A Systematic Review and Metanalysis on the Use of Hermetia illucens and Tenebrio molitor in Diets for Poultry
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
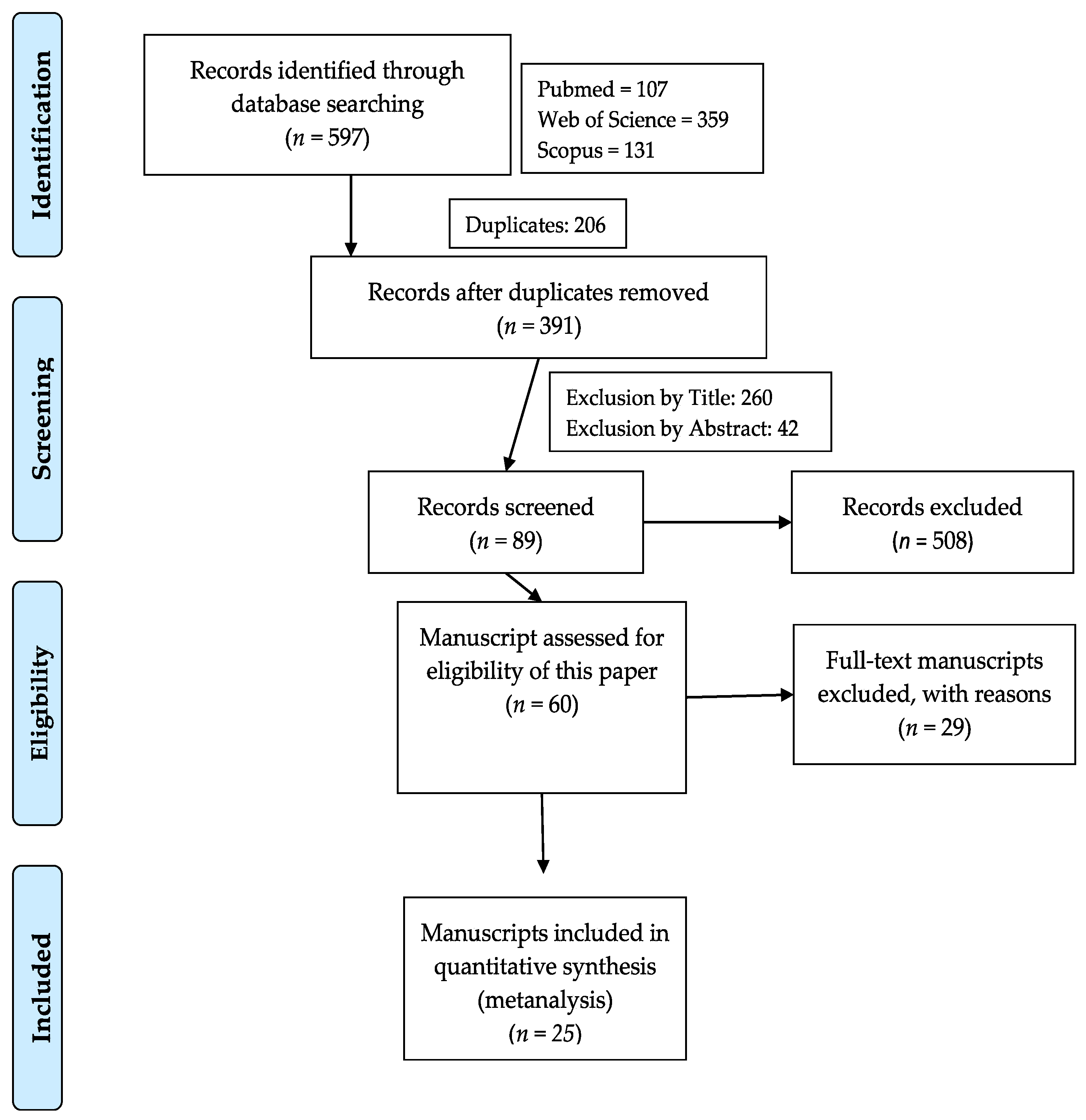
2.1. Systematic Review
2.2. Metanalysis
2.3. Statistical Analysis
3. Results
3.1. Systematic Review
3.2. Metanalysis
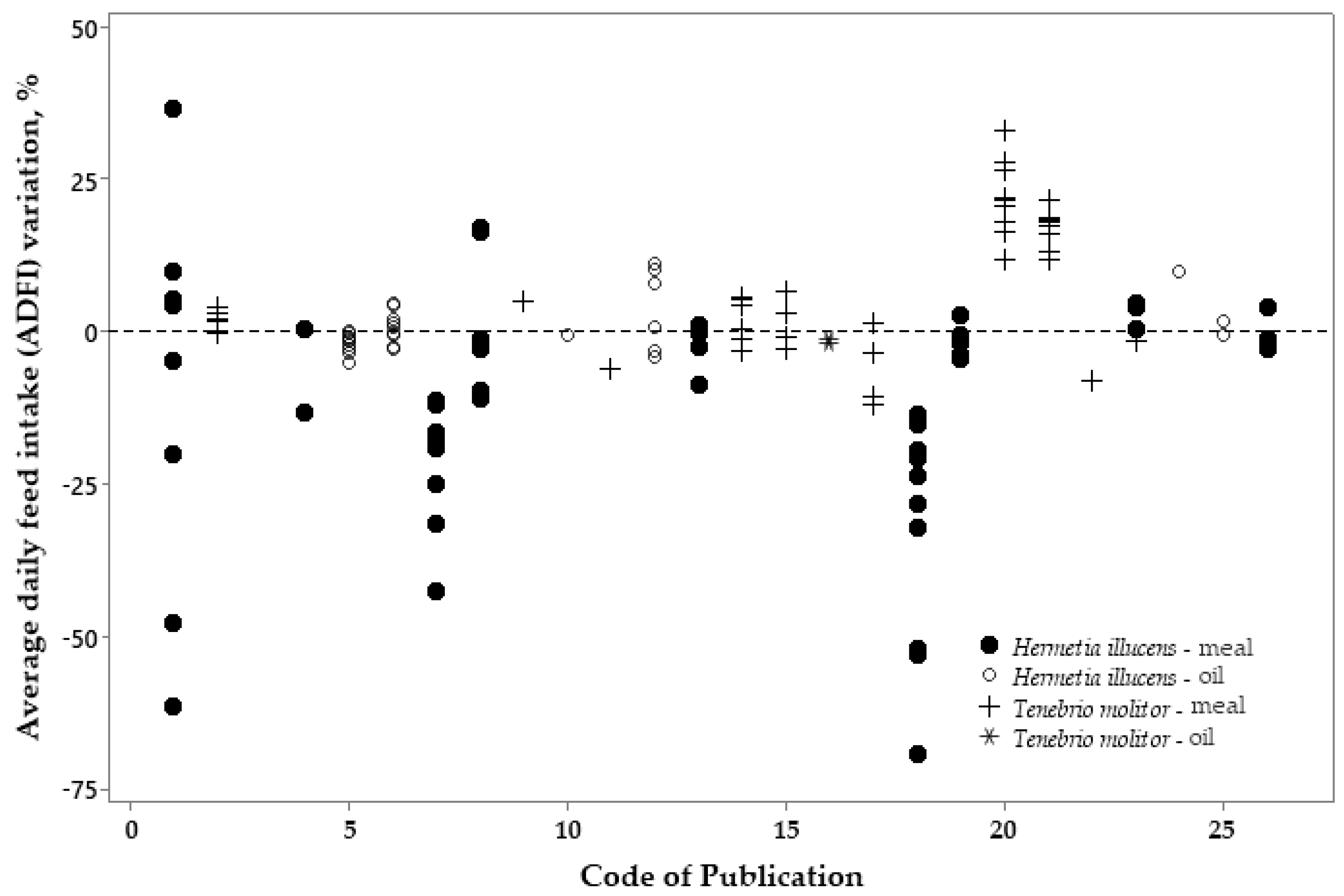

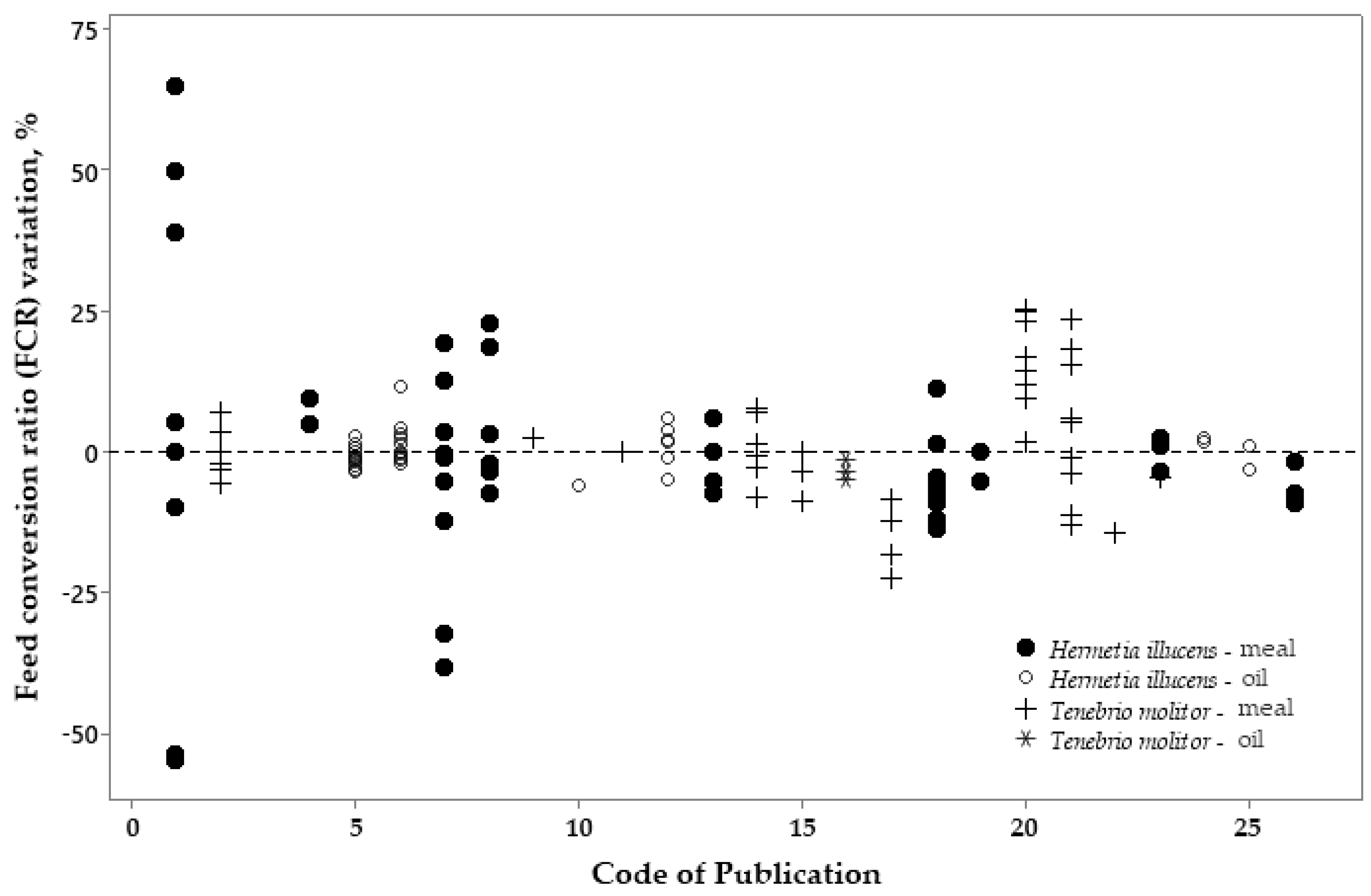
4. Discussion
4.1. Impacts of Dietary Inclusions of Insect Products on Poultry Health
4.2. Metanalysis: Exploratory Analyses of Insect Products on Broiler Performance
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Veldkamp, T.; van Duinkerken, G.; van Huis, A.; Lakemond, C.M.M.; Ottevanger, E.; Bosch, G.; Van Boekel, T. Insects as a Sustainable Feed Ingredient in Pig and Poultry Diets—A Feasibility Study; Wageningen UR Livestock Research: Lelystad, Holland, 2012. [Google Scholar]
- Marono, S.; Piccolo, G.; Loponte, R.; Di Meo, C.; Attia, Y.A.; Nizza, A. In Vitro Crude Protein Digestibility of Tenebrio molitor and Hermetia illucens Insect Meals and its Correlation with Chemical Composition Traits. Ital. J. Anim. Sci. 2015, 14, 3889. [Google Scholar] [CrossRef]
- Allegretti, G.; Talamini, E.; Schmidt, V.; Bogorni, P.C.; Ortega, E. Insect as Feed: An Emergy Assessment of Insect Meal as a Sustainable Protein Source for the Brazilian Poultry Industry. J. Clean. Prod. 2018, 171, 403–412. [Google Scholar] [CrossRef]
- Thrastardottir, R.; Olafsdottir, H.T.; Thorarinsdottir, R.I. Yellow Mealworm and Black Soldier Fly Larvae for Feed and Food Production in Europe, with Emphasis on Iceland. Foods 2021, 10, 2744. [Google Scholar] [CrossRef] [PubMed]
- Józefiak, D.; Józefiak, A.; Kierończyk, B.; Rawski, M.; Świątkiewicz, S.; Długosz, J.; Engberg, R.M. Insects—A Natural Nutrient Source for Poultry—A Review. Ann. Anim. Sci. 2016, 16, 297–313. [Google Scholar] [CrossRef]
- Kierończyk, B.; Sypniewski, J.; Rawski, M.; Czekała, W.; Swiatkiewicz, S.; Józefiak, D. From Waste to Sustainable Feed Material: The Effect of Hermetia illucens Oil on the Growth Performance, Nutrient Digestibility, and Gastrointestinal Tract Morphometry of Broiler Chickens. Ann. Anim. Sci. 2020, 20, 157–177. [Google Scholar] [CrossRef]
- Moula, N.; Scippo, M.L.; Douny, C.; Degand, G.; Dawans, E.; Cabaraux, J.F.; Hornick, J.L.; Medigo, R.C.; Leroy, P.; Francis, F.; et al. Performances of Local Poultry Breed Fed Black Soldier Fly Larvae Reared on Horse Manure. Anim. Nutr. 2018, 4, 73–78. [Google Scholar] [CrossRef] [PubMed]
- Ramos-Elorduy, J.; González, E.A.; Hernández, A.R.; Pino, J.M. Use of Tenebrio molitor (Coleoptera: Tenebrionidae) to Recycle Organic Wastes and as Feed for Broiler Chickens. J. Econ. Entomol. 2002, 95, 214–220. [Google Scholar] [CrossRef] [PubMed]
- van Huis, A.; Oonincx, D.G.A.B. The Environmental Sustainability of Insects as Food and Feed. A Review. Agron. Sustain. Dev. 2017, 37, 43. [Google Scholar] [CrossRef]
- Koutsos, E.; Modica, B.; Freel, T. Immunomodulatory Potential of Black Soldier Fly Larvae: Applications beyond Nutrition in Animal Feeding Programs. Transl. Anim. Sci. 2022, 6, txac084. [Google Scholar] [CrossRef]
- Zeitz, J.O.; Fennhoff, J.; Kluge, H.; Stangl, G.I.; Eder, K. Effects of Dietary Fats Rich in Lauric and Myristic Acid on Performance, Intestinal Morphology, Gut Microbes, and Meat Quality in Broilers. Poult. Sci. 2015, 94, 2404–2413. [Google Scholar] [CrossRef]
- Shin, C.S.; Kim, D.Y.; Shin, W.S. Characterization of Chitosan Extracted from Mealworm Beetle (Tenebrio molitor, Zophobas Morio) and Rhinoceros Beetle (Allomyrina Dichotoma) and Their Antibacterial Activities. Int. J. Biol. Macromol. 2019, 125, 72–77. [Google Scholar] [CrossRef] [PubMed]
- Józefiak, A.; Engberg, R.M. Insect Proteins as a Potential Source of Antimicrobial Peptides in Livestock Production. A Review. J. Anim. Feed Sci. 2017, 26, 87–99. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; Antes, G.; Atkins, D.; Barbour, V.; Barrowman, N.; Berlin, J.A.; Clark, J.; et al. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef] [PubMed]
- Richardson, W.S.; Wilson, M.C.; Nishikawa, J.; Hayward, R.S. The well-built clinical question: A key to evidence-based decisions. ACP J. Club. 1995, 123, A12–A13. [Google Scholar] [CrossRef] [PubMed]
- Van Milgen, J.; Gloaguen, M.; Le Floc’H, N.; Brossard, L.; Primot, Y.; Corrent, E. Meta-Analysis of the Response of Growing Pigs to the Isoleucine Concentration in the Diet. Animal 2012, 6, 1601–1608. [Google Scholar] [CrossRef] [PubMed]
- Mat, K.; Abdul Kari, Z.; Rusli, N.D.; Rahman, M.M.; Che Harun, H.; Al-Amsyar, S.M.; Mohd Nor, M.F.; Dawood, M.A.O.; Hassan, A.M. Effects of the Inclusion of Black Soldier Fly Larvae (Hermetia illucens) Meal on Growth Performance and Blood Plasma Constituents in Broiler Chicken (Gallus Gallus Domesticus) Production. Saudi J. Biol. Sci. 2022, 29, 809–815. [Google Scholar] [CrossRef] [PubMed]
