Clinical Application of Platelet Concentrates in Bovine Practice: A Systematic Review
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Search Strategy
2.2. Inclusion Criteria
2.3. Exclusion Criteria
2.4. Study Selection
2.5. Data Extraction
3. Results
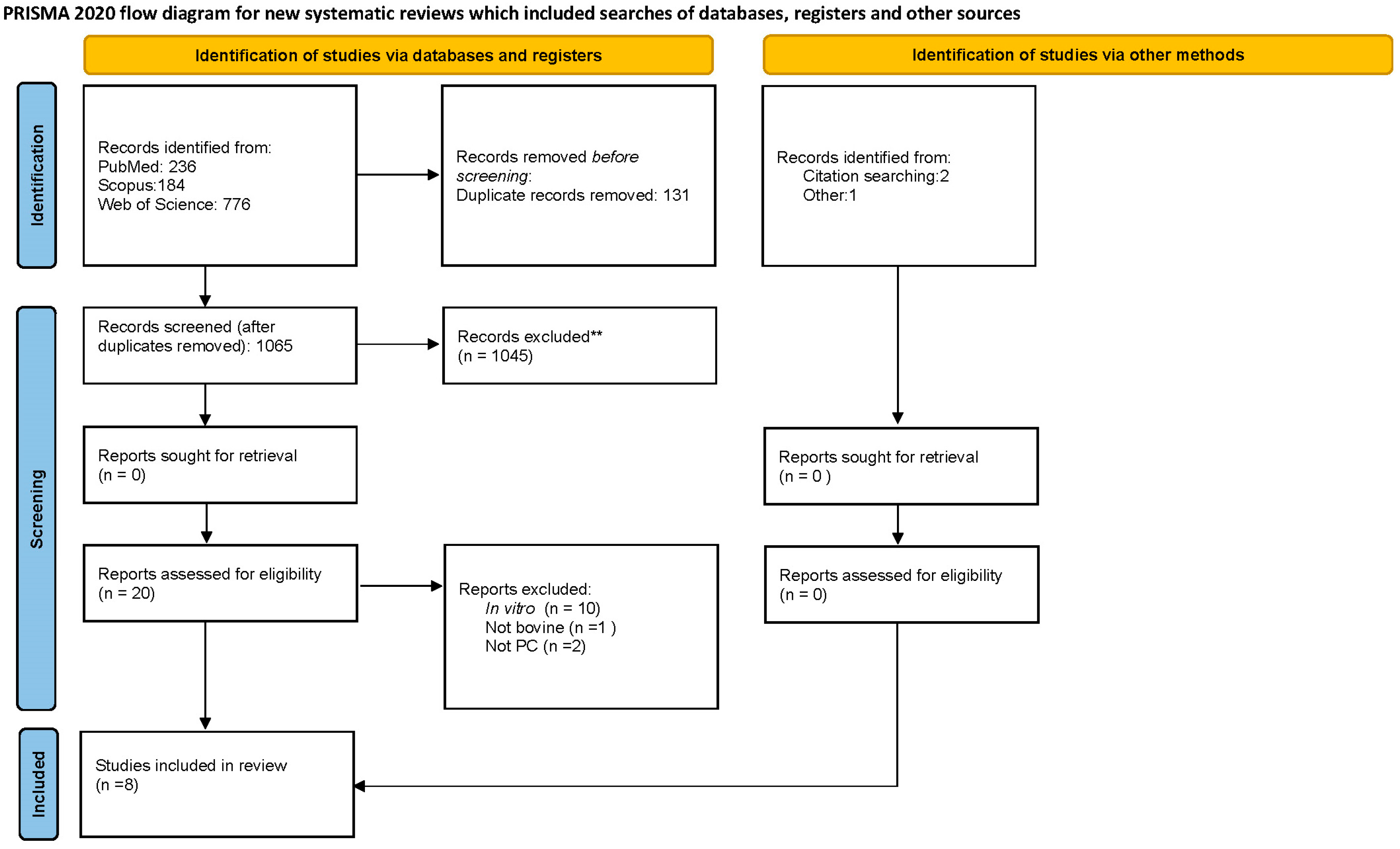
3.1. Database Review
3.2. Centrifugation Protocols
3.3. Mastitis
3.4. Uterine Dysfunctions
3.5. Ovarian Dysfunctions
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Choukroun, J.; Adda, F.; Schoeffler, C.; Vervelle, A. Une opportunité en paro-implantologie: Le PRF. Implantodontie 2001, 42, 55–62. [Google Scholar]
- Miron, R.J.; Fujioka-Kobayashi, M.; Bishara, M.; Zhang, Y.; Hernandez, M.; Choukroun, J. Platelet-Rich Fibrin and Soft Tissue Wound Healing: A Systematic Review. Tissue Eng.-Part B Rev. 2017, 23, 83–99. [Google Scholar] [CrossRef] [PubMed]
- Amable, P.R.; Carias, R.B.V.; Teixeira, M.V.T.; Da Cruz Pacheco, Í.; Corrêa Do Amaral, R.J.F.; Granjeiro, J.M.; Borojevic, R. Platelet-rich plasma preparation for regenerative medicine: Optimization and quantification of cytokines and growth factors. Stem Cell Res. Ther. 2013, 4, 67. [Google Scholar] [CrossRef] [PubMed]
- Lange-Consiglio, A.; Cazzaniga, N.; Garlappi, R.; Spelta, C.; Pollera, C.; Perrini, C.; Cremonesi, F. Platelet concentrate in bovine reproduction: Effects on in vitro embryo production and after intrauterine administration in repeat breeder cows. Reprod. Biol. Endocrinol. 2015, 13, 65. [Google Scholar] [CrossRef] [PubMed]
- Cremonesi, F.; Bonfanti, S.; Idda, A.; Lange-Consiglio, A. Platelet Rich Plasma for Regenerative Medicine Treatment of Bovine Ovarian Hypofunction. Front. Vet. Sci. 2020, 7, 517. [Google Scholar] [CrossRef]
- Cremonesi, F.; Bonfanti, S.; Idda, A.; Anna, L.C. Improvement of embryo recovery in holstein cows treated by intra-ovarian platelet rich plasma before superovulation. Vet. Sci. 2020, 7, 16. [Google Scholar] [CrossRef]
- Lange-Consiglio, A.; Spelta, C.; Garlappi, R.; Luini, M.; Cremonesi, F. Intramammary administration of platelet concentrate as an unconventional therapy in bovine mastitis: First clinical application. J. Dairy. Sci. 2014, 97, 6223–6230. [Google Scholar] [CrossRef]
- Duque-Madrid, P.C.; Velasco-Bolaños, J.; Ceballos-Márquez, A.; López, C.; Carmona, J.U. Intramammary treatment using allogeneic pure platelet-rich plasma in cows with subclinical mastitis caused by Gram-positive bacteria. Sci. Rep. 2021, 11, 23737. [Google Scholar] [CrossRef]
- Dohan, D.M.; Choukroun, J.; Diss, A.; Dohan, S.L.; Dohan, A.J.J.; Mouhyi, J.; Gogly, B. Platelet-rich fibrin (PRF): A second-generation platelet concentrate. Part II: Platelet-related biologic features. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontol. 2006, 101, e45–e50. [Google Scholar] [CrossRef]
