranges | Phenolic compounds | 1.5 # | 0.2 | ND |
| Glycolipids | 2.4 # | 0.3 | ND | |
| L-PC | ND | - | ND | |
| SM | 58.5 | 3.8 | ND | |
| PC/ PAF-like molecules | 6.3 * | 0.9 | ND | |
| Fatty Acids | 122.6 | 21.5 | ND | |
| MAG and DAG | 167.8 | 7.3 | ND | |
| Terpenoids | 86.7 | 5.6 | ND | |
| Triglycerides | ND | - | ND | |
| Fatty/Sterol esters | ND | - | ND | |
| TLs from Fresh squeezed juice of Sanguine Oranges | Phenolic compounds | 1.3 # | 0.2 | ND |
| Glycolipids | 3.8 * | 0.6 | ND | |
| L-PC | ND | - | ND | |
| SM | 72.3 | 4.6 | ND | |
| PC/ PAF-like molecules | 6.5 * | 0.6 | ND | |
| Fatty Acids | 143.4 | 27.3 | ND | |
| MAG and DAG | 177.3 | 9.2 | ND | |
| Terpenoids | 82.3 | 8.3 | ND | |
| Triglycerides | ND | - | ND | |
| Fatty/Sterol esters | ND | - | ND | |
| TLs from Fresh squeezed juice of Clementines | Phenolic compounds | 1.2 # | 0.3 | ND |
| Glycolipids | 4.3 * | 0.8 | ND | |
| L-PC | ND | - | ND | |
| SM | 77.3 | 8.4 | ND | |
| PC/ PAF-like molecules | 8.8 * | 0.7 | ND | |
| Fatty Acids | 167.7 | 20.3 | ND | |
| MAG and DAG | 165.4 | 8.2 | ND | |
| Terpenoids | 78.9 | 6.4 | ND | |
| Triglycerides | ND | - | ND | |
| Fatty/Sterol esters | ND | - | ND | |
| TLs from peels’ wastes that remained after the production of the squeezed juice from Navalina Oranges | Phenolic compounds | 0.8 # | 0.1 | ND |
| Glycolipids | 8.3 * | 1.3 | ND | |
| L-PC | ND | - | ND | |
| SM | 49.5 | 4.4 | ND | |
| PC/ PAF-like molecules | 2.4 # | 0.8 | +/+ (112.6 ± 15.8) | |
| Fatty Acids | 111.4 | 11.6 | ND | |
| MAG and DAG | 153.2 | 4.2 | ND | |
| Essential oils | 53.4 | 4.8 | ND | |
| Triglycerides | 456.8 | 34.6 | ND | |
| Fatty/Sterol esters | 887.9 | 82.5 | ND | |
| TLs from peels’ wastes that remained after the production of the squeezed juice from Sanguine Oranges | Phenolic compounds | 1.1 # | 0.3 | ND |
| Glycolipids | 9.2 * | 0.8 | ND | |
| L-PC | ND | - | ND | |
| SM | 44.8 | 3.2 | ND | |
| PC/ PAF-like molecules | 2.1 # | 0.6 | +/+ (128.9 ± 22.5) | |
| Fatty Acids | 125.5 | 14.3 | ND | |
| MAG and DAG | 158.7 | 7.1 | ND | |
| Essential oils | 41.5 | 2.9 | ND | |
| Triglycerides | 552.7 | 41.5 | ND | |
| Fatty/Sterol esters | 832.4 | 88.3 | ND | |
| TLs from peels’ wastes that remained after the production of the squeezed juice from Clementines | Phenolic compounds | 1.0 # | 0.3 | ND |
| Glycolipids | 8.5 * | 0.9 | ND | |
| L-PC | ND | - | ND | |
| SM | 52.1 | 6.2 | ND | |
| PC/ PAF-like molecules | 1.6 # | 0.4 | +/+ (136.5 ± 24.9) 2 | |
| Fatty Acids | 135.8 | 15.3 | ND | |
| MAG and DAG | 158.9 | 7.5 | ND | |
| Essential oils | 40.3 | 4.8 | ND | |
| Triglycerides | 665.4 | 41.9 | ND | |
| Fatty/Sterol esters | 942.5 | 76.3 | ND |
