Epidemiological Factors Associated with Gross Diagnosis of Pulmonary Pathology in Feedyard Mortalities
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Case Collection and Gross Diagnosis
2.2. Retrospective Health Data
2.3. Statistical Analysis
3. Results
3.1. Population Descriptive Statistics
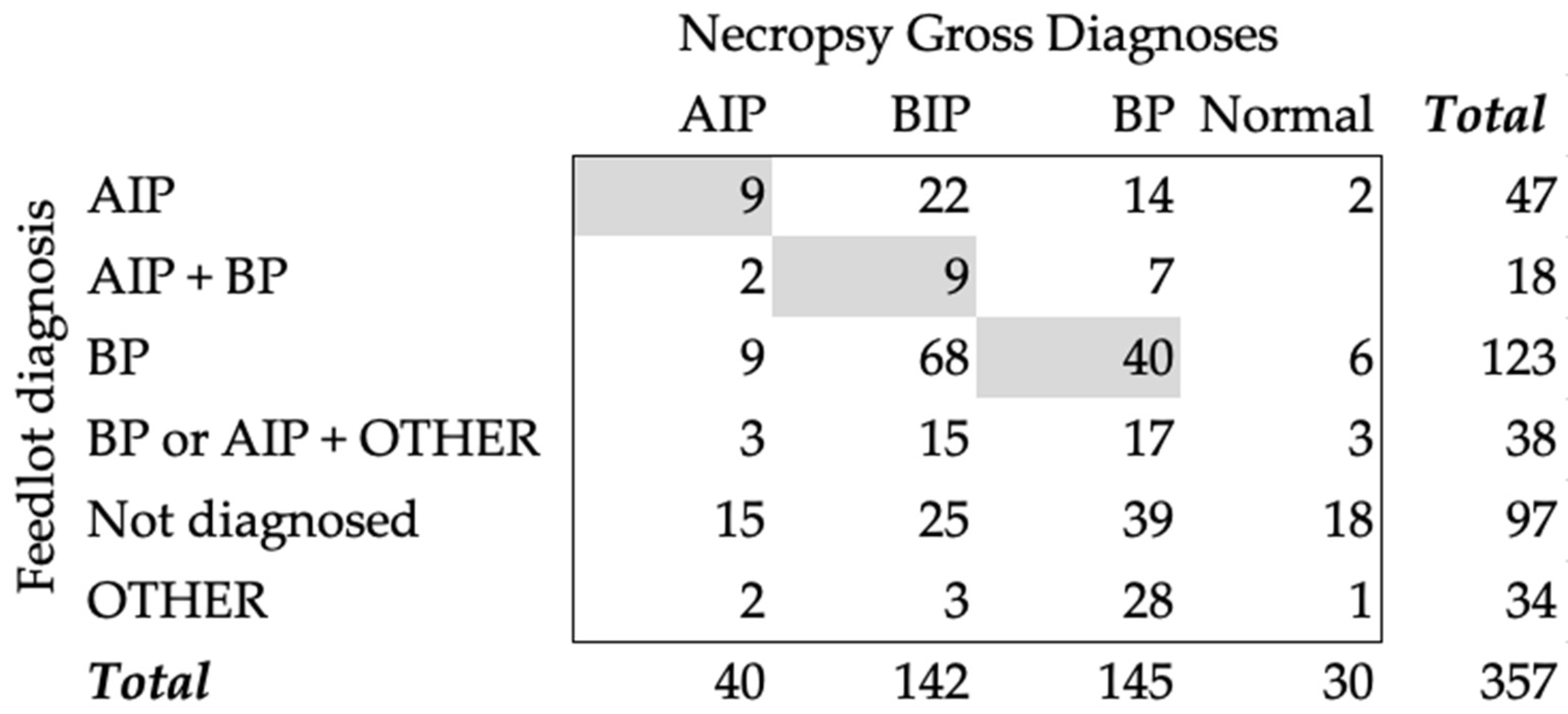
3.2. Necropsy Gross Diagnoses and Feedyard Diagnosis
3.3. Probabilities Models
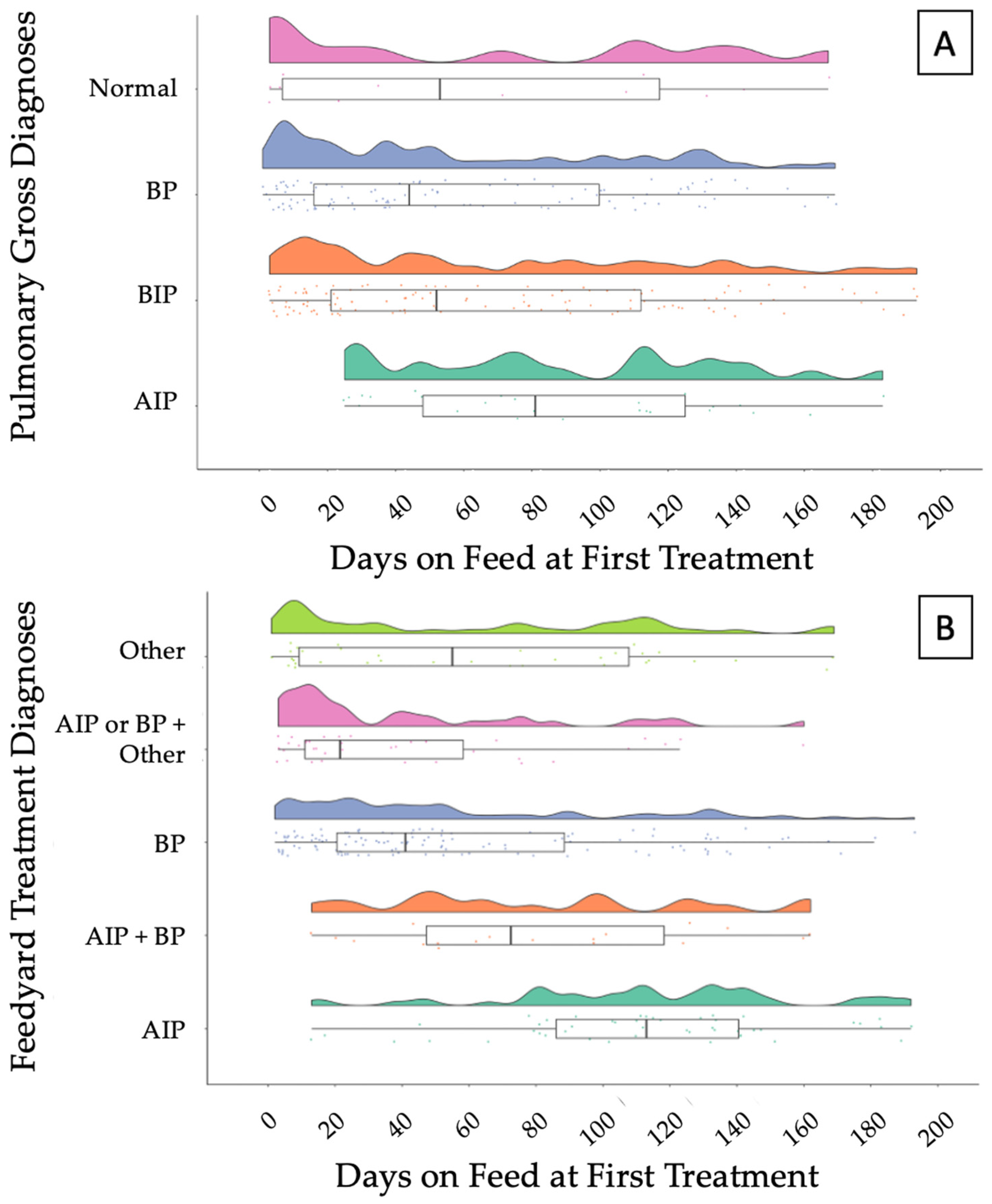
3.3.1. Acute Interstitial Pneumonia
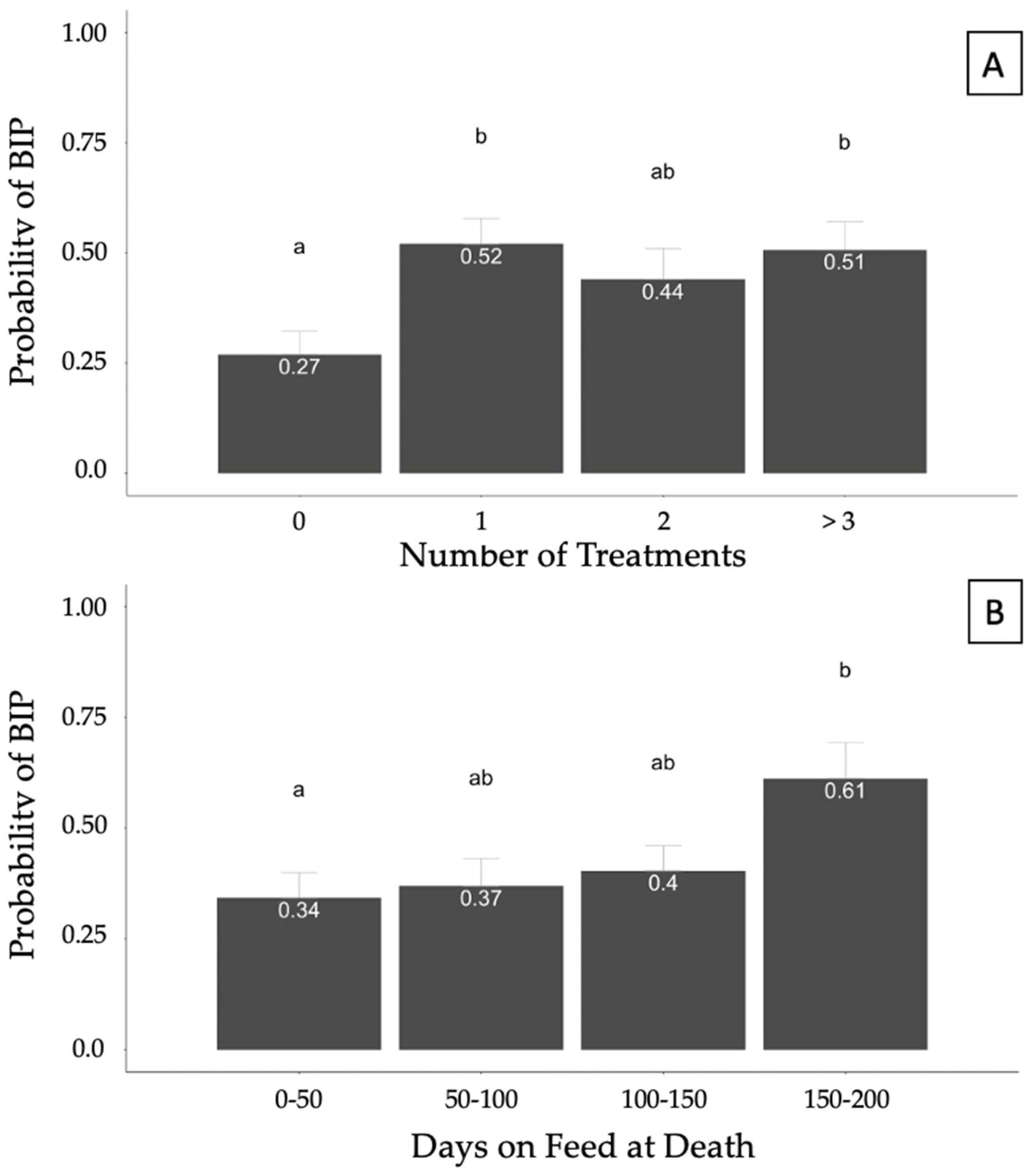
3.3.2. Bronchopneumonia with an Interstitial Pneumonia
3.3.3. Bronchopneumonia
3.3.4. Normal Pulmonary Tissue
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- USDA APHIS-VS. National Animal Health Monitoring System Beef Feedlot Study 2011. Types and Cost of Respiratory Disease Treatments in U.S. Feedlots; United States Department of Agriculture: Fort Collins, CO, USA, 2013.