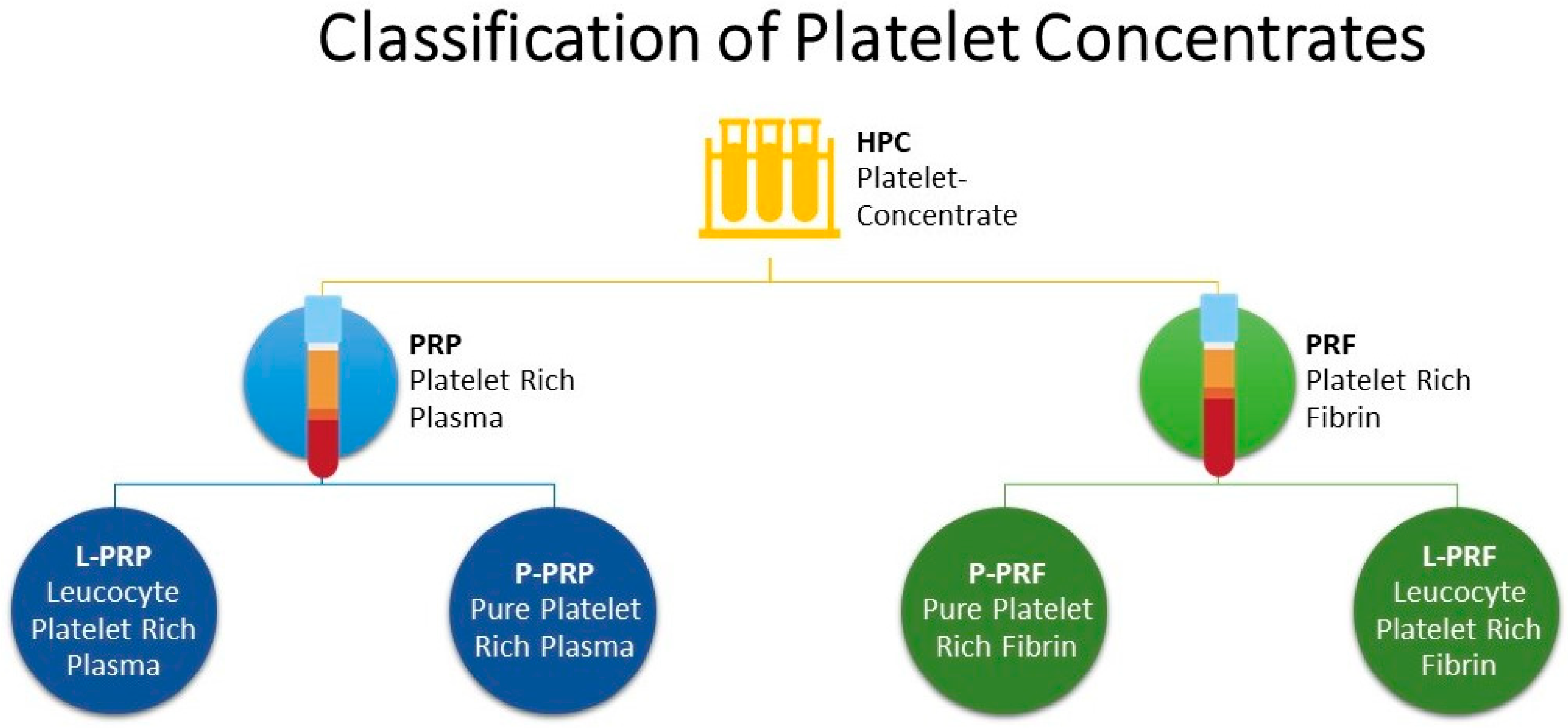
- Dohan Ehrenfest, D.M.; Rasmusson, L.; Albrektsson, T. Classification of platelet concentrates: From pure platelet-rich plasma (P-PRP) to leucocyte- and platelet-rich fibrin (L-PRF). Trends Biotechnol. 2009, 27, 158–167. [Google Scholar] [CrossRef]
- Harrison, P. The use of platelets in regenerative medicine and proposal for a new classification system: Guidance from the SSC of the ISTH. J. Thromb. Haemost. 2018, 16, 1895–1900. [Google Scholar] [CrossRef] [PubMed]
- Marini, M.G.; Perrini, C.; Esposti, P.; Corradetti, B.; Bizzaro, D.; Riccaboni, P.; Fantinato, E.; Urbani, G.; Gelati, G.; Cremonesi, F.; et al. Effects of platelet-rich plasma in a model of bovine endometrial inflammation in vitro. Reprod. Biol. Endocrinol. 2016, 14, 58. [Google Scholar] [CrossRef] [PubMed]
- Constant, C.; Desrochers, A.; Gagnon, C.A.; Provost, C.; Nichols, S.; Marchionatti, E.; Gara-Boivin, C. Single-step production of autologous bovine platelet concentrate for clinical applications in cattle. J. Dairy Sci. 2023, 106, 565–575. [Google Scholar] [CrossRef] [PubMed]
- Ramos-Deus, P.; Santos Nascimento, P.; Vieira, J.I.T.; Chaves, M.S.; Albuquerque, K.A.; Ferreira-Silva, J.C.; Viana Grázia, J.G.; ASantos Filho, A.S.; Mariano Batista, A.; Wanderley Teixeira, V.; et al. Application of platelet-rich plasma in the in vitro production of bovine embryos. Trop. Anim. Health Prod. 2020, 52, 2931–2936. [Google Scholar] [CrossRef] [PubMed]
- Tsuzuki, N.; Seo, J.-P.; Yamada, K.; Haneda, S.; Tabata, Y.; Sasaki, N. Effect of compound of gelatin hydrogel microsphere incorporated with platelet-rich-plasma and alginate on sole defect in cattle. J. Vet. Med. Sci. 2012, 74, 1041–1044. [Google Scholar] [CrossRef] [PubMed]
- Petrera, M.; De Croos, J.N.A.; Iu, J.; Hurtig, M.; Kandel, R.A.; Theodoropoulos, J.S. Supplementation with platelet-rich plasma improves the in vitro formation of tissue-engineered cartilage with enhanced mechanical properties. Arthrosc. J. Arthrosc. Relat. Surg. 2013, 29, 1685–1692. [Google Scholar] [CrossRef]
- Xie, X.; Ulici, V.; Alexander, P.G.; Jiang, Y.; Zhang, C.; Tuan, R.S. Platelet-Rich Plasma Inhibits Mechanically Induced Injury in Chondrocytes. Arthrosc. J. Arthrosc. Relat. Surg. 2015, 31, 1142–1150. [Google Scholar] [CrossRef]
- Tambella, A.M.; Attili, A.R.; Dupré, G.; Cantalamessa, A.; Martin, S.; Cuteri, V.; Marcazzan, S.; Del Fabbro, M. Platelet-rich plasma to treat experimentally-induced skin wounds in animals: A systematic review and meta-analysis. PLoS ONE 2018, 13, e0191093. [Google Scholar] [CrossRef]
