Duration of the Flaxseed Supplementation Affects Antioxidant Defence Mechanisms and the Oxidative Stress of Fattening Pigs
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals, Diets and Management
2.2. Sampling and Analysis of Blood Parameters
2.3. Sampling and Analysis of Tissue Oxidative Parameters
2.4. Statistical Analyses
3. Results
3.1. Total Antioxidant Capacity of the Blood of the Pigs
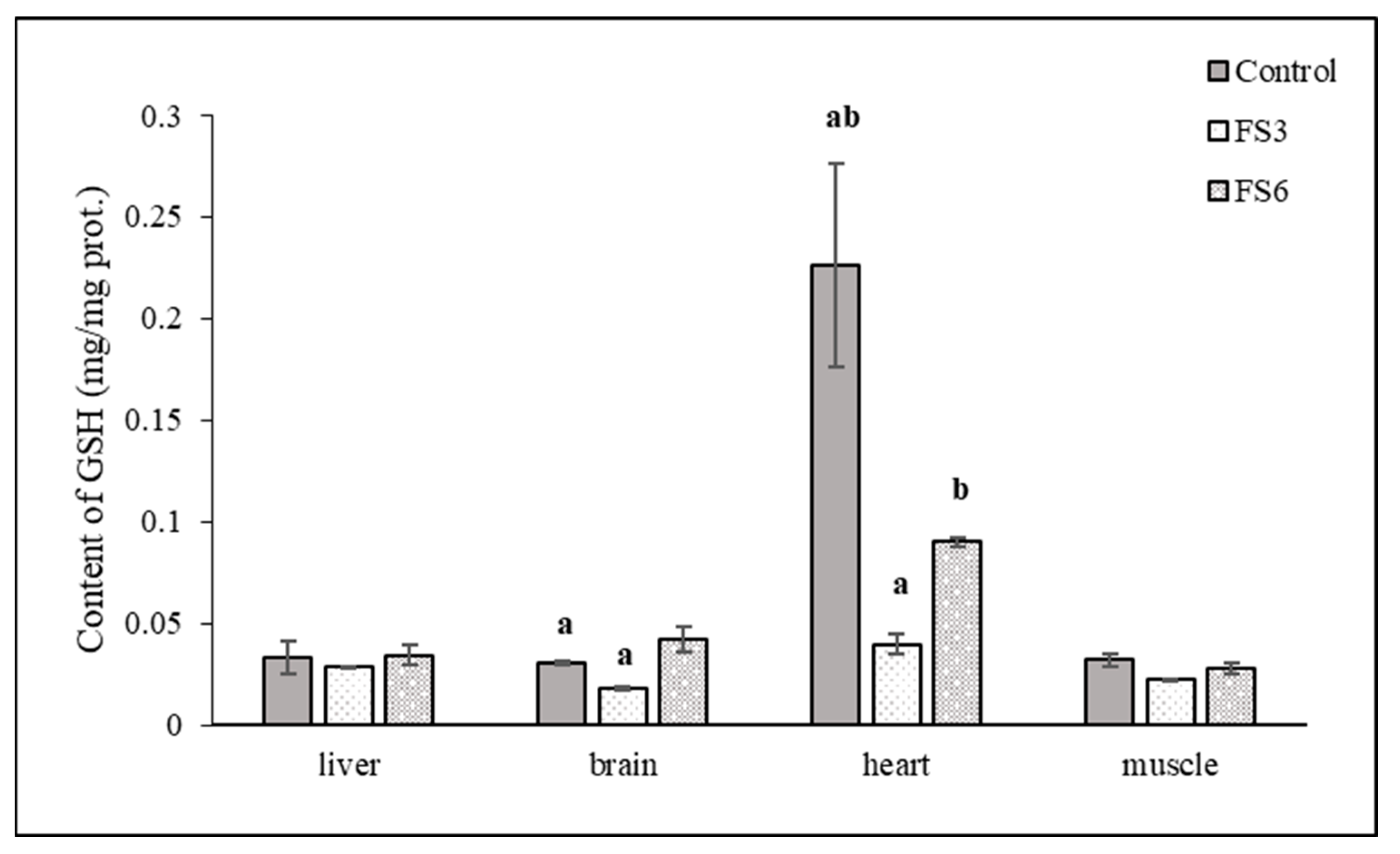
3.2. Antioxidant Parameters in the Tissues
3.3. Antioxidant Parameters in Tissues and Blood
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hirst, B.C.; Dibrov, E.; Hirst, S.D.; Pierce, G.N. Physiological and pathological considerations for the use of flaxseed as therapeutic dietary strategy. Rev. Cardiovasc. Med. 2023, 24, 149. [Google Scholar] [CrossRef]
- Bernacchia, R.; Preti, R.; Vinci, G. Chemical Composition and Health Benefits of Flaxseed. Austin J. Nutri. Food Sci. 2014, 2, id1045. [Google Scholar]
- Bekhit, A.E.-D.A.; Shavandi, A.; Jodjaja, T.; Birch, J.; Teh, S.; Mohamed, A.I.A.; Al-Juhaimi, F.Y.; Saeedi, P.; Bekhit, A.A. Flaxseed: Composition, detoxification, utilization, and opportunities. Biocatal. Agric. Biotechnol. 2018, 13, 129–152. [Google Scholar] [CrossRef]
- Qamar, H.; Ilyas, M.; Shabbir, G.; Irshad, G.; Nisar, F.; Abbas, S.M.; Ghias, M.; Arshad, A. Flax: Ancient to modern food. Pure Appl. Biol. 2019, 8, 2269–2276. [Google Scholar] [CrossRef]
- Safdar, B.; Pang, Z.; Liu, X.; Jatoi, M.A.; Mehmood, A.; Rashid, M.T.; Ali, N.; Naveed, M. Flaxseed gum: Extraction, bioactive composition, structural characterization, and its potential antioxidant activity. J. Food Biochem. 2019, 43, e13014. [Google Scholar] [CrossRef]
- Sicillia, T.; Niemeyer, H.B.; Honig, D.; Metzler, M. Identification and Stereochemical Characterization of Lignans in Flaxseed and Pumpkin Seeds. J. Agric. Food Chem. 2003, 51, 1181–1188. [Google Scholar] [CrossRef]
- Kasote, D.M. Flaxseed phenolics as natural antioxidants. Int. Food Res. J. 2013, 20, 27–34. [Google Scholar]
- Kuijsten, A.; Arts, I.C.W.; van’t Veer, P.; Hollman, P.C.H. The Relative Bioavailability of Enterolignans in Humans Is Enhanced by Milling and Crushing of Flaxseed. J. Nutr. 2005, 135, 2812–2816. [Google Scholar] [CrossRef]
- Oomah, B.D.; Mazza, G. Compositional changes during commercial processing of flaxseed. Ind. Crops Prod. 1998, 9, 29–37. [Google Scholar] [CrossRef]
- Kajla, P.; Sharma, A.; Sood, D.R. Flaxseed—A potential functional food source. J. Food Sci. Technol. 2015, 52, 1857–1871. [Google Scholar] [CrossRef]
