Feline Uroepithelial Cell Culture as a Novel Model of Idiopathic Cystitis: Investigations on the Effects of Norepinephrine on Inflammatory Response, Oxidative Stress, and Barrier Function
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents
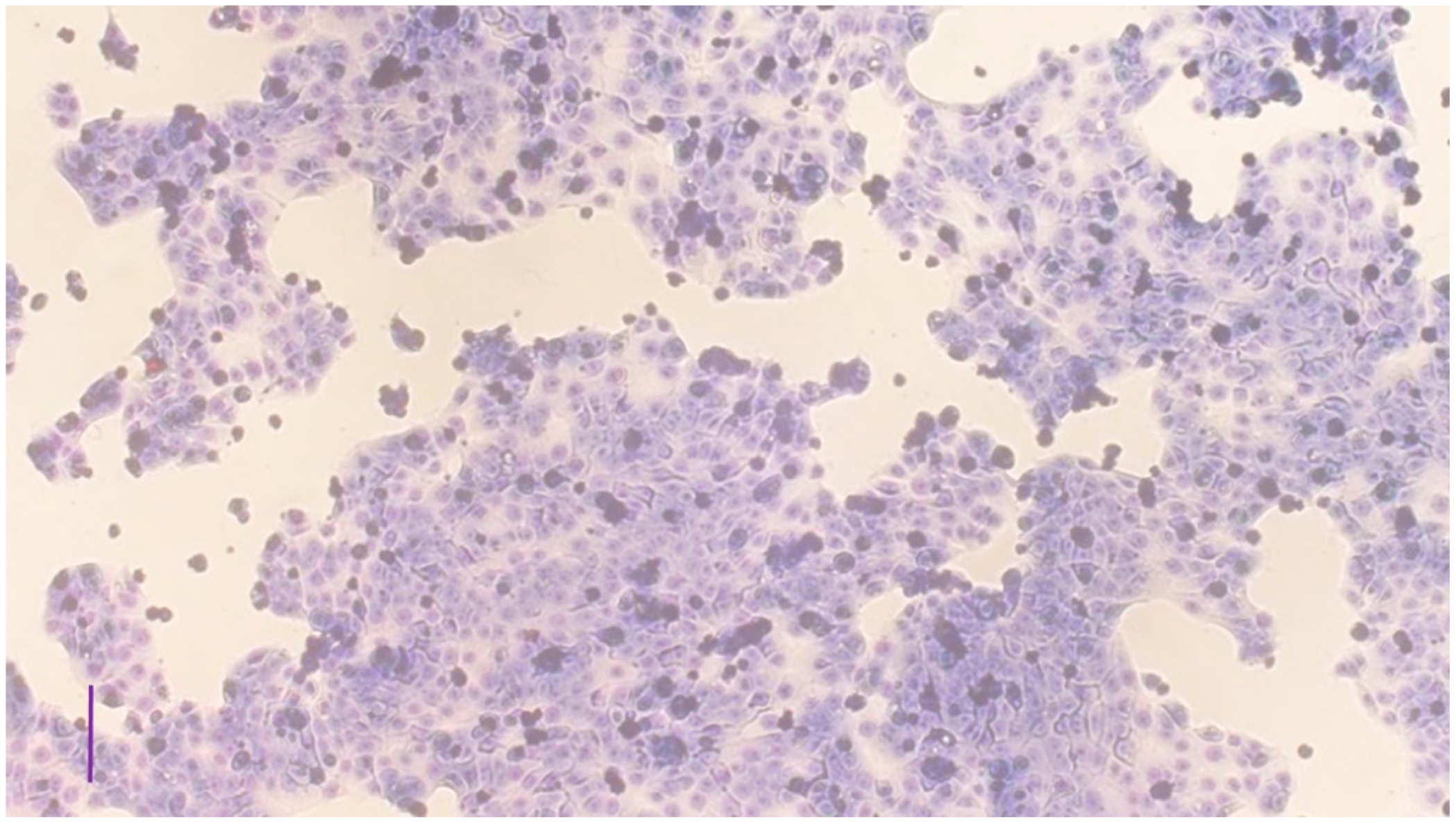
2.2. Isolation and Culturing of Epithelial Cells from Cat Bladder
2.3. Characterization of the Cell Culture
2.4. Norepinephrine Treatment of the Cultures
2.5. Laboratory Analyses
2.5.1. Assessment of Cellular Metabolic Activity and Cell Injury
2.5.2. Measurement of IL-6 and SDF-1 Concentrations
2.5.3. Assessment of the Redox State of the Cells
2.5.4. Investigation of Epithelial Barrier Function
2.6. Statistical Analysis
3. Results
3.1. Characterization of the Cell Cultures
3.2. Assessment of Cellular Metabolic Activity and Cell Injury
3.3. Measurement of IL-6 and SDF-1 Concentrations
3.4. Assessment of the Redox State of the Cells
3.5. Investigation of Epithelial Barrier Function
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chew, D.; Saim, D.A.; Buffington, C.A.T.; Acvn, D. Pandora Syndrome: It’s More than Just the Bladder. Conf. Am. Assoc. Feline. Pract. 2013, 1, 75–83. [Google Scholar]
- Lavelle, J.P.; Meyers, S.A.; Ruiz, W.G.; Buffington, C.A.T.; Zeidel, M.L.; Apodaca, G. Urothelial pathophysiological changes in feline interstitial cystitis: A human model. Am. J. Physiol. Renal. Physiol. 2000, 278, F540–F553. [Google Scholar] [CrossRef] [PubMed]
- Buffington, C.A.; Chew, D.J.; DiBartola, S.P. Interstitial cystitis in cats. Vet. Clin. North Am. Small Anim. Pract. 1996, 26, 317–326. [Google Scholar] [CrossRef] [PubMed]
- Apodaca, G. The uroepithelium: Not just a passive barrier. Traffic 2004, 5, 117–128. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.R.; Lin, J.H.; Walz, T.; Häner, M.; Yu, J.; Aebi, U.; Sun, T. Mammalian uroplakins. A group of highly conserved urothelial differentiation-related membrane proteins. J. Biol. Chem. 1994, 269, 13716–13724. [Google Scholar] [CrossRef]
- Wu, X.R.; Sun, T.T. Molecular cloning of a 47 kDa tissue-specific and differentiation-dependent urothelial cell surface glycoprotein. J. Cell Sci. 1993, 106 Pt 1, 31–43. [Google Scholar] [CrossRef]
- Yu, J.; Manabe, M.; Wu, X.R.; Xu, C.; Surya, B.; Sun, T.T. Uroplakin I: A 27-kD protein associated with the asymmetric unit membrane of mammalian urothelium. J. Cell Biol. 1990, 111, 1207–1216. [Google Scholar] [CrossRef]
