Effect of Aging on the Immune Response to Core Vaccines in Senior and Geriatric Dogs
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population and Study Protocol
2.2. Detection of Specific Antibodies to Core Vaccines by VacciCheck and Expression of the Results
2.3. Statistical Analysis
3. Results
3.1. Dog Population
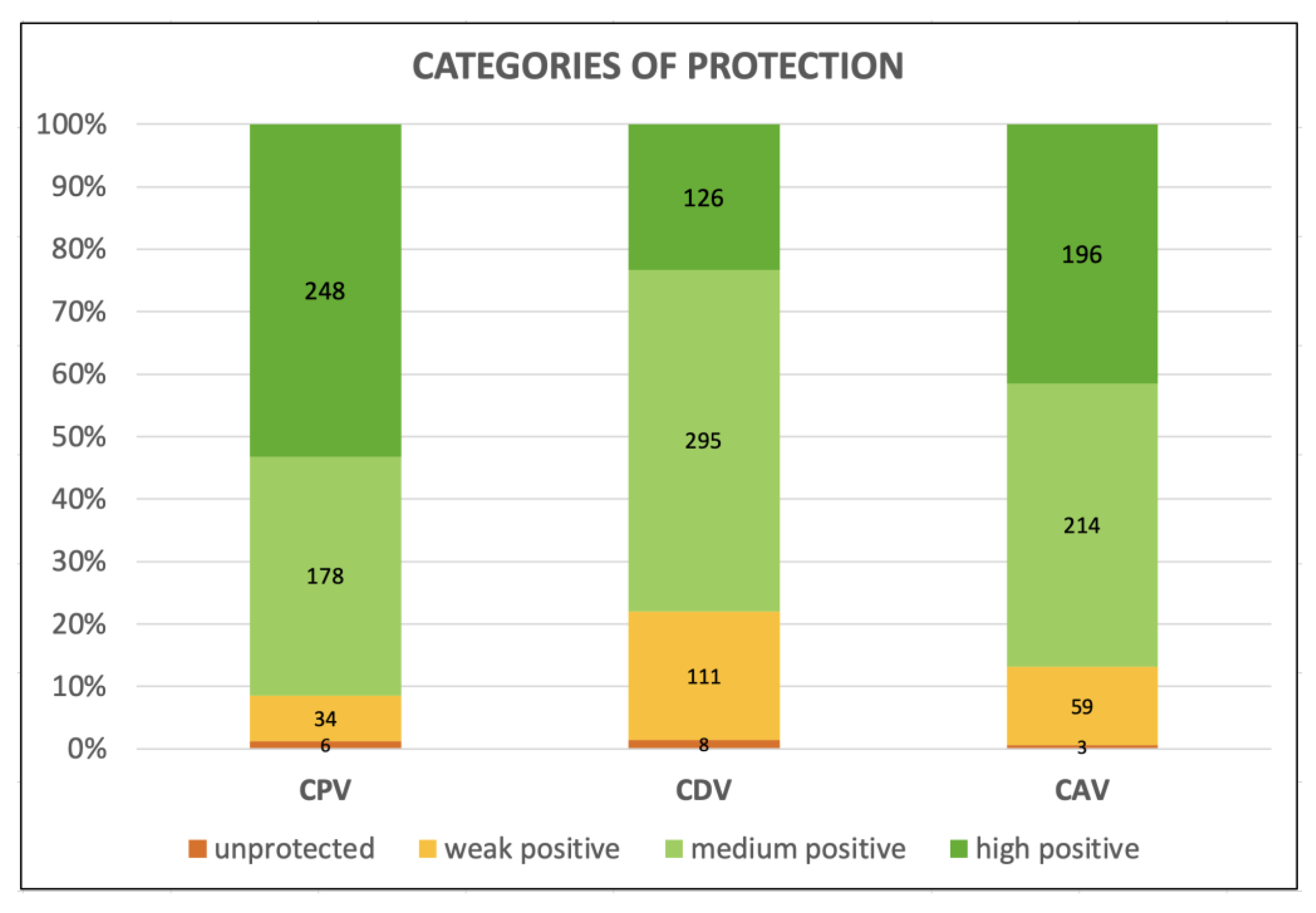
3.2. Antibody Titers and Protection of the Aging Dogs
3.3. Results According to the Different Variables
3.3.1. Age
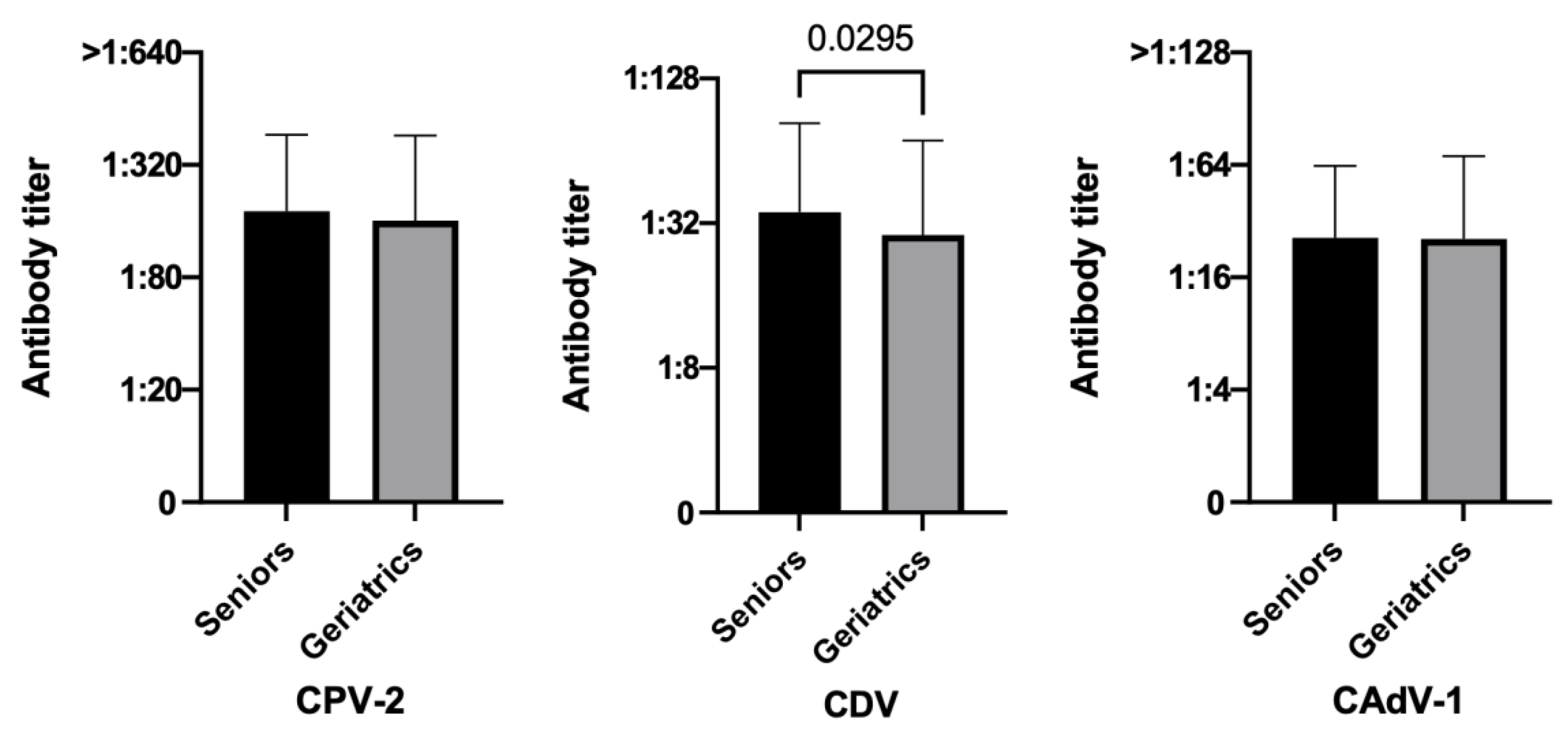
3.3.2. Size
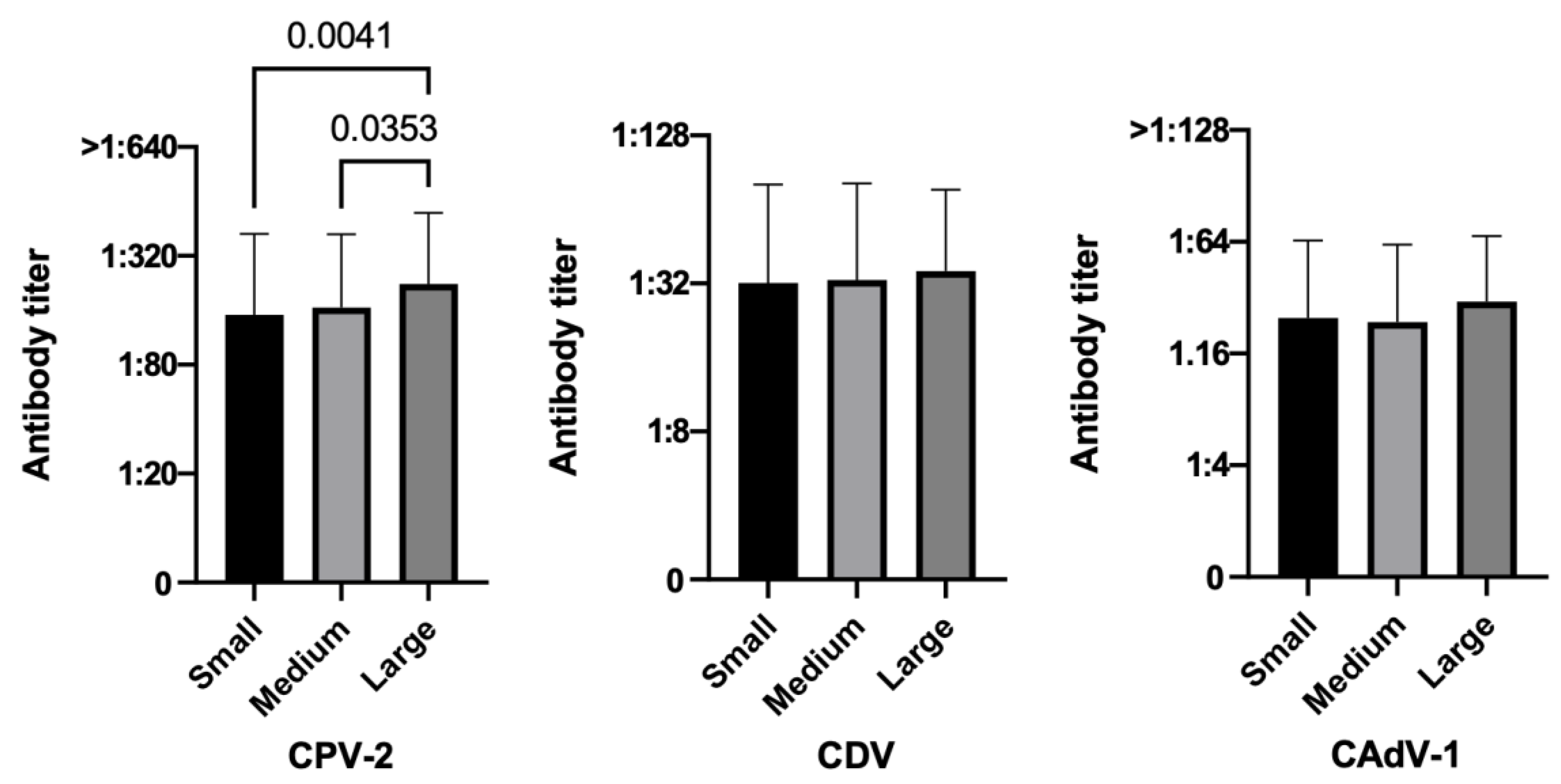