- Sedgh-Gooya, S.; Torki, M.; Darbemamieh, M.; Khamisabadi, H.; Abdolmohamadi, A. Growth Performance and Intestinal Morphometric Features of Broiler Chickens Fed on Dietary Inclusion of Yellow Mealworm (Tenebrio molitor) Larvae Powder. Vet. Med. Sci. 2022, 8, 2050–2058. [Google Scholar] [CrossRef]
- Kim, B.; Kim, H.R.; Baek, Y.C.; Ryu, C.H.; Ji, S.Y.; Jeong, J.Y.; Kim, M.; Jung, H.; Park, S.H. Evaluation of Microwave-Dried Black Soldier Fly (Hermetia illucens) Larvae Meal as a Dietary Protein Source in Broiler Chicken Diets. J. Insects Food Feed. 2022, 8, 977–987. [Google Scholar] [CrossRef]
- Hartinger, K.; Fröschl, K.; Ebbing, M.A.; Bruschek-Pfleger, B.; Schedle, K.; Schwarz, C.; Gierus, M. Suitability of Hermetia illucens Larvae Meal and Fat in Broiler Diets: Effects on Animal Performance, Apparent Ileal Digestibility, Gut Histology, and Microbial Metabolites. J. Anim. Sci. Biotechnol. 2022, 13, 50. [Google Scholar] [CrossRef]
- Murawska, D.; Daszkiewicz, T.; Sobotka, W.; Gesek, M.; Witkowska, D.; Matusevičius, P.; Bakuła, T. Partial and Total Replacement of Soybean Meal with Full-Fat Black Soldier Fly (Hermetia illucens L.) Larvae Meal in Broiler Chicken Diets: Impact on Growth Performance, Carcass Quality and Meat Quality. Animals 2021, 11, 2715. [Google Scholar] [CrossRef]
- Hartinger, K.; Greinix, J.; Thaler, N.; Ebbing, M.A.; Yacoubi, N.; Schedle, K.; Gierus, M. Effect of Graded Substitution of Soybean Meal by Hermetia illucens Larvae Meal on Animal Performance, Apparent Ileal Digestibility, Gut Histology and Microbial Metabolites of Broilers. Animals 2021, 11, 1628. [Google Scholar] [CrossRef] [PubMed]
- Elangovan, A.V.; Udayakumar, A.; Saravanakumar, M.; Awachat, V.B.; Mohan, M.; Yandigeri, M.S.; Krishnan, S.; Mech, A.; Rao, S.B.N.; Giridhar, K.; et al. Effect of Black Soldier Fly, Hermetia illucens (Linnaeus) Prepupae Meal on Growth Performance and Gut Development in Broiler Chicken. Int. J. Trop. Insect Sci. 2021, 41, 2077–2082. [Google Scholar] [CrossRef]
- Kim, Y.B.; Kim, D.H.; Jeong, S.B.; Lee, J.W.; Kim, T.H.; Lee, H.G.; Lee, K.W. Black Soldier Fly Larvae Oil as an Alternative Fat Source in Broiler Nutrition. Poult. Sci. 2020, 99, 3133–3143. [Google Scholar] [CrossRef]
- Pietras, M.; Orczewska-Dudek, S.; Szczurek, W.; Pieszka, M. Effect of Dietary Lupine Seeds (Lupinus luteus L.) and Different Insect Larvae Meals as Protein Sources in Broiler Chicken Diet on Growth Performance, Carcass, and Meat Quality. Livest. Sci. 2021, 250, 104537. [Google Scholar] [CrossRef]
- Dabbou, S.; Lauwaerts, A.; Ferrocino, I.; Biasato, I.; Sirri, F.; Zampiga, M.; Bergagna, S.; Pagliasso, G.; Gariglio, M.; Colombino, E.; et al. Modified Black Soldier Fly Larva Fat in Broiler Diet: Effects on Performance, Carcass Traits, Blood Parameters, Histomorphological Features and Gut Microbiota. Animals 2021, 11, 1837. [Google Scholar] [CrossRef] [PubMed]
- Mutisya, M.M.; Agbodzavu, M.K.; Kinyuru, J.N.; Tanga, C.M.; Gicheha, M.; Hailu, G.; Salifu, D.; Khan, Z.; Niassy, S. Can Black Soldier Fly Desmodium Intortum Larvae-Based Diets Enhance the Performance of Cobb500 Broiler Chickens and Smallholder Farmers’ Profit in Kenya? Poult. Sci. 2021, 100, 420–430. [Google Scholar] [CrossRef] [PubMed]
- Elahi, U.; Wang, J.; Ma, Y.B.; Wu, S.G.; Wu, J.; Qi, G.H.; Zhang, H.J. Evaluation of Yellow Mealworm Meal as a Protein Feedstuff in the Diet of Broiler Chicks. Animals 2020, 10, 224. [Google Scholar] [CrossRef]
- Benzertiha, A.; Kierończyk, B.; Kołodziejski, P.; Pruszyńska–Oszmałek, E.; Rawski, M.; Józefiak, D.; Józefiak, A. Tenebrio molitor and Zophobas Morio Full-Fat Meals as Functional Feed Additives Affect Broiler Chickens’ Growth Performance and Immune System Traits. Poult. Sci. 2020, 99, 196–206. [Google Scholar] [CrossRef]
- Benzertiha, A.; Rawski, M.; Józefiak, A.; Kozłowski, K.; Jankowski, J.; Józefiak, D. Tenebrio molitor and Zophobas Morio Full-Fat Meals in Broiler Chicken Diets: Effects on Nutrients Digestibility, Digestive Enzyme Activities, and Cecal Microbiome. Animals 2019, 9, 1128. [Google Scholar] [CrossRef]
- Khan, S.; Khan, R.U.; Alam, W.; Sultan, A. Evaluating the Nutritive Profile of Three Insect Meals and Their Effects to Replace Soya Bean in Broiler Diet. J. Anim. Physiol. Anim. Nutr. 2018, 102, e662–e668. [Google Scholar] [CrossRef]
- Brede, A.; Wecke, C.; Liebert, F. Does the Optimal Dietary Methionine to Cysteine Ratio in Diets for Growing Chickens Respond to High Inclusion Rates of Insect Meal from Hermetia illucens? Animals 2018, 8, 187. [Google Scholar] [CrossRef] [PubMed]
- Onsongo, V.O.; Osuga, I.M.; Gachuiri, C.K.; Wachira, A.M.; Miano, D.M.; Tanga, C.M.; Ekesi, S.; Nakimbugwe, D.; Fiaboe, K.K.M. Insects for Income Generation through Animal Feed: Effect of Dietary Replacement of Soybean and Fish Meal with Black Soldier Fly Meal on Broiler Growth and Economic Performance. J. Econ. Entomol. 2018, 111, 1966–1973. [Google Scholar] [CrossRef]