- Parikh, M.; Maddaford, T.G.; Austria, J.A.; Aliani, M.; Netticadan, T.; Pierce, G.N. Dietary Flaxseed as a Strategy for Improving Human Health. Nutrients 2019, 11, 1171. [Google Scholar] [CrossRef]
- Martinchik, A.N.; Baturin, A.K.; Zubtsov, V.V.; Molofeev, V. Nutritional value and functional properties of flaxseed. Vopr. Pitan. 2012, 81, 4–10. [Google Scholar]
- Pilar, B.; Güllich, A.; Oliviera, P.; Ströher, D.; Piccoli, J.; Manfredini, V. Protective role of flaxseed oil and flaxseed lignin secoisolariciresinol diglucoside against oxidative stress in rats with metabolic syndrome. J. Food Sci. 2017, 82, 3029–3036. [Google Scholar] [CrossRef] [PubMed]
- James, M.; Gibson, R.A.; Cleland, I.G. Dietary polyunsaturated fatty acids and inflammatory mediator production. Am. J. Clin. Nutr. 2000, 71, 3435–3485. [Google Scholar] [CrossRef] [PubMed]
- Simpoulos, A.P. Evolutionary aspects of diet: The omega-6/omega-3 ratio and the brain. Mol. Neurobiol. 2011, 44, 203–215. [Google Scholar] [CrossRef] [PubMed]
- Reda, F.M.; El-Kholy, M.S.; Abd-El-Hack, M.E.; Taha, A.E.; Othman, S.I.; Allam, A.A.; Alagawamy, M. Does the use of different oil sources in quail diet impact their productive and reproductive performance, egg quality, and blood constituents? Poultry Sci. 2020, 99, 3511–3518. [Google Scholar] [CrossRef]
- Spitalniak-Bajerska, K.; Szumny, A.; Pogoda-Sewerniak, K.; Kupczynski, R. Effect of n-3 fatty acids on growth, antioxidant status, and immunity of preweaned dairy calves. J. Dairy Sci. 2020, 103, 2864–2876. [Google Scholar] [CrossRef]
- Martini, S.; Tagliazucchi, D.; Minelli, G.; Fiego, D.P.L. Influence of linseed and antioxidant-rich diets in pig nutrition on lipid oxidation during cooking and in vitro digestion of pork. Food Res. Int. 2020, 137, 109528. [Google Scholar] [CrossRef]
- Palla, A.H.; Gilani, A.-H. Dual effectiveness of flaxseed in constipation and diarrhea: Possible mechanism. J. Ethnopharmacol. 2015, 169, 60–68. [Google Scholar] [CrossRef]
- Che, L.; Zhou, Q.; Liu, Y.; Hu, L.; Peng, X.; Wu, C.; Zhang, R.; Tang, J.; Wu, F.; Fang, Z.; et al. Flaxseed oil supplementation improves intestinal function and immunity, associated with altered intestinal microbiome and fatty acid profile in pigs with intrauterine growth retardation. Food Funct. 2019, 12, 8149–8160. [Google Scholar] [CrossRef]
- Singh, M.; Mollier, R.T.; Sharma, P.R.; Kadirvel, G.; Doley, S.; Snajukta, R.K.; Rajkhowa, D.J.; Kandpal, B.K.; Kumar, K.; Khan, M.H.; et al. Dietary flaxseed oil improve boar semen quality, antioxidant status and in-vivo fertility in humid sub-tropical region of North East India. Theriogenology 2021, 159, 123–131. [Google Scholar] [CrossRef] [PubMed]