3.3.3. Sex and Reproductive Status
3.3.4. Health Status
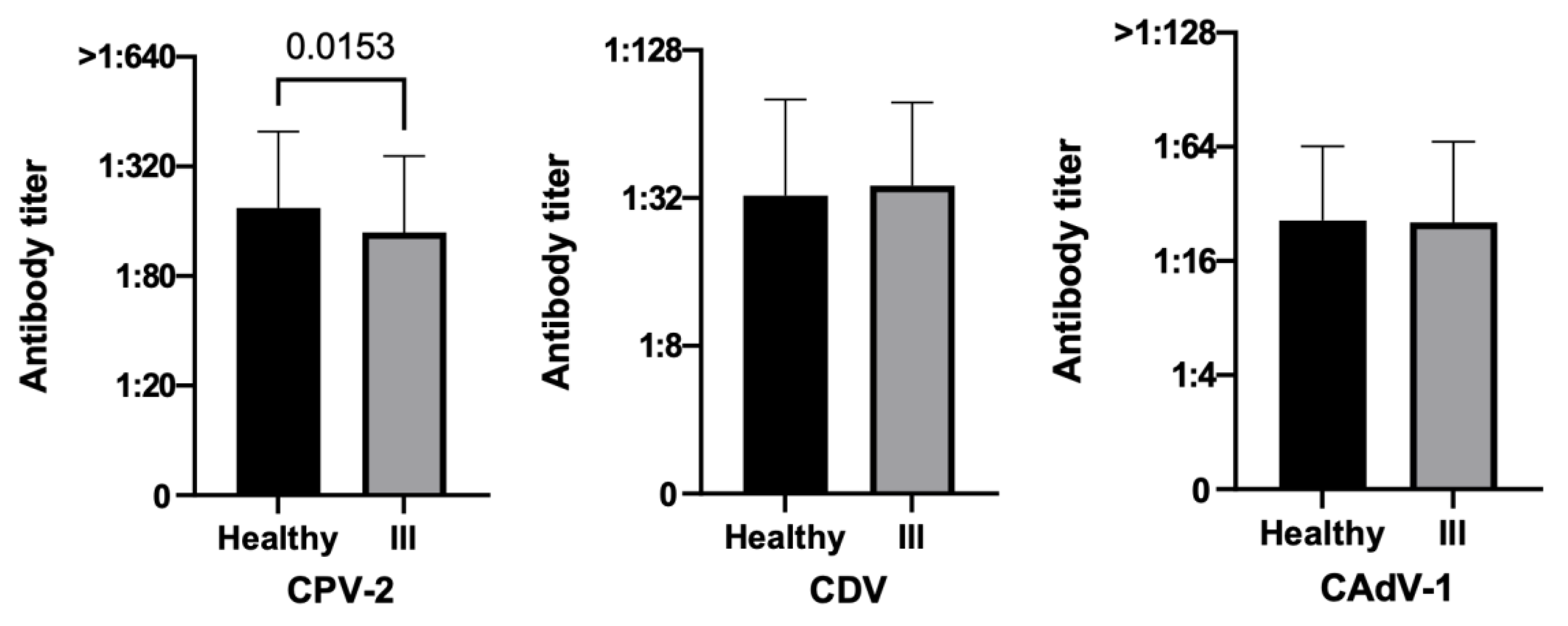
3.3.5. Time Elapsed since the Last Vaccination
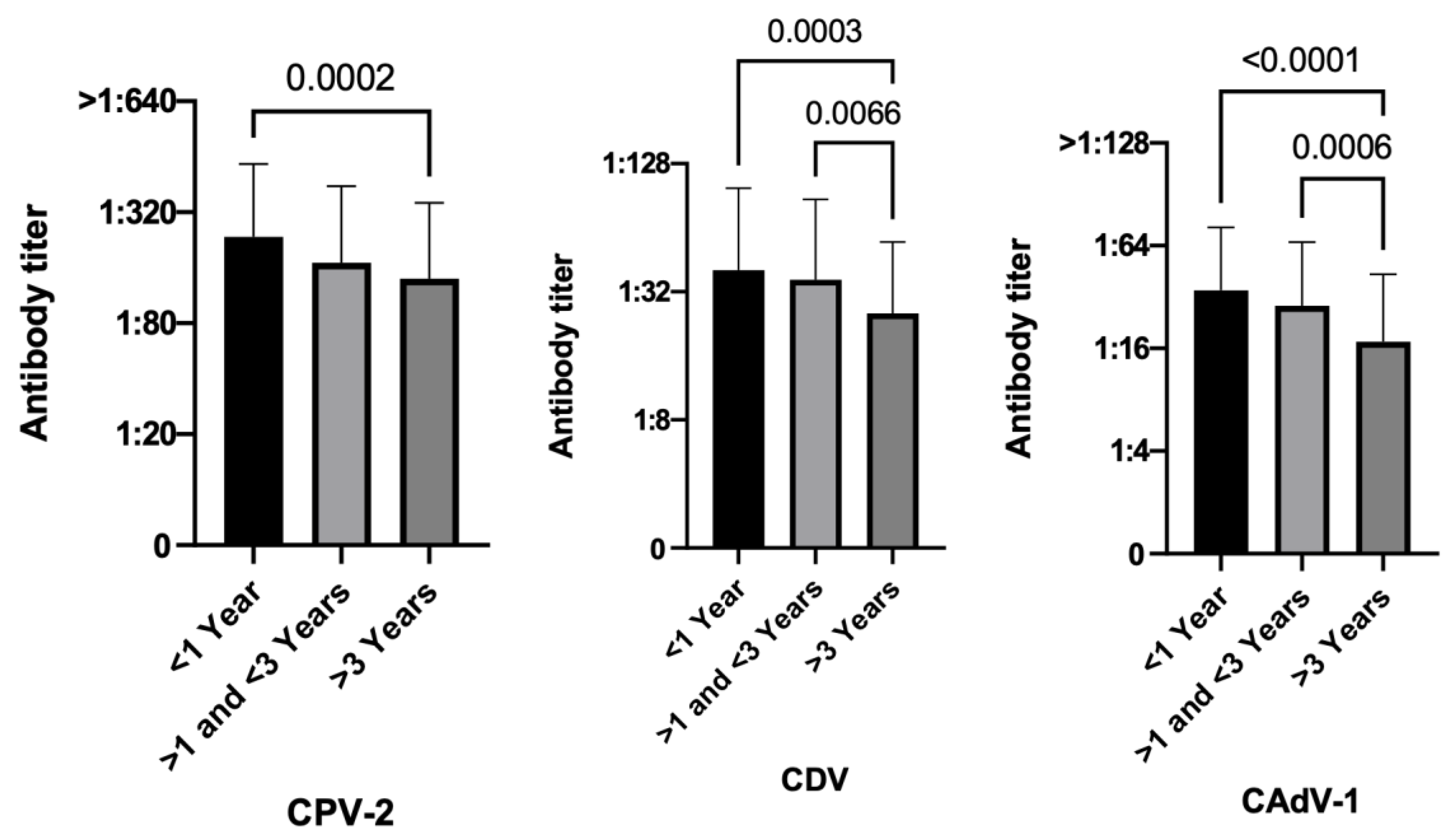
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dhaliwal, R.; Boynton, E.; Carrera-Justiz, S.; Cruise, N.; Gardner, M.; Huntingford, J.; Lobprise, H.; Rozanski, E. 2023 AAHA Senior Care Guidelines for Dogs and Cats. J. Am. Anim. Hosp. Assoc. 2023, 59, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Fortney, W.D. Implementing a Successful Senior/Geriatric Health Care Program for Veterinarians, Veterinary Technicians, and Office Managers. Vet. Clin. N. Am. Small Anim. Pract. 2012, 42, 823–834. [Google Scholar] [CrossRef] [PubMed]
- Creevy, K.E.; Grady, J.; Little, S.E.; Moore, G.E.; Strickler, B.G.; Thompson, S.; Webb, J.A. 2019 AAHA Canine Life Stage Guidelines. J. Am. Anim. Hosp. Assoc. 2019, 55, 267–290. [Google Scholar] [CrossRef]
- Market Research Pet Population and Ownership Trends in the U.S. 6th Edition. Available online: https://www.marketresearch.com/Packaged-Facts-v768/Pet-Population-Ownership-Trends-Edition-32315070/ (accessed on 15 April 2023).