- Biasato, I.; Gasco, L.; De Marco, M.; Renna, M.; Rotolo, L.; Dabbou, S.; Capucchio, M.T.; Biasibetti, E.; Tarantola, M.; Sterpone, L.; et al. Yellow Mealworm Larvae (Tenebrio molitor) Inclusion in Diets for Male Broiler Chickens: Effects on Growth Performance, Gut Morphology, and Histological Findings. Poult. Sci. 2018, 97, 540–548. [Google Scholar] [CrossRef] [PubMed]
- Biasato, I.; Gasco, L.; De Marco, M.; Renna, M.; Rotolo, L.; Dabbou, S.; Capucchio, M.T.; Biasibetti, E.; Tarantola, M.; Bianchi, C.; et al. Effects of Yellow Mealworm Larvae (Tenebrio molitor) Inclusion in Diets for Female Broiler Chickens: Implications for Animal Health and Gut Histology. Anim. Feed Sci. Technol. 2017, 234, 253–263. [Google Scholar] [CrossRef]
- Bovera, F.; Loponte, R.; Marono, S.; Piccolo, G.; Parisi, G.; Iaconisi, V.; Gasco, L.; Nizza, A. Use of Tenebrio molitor Larvae Meal as Protein Source in Broiler Diet: Effect on Growth Performance, Nutrient Digestibility, and Carcass and Meat Traits. J. Anim. Sci. 2016, 94, 639–647. [Google Scholar] [CrossRef]
- Józefiak, A.; Kierończyk, B.; Rawski, M.; Mazurkiewicz, J.; Benzertiha, A.; Gobbi, P.; Nogales-Mérida, S.; Świątkiewicz, S.; Józefiak, D. Full-Fat Insect Meals as Feed Additive—The Effect on Broiler Chicken Growth Performance and Gastrointestinal Tract Microbiota. J. Anim. Feed Sci. 2018, 27, 131–139. [Google Scholar] [CrossRef]
- Schiavone, A.; Cullere, M.; De Marco, M.; Meneguz, M.; Biasato, I.; Bergagna, S.; Dezzutto, D.; Gai, F.; Dabbou, S.; Gasco, L.; et al. Partial or Total Replacement of Soybean Oil by Black Soldier Fly Larvae (Hermetia illucens L.) Fat in Broiler Diets: Effect on Growth Performances, Feed-Choice, Blood Traits, Carcass Characteristics and Meat Quality. Ital. J. Anim. Sci. 2017, 16, 93–100. [Google Scholar] [CrossRef]
- Schiavone, A.; Dabbou, S.; De Marco, M.; Cullere, M.; Biasato, I.; Biasibetti, E.; Capucchio, M.T.; Bergagna, S.; Dezzutto, D.; Meneguz, M.; et al. Black Soldier Fly Larva Fat Inclusion in Finisher Broiler Chicken Diet as an Alternative Fat Source. Animal 2018, 12, 2032–2039. [Google Scholar] [CrossRef]
- Kareem, K.Y.; Abdulla, N.R.; Foo, H.L.; Zamri, A.N.M.; Shazali, N.; Loh, T.C.; Alshelmani, M.I. Effect of Feeding Larvae Meal in the Diets on Growth Performance, Nutrient Digestibility and Meat Quality in Broiler Chicken. Indian J. Anim. Sci. 2018, 88, 1180–1185. [Google Scholar] [CrossRef]
- Ipema, A.F.; Bokkers, E.A.M.; Gerrits, W.J.J.; Kemp, B.; Elizabeth Bolhuis, J. Provision of Black Soldier Fly Larvae (Hermetia illucens) in Different Ways Benefits Broiler Welfare and Performance, with Largest Effects of Scattering Live Larvae. Physiol. Behav. 2022, 257, 113999. [Google Scholar] [CrossRef]
- Bongiorno, V.; Gariglio, M.; Zambotto, V.; Cappone, E.E.; Biasato, I.; Renna, M.; Forte, C.; Coudron, C.; Bergagna, S.; Gai, F.; et al. Black Soldier Fly Larvae Used for Environmental Enrichment Purposes: Can They Affect the Growth, Slaughter Performance, and Blood Chemistry of Medium-Growing Chickens? Front. Vet. Sci. 2022, 9, 1064017. [Google Scholar] [CrossRef] [PubMed]
- Kierończyk, B.; Rawski, M.; Mikołajczak, Z.; Leciejewska, N.; Józefiak, D. Hermetia illucens Fat Affects the Gastrointestinal Tract Selected Microbial Populations, Their Activity, and the Immune Status of Broiler Chickens. Ann. Anim. Sci. 2022, 22, 663–675. [Google Scholar] [CrossRef]
- Zhang, Y.; Yang, C.Y.; Li, C.; Xu, Z.; Peng, P.; Xue, C.; Tomberlin, J.K.; Hu, W.; Cao, Y. Black Soldier Fly (Hermetia illucens L.) Larval Diet Improves CD8+ Lymphocytes Proliferation to Eliminate Chicken Coronavirus at an Early Infection Stage. Vet. Microbiol. 2021, 260, 109151. [Google Scholar] [CrossRef] [PubMed]
- de Souza Vilela, J.; Andronicos, N.M.; Kolakshyapati, M.; Hilliar, M.; Sibanda, T.Z.; Andrew, N.R.; Swick, R.A.; Wilkinson, S.; Ruhnke, I. Black Soldier Fly Larvae in Broiler Diets Improve Broiler Performance and Modulate the Immune System. Anim. Nutr. 2021, 7, 695–706. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.; Bang, H.T.; Jeong, J.Y.; Kim, M.; Kim, K.H.; Chun, J.L.; Ji, S.Y. Effects of Dietary Supplementation of Black Soldier Fly (Hermetia illucens) Larvae Oil on Broiler Health. J. Poult. Sci. 2021, 58, 222–229. [Google Scholar] [CrossRef] [PubMed]
- Biasato, I.; Ferrocino, I.; Dabbou, S.; Evangelista, R.; Gai, F.; Gasco, L.; Cocolin, L.; Capucchio, M.T.; Schiavone, A. Black Soldier Fly and Gut Health in Broiler Chickens: Insights into the Relationship between Cecal Microbiota and Intestinal Mucin Composition. J. Anim. Sci. Biotechnol. 2020, 11, 11. [Google Scholar] [CrossRef] [PubMed]
- Ipema, A.F.; Bokkers, E.A.M.; Gerrits, W.J.J.; Kemp, B.; Bolhuis, J.E. Long-Term Access to Live Black Soldier Fly Larvae (Hermetia illucens) Stimulates Activity and Reduces Fearfulness of Broilers, without Affecting Health. Sci. Rep. 2020, 10, 17428. [Google Scholar] [CrossRef]