- Salami, S.A.; Majokaa, M.A.; Saha, S.; Garbera, A.; Gabaroua, J.F. Efficacy of dietary antioxidants on broiler oxidative stress, performance and meat quality: Science and market. Aviar. Biol. Res. 2015, 8, 65–78. [Google Scholar] [CrossRef]
- Musazadeh, V.; Jafarzadeh, J.; Karzamati, M.; Zarezadeh, M.; Ahmadi, M.; Farrokhian, Z.; Astadrahimi, A. Flaxseed oil supplementation augments antioxidant capacity and alleviate oxidative stress: A systemic review and meta-analysis of randomized controlled trials. Evid. Baseds Complement. Altern. Med. 2021, 2021, 4438613. [Google Scholar] [CrossRef] [PubMed]
- Shahid, M.S.; Wu, S.; Xiao, Z.; Raza, T.; Dong, X.; Yua, J. Duration of the flaxseed diet promotes deposition of n-3 fatty acids in the meat and skin of Peking ducks. Food Nutr. Res. 2019, 63, 3590. [Google Scholar] [CrossRef]
- Konvičná, J.; Vargová, M.; Paulíková, I.; Kováč, G.; Kostecká, Z. Oxidative stress and antioxidant status in dairy cows during prepartal and postpartal periods. Acta Vet. Brno. 2015, 84, 133–140. [Google Scholar] [CrossRef]
- Benzie, I.F.; Strain, J.J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef]
- Miller, N.J.; Rice-Evans, C.; Davies, M.J.; Gopinathan, V.; Milner, A. A novel method for measuring antioxidant capacity and its application to monitoring the antioxidant status in premature neonates. Clin. Sci. 1993, 84, 407–412. [Google Scholar] [CrossRef]
- Paglia, D.E.; Valentine, W.N. Studies on the quantitative and qualitative characterization of erythrocyte glutathione peroxidase. J. Lab. Clin. Med. 1967, 70, 158–169. [Google Scholar]
- Flohé, L.; Ötting, F. SOD assays. Method Enzymol. 1984, 105, 93–104. [Google Scholar] [CrossRef]
- Sizer, I.W.; Beers, R.F., Jr. A spectrophotometric method for measuring the breakdown of hydrogen peroxide by catalase. J. Biol. Chem. 1952, 195, 133–139. [Google Scholar]
- Flohé, L.; Günzler, W.A. Assays of glutathione peroxidase. Methods Enzymol. 1984, 105, 114–121. [Google Scholar] [CrossRef]
- Pinto, M.C.; Mata, A.M.; López-Barea, J. Reversible inactivation of Saccharomyces cerevisiae glutathione reductase under reducing conditions. Arch. Biochem. Biophys. 1984, 228, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Habig, W.H.; Jakoby, W.B. Assays for differentiation of glutathione-S-transferases. Method Enzymol. 1981, 77, 398–405. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantification of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef] [PubMed]
