Meta-Analysis on the Global Prevalence of Tetracycline Resistance in Escherichia coli Isolated from Beef Cattle
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Search Strategy
2.2. Inclusion and Exclusion Criteria
2.3. Data Extraction and Quality Assessment
2.4. Outcomes and Variables
2.5. Meta-Analysis
3. Results
3.1. Study Selection
3.2. Study Characteristics
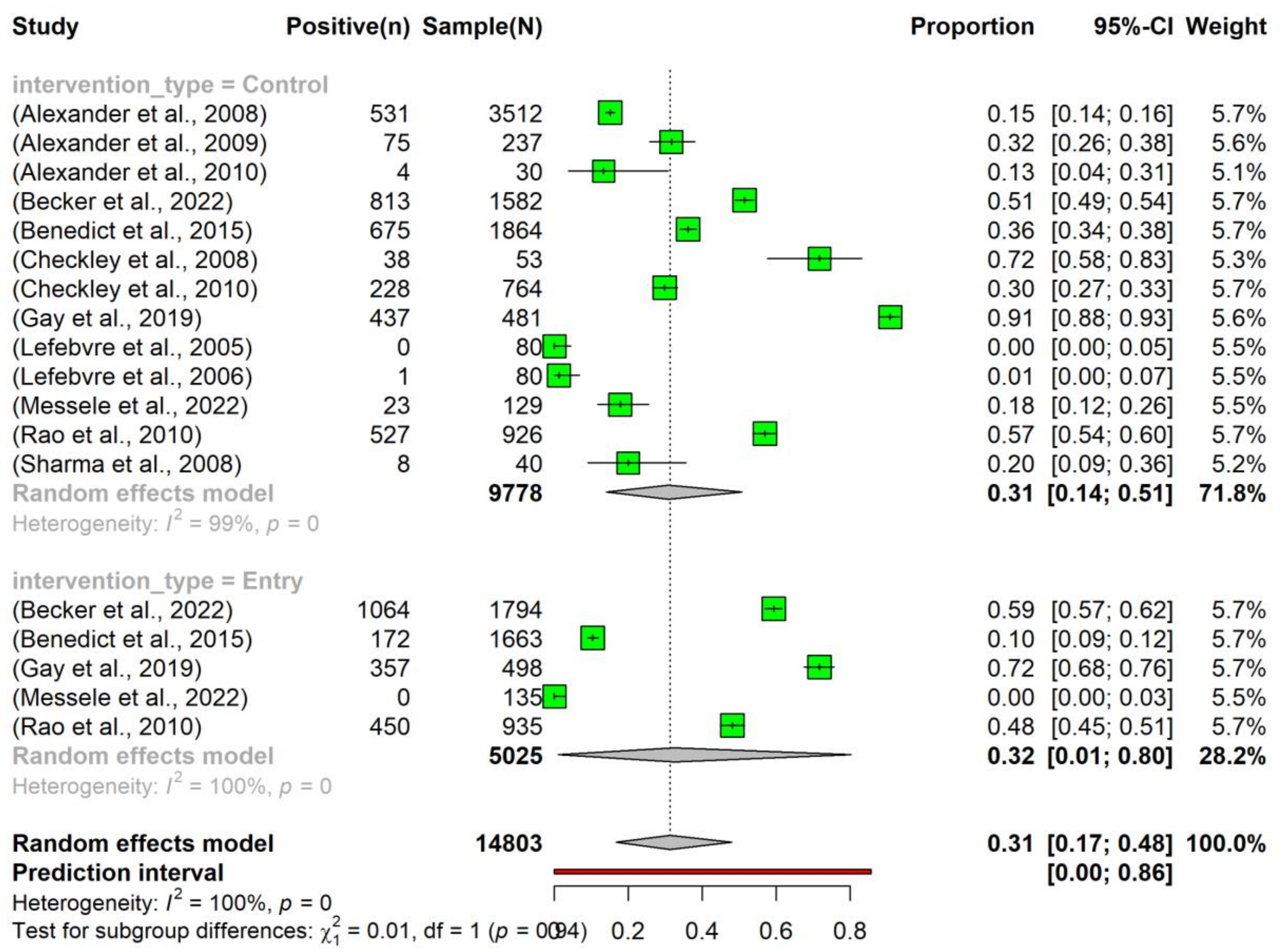
3.3. Prevalence of Tetracycline Resistance in Escherichia coli Isolated from Beef Cattle without Intervention
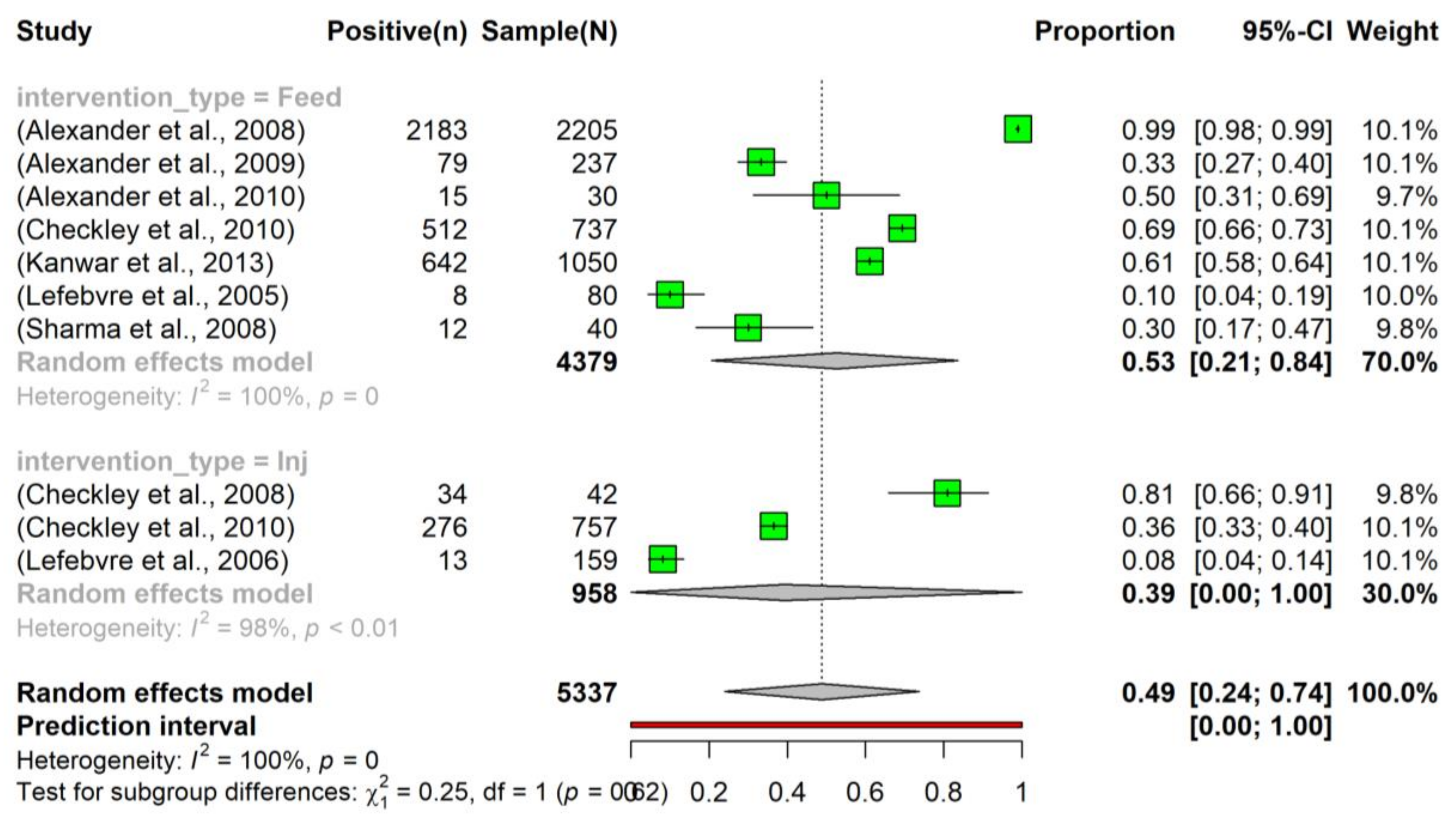
3.4. Prevalence of Tetracycline Resistance in Escherichia coli Isolated from Beef Cattle after Intervention
3.5. Heterogeneity and Subgroup Analysis
3.6. Sensitivity and Publication Bias Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kasimanickam, V.; Kasimanickam, M.; Kasimanickam, R. Antibiotics Use in Food Animal Production: Escalation of Antimicrobial Resistance: Where Are We Now in Combating AMR? Med. Sci. 2021, 9, 14. [Google Scholar] [CrossRef] [PubMed]
- Nicola, I.; Cerutti, F.; Grego, E.; Bertone, I.; Gianella, P.; D’Angelo, A.; Peletto, S.; Bellino, C. Characterization of the upper and lower respiratory tract microbiota in Piedmontese calves. Microbiome 2017, 5, 152. [Google Scholar] [CrossRef] [PubMed]
- Snowder, G.D.; Van Vleck, L.D.; Cundiff, L.V.; Bennett, G.L. Bovine respiratory disease in feedlot cattle: Environmental, genetic, and economic factors. J. Anim. Sci. 2006, 84, 1999–2008. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Noyes, N.R.; Benedict, K.M.; Gow, S.P.; Booker, C.W.; Hannon, S.J.; McAllister, T.A.; Morley, P.S. Mannheimia haemolytica in feedlot cattle: Prevalence of recovery and associations with antimicrobial use, resistance, and health outcomes. J. Vet. Intern. Med. 2015, 29, 705–713. [Google Scholar] [CrossRef] [PubMed]
- Maples, W.E.; Brorsen, B.W.; Peel, D.; Hicks, B. Observational study of the effect of metaphylaxis treatment on feedlot cattle productivity and health. Front. Vet. Sci. 2022, 9, 947585. [Google Scholar] [CrossRef]
- Urban-Chmiel, R.; Grooms, D. Prevention and control of bovine respiratory disease. J. Livest. Sci. 2012, 3, 27–36. [Google Scholar]