- Lee, J.; Kim, Y.M.; Park, Y.K.; Yang, Y.C.; Jung, B.G.; Lee, B.J. Black Soldier Fly (Hermetia illucens) Larvae Enhances Immune Activities and Increases Survivability of Broiler Chicks against Experimental Infection of Salmonella Gallinarum. J. Vet. Med. Sci. 2018, 80, 736–740. [Google Scholar] [CrossRef]
- Dabbou, S.; Gai, F.; Biasato, I.; Capucchio, M.T.; Biasibetti, E.; Dezzutto, D.; Meneguz, M.; Plachà, I.; Gasco, L.; Schiavone, A. Black Soldier Fly Deffated Meal as a Dietary Protein Source for Broiler Chickens: Effects on Growth Performance, Blood Tratis, Gut Mophology and Histological Features. J. Anim. Sci. Biotechnol. 2018, 9, 49. [Google Scholar] [CrossRef]
- Sedgh-Gooya, S.; Torki, M.; Darbemamieh, M.; Khamisabadi, H.; Karimi Torshizi, M.A.; Abdolmohamadi, A. Yellow Mealworm, Tenebrio molitor (Col: Tenebrionidae), Larvae Powder as Dietary Protein Sources for Broiler Chickens: Effects on Growth Performance, Carcass Traits, Selected Intestinal Microbiota and Blood Parameters. J. Anim. Physiol. Anim. Nutr. 2021, 105, 119–128. [Google Scholar] [CrossRef]
- Biasato, I.; Ferrocino, I.; Grego, E.; Dabbou, S.; Gai, F.; Gasco, L.; Cocolin, L.; Capucchio, M.T.; Schiavone, A. Yellow Mealworm Inclusion in Diets for Heavy-Size Broiler Chickens: Implications for Intestinal Microbiota and Mucin Dynamics. Animals 2020, 10, 1909. [Google Scholar] [CrossRef] [PubMed]
- Józefiak, A.; Benzertiha, A.; Kierończyk, B.; Łukomska, A.; Wesołowska, I.; Rawski, M. Improvement of Cecal Commensal Microbiome Following the Insect Additive into Chicken Diet. Animals 2020, 10, 577. [Google Scholar] [CrossRef] [PubMed]
- Biasato, I.; Ferrocino, I.; Grego, E.; Dabbou, S.; Gai, F.; Gasco, L.; Cocolin, L.; Capucchio, M.T.; Schiavone, A. Gut Microbiota and Mucin Composition in Female Broiler Chickens Fed Diets Including Yellow. Animals 2019, 9, 213. [Google Scholar] [CrossRef] [PubMed]
- Benzertiha, A.; Kierończyk, B.; Rawski, M.; Kołodziejski, P.; Bryszak, M.; Józefiak, D. Insect Oil as an Alternative to Palm Oil and Poultry Fat in Broiler Chicken Nutrition. Animals 2019, 9, 116. [Google Scholar] [CrossRef] [PubMed]
- Loponte, R.; Bovera, F.; Piccolo, G.; Gasco, L.; Secci, G.; Iaconisi, V.; Parisi, G. Fatty Acid Profile of Lipids and Caeca Volatile Fatty Acid Production of Broilers Fed a Full Fat Meal from Tenebrio molitor Larvae. Ital. J. Anim. Sci. 2019, 18, 168–173. [Google Scholar] [CrossRef]
- Biasato, I.; Ferrocino, I.; Biasibetti, E.; Grego, E.; Dabbou, S.; Sereno, A.; Gai, F.; Gasco, L.; Schiavone, A.; Cocolin, L.; et al. Modulation of Intestinal Microbiota, Morphology and Mucin Composition by Dietary Insect Meal Inclusion in Free-Range Chickens. BMC Vet. Res. 2018, 14, 383. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.M.; Yang, C.J. Efficacy of Mealworm and Super Mealworm Larvae Probiotics as an Alternative to Antibiotics Challenged Orally with Salmonella and E. Coli Infection in Broiler Chicks. Poult. Sci. 2017, 96, 27–34. [Google Scholar] [CrossRef] [PubMed]
- Bellezza Oddon, S.; Biasato, I.; Imarisio, A.; Pipan, M.; Dekleva, D.; Colombino, E.; Capucchio, M.T.; Meneguz, M.; Bergagna, S.; Barbero, R.; et al. Black Soldier Fly and Yellow Mealworm Live Larvae for Broiler Chickens: Effects on Bird Performance and Health Status. J. Anim. Physiol. Anim. Nutr. 2021, 105, 10–18. [Google Scholar] [CrossRef]
- Colombino, E.; Biasato, I.; Ferrocino, I.; Oddon, S.B.; Caimi, C.; Gariglio, M.; Dabbou, S.; Caramori, M.; Battisti, E.; Zanet, S.; et al. Effect of Insect Live Larvae as Environmental Enrichment on Poultry Gut Health: Gut Mucin Composition, Microbiota and Local Immune Response Evaluation. Animals 2021, 11, 2819. [Google Scholar] [CrossRef]
- Kierończyk, B.; Rawski, M.; Józefiak, A.; Mazurkiewicz, J.; Świątkiewicz, S.; Siwek, M.; Bednarczyk, M.; Szumacher-Strabel, M.; Cieślak, A.; Benzertiha, A.; et al. Effects of Replacing Soybean Oil with Selected Insect Fats on Broilers. Anim. Feed Sci. Technol. 2018, 240, 170–183. [Google Scholar] [CrossRef]
- Tabata, E.; Kashimura, A.; Wakita, S.; Ohno, M.; Sakaguchi, M.; Sugahara, Y.; Kino, Y.; Matoska, V.; Bauer, P.O.; Oyama, F. Gastric and Intestinal Proteases Resistance of Chicken Acidic Chitinase Nominates Chitin-Containing Organisms for Alternative Whole Edible Diets for Poultry. Sci. Rep. 2017, 7, 6662. [Google Scholar] [CrossRef]
- Vogel, H.; Müller, A.; Heckel, D.G.; Gutzeit, H.; Vilcinskas, A. Nutritional Immunology: Diversification and Diet-Dependent Expression of Antimicrobial Peptides in the Black Soldier Fly Hermetia illucens. Dev. Comp. Immunol. 2018, 78, 141–148. [Google Scholar] [CrossRef]
- Saviane, A.; Tassoni, L.; Naviglio, D.; Lupi, D.; Savoldelli, S.; Bianchi, G.; Cortellino, G.; Bondioli, P.; Folegatti, L.; Casartelli, M.; et al. Mechanical Processing of Hermetia illucens Larvae and Bombyx Mori Pupae Produces Oils with Antimicrobial Activity. Animals 2021, 11, 783. [Google Scholar] [CrossRef]
- Manniello, M.D.; Moretta, A.; Salvia, R.; Scieuzo, C.; Lucchetti, D.; Vogel, H.; Sgambato, A.; Falabella, P. Insect Antimicrobial Peptides: Potential Weapons to Counteract the Antibiotic Resistance. Cell. Mol. Life Sci. 2021, 78, 4259–4282. [Google Scholar] [CrossRef]