- Sprinkle, D. Seniors’ Are a Growing Part of the Pet Population. Available online: https://globalpetindustry.com/article/seniors-are-growing-part-pet-population#:~:text=Over%20the%20past%20decade%2C%20the,as%20pets%20aged%207%2B (accessed on 15 April 2023).
- McKenzie, B.A.; Chen, F.; LaCroix-Fralish, M.L. The Phenotype of Aging in the Dog: How Aging Impacts the Health and Well-Being of Dogs and Their Caregivers. J. Am. Vet. Med. Assoc. 2022, 260, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Day, M.J. Ageing, Immunosenescence and Inflammageing in the Dog and Cat. J. Comp. Pathol. 2010, 142, S60–S69. [Google Scholar] [CrossRef]
- Eldredge, D.M.; Carlson, L.D.; Carlson, D.G.; Giffin, J.M.; Adelman, B. Comparative Age of Dogs and Humans. In Dog Owner’s Home Veterinary Handbook; Wiley Publishing Inc.: Hoboken, NJ, USA, 2007; pp. 575–576. [Google Scholar]
- Kraus, C.; Pavard, S.; Promislow, D.E.L. The Size–Life Span Trade-Off Decomposed: Why Large Dogs Die Young. Am. Nat. 2013, 181, 492–505. [Google Scholar] [CrossRef]
- Montoya, M.; Morrison, J.A.; Arrignon, F.; Spofford, N.; Charles, H.; Hours, M.-A.; Biourge, V. Life Expectancy Tables for Dogs and Cats Derived from Clinical Data. Front. Vet. Sci. 2023, 10, 1082102. [Google Scholar] [CrossRef]
- Miller, R.A.; Austad, S.N. Growth and Aging. In Handbook of the Biology of Aging; Elsevier: Amsterdam, The Netherlands, 2005; pp. 512–533. [Google Scholar]
- AAHA My Pet’s Physiological Age. Available online: https://www.aaha.org/globalassets/02-guidelines/canine-life-stage-2019/canine_and_feline_age_chart_poster.pdf (accessed on 16 April 2023).
- Metzger, F.L. How Old Is Your Pet in People Years? Your Pet’s Health Can Rapidly with Age. Available online: https://www.idexx.com/files/09-71400-02-PHNPostersCatandDog-L.pdf (accessed on 18 April 2023).
- Hayek, M.G. Interaction between Nutrition and Immune Response: Implication for Canine and Feline Health. In Proceedings of the Recent Advances in Canine and Feline Nutrition; IAMS Nutrition Symposium Proceedings-Vol. II, Ed.; Orange Frazer Press: Wilmington, OH, USA, 1998; pp. 499–512. [Google Scholar]
- Dall’Ara, P. Immune System and Ageing in the Dog: Possible Consequences and Control Strategies. Vet. Res. Commun. 2003, 27, 535–542. [Google Scholar] [CrossRef]
- HogenEsch, H.; Thompson, S. Effect of Ageing on the Immune Response of Dogs to Vaccines. J. Comp. Pathol. 2010, 142, S74–S77. [Google Scholar] [CrossRef]
- Ginaldi, L.; Loreto, M.F.; Corsi, M.P.; Modesti, M.; De Martinis, M. Immunosenescence and Infectious Diseases. Microbes Infect. 2001, 3, 851–857. [Google Scholar] [CrossRef]
- Čičin-Šain, L.; Smyk-Paerson, S.; Currier, N.; Byrd, L.; Koudelka, C.; Robinson, T.; Swarbrick, G.; Tackitt, S.; Legasse, A.; Fischer, M.; et al. Loss of Naive T Cells and Repertoire Constriction Predict Poor Response to Vaccination in Old Primates. J. Immunol. 2010, 184, 6739–6745. [Google Scholar] [CrossRef]
- Gruver, A.; Hudson, L.; Sempowski, G. Immunosenescence of Ageing. J. Pathol. 2007, 211, 144–156. [Google Scholar] [CrossRef]
- Franceschi, C.; Santoro, A.; Capri, M. The Complex Relationship between Immunosenescence and Inflammaging: Special Issue on the New Biomedical Perspectives. Semin. Immunopathol. 2020, 42, 517–520. [Google Scholar] [CrossRef]
- Suchard, M.S. Immunosenescence: Ageing of the Immune System. S. Afr. Pharm. J. 2015, 82, 28–31. [Google Scholar]
- Ellis, J.; Gow, S.; Rhodes, C.; Lacoste, S.; Kong, L.; Musil, K.; Snead, E. Article Serum Antibody Responses to Vaccinal Antigens in Lean and Obese Geriatric Dogs. Can. Vet. J. 2016, 57, 531. [Google Scholar]
- HogenEsch, H.; Thompson, S.; Dunham, A.; Ceddia, M.; Hayek, M. Effect of Age on Immune Parameters and the Immune Response of Dogs to Vaccines: A Cross-Sectional Study. Vet. Immunol. Immunopathol. 2004, 97, 77–85. [Google Scholar] [CrossRef]
- Schultz, R.D.; Thiel, B.; Mukhtar, E.; Sharp, P.; Larson, L.J. Age and Long-Term Protective Immunity in Dogs and Cats. J. Comp. Pathol. 2010, 142, S102–S108. [Google Scholar] [CrossRef]
- Blount, D.G.; Pritchard, D.I.; Heaton, P.R. Age-Related Alterations to Immune Parameters in Labrador Retriever Dogs. Vet. Immunol. Immunopathol. 2005, 108, 399–407. [Google Scholar] [CrossRef]
- Pereira, M.; Valério-Bolas, A.; Saraiva-Marques, C.; Alexandre-Pires, G.; Pereira da Fonseca, I.; Santos-Gomes, G. Development of Dog Immune System: From in Uterus to Elderly. Vet. Sci. 2019, 6, 83. [Google Scholar] [CrossRef]
- Fulop, T.; Larbi, A.; Dupuis, G.; Le Page, A.; Frost, E.H.; Cohen, A.A.; Witkowski, J.M.; Franceschi, C. Immunosenescence and Inflamm-Aging As Two Sides of the Same Coin: Friends or Foes? Front. Immunol. 2018, 8, 1960. [Google Scholar] [CrossRef]
- Holder, A.; Mella, S.; Palmer, D.B.; Aspinall, R.; Catchpole, B. An Age-Associated Decline in Thymic Output Differs in Dog Breeds According to Their Longevity. PLoS ONE 2016, 11, e0165968. [Google Scholar] [CrossRef] [PubMed]