- Muir, A.J.T.; Niehaus, A.J.; Lozier, J.W.; Cole, S.L.; Belacic, Z.A.; Ballash, G.A.; Durgam, S.S. Autologous platelet-rich plasma effects on Staphylococcus aureus-induced chondrocyte death in an in vitro bovine septic arthritis model. Am. J. Vet. Res. 2021, 83, 119–126. [Google Scholar] [CrossRef]
- Gutiérrez, C.M.; López, C.; Giraldo, C.E.; Carmona, J.U. Study of a Two-Step Centrifugation Protocol for Concentrating Cells and Growth Factors in Bovine Platelet-Rich Plasma. Vet. Med. Int. 2017, 2017, 1950401. [Google Scholar] [CrossRef]
- Lange-Consiglio, A.; Gaspari, G.; Riccaboni, P.; Canesi, S.; Bosi, G.; Vigo, D.; Cremonesi, F. Platelet-rich plasma and ovarian quiescence: A bovine in vitro model for regeneration of the ovary. Reprod. Fertil. Dev. 2023, 35, 433–444. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef] [PubMed]
- Campbell, M.; McKenzie, J.E.; Sowden, A.; Katikireddi, S.V.; Brennan, S.E.; Ellis, S.; Hartmann-Boyce, J.; Ryan, R.; Shepperd, S.; Thomas, J.; et al. Synthesis without meta-analysis (SWiM) in systematic reviews: Reporting guideline. BMJ 2020, 368, l6890. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.M.; Mazzilli, M.; Pennarossa, G.; Brevini, T.A.L.; Zecconi, A.; Gandolfi, F. Chronic mastitis is associated with altered ovarian follicle development in dairy cattle. J. Dairy Sci. 2012, 95, 1885–1893. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.Z.; Khan, A. Basic facts of mastitis in dairy animals: A review. Pak. Vet. J. 2006, 26, 204. [Google Scholar]
- Kibebew, K. Bovine mastitis: A review of causes and epidemiological point of view. J. Biol. Agric. Healthc. 2017, 7, 1699–1713. [Google Scholar]
- Lam, T.; Van Den Borne, B.H.P.; Jansen, J.; Huijps, K.; Van Veersen, J.C.L.; Van Schaik, G.; Hogeveen, H. Improving bovine udder health: A national mastitis control program in the Netherlands. J. Dairy Sci. 2013, 96, 1301–1311. [Google Scholar] [CrossRef]
- Sheldon, I.M.; Lewis, G.S.; LeBlanc, S.; Gilbert, R.O. Defining postpartum uterine disease in cattle. Theriogenology 2006, 65, 1516–1530. [Google Scholar] [CrossRef] [PubMed]
- Fourichon, C.; Seegers, H.; Malher, X. Effect of disease on reproduction in the dairy cow: A meta-analysis. Theriogenology 2000, 53, 1729–1759. [Google Scholar] [CrossRef]
- Sheldon, I.M.; Cronin, J.; Goetze, L.; Donofrio, G.; Schuberth, H.-J. Defining postpartum uterine disease and the mechanisms of infection and immunity in the female reproductive tract in cattle. Biol. Reprod. 2009, 81, 1025–1032. [Google Scholar] [CrossRef]
- Mari, G.; Iacono, E.; Toni, F.; Predieri, P.G.; Merlo, B. Evaluation of the effectiveness of intrauterine treatment with formosulphathiazole of clinical endometritis in postpartum dairy cows. Theriogenology 2012, 78, 189–200. [Google Scholar] [CrossRef] [PubMed]