- Ives, S.E.; Richeson, J.T. Use of antimicrobial metaphylaxis for the control of bovine respiratory disease in high-risk cattle. Vet. Clin. Food Anim. Pract. 2015, 31, 341–350. [Google Scholar] [CrossRef]
- Nickell, J.S.; White, B.J. Metaphylactic antimicrobial therapy for bovine respiratory disease in stocker and feedlot cattle. Vet. Clin. Food Anim. Pract. 2010, 26, 285–301. [Google Scholar] [CrossRef]
- Horton, L.M.; Depenbusch, B.E.; Dewsbury, D.M.; McAtee, T.B.; Betts, N.B.; Renter, D.G. Comprehensive outcomes affected by antimicrobial metaphylaxis of Feedlot Calves at Medium-Risk for bovine respiratory disease from a randomized controlled trial. Vet. Sci. 2023, 10, 67. [Google Scholar] [CrossRef]
- Granados-Chinchilla, F.; Rodriguez, C. Tetracyclines in Food and Feedingstuffs: From Regulation to Analytical Methods, Bacterial Resistance, and Environmental and Health Implications. J. Anal. Methods Chem. 2017, 2017, 1315497. [Google Scholar] [CrossRef]
- Masse, D.I.; Saady, N.M.; Gilbert, Y. Potential of Biological Processes to Eliminate Antibiotics in Livestock Manure: An Overview. Animals 2014, 4, 146–163. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Andersson, D.I.; Hughes, D. Microbiological effects of sublethal levels of antibiotics. Nat. Rev. Microbiol. 2014, 12, 465–478. [Google Scholar] [CrossRef] [PubMed]
- Samtiya, M.; Matthews, K.R.; Dhewa, T.; Puniya, A.K. Antimicrobial Resistance in the Food Chain: Trends, Mechanisms, Pathways, and Possible Regulation Strategies. Foods 2022, 11, 2966. [Google Scholar] [CrossRef]
- Gallo, G.F.; Berg, J.L. Efficacy of a feed-additive antibacterial combination for improving feedlot cattle performance and health. Can. Vet. J. 1995, 36, 223–229. [Google Scholar]
- Serwecińska, L. Antimicrobials and Antibiotic-Resistant Bacteria: A Risk to the Environment and to Public Health. Water 2020, 12, 3313. [Google Scholar] [CrossRef]
- Prestinaci, F.; Pezzotti, P.; Pantosti, A. Antimicrobial resistance: A global multifaceted phenomenon. Pathog. Glob. Health 2015, 109, 309–318. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. Int. J. Surg. 2021, 88, 105906. [Google Scholar] [CrossRef]