- George, L.; Ellman, G.L. Tissue sulfhydryl groups. Arch. Biochem. Biophys. 1959, 82, 70–77. [Google Scholar] [CrossRef]
- Gutteridge, J.M.C. Ferrous ion-EDTA-stimulated phospholipid peroxidation. Biochem. J. 1984, 224, 697–701. [Google Scholar] [CrossRef]
- Barthet, V.J.; Klensporf-Pawlik, D.; Przybylski, R. Antioxidant activity of flaxseed meal components. Can. J. Plant Sci. 2014, 94, 593–602. [Google Scholar] [CrossRef]
- Przybylski, R.; Daun, J.K. Additional data on the storage stability of milled flaxseed. J. Am. Oil Chem. Soc. 2001, 78, 105–106. [Google Scholar] [CrossRef]
- Liang, S.; Liao, W.; Ma, X.; Li, X.; Wang, Y. H2O2 oxidative preparation, characterization and antiradical activity of a novel oligosaccharide derived from flaxseed gum. Food Chem. 2017, 230, 135–144. [Google Scholar] [CrossRef]
- Sembratowicz, I.; Zięba, G.; Cholewinska, E.; Czech, A. Effect of dietary flaxseed oil supplementation on the redox status, haematological and biochemical parameters of horses´ blood. Animals 2020, 10, 2244. [Google Scholar] [CrossRef]
- Króliczewska, B.; Miśta, D.; Ziarnik, A. The effects of seed from Linum usitatissimum cultivar with increased phenylpropanoid compounds and hydrolysable tannin in a high cholesterol-fed rabbit. Lipids Health Dis. 2018, 17, 76. [Google Scholar] [CrossRef] [PubMed]
- Hosseinian, F.S.; Muir, A.D.; Westcott, N.D.; Krol, E.S. Antioxidant capacity of flax seed lignans in two model systems. J. Am. Oil Chem. Soc. 2006, 83, 835–840. [Google Scholar] [CrossRef]
- Chera, E.I.; Pop, R.M.; Pârvu, M.; Sorițău, O.; Uifălean, A.; Cătoi, F.A.; Cecan, A.; Negoescu, A.G.; Achimaș-Cadariu, P.; Pârvu, A.E. Flaxseed Ethanol Extracts’ Antitumor, Antioxidant, and Anti-Inflammatory Potential. Antioxidants 2022, 11, 892. [Google Scholar] [CrossRef] [PubMed]
- Cheng, C.; Yu, X.; McClements, D.J.; Huang, Q.; Tang, H.; Yu, K.; Xiang, X.; Chen, P.; Wang, X.; Deng, Q. Effect of flaxseed polyphenols on physical stability and oxidative stability of flaxseed oil-in-water nanoemulsions. Food Chem. 2019, 301, 125207. [Google Scholar] [CrossRef]
- Naik, H.S.; Srilatha, C.; Sujatha, K.; Sreedevi, B.; Prasad, T.N.V.K.V. Supplementation of whole grain flaxseeds (Linum usitatissimum) along with high cholesterol diet and its effect on hyperlipidemia and initiated atherosclerosis in Wistar albino male rats. Vet. World 2018, 11, 1433–1439. [Google Scholar] [CrossRef]
- Chaudière, J.; Ferrari-Iliou, R. Intracellular antioxidants: From chemical to biochemical mechanisms. Food Chem. Toxicol. 1999, 37, 949–962. [Google Scholar] [CrossRef]