- Oakley, B.B.; Lillehoj, H.S.; Kogut, M.H.; Kim, W.K.; Maurer, J.J.; Pedroso, A.; Lee, M.D.; Collett, S.R.; Johnson, T.J.; Cox, N.A. The Chicken Gastrointestinal Microbiome. FEMS Microbiol. Lett. 2014, 360, 100–112. [Google Scholar] [CrossRef]
- Wei, S.; Morrison, M.; Yu, Z. Bacterial Census of Poultry Intestinal Microbiome. Poult. Sci. 2013, 92, 671–683. [Google Scholar] [CrossRef]
- Apajalahti, J.; Vienola, K. Interaction between Chicken Intestinal Microbiota and Protein Digestion. Anim. Feed Sci. Technol. 2016, 221, 323–330. [Google Scholar] [CrossRef]
- Li, Z.; Wang, W.; Liu, D.; Guo, Y. Effects of Lactobacillus Acidophilus on Gut Microbiota Composition in Broilers Challenged with Clostridium Perfringens. PLoS ONE 2017, 12, e0188634. [Google Scholar] [CrossRef]
- Jung, T.H.; Park, J.H.; Jeon, W.M.; Han, K.S. Butyrate Modulates Bacterial Adherence on LS174T Human Colorectal Cells by Stimulating Mucin Secretion and MAPK Signaling Pathway. Nutr. Res. Pract. 2015, 9, 343–349. [Google Scholar] [CrossRef]
- Borrelli, L.; Coretti, L.; Dipineto, L.; Bovera, F.; Menna, F.; Chiariotti, L.; Nizza, A.; Lembo, F.; Fioretti, A. Insect-Based Diet, a Promising Nutritional Source, Modulates Gut Microbiota Composition and SCFAs Production in Laying Hens. Sci. Rep. 2017, 7, 16269. [Google Scholar] [CrossRef]
- Foysal, M.J.; Fotedar, R.; Tay, C.Y.; Gupta, S.K. Dietary Supplementation of Black Soldier Fly (Hermetica Illucens) Meal Modulates Gut Microbiota, Innate Immune Response and Health Status of Marron (Cherax Cainii, Austin 2002) Fed Poultry-by-Product and Fishmeal Based Diets. PeerJ 2019, 7, e6891. [Google Scholar] [CrossRef]
- Li, Y.; Kortner, T.M.; Chikwati, E.M.; Munang’andu, H.M.; Lock, E.J.; Krogdahl, Å. Gut Health and Vaccination Response in Pre-Smolt Atlantic Salmon (Salmo Salar) Fed Black Soldier Fly (Hermetia illucens) Larvae Meal. Fish Shellfish. Immunol. 2019, 86, 1106–1113. [Google Scholar] [CrossRef] [PubMed]
- Su, J.; Gong, Y.; Cao, S.; Lu, F.; Han, D.; Liu, H.; Jin, J.; Yang, Y.; Zhu, X.; Xie, S. Effects of Dietary Tenebrio molitor Meal on the Growth Performance, Immune Response and Disease Resistance of Yellow Catfish (Pelteobagrus Fulvidraco). Fish Shellfish Immunol. 2017, 69, 59–66. [Google Scholar] [CrossRef] [PubMed]
- Tang, Q.; Xu, E.; Wang, Z.; Xiao, M.; Cao, S.; Hu, S.; Wu, Q.; Xiong, Y.; Jiang, Z.; Wang, F.; et al. Dietary Hermetia illucens Larvae Meal Improves Growth Performance and Intestinal Barrier Function of Weaned Pigs Under the Environment of Enterotoxigenic Escherichia coli K88. Front. Nutr. 2022, 8, 812011. [Google Scholar] [CrossRef]
- Yu, M.; Li, Z.; Chen, W.; Rong, T.; Wang, G.; Ma, X. Hermetia illucens Larvae as a Potential Dietary Protein Source Altered the Microbiota and Modulated Mucosal Immune Status in the Colon of Finishing Pigs. J. Anim. Sci. Biotechnol. 2019, 10, 50. [Google Scholar] [CrossRef]
- Valdés, F.; Villanueva, V.; Durán, E.; Campos, F.; Avendaño, C.; Sánchez, M.; Domingoz-araujo, C.; Valenzuela, C. Insects as Feed for Companion and Exotic Pets: A Current Trend. Animals 2022, 12, 1450. [Google Scholar] [CrossRef]
- Lei, X.J.; Kim, T.H.; Park, J.H.; Kim, I.H. Evaluation of Supplementation of Defatted Black Soldier Fly (Hermetia illucens) Larvae Meal in Beagle Dogs. Ann. Anim. Sci. 2019, 19, 767–777. [Google Scholar] [CrossRef]
- Bosch, G.; Swanson, K.S. Effect of Using Insects as Feed on Animals: Pet Dogs and Cats. J. Insects as Food Feed 2021, 7, 795–805. [Google Scholar] [CrossRef]
- Moula, N.; Detilleux, J. A Meta-Analysis of the Effects of Insects in Feed on Poultry Growth Performances. Animals 2019, 9, 201. [Google Scholar] [CrossRef]
- Martínez Marín, A.L.; Gariglio, M.; Biasato, I.; Gasco, L.; Schiavone, A. Meta-Analysis of the Effect of Black Soldier Fly Larvae Meal in Diet on Broiler Performance and Prediction of Its Metabolisable Energy Value. Ital. J. Anim. Sci. 2023, 22, 379–387. [Google Scholar] [CrossRef]
- Mahmoud, A.E.; Morel, P.C.H.; Potter, M.A.; Ravindran, V. The Apparent Metabolisable Energy and Ileal Amino Digestibility of Black Soldier Fly (Hermetia illucens) Larvae Meal for Broiler Chickens. Br. Poult. Sci. 2023, 64, 377–383. [Google Scholar] [CrossRef] [PubMed]
- Dalmoro, Y.K.; Adams, C.B.; Haetinger, V.S.; Bairros, L.; Yacoubi, N.; Stefanello, C. Energy Values of Tenebrio molitor Larvae Meal and Tilapia Byproduct Meal for Broiler Chickens Determined Using the Regression Method. Anim. Feed Sci. Technol. 2021, 272, 114784. [Google Scholar] [CrossRef]
| Reference | Code of Publication | Insect Used 1 | Feeding Phases 2 | Genetic/Sex 3 | Protein/Oil Source 4 | n 5 |
|---|---|---|---|---|---|---|
| Mat et al. [17] | 1 | HI | Str, Grw, Fin | Cobb/male | FM, SBM | 360 |
| Sedgh-Gooya et al. [18] | 2 | TM | Str, Fin | Arbor Acres | FM, SBM | 180 |
| Kim et al. [19] | 3 | HI | Str, Grw | Ross/male | SBM, CGM | 126 |