- Kaufmann, O.; Volmerig, J.; Dietel, M. Uroplakin III is a highly specific and moderately sensitive immunohistochemical marker for primary and metastatic urothelial carcinomas. Am. J. Clin. Pathol. 2000, 113, 683–687. [Google Scholar] [CrossRef]
- Truschel, S.T.; Ruiz, W.G.; Shulman, T.; Pilewski, J.; Sun, T.T.; Zeidel, M.L.; Apodaca, G. Primary uroepithelial cultures. A model system to analyze umbrella cell barrier function. J. Biol. Chem. 1999, 274, 15020–15029. [Google Scholar] [CrossRef]
- González-Mariscal, L.; Betanzos, A.; Nava, P.; Jaramillo, B.E. Tight junction proteins. Prog. Biophys. Mol. Biol. 2003, 81, 1–44. [Google Scholar] [CrossRef]
- Parsons, C.L.; Boychuk, D.; Jones, S.; Hurst, R.; Callahan, H. Bladder surface glycosaminoglycans: An epithelial permeability barrier. J. Urol. 1990, 143, 139–142. [Google Scholar] [CrossRef]
- Hauser, P.J.; VanGordon, S.B.; Seavey, J.; Sofinowski, T.M.; Ramadan, M.; Abdullah, S.; Buffington, C.A.T.; Hurst, R.E. Abnormalities in Expression of Structural, Barrier and Differentiation Related Proteins, and Chondroitin Sulfate in Feline and Human Interstitial Cystitis. J. Urol. 2015, 194, 571–577. [Google Scholar] [CrossRef]
- Buffington, C.A.; Pacak, K. Increased plasma norepinephrine concentration in cats with interstitial cystitis. J. Urol. 2001, 165, 2051–2054. [Google Scholar] [CrossRef]
- Westropp, J.L.; Kass, P.H.; Buffington, C.A.T. Evaluation of the effects of stress in cats with idiopathic cystitis. Am. J. Vet. Res. 2006, 67, 731–736. [Google Scholar] [CrossRef]
- Stein, P.C.; Torri, A.; Parsons, C.L. Elevated urinary norepinephrine in interstitial cystitis. Urology 1999, 53, 1140–1143. [Google Scholar] [CrossRef]
- Brown, M.R.; Fisher, L.A. Glucocorticoid suppression of the sympathetic nervous system and adrenal medulla. Life Sci. 1986, 39, 1003–1012. [Google Scholar] [CrossRef]
- Westropp, J.L.; Welk, K.A.; Buffington, C.A.T. Small adrenal glands in cats with feline interstitial cystitis. J. Urol. 2003, 170, 2494–2497. [Google Scholar] [CrossRef]
- Erickson, D.R.; Kunselman, A.R.; Bentley, C.M.; Peters, K.M.; Rovner, E.S.; Demers, L.M.; Wheeler, M.A.; Keay, S.K. Changes in urine markers and symptoms after bladder distention for interstitial cystitis. J. Urol. 2007, 177, 556–560. [Google Scholar] [CrossRef]
- Malley, S.E.; Vizzard, M.A. Changes in urinary bladder cytokine mRNA and protein after cyclophosphamide-induced cystitis. Physiol. Genom. 2002, 9, 5–13. [Google Scholar] [CrossRef] [PubMed]