| Samples | IC50-Valuesa against PAF-CPT 1 | IC50-Valuesa against Lyso-PAF-AT 1 | |
|---|---|---|---|
| Freshly dissolved Vitamin C supplement | - | ND | ND |
| Fresh squeezed juice of Navalina Oranges | TL | 56.2 ± 15.0 | 68.2 ± 14.2 |
| Phenolic Compounds | 11.1 ± 2.2 | 15.2 ± 3.3 | |
| Glycolipids | 16.4 ± 5.1 | 18.4 ± 4.7 | |
| PC | 9.8 ± 1.0 | 11.2 ± 3.1 | |
| Terpenoids | ND | ND | |
| Fresh squeezed juice of Sanguine Oranges | TL | 53.4 ± 14.2 | 62.3 ± 13.8 |
| Phenolic Compounds | 13.4 ± 2.1 | 18.2 ± 4.3 | |
| Glycolipids | 16.4 ± 5.2 | 20.5 ± 5.1 | |
| PC | 8.2 ± 1.1 | 9.1 ± 1.2 | |
| Terpenoids | ND | ND | |
| Fresh squeezed juice of Clementines | TL | 71.5 ± 13.1 | 76.9 ± 12.9 |
| Phenolic Compounds | 17.1 ± 5.1 | 18.1 ± 4.3 | |
| Glycolipids | 19.1 ± 3.9 | 22.4 ± 4.3 | |
| PC | 10.5 ± 1.5 | 12.2 ± 1.2 | |
| Terpenoids | ND | ND | |
| TLs from peels’ wastes that remained after the production of the squeezed juice from Navalina Oranges | TL | 38 ± 4.5 | 43.6 ± 4.4 |
| Phenolic Compounds | 7.2 ± 1.0 | 8.5 ± 1.2 | |
| Glycolipids | 16.5 ± 1.5 | 18.4 ± 3.3 | |
| PC | 6.5 ± 1.1 | 8.2 ± 1.2 | |
| Terpenoids | ND | ND | |
| TLs from peels’ wastes that remained after the production of the squeezed juice from Sanguine Oranges | TL | 39 ± 5.1 | 42.2 ± 7.3 |
| Phenolic Compounds | 8.4 ± 1.1 | 9.1 ± 1.4 | |
| Glycolipids | 16.3 ± 1.8 | 18.4 ± 1.4 | |
| PC | 5.3 ± 0.8 | 6.5 ± 1.2 | |
| Terpenoids | ND | ND | |
| TLs from peels’ wastes that remained after the production of the squeezed juice from Clementines | TL | 34 ± 5.3 | 39.5 ± 4.6 |
| Phenolic Compounds | 4.9 ± 0.7 | 5.6 ± 1.4 | |
| Glycolipids | 14.3 ± 3.2 | 17.2 ± 4.8 | |
| PC | 5.3 ± 0.8 | 4.3 ± 0.7 | |
| Terpenoids | ND | ND |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tsoupras, A. The Anti-Inflammatory and Antithrombotic Properties of Bioactives from Orange, Sanguine and Clementine Juices and from Their Remaining By-Products. Beverages 2022, 8, 39. https://doi.org/10.3390/beverages8030039
Tsoupras A. The Anti-Inflammatory and Antithrombotic Properties of Bioactives from Orange, Sanguine and Clementine Juices and from Their Remaining By-Products. Beverages. 2022; 8(3):39. https://doi.org/10.3390/beverages8030039
Chicago/Turabian StyleTsoupras, Alexandros. 2022. "The Anti-Inflammatory and Antithrombotic Properties of Bioactives from Orange, Sanguine and Clementine Juices and from Their Remaining By-Products" Beverages 8, no. 3: 39. https://doi.org/10.3390/beverages8030039
APA StyleTsoupras, A. (2022). The Anti-Inflammatory and Antithrombotic Properties of Bioactives from Orange, Sanguine and Clementine Juices and from Their Remaining By-Products. Beverages, 8(3), 39. https://doi.org/10.3390/beverages8030039