- LeBlanc, S.J.; Duffield, T.F.; Leslie, K.E.; Bateman, K.G.; Keefe, G.P.; Walton, J.S.; Johnson, W.H. Defining and diagnosing postpartum clinical endometritis and its impact on reproductive performance in dairy cows. J. Dairy Sci. 2002, 85, 2223–2236. [Google Scholar] [CrossRef] [PubMed]
- Galvão, K.N.; Bicalho, R.C.; Jeon, S.J. Symposium review: The uterine microbiome associated with the development of uterine disease in dairy cows. J. Dairy Sci. 2019, 102, 11786–11797. [Google Scholar] [CrossRef] [PubMed]
- Anitua, E.; Sánchez, M.; Zalduendo, M.M.; De La Fuente, M.; Prado, R.; Orive, G.; Andía, I. Fibroblastic response to treatment with different preparations rich in growth factors. Cell Prolif. 2009, 42, 162–170. [Google Scholar] [CrossRef] [PubMed]
- Lonergan, P.; O’hara, L.; Forde, N. Role of diestrus progesterone on endometrial function and conceptus development in cattle. Anim. Reprod. 2018, 10, 223–227. [Google Scholar]
- López-Gatius, F.; Santolaria, P.; Yániz, J.; Rutlant, J.; López-Béjar, M. Persistent ovarian follicles in dairy cows: A therapeutic approach. Theriogenology 2001, 56, 649–659. [Google Scholar] [CrossRef]
- Arnoczky, S.P.; Shebani-Rad, S. The basic science of platelet-rich plasma (PRP): What clinicians need to know. Sports Med. Arthrosc. 2013, 21, 180–185. [Google Scholar] [CrossRef] [PubMed]
- Dohan Ehrenfest, D.M.; Andia, I.; Zumstein, M.A.; Zhang, C.Q.; Pinto, N.R.; Bielecki, T. Classification of platelet concentrates (Platelet-Rich Plasma-PRP, platelet-rich fibrin-PRF) for topical and infiltrative use in orthopedic and sports medicine: Current consensus, clinical implications and perspectives. Muscles Ligaments Tendons J. 2014, 4, 3–9. [Google Scholar] [CrossRef] [PubMed]
- Pensato, R.; Al-Amer, R.; La Padula, S. The Impact of Duration and Force of Centrifugation on Platelet Content and Mass in the Preparation of Platelet-Rich Plasma. Aesthetic Plast. Surg. 2019, 43, 1074–1078. [Google Scholar]
- Mann, K.G. Biochemistry and physiology of blood coagulation. Thromb. Haemost. 1999, 82, 165–174. [Google Scholar] [CrossRef]
- Han, B.; Woodell-May, J.; Ponticiello, M.; Yang, Z.; Nimni, M. The effect of thrombin activation of platelet-rich plasma on demineralized bone matrix osteoinductivity. J. Bone Jt. Surg. Am. 2009, 91, 1459–1470. [Google Scholar] [CrossRef]
- Ranly, D.M.; Lohmann, C.H.; Andreacchio, D.; Boyan, B.D.; Schwartz, Z. Platelet-rich plasma inhibits demineralized bone matrix-induced bone formation in nude mice. J. Bone Jt. Surg. Am. 2007, 89, 139–147. [Google Scholar] [CrossRef]
- Klaas, I.C.; Zadoks, R.N. An update on environmental mastitis: Challenging perceptions. Transbound. Emerg. Dis. 2018, 65, 166–185. [Google Scholar] [CrossRef]