| Kierończyk et al. [6] | 4 | HI oil | Str, Grw | Ross | SBM | 960 |
| Hartinger et al. [20] | 5 | HI | Str, Grw | Ross/male | SBM | 432 |
| Murawska et al. [21] | 6 | HI | Str, Grw, Fin | Ross/male | SBM | 384 |
| Hartinger et al. [22] | 7 | HI | Str, Grw | Ross/mixed | SBM | 216 |
| Elangovan et al. [23] | 8 | HI | Str | Cobb/mixed | SBM | 90 |
| Kim et al. [24] | 9 | HI oil | Str, Grw | Ross/male | SBM, CGM | 300 |
| Pietras et al. [25] | 10 | TM | Fin | Ross/male | SBM, LM | 64 |
| Dabbou et al. [26] | 11 | HI oil | Str, Grw | Ross/male | SBM, CGM | 200 |
| Mutisya et al. [27] | 12 | HI | Str, Fin | Cobb/mixed | FM | 120 |
| Elahi et al. [28] | 13 | TM | Str, Fin | Ross/male | SBM | 700 |
| Benzertiha et al. [29] | 14 | TM | Str, Grw | Ross/female | SBM, RS, FM | 300 |
| Benzertiha et al. [30] | 15 | TM oil | Str, Grw | Ross/female | SBM | 48 |
| Khan et al. [31] | 16 | TM | Str, Grw | Ross | SBM, CM, GM, SM | 100 |
| Brede et al. [32] | 17 | HI | Str, Grw | Ross/male | SBM | 240 |
| Onsongo et al. [33] | 18 | HI | Str, Fin | Cobb/male | SBM, FM | 288 |
| Biasato et al. [34] | 19 | TM | Str, Grw, Fin | Ross/male | SBM, CGM | 160 |
| Biasato et al. [35] | 20 | TM | Str, Grw, Fin | Ross/female | SBM, CGM | 160 |
| Bovera et al. [36] | 21 | TM | Fin | Shaver Brown/male | SBM | 80 |
| Józefiak et al. [37] | 22 | HI and TM | Str, Grw | Ross/female | SBM, RS, rye, FM | 300 |
| Schiavone et al. [38] | 23 | HI oil | Str, Grw, Fin | Ross/male | SBM, GLM | 150 |
| Schiavone et al. [39] | 24 | HI oil | Fin | Ross/male | SBM, GLM | 120 |
| Kareem et al. [40] | 25 | HI | Str, Grw, Fin | Cobb/female | SBM, FM | 216 |
| Reference | Overall 1 | Inclusion | Main Effects 3 |
|---|---|---|---|
| Ipema et al. [41] | Replacement of 8% of the diet in DM with a different provision of HI (meal + oil, live scattered on litter, whole dried in the feeder and whole dried scattered on litter), and impacts on broiler health and behavior. | 5.76% | No difference was found for feather corticosterone, IgG, and IgM against keyhole limpet hemocyanin in plasma. Footpad dermatitis and litter quality were affected by the treatments. |
| Bongiorno et al. [42] | HI live larvae for local poultry and the effects on blood parameters. | 10% live larvae supplementation on the ADFI 2 | HI reduced leukocytes levels and increased monocytes and cholesterol levels. Higher gamma glutamyl transferase in blood samples, indicating an improvement in liver health status. |
| Mat et al. [17] | Different replacement levels of fishmeal with defatted HI meal for broilers and the effects on blood parameters. | 4%, 8%, and 12% | Corpuscular volume, monocytes, red blood cells, granulocytes, hematocrits, hemoglobin, corpuscular hemoglobin concentration, red cell distribution width, platelet volume, and lymphocytes were affected by HI meal. |
| Kierończyk et al. [43] | HI oil replacing soybean oil and the effects on gut microbiota and immune characteristics. | 1.67% and 3.34% | 100% replacement increased the total number of Clostridium leptum subgroups and Enterobacteriaceae and Lactobacillus groups in crop. No effects on jejunal microbiota. Reduced C. perfringens and all other microorganisms except Bacteroides with 50% HI replacement. Reduced cholesterol and ALT in plasma. No effects on plasma immunoglobulins and IL. |
| Hartinger et al. [20] | HI oil replacing 50% or 100% of soybean oil and HI meal replacing a 15% CP diet for broilers. Evaluated the effects on ileum histomorphology and caeca microbial metabolites. | 5.2%, 4.64% and 4% of HI meal and/or 0.75%, 1.5%, 0.64% and 1.28% of HI oil | HI meal decreased the biogenic amines Agmatine, Spermidine, Spermine, and ammonia in caeca contents. Ethanolamine was higher with the HI oil. HI oil increased the concentration of Agmatine in colon contents. HI meal and 50 or 100% oil replacement increased jejunal villus area and width compared to control diets. |
| Kim et al. [19] | Microwave-dried HI meal replacing 25% and 50% of SBM. | Starter: 7.5% and 15%. Grower: 7% and 14%. Finisher: 6.5% and 13% | SCFAs in caeca were higher in HI treatments (except butyrate), especially with 50% replacement. Blood triglycerides, monocytes, and red blood cell distribution were higher in 50% replacement. Decreased low-density lipoprotein was obtained. |
| Zhang et al. [44] | Immune responses using HI larvae meal for broilers experimentally infected with IBV. | 1%, 5%, and 10% | Reduced IBV symptoms with 10% inclusion. At the tissue level, reduced IBV infection and lesions were presented. Increased survival rate of chicks. Improved proliferation of CD8+ lymphocytes. |
| Hartinger et al. [22] | 0, 15%, and 30% of CP from SBM replaced with HI meal. | 0, 4%, 10% | No differences in intestine morphology parameters. |
| de Souza Vilela et al. [45] | Increasing levels of full-fat HI meal for broilers and the effects on immune responses. | Starter: 2.5%, 5%, 7.5%, and 10%. Grower/Finisher: 5%, 10%, 15%, and 20% | Decreased blood lymphocytes at 21 d and decreased cytotoxic T cell CD3+CD8+ in jejunum. |