- Viechtbauer, W. Conducting Meta-Analyses inRwith themetaforPackage. J. Stat. Softw. 2010, 36, 25. [Google Scholar] [CrossRef] [Green Version]
- Olkin, I.; Dahabreh, I.J.; Trikalinos, T.A. GOSH—A graphical display of study heterogeneity. Res. Synth. Methods 2012, 3, 214–223. [Google Scholar] [CrossRef]
- Ester, M.; Kriegel, H.-P.; Sander, J.; Xu, X. A density-based algorithm for discovering clusters in large spatial databases with noise. In Proceedings of the KDD, Portland, OR, USA, 2–4 August 1996; pp. 226–231. [Google Scholar]
- Viechtbauer, W.; Cheung, M.W. Outlier and influence diagnostics for meta-analysis. Res. Synth. Methods 2010, 1, 112–125. [Google Scholar] [CrossRef]
- Peters, J.L.; Sutton, A.J.; Jones, D.R.; Abrams, K.R.; Rushton, L. Comparison of two methods to detect publication bias in meta-analysis. JAMA 2006, 295, 676–680. [Google Scholar] [CrossRef] [Green Version]
- Sterne, J.A.; Egger, M.; Moher, D. Addressing reporting biases. In Cochrane Handbook for Systematic Reviews of Interventions; Cochrane Book Series; John Wiley & Sons: Chichester, UK, 2008; pp. 297–333. [Google Scholar]
- Alexander, T.W.; Inglis, G.D.; Yanke, L.J.; Topp, E.; Read, R.R.; Reuter, T.; McAllister, T.A. Farm-to-fork characterization of Escherichia coli associated with feedlot cattle with a known history of antimicrobial use. Int. J. Food Microbiol. 2010, 137, 40–48. [Google Scholar] [CrossRef]
- Alexander, T.W.; Reuter, T.; Sharma, R.; Yanke, L.J.; Topp, E.; McAllister, T.A. Longitudinal characterization of resistant Escherichia coli in fecal deposits from cattle fed subtherapeutic levels of antimicrobials. Appl. Environ. Microbiol. 2009, 75, 7125–7134. [Google Scholar] [CrossRef] [Green Version]