- Haydock, L.A.J.; Fenton, R.K.; Sergejewich, L.; Squires, E.J.; Caswell, J.L. Acute Interstitial Pneumonia and the Biology of 3-Methylindole in Feedlot Cattle. Anim. Health Res. Rev. 2022, 23, 72–81. [Google Scholar] [CrossRef]
- White, B.J.; Larson, B.L. Impact of Bovine Respiratory Disease in U.S. Beef Cattle. Anim. Health Res. Rev. 2020, 21, 132–134. [Google Scholar] [CrossRef] [PubMed]
- Taylor, J.D.; Fulton, R.W.; Lehenbauer, T.W.; Step, D.L.; Confer, A.W. The Epidemiology of Bovine Respiratory Disease: What Is the Evidence for Predisposing Factors? Can. Vet. J. 2010, 51, 1095–1102. [Google Scholar] [PubMed]
- Srikumaran, S.; Kelling, C.L.; Ambagala, A. Immune Evasion by Pathogens of Bovine Respiratory Disease Complex. Anim. Health Res. Rev. 2007, 8, 215–229. [Google Scholar] [CrossRef] [PubMed]
- Jones, C.; Chowdhury, S. A Review of the Biology of Bovine Herpesvirus Type 1 (BHV-1), Its Role as a Cofactor in the Bovine Respiratory Disease Complex and Development of Improved Vaccines. Anim. Health Res. Rev. 2007, 8, 187–205. [Google Scholar] [CrossRef]
- Hägglund, S.; Svensson, C.; Emanuelson, U.; Valarcher, J.F.; Alenius, S. Dynamics of Virus Infections Involved in the Bovine Respiratory Disease Complex in Swedish Dairy Herds. Vet. J. 2006, 172, 320–328. [Google Scholar] [CrossRef]
- Theurer, M.E.; Fox, J.T.; Bryant, L.K.; Nickell, J.S.; Hutcheson, J.P. Treatment Efficacy of Tildipirosin or Tulathromycin for First Treatment of Naturally Occurring Bovine Respiratory Disease in a Commercial Feedlot. Bov. Pract. 2018, 52, 154–159. [Google Scholar] [CrossRef]
- Valles, J.A.; Apley, M.D.; Reinhardt, C.D.; Bartle, S.J.; Thomson, D.U. Pathologies of Acute Interstitial Pneumonia in Feedlot Cattle. Am. J. Anim. Vet. Sci. 2016, 11, 1–7. [Google Scholar] [CrossRef][Green Version]
- Edwards, A. Respiratory Diseases of Feedlot Cattle in Central USA. Bov. Pract. 1996, 30, 5–7. [Google Scholar] [CrossRef]
- Alexander, B.H.; MacVean, D.W.; Salman, M.D. Risk Factors for Lower Respiratory Tract Disease in a Cohort of Feedlot Cattle. J. Am. Med. Vet. Assoc. 1989, 195, 207–2011. [Google Scholar]
- Loneragan, G.H.; Gould, D.H.; Collins, F. Acute Interstitial Pneumonia in Feedlot Cattle. In Proceedings of the American Association of Bovine Practitioners Conference Proceedings, Rapid City, SD, USA, 21–23 September 2000. [Google Scholar]
- Loneragan, G.H.; Gould, D.H.; Mason, G.L.; Garry, F.B.; Yost, G.S.; Lanza, D.L.; Miles, D.G.; Hoffman, B.W.; Mills, L.J. Association of 3-Methyleneindolenine, a Toxic Metabolite of 3-Methylindole, with Acute Interstitial Pneumonia in Feedlot Cattle. Am. J. Vet. Res. 2001, 62, 1525–1530. [Google Scholar] [CrossRef] [PubMed]
- Woolums, A.R. Feedlot Acute Interstitial Pneumonia. Vet. Clin. North Am. Food Anim. Pract. 2015, 31, 381–389. [Google Scholar] [CrossRef] [PubMed]