- Gomes, F.; Henriques, M. Control of bovine mastitis: Old and recent therapeutic approaches. Curr. Microbiol. 2016, 72, 377–382. [Google Scholar] [CrossRef] [PubMed]
- Guccione, J.; D’Andrea, L.; Pesce, A.; Toni, F.; Borriello, G.; Salzano, C.; Diuccio, F.; Pascale, M.; Ciaramella, P. Antibiotic dry buffalo therapy: Effect of intramammary administration of benzathine cloxacillin against Staphylococcus aureus mastitis in dairy water buffalo. BMC Vet. Res. 2020, 16, 191. [Google Scholar] [CrossRef] [PubMed]
- Hogeveen, H.; Steeneveld, W.; Wolf, C.A. Production diseases reduce the efficiency of dairy production: A review of the results, methods, and approaches regarding the economics of mastitis. Annu. Rev. Resour. Econ. 2019, 11, 289–312. [Google Scholar] [CrossRef]
- Oliver, S.P.; Murinda, S.E. Antimicrobial resistance of mastitis pathogens. Vet. Clin. Food Anim. Pract. 2012, 28, 165–185. [Google Scholar] [CrossRef]
- Ruegg, P.L. A 100-Year Review: Mastitis detection, management, and prevention. J. Dairy Sci. 2017, 100, 10381–10397. [Google Scholar] [CrossRef] [PubMed]
- Cheng, W.N.; Han, S.G. Bovine mastitis: Risk factors, therapeutic strategies, and alternative treatments—A review. Asian-Australas. J. Anim. Sci. 2020, 33, 1699. [Google Scholar] [CrossRef]
- Abebe, R.; Hatiya, H.; Abera, M.; Megersa, B.; Asmare, K. Bovine mastitis: Prevalence, risk factors and isolation of Staphylococcus aureus in dairy herds at Hawassa milk shed, South Ethiopia. BMC Vet. Res. 2016, 12, 270. [Google Scholar] [CrossRef]
- Biggs, A. Update on dry cow therapy 1. antibiotic v non-antibiotic approaches. Practice 2017, 39, 328–333. [Google Scholar] [CrossRef]
- EFSA Panel on Biological Hazards (BIOHAZ); Ricci, A.; Allende, A.; Bolton, D.; Chemaly, M.; Davies, R.; Fernández Escámez, P.S.; Girones, R.; Koutsoumanis, K.; Lindqvist, R.; et al. Risk for the development of Antimicrobial Resistance (AMR) due to feeding of calves with milk containing residues of antibiotics. Efsa J. 2017, 15, e04665. [Google Scholar] [PubMed]
- Hossain, M.K.; Paul, S.; Hossain, M.M.; Islam, M.R.; Alam, M.G.S. Bovine mastitis and its therapeutic strategy doing antibiotic sensitivity test. Austin J. Vet. Sci. Anim. Husb. 2017, 4, 1030. [Google Scholar]
- Sheffield, L.G. Mastitis increases growth factor messenger ribonucleic acid in bovine mammary glands. J. Dairy Sci. 1997, 80, 2020–2024. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Báez, J.; Silva, T.V.; Risco, C.A.; Chebel, R.C.; Cunha, F.; De Vries, A.; Santos, J.E.P.; Lima, F.S.; Pinedo, P.; Schuenemann, G.M.; et al. The economic cost of metritis in dairy herds. J. Dairy Sci. 2021, 104, 3158–3168. [Google Scholar] [CrossRef] [PubMed]
- Armengol, R.; Fraile, L. Comparison of two treatment strategies for cows with metritis in high-risk lactating dairy cows. Theriogenology 2015, 83, 1344–1351. [Google Scholar] [CrossRef]