- Alexander, T.W.; Yanke, L.J.; Topp, E.; Olson, M.E.; Read, R.R.; Morck, D.W.; McAllister, T.A. Effect of subtherapeutic administration of antibiotics on the prevalence of antibiotic-resistant Escherichia coli bacteria in feedlot cattle. Appl. Environ. Microbiol. 2008, 74, 4405–4416. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Checkley, S.L.; Campbell, J.R.; Chirino-Trejo, M.; Janzen, E.D.; McKinnon, J.J. Antimicrobial resistance in generic fecal Escherichia coli obtained from beef cattle on arrival at the feedlot and prior to slaughter, and associations with volume of total individual cattle antimicrobial treatments in one western Canadian feedlot. Can. J. Vet. Res. 2008, 72, 101–108. [Google Scholar] [PubMed]
- Checkley, S.L.; Campbell, J.R.; Chirino-Trejo, M.; Janzen, E.D.; Waldner, C.L. Associations between antimicrobial use and the prevalence of antimicrobial resistance in fecal Escherichia coli from feedlot cattle in western Canada. Can. Vet. J. 2010, 51, 853–861. [Google Scholar]
- Lefebvre, B.; Diarra, M.S.; Giguere, K.; Roy, G.; Michaud, S.; Malouin, F. Antibiotic resistance and hypermutability of Escherichia coli O157 from feedlot cattle treated with growth-promoting agents. J. Food Prot. 2005, 68, 2411–2419. [Google Scholar] [CrossRef]
- Sharma, R.; Munns, K.; Alexander, T.; Entz, T.; Mirzaagha, P.; Yanke, L.J.; Mulvey, M.; Topp, E.; McAllister, T. Diversity and distribution of commensal fecal Escherichia coli bacteria in beef cattle administered selected subtherapeutic antimicrobials in a feedlot setting. Appl. Environ. Microbiol. 2008, 74, 6178–6186. [Google Scholar] [CrossRef] [Green Version]
- Becker, J.; Perreten, V.; Steiner, A.; Stucki, D.; Schupbach-Regula, G.; Collaud, A.; Rossano, A.; Wuthrich, D.; Muff-Hausherr, A.; Meylan, M. Antimicrobial susceptibility in E. coli and Pasteurellaceae at the beginning and at the end of the fattening process in veal calves: Comparing ‘outdoor veal calf’ and conventional operations. Vet. Microbiol. 2022, 269, 109419. [Google Scholar] [CrossRef]
- Benedict, K.M.; Gow, S.P.; McAllister, T.A.; Booker, C.W.; Hannon, S.J.; Checkley, S.L.; Noyes, N.R.; Morley, P.S. Antimicrobial Resistance in Escherichia coli Recovered from Feedlot Cattle and Associations with Antimicrobial Use. PLoS ONE 2015, 10, e0143995. [Google Scholar] [CrossRef]