- Arms, L.; Girard, B.M.; Vizzard, M.A. Expression and function of CXCL12/CXCR4 in rat urinary bladder with cyclophosphamide-induced cystitis. Am. J. Physiol. Renal. Physiol. 2010, 298, F589–F600. [Google Scholar] [CrossRef]
- Parys, M.; Yuzbasiyan-Gurkan, V.; Kruger, J.M. Serum Cytokine Profiling in Cats with Acute Idiopathic Cystitis. J. Vet. Intern. Med. 2018, 32, 274–279. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Forrester, S.J.; Kikuchi, D.S.; Hernandes, M.S.; Xu, Q.; Griendling, K.K. Reactive Oxygen Species in Metabolic and Inflammatory Signaling. Circ. Res. 2018, 122, 877–902. [Google Scholar] [CrossRef] [PubMed]
- Treutlein, G.; Dorsch, R.; Euler, K.N.; Hauck, S.M.; Amann, B.; Hartmann, K.; Deeg, C.A. Novel Potential Interacting Partners of Fibronectin in Spontaneous Animal Model of Interstitial Cystitis. PLoS ONE 2012, 7, e51391. [Google Scholar] [CrossRef] [PubMed]
- Miyata, Y.; Matsuo, T.; Mitsunari, K.; Asai, A.; Ohba, K.; Sakai, H. A review of oxidative stress and urinary dysfunction caused by bladder outlet obstruction and treatments using antioxidants. Antioxidants 2019, 8, 132. [Google Scholar] [CrossRef]
- Homan, T.; Tsuzuki, T.; Dogishi, K.; Shirakawa, H.; Oyama, T.; Nakagawa, T.; Kaneko, S. Novel mouse model of chronic inflammatory and overactive bladder by a single intravesical injection of hydrogen peroxide. J. Pharmacol. Sci. 2013, 121, 327–337. [Google Scholar] [CrossRef]
- Buffington, C.A.T.; Teng, B.; Somogyi, G.T. Norepinephrine content and adrenoceptor function in the bladder of cats with feline interstitial cystitis. J. Urol. 2002, 167, 1876–1880. [Google Scholar] [CrossRef]
- Yang, R.; Lin, Q.; Gao, H.B.; Zhang, P. Stress-related hormone norepinephrine induces interleukin-6 expression in GES-1 cells. Brazilian J. Med. Biol. Res.=Rev. Bras. Pesqui. Med. Biol. 2014, 47, 101–109. [Google Scholar] [CrossRef]
- Lutgendorf, S.K.; Kreder, K.J.; Rothrock, N.E.; Ratliff, T.L.; Zimmerman, B. Stress and symptomatology in patients with interstitial cystitis: A laboratory stress model. J. Urol. 2000, 164, 1265–1269. [Google Scholar] [CrossRef]
- Kodama, D.; Togari, A. Noradrenaline stimulates cell proliferation by suppressing potassium channels via G(i/o)-protein-coupled α(1B)-adrenoceptors in human osteoblasts. Br. J. Pharmacol. 2013, 168, 1230–1239. [Google Scholar] [CrossRef]