| Kim et al. [46] | Replacement of 50% and 100% of soybean oil with HI oil and the effects on intestinal health and blood profile. | 1.5% and 3% | Higher ileum villus height with 50% replacement. Higher butyrate levels in caeca content with HI oil. Lowered lipase levels in blood samples. |
| Biasato et al. [47] | Use of defatted HI meal in broilers and the impact on gut health. | 5%, 10%, and 15% | HI did not affect the relative abundance of Firmicutes and Bacteroidetes and the Firmicutes/Bacteroidetes ratio, but 15% HI caused higher relative abundance of Proteobacteria. L-Ruminococcus (Ruminococcus from Lachnospiraceae family), Faecalibacterium, Blautia, Clostridium, Bacteroides, Roseburia, and Helicobacter genera. Lactobacillus and Ruminococcus improved in 10% HI diet. The mucin staining was not affected by HI. |
| Kierończyk et al. [6] | Replacement of 25%, 50%, 75% and 100% of soybean oil with HI oil and the effects on broiler gut histomorphology. | Starter: 0.83%, 1.67%, 2.34%, and 3.34%. Finisher: 1.23%, 2.46%, 3.44% and 4.92% | No effects on histomorphology of duodenum, jejunum, and ileum. The HI oil reduced the jejunum and ileum weights relative to BW. |
| Ipema et al. [48] | Use of live larvae and impacts on health and behavior of broilers. | 5% and 10% of ADFI 2 in DM with live larvae | No effects on hock burns, lameness, cleanliness, thigh scratches, tibia length, tibia fluctuating asymmetry, and tibia breaking strength. Only reduced the tibia width with 10% replacement. |
| Lee et al. [49] | Immune activity of broilers experimentally infected with Salmonella Gallinarum fed with HI larvae. | 1%, 2%, and 3% | Higher presence of CD3+CD4+ T lymphocytes in spleen. Amplified spleen lymphocyte proliferation, increased lysozyme activity in serum, and increased survival rate of chicks with 3% inclusion. |
| Dabbou et al. [50] | HI defatted meal and impacts on blood traits, gut morphology, and histological features. | 5%, 10%, and 15% | No effects on hematological and serum parameters. Diet with 15% HI decreased villi height, crypt depth, and V:C ratio compared to the other diets. |
| Schiavone et al. [39] | Replacement of 50% and 100% of soybean oil with HI oil for broilers (finisher phase) and effects on blood and gut morphology parameters. | 3.43% and 6.87% | No differences in blood and histomorphology parameters. |
| Schiavone et al. [38] | 50% and 100% of soybean oil replaced with HI oil and the effects on blood parameters. | 2.91% and 5.85% | No differences observed in blood analyses. |
| Reference | Overall 1 | Inclusion | Main Effects 2 |
|---|---|---|---|
| Sedgh-Gooya et al. [18] | Effects on histomorphology of broilers fed full-fat TM meal. | 2.5% and 5% | No effects on histomorphology. |
| Sedgh-Gooya et al. [51] | Inclusion of TM meal in broiler diets and effects on caeca microbiota and blood parameters. | 2.5% and 5% | Decreased blood albumin/globulin ratio with TM diets. Escherichia coli reduced with 5% TM inclusion. |
| Benzertiha et al. [29] | Full-fat TM meal supplemented in broiler diets and impacts on bird immune system. | 0.2% and 0.3% | TM meal diets had same IgY and IgM levels as diets with salinomycin, but had increased IL-2 and TNF-α with 0.3% TM inclusion. |
| Biasato et al. [52] | TM meal and the effects on broiler intestinal microbiota and mucin. | 5%, 10%, and 15% | Decreased relative abundance of Firmicutes/Bacteroidetes ratio and the relative abundance of Clostridium, Coprococcus, L-Ruminococcus, and Ruminococcus with 15% TM. Higher mucin staining with 5% TM. |
| Elahi et al. [28] | Different levels of dried TM meal and usage of fresh TM meal. | 2%, 4%, 8%, and 10.48% as fresh matter that correspond to 4% of dried TM inclusion | Linear increase in ALT in blood. Reduced total protein. Higher malondialdehyde and lower total antioxidant capacity. Higher uric acid in blood. Decreased levels of lysozyme with fresh TM. |
| Józefiak et al. [53] | Full-fat TM meal supplemented in broiler diets and effects on caeca microbiome. | 0.2% and 0.3% | Phylum: Decreased relative number of Actinobacteria with 0.2% TM and Proteobacteria in both treatments. Increased Bacteroidetes with TM addition. Class: 0.3% TM decreased Clostridia and 0.2% TM increased Clostridia and decreased Actinobacteria. Order: 0.2% TM increased Clostridiales, but there was a decrease with 0.3% TM, and 0.2% TM decreased Lactobacilales. Family: 0.3% TM reduced Ruminococcaceae, Enterobacteriaceae and Bifidobacteriaceae. Genus: 0.3% TM stimulated growth of Ruminococcus and Bifidobacterium, and 0.2% TM decreased Lactobacillus. |
| Biasato et al. [54] | TM full-fat meal inclusion in broiler diets and effects on caeca microbiota and health. | 5% and 15% | Higher inclusion of TM meal decreased relative abundance of Firmicutes phylum and Firmicutes/Bacteroidetes ratio. Higher inclusion of TM meal decreased villi mucin staining. |