- Kay, M.M.B. The Thymus: Clock for Immunologic Aging? J. Investig. Dermatol. 1979, 73, 29–38. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.-J.; Hong, S.-J.; Kim, S.-S.; Kwon, Y.-Y.; Choi, B.-H.; Choi, K.-M.; Sheen, S.-H.; Lee, M.-J.; Hwang, S.-Y.; Park, K.; et al. CD4+/CD8+ Ratio and Growth Differentiation Factor 8 Levels in Peripheral Blood of Large Canine Males Are Useful Parameters to Build an Age Prediction Model. World J. Mens. Health 2022, 40, 316. [Google Scholar] [CrossRef] [PubMed]
- Belizário, J.E.; Garay-Malpartida, M. Growth Hormone, Immunosenescence and Vaccination Failure in the Elderly. Clin. Immunol. Commun. 2023, 3, 51–57. [Google Scholar] [CrossRef]
- Horzinek, M.C. Vaccination Protocols for Companion Animals: The Veterinarian’s Perspective. J. Comp. Pathol. 2010, 142, S129–S132. [Google Scholar] [CrossRef]
- Day, M.J.; Horzinek, M.C.; Schultz, R.D.; Squires, R.A. WSAVA Guidelines for the Vaccination of Dogs and Cats. J. Small Anim. Pract. 2016, 57, E1–E45. [Google Scholar] [CrossRef]
- Ellis, J.; Marziani, E.; Aziz, C.; Brown, C.M.; Cohn, L.A.; Lea, C.; Moore, G.E.; Taneja, N. 2022 AAHA Canine Vaccination Guidelines. J. Am. Anim. Hosp. Assoc. 2022, 58, 213–230. [Google Scholar] [CrossRef]
- Australian Veterinary Association (AVA) Vaccination of Dogs and Cats. Available online: https://www.ava.com.au/policy-advocacy/policies/companion-animals-health/vaccination-of-dogs-and-cats/ (accessed on 16 January 2023).
- Joint American Veterinary Medical Association (AVMA)-Federation of Veterinarians of Europe (FVE)-Canadian Veterinary Medical Association (CVMA) Statement on the Benefits of Animal Vaccination Programs in Advancing Animal and Human Health. Available online: https://fve.org/cms/wp-content/uploads/AVMA-CVMA-FVE_vacconation_joint-paper.docx.pdf (accessed on 18 February 2023).
- British Veterinary Association (BVA) Getting Your Pet Vaccinated. Available online: https://www.bva.co.uk/media/2649/client_leaflet_9_-_getting_your_pet_vaccinated.pdf (accessed on 4 January 2023).
- Dall’Ara, P. Vaccini e Vaccinazioni degli Animali da Compagnia, 1st ed.; Dall’Ara, P., Ed.; EDRA: Milano, Italy, 2020. [Google Scholar]
- Decaro, N.; Cirone, F.; Desario, C.; Elia, G.; Lorusso, E.; Colaianni, M.L.; Martella, V.; Buonavoglia, C. Severe Parvovirus in a 12-Year-Old Dog That Had Been Repeatedly Vaccinated. Vet. Rec. 2009, 164, 593–595. [Google Scholar] [CrossRef]
- Rossato, C.K.; Martins, D.B. Hepatite Infecciosa Canina Em Um Cão Geriátrico Naturalmente Infectado. Acta Sci. Vet. 2015, 43, 105–108. [Google Scholar]
- Amude, A.M.; Alfieri, A.A.; Arias, M.V.B.; Alfieri, A.F. Clinical Syndromes of Nervous Distemper in Dogs Initially Presented without Conventional Evidences of CDV Infection. Semin. Cienc. Agrar. 2012, 33, 2347–2358. [Google Scholar] [CrossRef]
- Metzger, F.L. Senior and Geriatric Care Programs for Veterinarians. Vet. Clin. N. Am. Small Anim. Pract. 2005, 35, 743–753. [Google Scholar] [CrossRef]
- Gardner, M.; McVety, D. Caring for Geriatric Pets in Your Pracrice. Today’s Vet. Pract. 2016, 6, 103–108. [Google Scholar]
- Davies, M. Focusing on Geriatric Pets. Practice 2016, 38, 39–42. [Google Scholar] [CrossRef]
- Dall’Ara, P.; Lauzi, S.; Filipe, J.; Caseri, R.; Beccaglia, M.; Desario, C.; Cavalli, A.; Aiudi, G.G.; Buonavoglia, C.; Decaro, N. Discrepancy Between In-Clinic and Haemagglutination-Inhibition Tests in Detecting Maternally-Derived Antibodies Against Canine Parvovirus in Puppies. Front. Vet. Sci. 2021, 8, 630809. [Google Scholar] [CrossRef]
- Day, M.J. Small Animal Vaccination: A Practical Guide for Vets in the UK. Practice 2017, 39, 110–118. [Google Scholar] [CrossRef]
- Waner, T.; Mazar, S.; Keren-Kornblatt, E. Application of a Dot Enzyme-Linked Immunosorbent Assay for Evaluation of the Immune to Canine Parvovirus and Distemper Virus in Adult Dogs before Revaccination. J. Vet. Diagn. Investig. 2006, 18, 267–270. [Google Scholar] [CrossRef]
- Salomon, K.; de Lange, T.; Calis, A.; Radier, O.; Krosse, J. In-Clinic Canine IgG Antibody Titer Test Comparative Study: Results from Five Clinics. Isr. J. Vet. Med. 2022, 77, 7–14. [Google Scholar]
- Meazzi, S.; Filipe, J.; Fiore, A.; Di Bella, S.; Mira, F.; Dall’Ara, P. Agreement between In-Clinics and Virus Neutralization Tests in Detecting Antibodies against Canine Distemper Virus (CDV). Viruses 2022, 14, 517. [Google Scholar] [CrossRef]
- Dall’Ara, P.; Lauzi, S.; Zambarbieri, J.; Servida, F.; Barbieri, L.; Rosenthal, R.; Turin, L.; Scarparo, E.; Filipe, J. Prevalence of Serum Antibody Titers against Core Vaccine Antigens in Italian Dogs. Life 2023, 13, 587. [Google Scholar] [CrossRef]
- Killey, R.; Mynors, C.; Pearce, R.; Nell, A.; Prentis, A.; Day, M.J. Long-Lived Immunity to Canine Core Vaccine Antigens in UK Dogs as Assessed by an in-Practice Test Kit. J. Small Anim. Pract. 2018, 59, 27–31. [Google Scholar] [CrossRef]
- DiGangi, B.A.; Dingman, P.A.; Grijalva, C.J.; Belyeu, M.; Tucker, S.; Isaza, R. Prevalence and Risk Factors for the Presence of Serum Antibodies against Canine Distemper, Canine Parvovirus, and Canine Adenovirus in Communities in Mainland Ecuador. Vet. Immunol. Immunopathol. 2019, 218, 109933. [Google Scholar] [CrossRef] [PubMed]
- Dodds, J. Vaccination and Antibody Tests: 2018 Update. Available online: https://www.vaccicheck.nl/sites/default/files/documents/serology_testing_clinical_review_dr_dodds_march_2018.pdf (accessed on 8 January 2023).