- Mackei, M.; Molnár, A.; Nagy, S.; Pál, L.; Kővágó, C.; Gálfi, P.; Dublecz, K.; Husvéth, F.; Neogrády, Z.; Mátis, G. Effects of Acute Heat Stress on a Newly Established Chicken Hepatocyte-Nonparenchymal Cell Co-Culture Model. Animals 2020, 10, 409. [Google Scholar] [CrossRef]
- Bürger, A.; Benicke, M.; Deten, A.; Zimmer, H.G. Catecholamines stimulate interleukin-6 synthesis in rat cardiac fibroblasts. Am. J. Physiol. Heart Circ. Physiol. 2001, 281, H14–H21. [Google Scholar] [CrossRef] [Green Version]
- Lamale, L.M.; Lutgendorf, S.K.; Zimmerman, M.B.; Kreder, K.J. Interleukin-6, histamine, and methylhistamine as diagnostic markers for interstitial cystitis. Urology 2006, 68, 702–706. [Google Scholar] [CrossRef]
- Mohamaden, W.I.; Hamad, R.; Bahr, H.I. Alterations of pro-inflammatory cytokines and tissue protein expressions in cats with interstitial cystitis. Pak. Vet. J. 2020, 39, 151–156. [Google Scholar] [CrossRef]
- Corcoran, A.T.; Yoshimura, N.; Tyagi, V.; Jacobs, B.; Leng, W.; Tyagi, P. Mapping the cytokine profile of painful bladder syndrome/interstitial cystitis in human bladder and urine specimens. World J. Urol. 2013, 31, 241–246. [Google Scholar] [CrossRef]
- Dizdaroglu, M. Facts about the artifacts in the measurement of oxidative DNA base damage by gas chromatography-mass spectrometry. Free Radic. Res. 1998, 29, 551–563. [Google Scholar] [CrossRef]
- Valente, V.B.; de Melo Cardoso, D.; Kayahara, G.M.; Nunes, G.B.; Tjioe, K.C.; Biasoli, R.; Miyahara, G.I.; Oliveira, S.H.P.; Mingoti, G.Z.; Bernabé, D.G. Stress hormones promote DNA damage in human oral keratinocytes. Sci. Rep. 2021, 11, 19701. [Google Scholar] [CrossRef]
- Adachi, S.; Kawamura, K.; Takemoto, K. Oxidative damage of nuclear DNA in liver of rats exposed to psychological stress. Cancer Res. 1993, 53, 4153–4155. [Google Scholar]
- Lin, H.-Y.; Lu, J.-H.; Chuang, S.-M.; Chueh, K.-S.; Juan, T.-J.; Liu, Y.-C.; Juan, Y.-S. Urinary Biomarkers in Interstitial Cystitis/Bladder Pain Syndrome and Its Impact on Therapeutic Outcome. Diagnostics 2021, 12, 75. [Google Scholar] [CrossRef]
- Dokumacioglu, E.; Demiray, O.; Dokumacioglu, A.; Sahin, A.; Sen, T.M.; Cankaya, S. Measuring urinary 8-hydroxy-2′-deoxyguanosine and malondialdehyde levels in women with overactive bladder. Investig Clin. Urol. 2018, 59, 252–256. [Google Scholar] [CrossRef]
- Dalle-Donne, I.; Rossi, R.; Giustarini, D.; Milzani, A.; Colombo, R. Protein carbonyl groups as biomarkers of oxidative stress. Clin. Chim. Acta 2003, 329, 23–38. [Google Scholar] [CrossRef]