- Basbas, C.; Garzon, A.; Silva-del-Rio, N.; Byrne, B.A.; Karle, B.; Aly, S.S.; Champagne, J.D.; Williams, D.R.; Lima, F.S.; Machado, V.S.; et al. Evaluation of antimicrobial resistance and risk factors for recovery of intrauterine Escherichia coli from cows with metritis on California commercial dairy farms. Sci. Rep. 2022, 12, 13937. [Google Scholar] [CrossRef]
- Goshen, T.; Shpigel, N.Y. Evaluation of intrauterine antibiotic treatment of clinical metritis and retained fetal membranes in dairy cows. Theriogenology 2006, 66, 2210–2218. [Google Scholar] [CrossRef] [PubMed]
- Farimani, M.; Poorolajal, J.; Rabiee, S.; Bahmanzadeh, M. Successful pregnancy and live birth after intrauterine administration of autologous platelet-rich plasma in a woman with recurrent implantation failure: A case report. Int. J. Reprod. BioMedicine 2017, 15, 803–806. [Google Scholar] [CrossRef]
- Aghajanzadeh, F.; Esmaeilzadeh, S.; Basirat, Z.; Mahouti, T.; Heidari, F.N.; Golsorkhtabaramiri, M. Using autologous intrauterine platelet-rich plasma to improve the reproductive outcomes of women with recurrent implantation failure. JBRA Assist Reprod. 2020, 24, 30–33. [Google Scholar] [CrossRef]
- Mehrafza, M.; Kabodmehri, R.; Nikpouri, Z.; Pourseify, G.; Raoufi, A.; Eftekhari, A.; Samadnia, S.; Hosseini, A. Comparing the Impact of Autologous Platelet-rich Plasma and Granulocyte Colony Stimulating Factor on Pregnancy Outcome in Patients with Repeated Implantation Failure. J. Reprod. Infertil. 2019, 20, 35–41. [Google Scholar] [PubMed]
- Siess, W. Molecular mechanisms of platelet activation. Physiol. Rev. 1989, 69, 58–178. [Google Scholar] [CrossRef] [PubMed]
- Larson, R.C.; Ignotz, G.G.; Currie, W.B. Transforming growth factor beta and basic fibroblast growth factor synergistically promote early bovine embryo development during the fourth cell cycle. Mol. Reprod. Dev. 1992, 33, 432–435. [Google Scholar] [CrossRef]
- Larson, R.C.; Ignotz, G.G.; Currie, W.B. Platelet derived growth factor (PDGF) stimulates development of bovine embryos during the fourth cell cycle. Development 1992, 115, 821–826. [Google Scholar] [CrossRef]
- Castro-Rendón, W.A.; Castro-Alvarez, J.F.; Guzmán-Martinez, C.; Bueno-Sanchez, J.C. Blastocyst-endometrium interaction: Intertwining a cytokine network. Braz. J. Med. Biol. Res. 2006, 39, 1373–1385. [Google Scholar] [CrossRef] [PubMed]
- Katagiri, S.; Takahashi, Y. Changes in EGF concentrations during estrous cycle in bovine endometrium and their alterations in repeat breeder cows. Theriogenology 2004, 62, 103–112. [Google Scholar] [CrossRef] [PubMed]