- Gay, E.; Bour, M.; Cazeau, G.; Jarrige, N.; Martineau, C.; Madec, J.Y.; Haenni, M. Antimicrobial Usages and Antimicrobial Resistance in Commensal Escherichia coli From Veal Calves in France: Evolution During the Fattening Process. Front. Microbiol. 2019, 10, 792. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lefebvre, B.; Malouin, F.; Roy, G.; Giguere, K.; Diarra, M.S. Growth performance and shedding of some pathogenic bacteria in feedlot cattle treated with different growth-promoting agents. J. Food Prot. 2006, 69, 1256–1264. [Google Scholar] [CrossRef] [PubMed]
- Messele, Y.E.; Alkhallawi, M.; Veltman, T.; Trott, D.J.; McMeniman, J.P.; Kidd, S.P.; Low, W.Y.; Petrovski, K.R. Phenotypic and Genotypic Analysis of Antimicrobial Resistance in Escherichia coli Recovered from Feedlot Beef Cattle in Australia. Animals 2022, 12, 2256. [Google Scholar] [CrossRef] [PubMed]
- Rao, S.; Van Donkersgoed, J.; Bohaychuk, V.; Besser, T.; Song, X.M.; Wagner, B.; Hancock, D.; Renter, D.; Dargatz, D.; Morley, P.S. Antimicrobial drug use and antimicrobial resistance in enteric bacteria among cattle from Alberta feedlots. Foodborne Pathog. Dis. 2010, 7, 449–457. [Google Scholar] [CrossRef] [Green Version]
- Kanwar, N.; Scott, H.M.; Norby, B.; Loneragan, G.H.; Vinasco, J.; Cottell, J.L.; Chalmers, G.; Chengappa, M.M.; Bai, J.; Boerlin, P. Impact of treatment strategies on cephalosporin and tetracycline resistance gene quantities in the bovine fecal metagenome. Sci. Rep. 2014, 4, 5100. [Google Scholar] [CrossRef] [Green Version]
- Hu, X.; Zhang, Y.; Chen, Z.; Gao, Y.; Teppen, B.; Boyd, S.A.; Zhang, W.; Tiedje, J.M.; Li, H. Tetracycline accumulation in biofilms enhances the selection pressure on Escherichia coli for expression of antibiotic resistance. Sci. Total Environ. 2023, 857, 159441. [Google Scholar] [CrossRef]
- Zhang, Y.; Pei, M.; Zhang, B.; He, Y.; Zhong, Y. Changes of antibiotic resistance genes and bacterial communities in the advanced biological wastewater treatment system under low selective pressure of tetracycline. Water Res. 2021, 207, 117834. [Google Scholar] [CrossRef]