- Jensen, R.; Pierson, R.E.; Braddy, P.M.; Saari, D.A.; Lauerman, L.H.; England, J.J.; Benitez, A.; Horton, D.P.; McChesney, A.E. Atypical Interstitial Pneumonia in Yearling Feedlot Cattle. J. Am.Vet. Med. Assoc. 1976, 169, 507–510. [Google Scholar] [PubMed]
- Haydock, L.A.J.; Fenton, R.K.; Smerek, D.; Renaud, D.L.; Caswell, J.L. Bronchopneumonia with Interstitial Pneumonia in Feedlot Cattle: Epidemiologic Characteristics of Affected Animals. Vet. Pathol. 2023, 60, 226–234. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, P.H.; White, B.J.; Finley, A.; Bortoluzzi, E.M.; Depenbusch, B.E.; Mancke, M.; Brown, R.E.; Jensen, M.; Lancaster, P.A.; Larson, R.L. Determining Frequency of Common Pulmonary Gross and Histopathological Findings in Feedyard Fatalities. Vet. Sci. 2023, 10, 228. [Google Scholar] [CrossRef]
- Bortoluzzi, E.M.; Schmidt, P.H.; Brown, R.E.; Jensen, M.; Mancke, M.R.; Larson, R.L.; Lancaster, P.A.; White, B.J. Image Classification and Automated Machine Learning to Classify Lung Pathologies in Deceased Feedlot Cattle. Vet. Sci. 2023, 10, 113. [Google Scholar] [CrossRef]
- Haydock, L.A.J.; Fenton, R.K.; Sergejewich, L.; Veldhuizen, R.A.W.; Smerek, D.; Ojkic, D.; Caswell, J.L. Bronchopneumonia with Interstitial Pneumonia in Beef Feedlot Cattle: Characterization and Laboratory Investigation. Vet. Pathol. 2023, 60, 214–225. [Google Scholar] [CrossRef]
- Panciera, R.J.; Confer, A.W. Pathogenesis and Pathology of Bovine Pneumonia. Vet. Clin. North Am. Food Anim. Pract. 2010, 26, 191–214. [Google Scholar] [CrossRef]
- Curtis, R.; Thomson, R.; Sandals, W. Atypical Interstitial Pneumonia in Cattle. Can. Vet. J. 1979, 20, 141–142. [Google Scholar]
- Snowder, G.D.; Van Vleck, L.D.; Cundiff, L.V.; Bennett, G.L. Bovine Respiratory Disease in Feedlot Cattle: Environmental, Genetic, and Economic Factors. J. Anim. Sci. 2006, 84, 1999–2008. [Google Scholar] [CrossRef]
- Snowder, G.D.; Van Vleck, L.D.; Cundiff, L.V.; Bennett, G.L. Influence of Breed, Heterozygosity, and Disease Incidence on Estimates of Variance Components of Respiratory Disease in Preweaned Beef Calves. J. Anim. Sci. 2005, 83, 1247–1261. [Google Scholar] [CrossRef]
- Callan, R.J.; Garry, F.B. Biosecurity and Bovine Respiratory Disease. Vet. Clin. North Am. Food Anim. Pract. 2002, 18, 57–77. [Google Scholar] [CrossRef] [PubMed]
- Dorso, L.; Rouault, M.; Barbotin, C.; Chartier, C.; Assié, S. Infectious Bovine Respiratory Diseases in Adult Cattle: An Extensive Necropsic and Etiological Study. Animals 2021, 11, 2280. [Google Scholar] [CrossRef]
- Timsit, E.; Dendukuri, N.; Schiller, I.; Buczinski, S. Diagnostic Accuracy of Clinical Illness for Bovine Respiratory Disease (BRD) Diagnosis in Beef Cattle Placed in Feedlots: A Systematic Literature Review and Hierarchical Bayesian Latent-Class Meta-Analysis. Prev. Vet. Med. 2016, 135, 67–73. [Google Scholar] [CrossRef]
- Woolums, A.R.; Mason, G.L.; Hawkins, L.L.; Brown, C.C.; Williams, S.M.; Gould, J.A.; Fox, J.J.; Sturgeon, S.D.; Anderson, J.L.; Duggan, F.E.; et al. Microbiologic Findings in Feedlot Cattle with Acute Interstitial Pneumonia. Am. J. Vet. Res. 2004, 65, 1525–1532. [Google Scholar] [CrossRef]