- Kullmann, F.A.; McDonnell, B.M.; Wolf-Johnston, A.S.; Kanai, A.J.; Shiva, S.; Chelimsky, T.; Rodriguez, L.; Birder, L.A. Stress-induced autonomic dysregulation of mitochondrial function in the rat urothelium. Neurourol. Urodyn. 2019, 38, 572–581. [Google Scholar] [CrossRef] [PubMed]
- Da Costa, L.A.; Badawi, A.; El-Sohemy, A. Nutrigenetics and modulation of oxidative stress. Ann. Nutr. Metab. 2012, 60 (Suppl. 3), 27–36. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rao, R.V.; Peel, A.; Logvinova, A.; Del Rio, G.; Hermel, E.; Yokota, T.; Goldsmith, P.C.; Ellerby, L.M.; Ellerby, H.M.; Bredesen, D.E. Coupling endoplasmic reticulum stress to the cell death program: Role of the ER chaperone GRP78. FEBS Lett. 2002, 514, 122–128. [Google Scholar] [CrossRef] [PubMed]
- Vera, P.L.; Wang, X.; Bucala, R.J.; Meyer-Siegler, K.L. Intraluminal blockade of cell-surface CD74 and glucose regulated protein 78 prevents substance P-induced bladder inflammatory changes in the rat. PLoS ONE 2009, 4, e5835. [Google Scholar] [CrossRef]
- Mao, W.; Iwai, C.; Keng, P.C.; Vulapalli, R.; Liang, C.S. Norepinephrine-induced oxidative stress causes PC-12 cell apoptosis by both endoplasmic reticulum stress and mitochondrial intrinsic pathway: Inhibition of phosphatidylinositol 3-kinase survival pathway. Am. J. Physiol. Cell Physiol. 2006, 290, C1373–C1384. [Google Scholar] [CrossRef]
- Yang, C.; Zhou, J.-Y.; Zhong, H.-J.; Wang, H.-Y.; Yan, J.; Liu, Q.; Huang, S.-N.; Jiang, J.-X. Exogenous norepinephrine correlates with macrophage endoplasmic reticulum stress response in association with XBP-1. J. Surg Res. 2011, 168, 262–271. [Google Scholar] [CrossRef]
- Buffington, C.A.; Blaisdell, J.L.; Binns, S.P.J.; Woodworth, B.E. Decreased urine glycosaminoglycan excretion in cats with interstitial cystitis. J. Urol. 1996, 155, 1801–1804. [Google Scholar] [CrossRef]
- Liebert, M. The urothelial glycosaminoglycan layer revisited. J. Urol. 2009, 182, 2103–2104. [Google Scholar] [CrossRef]
- Acharya, P.; Beckel, J.; Ruiz, W.G.; Wang, E.; Rojas, R.; Birder, L.; Apodaca, G. Distribution of the tight junction proteins ZO-1, occludin, and claudin-4, -8, and -12 in bladder epithelium. Am. J. Physiol. Renal. Physiol. 2004, 287, F305–F318. [Google Scholar] [CrossRef]
- Tsukita, S.; Furuse, M.; Itoh, M. Multifunctional strands in tight junctions. Nat. Rev. Mol. Cell Biol. 2001, 2, 285–293. [Google Scholar] [CrossRef]