- Mirzaagha, P.; Louie, M.; Sharma, R.; Yanke, L.J.; Topp, E.; McAllister, T.A. Distribution and characterization of ampicillin- and tetracycline-resistant Escherichia coli from feedlot cattle fed subtherapeutic antimicrobials. BMC Microbiol. 2011, 11, 78. [Google Scholar] [CrossRef] [Green Version]
- Galland, J.C.; Hyatt, D.R.; Crupper, S.S.; Acheson, D.W. Prevalence, antibiotic susceptibility, and diversity of Escherichia coli O157:H7 isolates from a longitudinal study of beef cattle feedlots. Appl. Environ. Microbiol. 2001, 67, 1619–1627. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morris, C.; Wickramasingha, D.; Abdelfattah, E.M.; Pereira, R.V.; Okello, E.; Maier, G. Prevalence of antimicrobial resistance in fecal Escherichia coli and Enterococcus spp. isolates from beef cow-calf operations in northern California and associations with farm practices. Front. Microbiol. 2023, 14, 1086203. [Google Scholar] [CrossRef]
- Gaze, W.H.; Krone, S.M.; Larsson, D.G.; Li, X.Z.; Robinson, J.A.; Simonet, P.; Smalla, K.; Timinouni, M.; Topp, E.; Wellington, E.M.; et al. Influence of humans on evolution and mobilization of environmental antibiotic resistome. Emerg. Infect. Dis. 2013, 19, e120871. [Google Scholar] [CrossRef]
- Anderson, G.G.; O’Toole, G.A. Innate and induced resistance mechanisms of bacterial biofilms. Curr. Top. Microbiol. Immunol. 2008, 322, 85–105. [Google Scholar] [CrossRef]
- Tao, S.; Chen, H.; Li, N.; Wang, T.; Liang, W. The Spread of Antibiotic Resistance Genes In Vivo Model. Can. J. Infect. Dis. Med. Microbiol. 2022, 2022, 3348695. [Google Scholar] [CrossRef] [PubMed]
- Zhao, F.; Wang, B.; Huang, K.; Yin, J.; Ren, X.; Wang, Z.; Zhang, X.-X. Correlations among antibiotic resistance genes, mobile genetic elements and microbial communities in municipal sewage treatment plants revealed by high-throughput sequencing. Int. J. Environ. Res. Public Health 2023, 20, 3593. [Google Scholar] [CrossRef] [PubMed]
- Wales, A.D.; Davies, R.H. Co-Selection of Resistance to Antibiotics, Biocides and Heavy Metals, and Its Relevance to Foodborne Pathogens. Antibiotics 2015, 4, 567–604. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stogiannis, D.; Siannis, F.; Androulakis, E. Heterogeneity in meta-analysis: A comprehensive overview. Int. J. Biostat. 2023. online ahead of print. [Google Scholar] [CrossRef] [PubMed]