- Pantos, K.; Simopoulou, M.; Pantou, A.; Rapani, A.; Tsioulou, P.; Nitsos, N.; Syrkos, S.; Pappas, A.; Koutsilieris, M.; Sfakianoudis, K. A Case Series on Natural Conceptions Resulting in Ongoing Pregnancies in Menopausal and Prematurely Menopausal Women Following Platelet-Rich Plasma Treatment. Cell Transplant. 2019, 28, 1333–1340. [Google Scholar] [CrossRef]
- Sills, E.S.; Rickers, N.S.; Li, X.; Palermo, G.D. First data on in vitro fertilization and blastocyst formation after intraovarian injection of calcium gluconate-activated autologous platelet rich plasma. Gynecol. Endocrinol. 2018, 34, 756–760. [Google Scholar] [CrossRef] [PubMed]
- Hsu, C.-C.; Hsu, L.; Hsu, I.; Chiu, Y.-J.; Dorjee, S. Live Birth in Woman with Premature Ovarian Insufficiency Receiving Ovarian Administration of Platelet-Rich Plasma (PRP) in Combination with Gonadotropin: A Case Report. Front. Endocrinol. 2020, 11, 50. [Google Scholar] [CrossRef]
- Stojkovska, S.; Dimitrov, G.; Stamenkovska, N.; Hadzi-Lega, M.; Petanovski, Z. Live Birth Rates in Poor Responders’ Group after Previous Treatment with Autologous Platelet-Rich Plasma and Low Dose Ovarian Stimulation Compared with Poor Responders Used Only Low Dose Ovarian Stimulation Before in Vitro Fertilization. Open Access Maced. J. Med. Sci. 2019, 7, 3184–3188. [Google Scholar] [CrossRef]
| Authors | Year | N. Cases | Breed | Site of Blood Collection | Centrifugation Protocol | Aim | Outcome | JBI |
|---|---|---|---|---|---|---|---|---|
| Lange-Consiglio | 2014 | 229 | Not specified | Subcutaneous mammary vein | Double centrifugation method | Evaluate the effect of PRP, alone or associated with antibiotic in the control of clinical acute and chronic mastitis | PPR and antibiotic showed significantly superior performance but PRP alone showed better results in chronic mastitis | Yes, include |
| Lange-Consiglio | 2015 | 65 | Holstein-Friesian | Subcutaneous mammary vein | Double centrifugation method | The in vivo experiment evaluate embryo implantation and development in repeat breeder cows after intrauterine administration of PRP at 48 h following artificial insemination | administration of PPR 48 h after AI, it is supposed to be the ideal time in order to not disturb spermatozoa progression and before the embryo reaches the uterus, making the uterus more favourable to embryo implantation | Yes, include |
| Marini | 2016 | 14 | Holstein-Friesian | Subcutaneous mammary vein | Double centrifugation method | Evaluate the effect of PRP in vivo and in vitro, on a model of healthy (in vivo) or endotoxin lipopolysaccharide stressed bovine endometrial cells | PRP might be helpful in maintaining and/or increasing the number of progesterone receptors | Yes, include |