- Kullmann, F.A.; McDonnell, B.M.; Wolf-Johnston, A.S.; Lynn, A.M.; Getchell, S.E.; Ruiz, W.G.; Zabbarova, I.V.; Ikeda, Y.; Kanai, A.J.; Roppolo, J.R. Inflammation and Tissue Remodeling in the Bladder and Urethra in Feline Interstitial Cystitis. Front. Syst. Neurosci. 2018, 12, 13. [Google Scholar] [CrossRef]
- Lee, J.-D.; Lee, M.-H. Decreased expression of zonula occludens-1 and occludin in the bladder urothelium of patients with interstitial cystitis/painful bladder syndrome. J. Formos. Med. Assoc. 2014, 113, 17–22. [Google Scholar] [CrossRef] [Green Version]
- Kruger, J.M.; Osborne, C.A.; Lulich, J.P. Changing paradigms of feline idiopathic cystitis. Vet. Clin. North Am. Small Anim. Pract. 2009, 39, 15–40. [Google Scholar] [CrossRef]
- Warland, J.; Bestwick, J. Canine cystitis–diseases, causes and treatments. Vet. Times 2017, 47, 8–10. [Google Scholar]
- Ling, G.V. Therapeutic strategies involving antimicrobial treatment of the canine urinary tract. J. Am. Vet. Med. Assoc. 1984, 185, 1162–1164. [Google Scholar]











Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hatala, P.; Lajos, A.; Mackei, M.; Sebők, C.; Tráj, P.; Vörösházi, J.; Neogrády, Z.; Mátis, G. Feline Uroepithelial Cell Culture as a Novel Model of Idiopathic Cystitis: Investigations on the Effects of Norepinephrine on Inflammatory Response, Oxidative Stress, and Barrier Function. Vet. Sci. 2023, 10, 132. https://doi.org/10.3390/vetsci10020132
Hatala P, Lajos A, Mackei M, Sebők C, Tráj P, Vörösházi J, Neogrády Z, Mátis G. Feline Uroepithelial Cell Culture as a Novel Model of Idiopathic Cystitis: Investigations on the Effects of Norepinephrine on Inflammatory Response, Oxidative Stress, and Barrier Function. Veterinary Sciences. 2023; 10(2):132. https://doi.org/10.3390/vetsci10020132
Chicago/Turabian StyleHatala, Patrícia, Andrea Lajos, Máté Mackei, Csilla Sebők, Patrik Tráj, Júlia Vörösházi, Zsuzsanna Neogrády, and Gábor Mátis. 2023. "Feline Uroepithelial Cell Culture as a Novel Model of Idiopathic Cystitis: Investigations on the Effects of Norepinephrine on Inflammatory Response, Oxidative Stress, and Barrier Function" Veterinary Sciences 10, no. 2: 132. https://doi.org/10.3390/vetsci10020132
APA StyleHatala, P., Lajos, A., Mackei, M., Sebők, C., Tráj, P., Vörösházi, J., Neogrády, Z., & Mátis, G. (2023). Feline Uroepithelial Cell Culture as a Novel Model of Idiopathic Cystitis: Investigations on the Effects of Norepinephrine on Inflammatory Response, Oxidative Stress, and Barrier Function. Veterinary Sciences, 10(2), 132. https://doi.org/10.3390/vetsci10020132