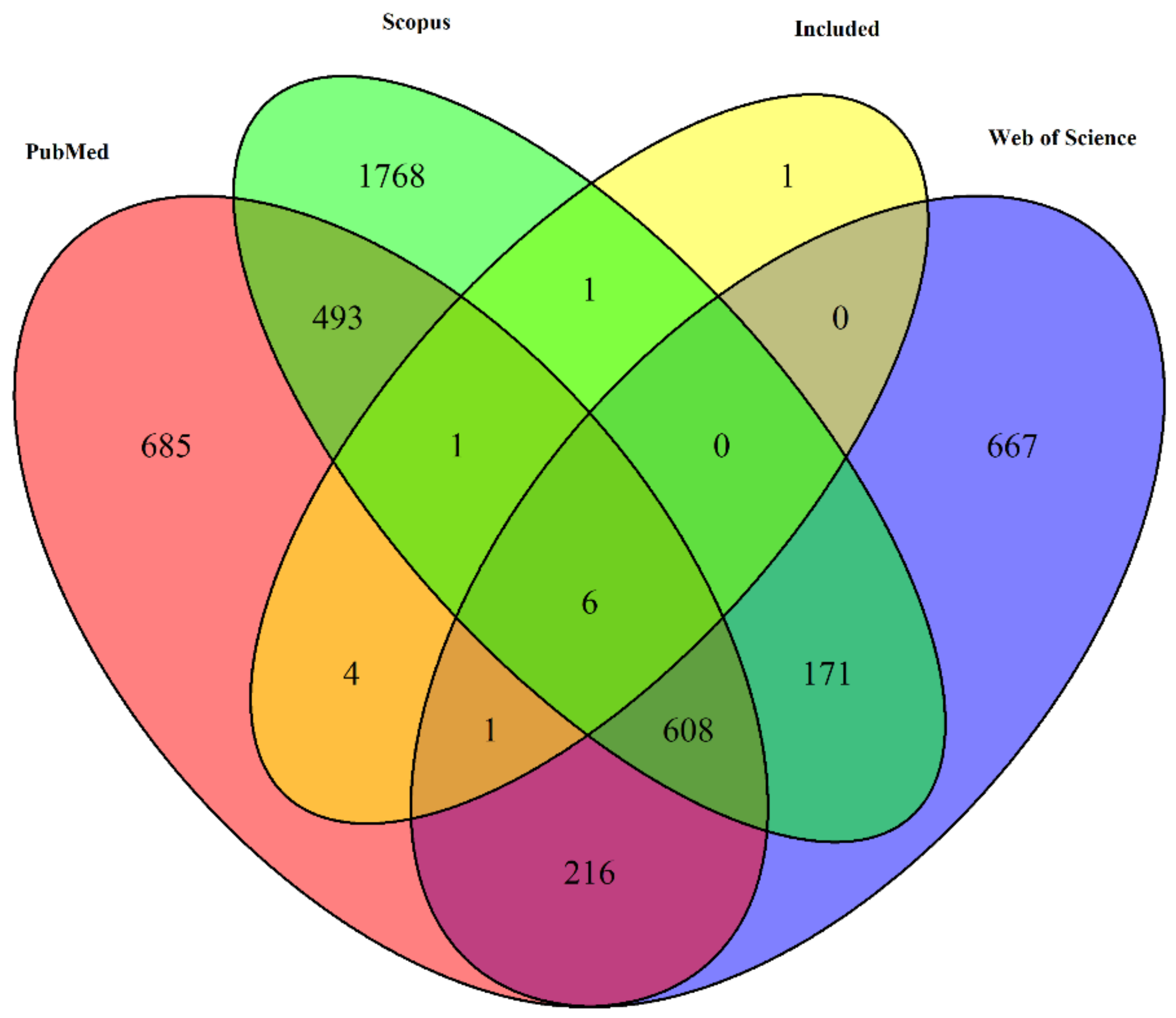
| Criteria | Inclusion Criteria | Exclusion Criteria |
|---|---|---|
| Study design | observational (cohort, case-control) | Reviews, editorials, commentaries, and non-observational studies (e.g., experimental, or interventional studies |
| Target bacteria | Escherichia coli, Enterococcus and Salmonella | Studies not specifically examining Escherichia coli, Enterococcus spp. or Salmonella spp. |
| Sample size | Greater than 30 samples | Less than 30 samples |
| Target animal | Beef cattle | Studies focused on dairy cattle, buffalo, or other species |
| Publication type | Peer review | non-peer-reviewed articles, conference abstracts, or unpublished data |
| Language | English | Non-English-language publications |
| Sample source | Fecal samples | Studies that use non-fecal samples, such as tissue, blood, or environmental samples. |
| Antimicrobial type | tetracycline, oxytetracycline, chlortetracycline | Studies using antimicrobials other than tetracyclines |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Messele, Y.E.; Werid, G.M.; Petrovski, K. Meta-Analysis on the Global Prevalence of Tetracycline Resistance in Escherichia coli Isolated from Beef Cattle. Vet. Sci. 2023, 10, 479. https://doi.org/10.3390/vetsci10070479
Messele YE, Werid GM, Petrovski K. Meta-Analysis on the Global Prevalence of Tetracycline Resistance in Escherichia coli Isolated from Beef Cattle. Veterinary Sciences. 2023; 10(7):479. https://doi.org/10.3390/vetsci10070479
Chicago/Turabian StyleMessele, Yohannes E., Gebremeskel Mamu Werid, and Kiro Petrovski. 2023. "Meta-Analysis on the Global Prevalence of Tetracycline Resistance in Escherichia coli Isolated from Beef Cattle" Veterinary Sciences 10, no. 7: 479. https://doi.org/10.3390/vetsci10070479
APA StyleMessele, Y. E., Werid, G. M., & Petrovski, K. (2023). Meta-Analysis on the Global Prevalence of Tetracycline Resistance in Escherichia coli Isolated from Beef Cattle. Veterinary Sciences, 10(7), 479. https://doi.org/10.3390/vetsci10070479