- den Besten, R. An Analysis of Titer Testing as Part of the Vaccination Guideline for Dogs. Master of Veterinary Medicine, Utrecht University, Faculty of Veterinary Medicine, Utrecht, The Netherlands, 2018. [Google Scholar]
- Kim, S.-G.; Kang, M.-H.; Park, H.-M. Comparative Study of Two Point-of-Care Enzyme-Linked Immunosorbent Assays for the Detection of Antibodies against Canine Parvovirus and Canine Distemper Virus. Pak. Vet. J. 2017, 37, 405–410. [Google Scholar]
- Gray, L.K.; Crawford, P.C.; Levy, J.K.; Dubovi, E.J. Comparison of Two Assays for Detection of Antibodies against Canine Parvovirus and Canine Distemper Virus in Dogs Admitted to a Florida Animal Shelter. JAVMA 2012, 9, 1084–1087. [Google Scholar] [CrossRef] [PubMed]
- Egerer, A.; Schaefer, Z.; Larson, L. A Point-of-Care Dot Blot ELISA Assay for Detection of Protective Antibody against Canine Adenovirus, Canine Parvovirus, and Canine Distemper Virus Is Diagnostically Accurate. J. Am. Vet. Med. Assoc. 2022, 260, 1928–1933. [Google Scholar] [CrossRef] [PubMed]
- Riedl, M.; Truyen, U.; Reese, S.; Hartmann, K. Prevalence of Antibodies to Canine Parvovirus and Reaction to Vaccination in Client-Owned, Healthy Dogs. Vet. Rec. 2015, 177, 597. [Google Scholar] [CrossRef]
- Decaro, N.; Buonavoglia, C.; Barrs, V.R. Canine Parvovirus Vaccination and Immunisation Failures: Are We Far from Disease Eradication? Vet. Microbiol. 2020, 247, 108760. [Google Scholar] [CrossRef]
- Carrai, M.; Decaro, N.; Van Brussel, K.; Dall’Ara, P.; Desario, C.; Fracasso, M.; Šlapeta, J.; Colombo, E.; Bo, S.; Beatty, J.A.; et al. Canine Parvovirus Is Shed Infrequently by Cats without Diarrhoea in Multi-Cat Environments. Vet. Microbiol. 2021, 261, 109204. [Google Scholar] [CrossRef]
- Clegg, S.R.; Coyne, K.P.; Dawson, S.; Spibey, N.; Gaskell, R.M.; Radford, A.D. Canine Parvovirus in Asymptomatic Feline Carriers. Vet. Microbiol. 2012, 157, 78–85. [Google Scholar] [CrossRef]
- Decaro, N.; Buonavoglia, D.; Desario, C.; Amorisco, F.; Colaianni, M.L.; Parisi, A.; Terio, V.; Elia, G.; Lucente, M.S.; Cavalli, A.; et al. Characterisation of Canine Parvovirus Strains Isolated from Cats with Feline Panleukopenia. Res. Vet. Sci. 2010, 89, 275–278. [Google Scholar] [CrossRef]
- Greene, C.E. Infectious Canine Hepatitis and Canine Acidophil Cell Hepatitis. In Infectious Diseases of the Dog and Cat; Greene, C.E., Ed.; Saunders Elsevier: St. Louis, MO, USA, 2012; pp. 42–48. [Google Scholar]
- Decaro, N.; Campolo, M.; Elia, G.; Buonavoglia, D.; Colaianni, M.L.; Lorusso, A.; Mari, V.; Buonavoglia, C. Infectious Canine Hepatitis: An “Old” Disease Reemerging in Italy. Res. Vet. Sci. 2007, 83, 269–273. [Google Scholar] [CrossRef]
- Mira, F.; Puleio, R.; Schirò, G.; Condorelli, L.; Di Bella, S.; Chiaramonte, G.; Purpari, G.; Cannella, V.; Balboni, A.; Randazzo, V.; et al. Study on the Canine Adenovirus Type 1 (CAdV-1) Infection in Domestic Dogs in Southern Italy. Pathogens 2022, 11, 1254. [Google Scholar] [CrossRef]
- Dall’Ara, P.; Turin, L. Immunology of the Canine Eye in Health and Disease: A Concise Review. Vet. Med. 2019, 64, 1–17. [Google Scholar] [CrossRef]
- Dall’Ara, P. Vaccini e Vaccinazioni Core del Cane. In Vaccini e Vaccinazioni degli Animali da Compagnia; Dall’Ara, P., Ed.; EDRA: Milano, Italy, 2020; pp. 153–174. [Google Scholar]
- Lechner, E.S.; Crawford, P.C.; Levy, J.K.; Edinboro, C.H.; Dubovi, E.J.; Caligiuri, R. Prevalence of Protective Antibody Titers for Canine Distemper Virus and Canine Parvovirus in Dogs Entering a Florida Animal Shelter. J. Am. Vet. Med. Assoc. 2010, 236, 1317–1321. [Google Scholar] [CrossRef]
- Parrish, C.R.; Sykes, J.E. Canine Parvovirus Infections and Other Viral Enteritides. In Greene’s Infectious Diseases of the Dog and Cat; Sikes, J.E., Ed.; Elsevier: St. Louis, MO, USA, 2022; pp. 341–351. [Google Scholar]
- Greene, C.E.; Decaro, N. Canine Viral Enteritis. In Infectious Diseases of the Dog and Cat; Greene, C.E., Ed.; Elsevier: St Louis, MO, USA, 2012; pp. 67–80. [Google Scholar]
- Schultz, R.D. Duration of Immunity for Canine and Feline Vaccines: A Review. Vet. Microbiol. 2006, 117, 75–79. [Google Scholar] [CrossRef]