| Cremonesi | 2020 | 8 | Holstein-Friesian | Mammary vein | Double centrifugation method | Evaluate if administration of autologous PRP inside the bovine ovary before gonadotropin treatment could increase the number of follicles responsive to superovulation in order to improve embryo recovery from eight donor cows | Results show that after the superovulation protocol, a statistically significant difference (p ≤ 0.05) was detected between PRP treated and control ovaries | Yes, include |
| Cremonesi | 2020 | 12 | Holstein-Friesian | Subcutaneous mammary vein | Double centrifugation method | Evaluate the effect of PRP in restoring ovarian function in cows with ovarian failure through the measurement of progesterone levels as a marker for restored ovarian cycling and the monitoring of oestrus to perform AI | after intraovarian PRP administration (week 0), the level of PRG increased between 3- and 11-fold during the subsequent 4 weeks | Yes, include |
| Dunque-Madrid | 2021 | 103 | Creole Colombian bovine | Jugular vein | 1600 g × 8 min | Compare the cure risk of intramammary treatment of pure platelet rich plasma (P-PRP) or cefquinome sulphate (CS) in cows with subclinical mastitis caused by Gram-positive bacteria, evaluated via somatic cell count and the microbiological analysis of milk; to compare the inflammatory/anti-inflammatory response of mammary gland to both treatments through the analyses of interleukins (IL), interferon gamma (IFN-γ), and tumour necrosis factor alpha (TNF-α) in milk | The group of SCM cows treated with PRP presented a lower rate of bacteriologic cure when compared to SCM animals treated with CS | Yes, include |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Caterino, C.; Della Valle, G.; Aragosa, F.; Cavalli, S.; Guccione, J.; Lamagna, F.; Fatone, G. Clinical Application of Platelet Concentrates in Bovine Practice: A Systematic Review. Vet. Sci. 2023, 10, 686. https://doi.org/10.3390/vetsci10120686
Caterino C, Della Valle G, Aragosa F, Cavalli S, Guccione J, Lamagna F, Fatone G. Clinical Application of Platelet Concentrates in Bovine Practice: A Systematic Review. Veterinary Sciences. 2023; 10(12):686. https://doi.org/10.3390/vetsci10120686
Chicago/Turabian StyleCaterino, Chiara, Giovanni Della Valle, Federica Aragosa, Stefano Cavalli, Jacopo Guccione, Francesco Lamagna, and Gerardo Fatone. 2023. "Clinical Application of Platelet Concentrates in Bovine Practice: A Systematic Review" Veterinary Sciences 10, no. 12: 686. https://doi.org/10.3390/vetsci10120686
APA StyleCaterino, C., Della Valle, G., Aragosa, F., Cavalli, S., Guccione, J., Lamagna, F., & Fatone, G. (2023). Clinical Application of Platelet Concentrates in Bovine Practice: A Systematic Review. Veterinary Sciences, 10(12), 686. https://doi.org/10.3390/vetsci10120686