- Sorden, S.D.; Kerr, R.W.; Janzen, E.D. Interstitial Pneumonia in Feedlot Cattle: Concurrent Lesions and Lack of Immunohistochemical Evidence for Bovine Respiratory Syncytial Virus Infection. J. VET Diagn. Investig. 2000, 12, 510–517. [Google Scholar] [CrossRef] [PubMed]
- Ayroud, M.; Popp, J.D.; VanderKop, M.A.; Yost, G.S.; Haines, D.M.; Majak, W.; Karren, D.; Yanke, L.J.; McAllister, T.A. Characterization of Acute Interstitial Pneumonia in Cattle in Southern Alberta Feedyards. Can. Vet. J. 2000, 41, 8. [Google Scholar]
- Loneragan, G.H.; Gould, D.H.; Mason, G.L.; Garry, F.B.; Yost, G.S.; Miles, D.G.; Hoffman, B.W.; Mills, L.J. Involvement of Microbial Respiratory Pathogens in Acute Interstitial Pneumonia in Feedlot Cattle. Ajvr 2001, 62, 1519–1524. [Google Scholar] [CrossRef]

| Necropsy Pulmonary Gross Diagnoses | ||||
|---|---|---|---|---|
| AIP 1 | BIP 2 | BP 3 | Normal 4 | |
| Sex | ||||
| Heifer | 34 | 98 | 104 | 16 |
| Steer | 6 | 44 | 41 | 14 |
| Treatment count, n | ||||
| 0 | 15 | 25 | 39 | 18 |
| 1 | 12 | 52 | 36 | 5 |
| 2 | 6 | 26 | 31 | 4 |
| >3 | 7 | 39 | 39 | 3 |
| Antibiotic treatments, n | ||||
| 0 | 15 | 26 | 46 | 19 |
| 1 | 17 | 58 | 42 | 4 |
| 2 | 7 | 30 | 32 | 6 |
| >3 | 1 | 28 | 25 | 1 |
| Anti-inflammatory treatments, n | ||||
| 0 | 23 | 68 | 68 | 19 |
| 1 | 11 | 53 | 39 | 10 |
| 2 | 4 | 15 | 21 | 0 |
| >3 | 2 | 6 | 17 | 1 |
| Arrival weight categories, kg | ||||
| <272 | 8 | 18 | 16 | 0 |
| 272–317 | 6 | 36 | 28 | 3 |
| 317–363 | 16 | 47 | 58 | 17 |
| >363 | 10 | 41 | 43 | 10 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bortoluzzi, E.M.; White, B.J.; Schmidt, P.H.; Mancke, M.R.; Brown, R.E.; Jensen, M.; Lancaster, P.A.; Larson, R.L. Epidemiological Factors Associated with Gross Diagnosis of Pulmonary Pathology in Feedyard Mortalities. Vet. Sci. 2023, 10, 522. https://doi.org/10.3390/vetsci10080522
Bortoluzzi EM, White BJ, Schmidt PH, Mancke MR, Brown RE, Jensen M, Lancaster PA, Larson RL. Epidemiological Factors Associated with Gross Diagnosis of Pulmonary Pathology in Feedyard Mortalities. Veterinary Sciences. 2023; 10(8):522. https://doi.org/10.3390/vetsci10080522
Chicago/Turabian StyleBortoluzzi, Eduarda M., Brad J. White, Paige H. Schmidt, Maddie R. Mancke, Rachel E. Brown, Makenna Jensen, Phillip A. Lancaster, and Robert L. Larson. 2023. "Epidemiological Factors Associated with Gross Diagnosis of Pulmonary Pathology in Feedyard Mortalities" Veterinary Sciences 10, no. 8: 522. https://doi.org/10.3390/vetsci10080522
APA StyleBortoluzzi, E. M., White, B. J., Schmidt, P. H., Mancke, M. R., Brown, R. E., Jensen, M., Lancaster, P. A., & Larson, R. L. (2023). Epidemiological Factors Associated with Gross Diagnosis of Pulmonary Pathology in Feedyard Mortalities. Veterinary Sciences, 10(8), 522. https://doi.org/10.3390/vetsci10080522