- Wallace, R.M.; Pees, A.; Blanton, J.B.; Moore, S.M. Risk Factors for Inadequate Antibody Response to Primary Rabies Vaccination in Dogs under One Year of Age. PLoS Negl. Trop. Dis. 2017, 11, e0005761. [Google Scholar] [CrossRef]
- Kennedy, L.J.; Lunt, M.; Barnes, A.; McElhinney, L.; Fooks, A.R.; Baxter, D.N.; Ollier, W.E.R. Factors Influencing the Antibody Response of Dogs Vaccinated against Rabies. Vaccine 2007, 25, 8500–8507. [Google Scholar] [CrossRef]
- Mende, K.; Stuetzer, B.; Sauter-Louis, C.; Homeier, T.; Truyen, U.; Hartmann, K. Prevalence of Antibodies against Feline Panleukopenia Virus in Client-Owned Cats in Southern Germany. Vet. J. 2014, 199, 419–423. [Google Scholar] [CrossRef]
- Clegg, S.R.; Coyne, K.P.; Parker, J.; Dawson, S.; Godsall, S.A.; Pinchbeck, G.; Cripps, P.J.; Gaskell, R.M.; Radford, A.D. Molecular Epidemiology and Phylogeny Reveal Complex Spatial Dynamics in Areas Where Canine Parvovirus Is Endemic. J. Virol. 2011, 85, 7892–7899. [Google Scholar] [CrossRef]
- Hussain, K.; Khan, Y.; Ullah, Q.; Rabbani, A.H.; Naseer, O.; Raza, A.; Shahid, M.; Ali, S.; Ali, A. Canine Parvo Virus: A Review on Current Perspectives in Seroprevalence, Diagnostics and Therapeutics. Glob. Vet. 2021, 23, 113–126. [Google Scholar]
- Decaro, N.; Buonavoglia, C. Canine Parvovirus—A Review of Epidemiological and Diagnostic Aspects, with Emphasis on Type 2c. Vet. Microbiol. 2012, 155, 1–12. [Google Scholar] [CrossRef]
- Diakoudi, G.; Desario, C.; Lanave, G.; Salucci, S.; Ndiana, L.A.; Zarea, A.A.K.; Fouad, E.A.; Lorusso, A.; Alfano, F.; Cavalli, A.; et al. Feline Panleukopenia Virus in Dogs from Italy and Egypt. Emerg. Infect. Dis. 2022, 28, 1933–1935. [Google Scholar] [CrossRef] [PubMed]
- Stuetzer, B.; Hartmann, K. Feline Parvovirus Infection and Associated Diseases. Vet. J. 2014, 201, 150–155. [Google Scholar] [CrossRef] [PubMed]
- Big Dog Mom Large Breed Dogs Are NOT Right for Everyone–Are You a Good Fit? Available online: https://bigdogmom.com/big-dog-ownership/ (accessed on 6 May 2023).
- Slater, M.R.; Di Nardo, A.; Pediconi, O.; Villa, P.D.; Candeloro, L.; Alessandrini, B.; Del Papa, S. Cat and Dog Ownership and Management Patterns in Central Italy. Prev. Vet. Med. 2008, 85, 267–294. [Google Scholar] [CrossRef] [PubMed]
- Harris, M.B. Some Factors Influencing Selection and Naming of Pets. Psychol. Rep. 1983, 53, 1163–1170. [Google Scholar] [CrossRef]
- Twark, L.; Dodds, W.J. Clinical Use of Serum Parvovirus and Distemper Virus Antibody Titers for Determining Revaccination Strategies in Healthy Dogs. J. Am. Vet. Med. Assoc. 2000, 217, 1021–1024. [Google Scholar] [CrossRef]
| DOGS’ PHYSIOLOGICAL AGES IN HUMAN YEARS | ||||
|---|---|---|---|---|
| DOGS’ SIZES | ||||
| AGE (Years) | ≤9 kg | 10–22 kg | 23–41 kg | >41 kg |
| 5 | -- | -- | -- | 42 |
| 6 | -- | -- | 45 | 49 |
| 7 | 44 | 47 | 50 | 56 |
| 8 | 48 | 51 | 55 | 64 |
| 9 | 52 | 56 | 61 | 71 |
| 10 | 56 | 60 | 66 | 78 |
| 11 | 60 | 65 | 72 | 86 |
| 12 | 64 | 69 | 77 | 93 |
| 13 | 68 | 74 | 82 | 101 |
| 14 | 72 | 78 | 88 | 108 |
| 15 | 76 | 83 | 93 | 115 |
| 16 | 80 | 87 | 99 | 123 |
| 17 | 84 | 92 | 104 | -- |
| 18 | 88 | 96 | 109 | -- |
| 19 | 92 | 101 | 115 | -- |
| 20 | 96 | 105 | 120 | -- |
| Protective Antibody Titers (PATs)—% (n. of Dogs) | |||
|---|---|---|---|
| CPV-2 | CDV | CAdV-1 | |
| Threshold values | 1:80 | 1:32 | 1:16 |
| Protected dogs (with PATs) | 88.6 (310/350) | 66.0 (231/350) | 82.3 (288/350) |
| Unprotected dogs (under PATs) | 11.4 (40/350) | 34.0 (119/350) | 17.7 (62/350) |
| Age | |||
| Seniors (258) | 90.3 (233/258) | 69.4 (179/258) | 82.9 (214/258) |
| Geriatrics (92) | 83.7 (77/92) | 56.5 (52/92) | 80.4 (74/92) |
| Size | |||
| Small (<10 kg) (124) | 84.7 (105/124) | 61.3 (76/124) | 80.6 (100/124) |
| Medium (≥10–≤25 kg) (123) | 87.8 (108/123) | 65.0 (80/123) | 78.9 (97/123) |
| Large (>25 kg) (103) | 94.2 (97/103) | 72.8 (75/103) | 88.3 (91/103) |
| Sex and reproductive status | |||
| Intact females (89) | 86.5 (77/89) | 58.4 (52/89) | 68.5 (61/89) |
| Neutered females (95) | 87.4 (83/95) | 67.4 (64/95) | 86.3 (82/95) |
| Intact males (132) | 90.2 (119/132) | 69.7 (92/132) | 87.9 (116/132) |