- Gaber, D.A.; Badawy, W.A. Role of flaxseed oil and silymarin in amelioration of lead-induced kidney injury. Kasr. Al. Ainy. Med. J. 2019, 25, 29–37. [Google Scholar] [CrossRef]
- Bruce, K.D.; Zsombok, A.; Eckel, R.H. Lipid Processing in the Brain: A Key Regulator of Systemic Metabolism. Front. Endocrinol. 2017, 8, 60. [Google Scholar] [CrossRef]
- Bourre, J.M. Roles of unsaturated fatty acids (especially omega-3 fatty acids) in the brain at various ages and during ageing. J. Nutr. Health Aging 2004, 8, 163–174. [Google Scholar]
- Eckert, G.P.; Franke, C.; Nöldner, M.; Raur, O.; Wurglics, M.; Schubert-Zsilavecz, M.; Müller, W.E. Plant derived omega-3-fatty acids protect mitochondrial function in the brain. Pharmacol. Res. 2010, 61, 234–241. [Google Scholar] [CrossRef]
- Mila-Kierzenkowska, C.; Augustynska, B.; Wozniak, A.; Boraczynski, T.; Weselowski, R.; Sutkowy, P.; Sewcyzk-Golec, K. Effect of changes in ambient temperature on oxidative stress markers in blood of regular winter swimmers. Med. Ogólna Nauki Zdr. 2016, 22, 46–50. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, Q.; Wang, L.; Wang, H.; Sun, T.; Xia, H.; Yang, Y.; Zhang, L. The α-lipoic acid improves high-fat diet-induced cerebral damage through inhibition of oxidative stress and inflammatory reaction. Environ. Toxicol. Pharmacol. 2017, 56, 219–224. [Google Scholar] [CrossRef] [PubMed]
- Galal, A.M.; Walker, L.A.; Khan, I.A. Induction of GST and related events by dietary phytochemicals: Sources, chemistry, and possible contribution to chemoprevention. Curr. Top. Med. Chem. 2015, 14, 2802–2821. [Google Scholar] [CrossRef] [PubMed]
- Han, J.; Won, E.-J.; Hwang, D.-S.; Rhee, J.-S.; Kim, I.-C.; Lee, J.-S. Effect of copper exposure on GST activity and on the expression of four GSTs under oxidative stress condition in the monogonont rotifer, Brachionus koreanus. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2013, 158, 91–100. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.; Fang, Y.Z.; Yang, S.; Lupton, J.R.; Turner, N.D. Glutathione metabolism and its implications for health. J. Nutr. 2004, 134, 489–492. [Google Scholar] [CrossRef] [PubMed]
- Celi, P.; Trana, A.D.; Claps, S. Effect of plane of nutrition on oxidative stress in goats during the peripartum period. Vet. J. 2010, 184, 95–99. [Google Scholar] [CrossRef]
- Kumar, F.; Tyagi, P.K.; Mir, N.A.; Dev, K.; Begum, J.; Biswas, A.; Sheikh, S.A.; Tyagi, P.K.; Sharma, D.; Sahu, B.; et al. Dietary flaxseed and turmeric is a novel strategy to enrich chicken meat with long chain ω-3 polyunsaturated fatty acids with better oxidative stability and functional properties. Food Chem. 2020, 305, 125458. [Google Scholar] [CrossRef]