| Benzertiha et al. [30] | Full-fat TM meal supplementation in diets and effects on enzyme activity and microbiota. | 0.2% or 0.3% | TM meal at 0.3% decreased the caeca Bacteroides– Prevotella cluster. Also, 0.2 and 0.3% decreased C. perfringens. Salinomycin and TM treatments had the lowest extracellular β-glucuronidase and a higher α-glucosidase activity for caeca contents. |
| Benzertiha et al. [55] | TM oil and effects on pancreatic enzyme and blood parameters. | 5% | TM oil reduced amylase activity and triglycerides in blood, and triglycerides and total cholesterol in liver. |
| Loponte et al. [56] | Total replacement of SBM with full-fat TM meal in broiler diets and the effects on caeca VFAs. | 100% SBM replacement | Almost doubled Mmol/L in all VFAs, increased % of butyrate in total VFAs, and decreased acetate, propionate, and valerianate. |
| Biasato et al. [57] | TM meal and effects on intestinal microbiota, histomorphology, and mucin composition of free-range broilers. | 7.5% | Increased Sutterella, Ruminococcus, Oscillospira, Clostridium, Coprococcus, and Firmicutes/Bacteroidetes ratio. Increased mucin staining intensity on ileum. |
| Biasato et al. [35] | TM meal and the effects on broiler health. | 5%, 10%, and 15% | Increased levels of erythrocytes, linear decrease in albumin levels, and quadratic decrease in gamma glutamyl transferase in blood. No differences in histomorphology using TM meal. |
| Islam and Yang. [58] | TM probiotic supplementation for broilers experimentally challenged with Salmonella enteritis and E. coli. | 0.4% of TM probiotic | IgG and IgA levels were higher with the probiotic. Lower mortality of birds and lower presence of E. coli and Salmonella spp. in caeca microbiota with the probiotic. |
| Reference | Overall 1 | Inclusion | Main Effects |
|---|---|---|---|
| Bellezza Oddon et al. [59] | Live TM and HI larvae and impacts on health. | 5% of ADFI 2 with larvae of TM or HI | Gut histomorphology index and histopathological alterations were not influenced by HI and TM larvae. No effects on hematological and serum parameters. |
| Colombino et al. [60] | Live TM and HI larvae for broilers and effects on mucin, immune response, and caeca microbiota. | 5% of the expected ADFI | TM diets had lower interleukin-2 expression compared to HI diets. Mucin was not affected by live larvae. HI and TM influenced the relative abundance of the Victivillaceae family. Saccharibacteria and Clostridium increased HI, Collinsella was more abundant in TM treatment, and Eubacterium increased in both diets. |
| Józefiak et al. [37] | TM and HI full-fat meal supplementation and impact on microbiota. | 0.2% supplemented | Crop digesta: HI decreased C. leptum and increased C. coccoides–Eubacterium rectale cluster. Lactobacillus spp. and Enterococcus increased in TM and HI groups, while TM reduced Bacteroides–Prevotella. Ileal digesta: both insects increased the number of C. coccoides–E. rectale and Lactobacillus spp. Enterococcus spp. counts decreased in HI and increased in TM groups. Caeca digesta: Bacteroides–Prevotella, Streptococcus spp./Lactococcus spp., C. coccoides–E. rectale cluster, and Lactobacillus spp./Enterococcus spp. count increased with HI. |
| Variation, % 1 | Meal | p-Value 4 | Oil | p-Value | ||
|---|---|---|---|---|---|---|
| HI 2 | TM 3 | HI | TM | |||
| ADFI | −10.70 | 7.20 | <0.001 | 0.61 | −4.25 | 0.036 |
| ADG | −7.92 | 4.05 | 0.002 | −0.51 | 0.81 | 0.888 |
| FCR | −1.49 | 2.46 | 0.241 | 0.41 | −3.56 | 0.049 |
| Variation, % 1 | Meal 2 | p-Value 4 | Oil 3 | p-Value | ||||
|---|---|---|---|---|---|---|---|---|
| Control | Low | High | Control | Low | High | |||
| ADFI | 94.42 a | 95.98 a | 85.62 b | <0.001 | 98.44 | 97.85 | 97.17 | 0.620 |
| ADG | 92.46 a | 94.70 a | 83.41 b | <0.001 | 97.12 | 97.83 | 96.85 | 0.545 |
| FCR | 107.1 | 109.7 | 107.9 | 0.647 | 102.4 | 101.8 | 101.7 | 0.576 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dalmoro, Y.K.; Franceschi, C.H.; Stefanello, C. A Systematic Review and Metanalysis on the Use of Hermetia illucens and Tenebrio molitor in Diets for Poultry. Vet. Sci. 2023, 10, 702. https://doi.org/10.3390/vetsci10120702
Dalmoro YK, Franceschi CH, Stefanello C. A Systematic Review and Metanalysis on the Use of Hermetia illucens and Tenebrio molitor in Diets for Poultry. Veterinary Sciences. 2023; 10(12):702. https://doi.org/10.3390/vetsci10120702
Chicago/Turabian StyleDalmoro, Yuri Katagiri, Carolina H. Franceschi, and Catarina Stefanello. 2023. "A Systematic Review and Metanalysis on the Use of Hermetia illucens and Tenebrio molitor in Diets for Poultry" Veterinary Sciences 10, no. 12: 702. https://doi.org/10.3390/vetsci10120702
APA StyleDalmoro, Y. K., Franceschi, C. H., & Stefanello, C. (2023). A Systematic Review and Metanalysis on the Use of Hermetia illucens and Tenebrio molitor in Diets for Poultry. Veterinary Sciences, 10(12), 702. https://doi.org/10.3390/vetsci10120702