| Neutered males (34) | 91.2 (31/34) | 67.6 (23/34) | 85.3 (29/34) |
| Health status | |||
| Healthy (260) | 90.4 (235/260) | 64.2 (167/260) | 82.3 (214/260) |
| Unhealthy (90) | 83.3 (75/90) | 71.1 (64/90) | 82.2 (74/90) |
| Time after vaccination * | |||
| ≤1 year (115) | 92.2 (106/115) | 76.5 (88/115) | 90.4 (104/115) |
| >1–≤3 years (134) | 88.8 (119/134) | 70.1 (94/134) | 90.3 (121/134) |
| >3 years (92) | 87.0 (80/92) | 52.2 (48/92) | 66.3 (61/92) |
| CPV-2 | CDV | CAdV-1 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| PROTECTED | p-Value | PROTECTED | p-Value | PROTECTED | p-Value | |||||
| Statistical Variable (Number) | YES | NO | YES | NO | YES | NO | ||||
| Age | Seniors (258) | 90.3% (233) | 9.7% (25) | 0.0869 | 69.4% (179) | 30.6% (79) | 0.0254 | 82.9% (214) | 17.1% (44) | 0.5881 |
| Geriatrics (92) | 83.7% (77) | 16.3% (15) | 56.5% (52) | 43.5% (40) | 80.4% (74) | 19.6% (18) | ||||
| Size | Small (124) | 84.7% (105) | 15.3% (19) | 0.0772 | 61.3% (76) | 38.7% (48) | 0.1819 | 80.6% (100) | 19.4% (24) | 0.1483 |
| Medium (123) | 87.8% (108) | 12.2% (15) | 65.0% (80) | 35.0% (43) | 78.9% (97) | 21.1% (26) | ||||
| Large (103) | 94.2% (97) | 5.8% (6) | 72.8% (75) | 27.2% (28) | 88.3% (91) | 11.7% (12) | ||||
| Sex | Intact F (89) | 86.5% (77) | 13.5% (12) | 0.7866 | 58.4% (52) | 41.6% (37) | 0.3620 | 68.5% (61) | 31.5% (28) | 0.0013 |
| Neutered F (95) | 87.4% (83) | 12.6% (12) | 67.4% (64) | 32.7% (31) | 86.3% (82) | 13.7% (13) | ||||
| Intact M (132) | 90.2% (119) | 9.8% (13) | 69.7% (92) | 30.3% (40) | 87.9% (116) | 12.1% (16) | ||||
| Neutered M (34) | 91.2% (31) | 8.8% (3) | 67.6% (23) | 32.4% (11) | 85.3% (29) | 14.7% (5) | ||||
| Health status | Healthy (260) | 90.4% (235) | 9.6% (25) | 0.0835 | 64.2% (167) | 35.8% (93) | 0.2482 | 82.3% (214) | 17.7% (46) | 0.9999 |
| Unhealthy (90) | 83.3% (75) | 16.7% (15) | 71.1% (64) | 28.9% (26) | 82.2% (74) | 17.8% (16) | ||||
| Time after vaccination | ≤1 year (115) | 92.2% (106) | 7.8% (9) | 0.4565 | 76.5% (88) | 23.5% (27) | 0.0007 | 90.4% (104) | 9.6% (11) | <0.0001 |
| >1–≤3 years (134) | 88.8% (119) | 11.2% (15) | 70.1% (94) | 29.9% (40) | 90.3% (121) | 9.7% (13) | ||||
| >3 years (92) | 87.0% (80) | 13.0% (12) | 52.2% (48) | 47.8% (44) | 66.3% (61) | 33.7% (31) | ||||
| SENIORS | |||||||
|---|---|---|---|---|---|---|---|
| Age (Years) | Breed | Size | Sex | Health | CPV-2 1:80 | CDV 1:32 | CAdV-1 1:16 |
| 8 | Beagle | Medium | F | Unhealthy | 40 | 16 | 8 |
| 9 | American Bulldog | Large | F | Healthy | 160 | 16 | 8 |
| 8 | Crossbred | Small | NM | Unhealthy | 80 | 16 | 8 |
| 10.5 | Crossbred | Small | F | Healthy | 320 | 8 | 8 |
| GERIATRICS | |||||||
| Age (Years) | Breed | Size | Sex | Health | CPV-2 1:80 | CDV 1:32 | CAdV-1 1:16 |
| 12 | Crossbred | Medium | M | Healthy | 160 | 16 | 8 |
| 15 | Cocker Spaniel | Medium | NF | Healthy | 40 | 8 | 4 |
| 16 | Jack Russell | Small | NF | Unhealthy | 80 | 16 | 16 |
| 15 | Crossbred | Medium | M | Unhealthy | 40 | 32 | 16 |
| 14.5 | Crossbred | Small | F | Healthy | 0 | 8 | 4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dall’Ara, P.; Lauzi, S.; Turin, L.; Castaldelli, G.; Servida, F.; Filipe, J. Effect of Aging on the Immune Response to Core Vaccines in Senior and Geriatric Dogs. Vet. Sci. 2023, 10, 412. https://doi.org/10.3390/vetsci10070412
Dall’Ara P, Lauzi S, Turin L, Castaldelli G, Servida F, Filipe J. Effect of Aging on the Immune Response to Core Vaccines in Senior and Geriatric Dogs. Veterinary Sciences. 2023; 10(7):412. https://doi.org/10.3390/vetsci10070412
Chicago/Turabian StyleDall’Ara, Paola, Stefania Lauzi, Lauretta Turin, Giulia Castaldelli, Francesco Servida, and Joel Filipe. 2023. "Effect of Aging on the Immune Response to Core Vaccines in Senior and Geriatric Dogs" Veterinary Sciences 10, no. 7: 412. https://doi.org/10.3390/vetsci10070412
APA StyleDall’Ara, P., Lauzi, S., Turin, L., Castaldelli, G., Servida, F., & Filipe, J. (2023). Effect of Aging on the Immune Response to Core Vaccines in Senior and Geriatric Dogs. Veterinary Sciences, 10(7), 412. https://doi.org/10.3390/vetsci10070412