- Sarikaya, E.; Dogan, S. Glutathione Peroxidase in Health and Diseases. In Glutathione System and Oxidative Stress in Health and Disease; IntechOpen: London, UK, 2020. [Google Scholar] [CrossRef]
| Variable | SFM | Flaxseed | SFM + 10% FS |
|---|---|---|---|
| Crude protein, g/kg | 140.42 | 227.6 | 150.51 |
| Crude fat, g/kg | 18.07 | 307.25 | 42.23 |
| Crude fibre, g/kg | 35.69 | 232.58 | 68.29 |
| NDF, g/kg | 176.11 | 411.9 | 189.04 |
| ADF, g/kg | 42.27 | 278.9 | 74.47 |
| Ash, g/kg | 60.12 | 35.5 | 48.30 |
| Starch, g/kg | 574.62 | 42.32 | 510.28 |
| Ca, g/kg | 8.12 | 2.81 | 12.80 |
| Mg, g/kg | 3.35 | 4.11 | 3.59 |
| Na, g/kg | 1.45 | 4.33 | 1.52 |
| K, g/kg | 5.58 | 8.12 | 6.29 |
| P, g/kg | 4.80 | 2.71 | 5.84 |
| Cu, mg/kg | 40.82 | 33.55 | 57.40 |
| Zn, mg/kg | 131.83 | 48.7 | 103.45 |
| Mn, mg/kg | 150.57 | 43.83 | 125.80 |
| ME, MJ/kg | 13.26 | 12.87 | 13.36 |
| Item | Unit | Control | FS3 | FS6 |
|---|---|---|---|---|
| TAS | mmol/L | 1.26 ± 0.08 | 1.12 ± 0.04 | 1.15 ± 0.04 |
| FRAP | μmol/L | 217 ± 16 | 212 ± 14 | 256 ± 33 |
| dROMs | UCARR | 1289 ± 70 | 1123 ± 31 | 1134 ± 65 |
| PAT | 1.4 μmol/L ascorbic acid | 3196 ± 168 | 3288 ± 66 | 3368 ± 66 |
| Item | Unit | Control | FS3 | FS6 |
|---|---|---|---|---|
| Liver | ||||
| SOD | U·mg prot.−1 | 102 ±12 | 81± 5 | 95 ±17 |
| CAT | U·mg prot.−1 | 3650 ± 359 | 3930 ± 116 | 3820 ± 319 |
| GPx | U·mg prot.−1 | 0.073 ± 0.017 | 0.047 ± 0.008 | 0.042 ± 0.007 |
| GST | U·mg prot.−1 | 2.358 ± 0.237 | 1.451 ± 0.099 | 2.018 ± 0.223 |
| GR | U·mg prot.−1 | 0.140 ± 0.022 | 0.121 ± 0.007 | 0.091± 0.013 |
| TBARS | A535/mg prot. | 0.0079 ± 0.0009 | 0.0057 ± 0.0003 | 0.0070 ± 0.0010 |
| Brain | ||||
| SOD | U·mg prot.−1 | 43 ± 4 | 34 ± 2 | 39 ± 2 |
| CAT | U·mg prot.−1 | 11.8 ± 0.2 | 10.8 ± 0.2 | 14.4 ± 0.2 |
| GPx | U·mg prot.−1 | 0.015 ± 0.002 | 0.008 ± 0.002 | 0.014 ± 0.002 |
| GST | U·mg prot.−1 | 0.190 ± 0.009 | 0.154 ± 0.012 | 0.220 ± 0.007 |
| GR | U·mg prot.−1 | 0.0166 ±0.0012 | 0.0132 ±0.0006 | 0.0164 ± 0.0010 |
| TBARS | A535/mg prot. | 0.0078 ± 0.0002 a | 0.0019 ± 0.000 ab | 0.0098 ± 0.0007 b |
| Heart | ||||
| SOD | U·mg prot.−1 | 231 ± 43 ab | 46 ± 2 ac | 75± 8 bc |
| CAT | U·mg prot.−1 | 259 ± 41 ab | 79 ± 11 bc | 119 ± 7 ac |
| GPx | U·mg prot.−1 | 0.178 ± 0.042 ab | 0.032 ± 0.001 a | 0.063 ± 0.005 b |
| GST | U·mg prot.−1 | 0.216 ± 0.050 a | 0.369 ± 0.001 b | 0.077 ± 0.006 ab |
| GR | U·mg prot.−1 | 0.177 ± 0.038 ab | 0.038 ± 0.006 ac | 0.059 ± 0.003 bc |
| TBARS | A535/mg prot. | 0.038 ± 0.013 ab | 0.005 ± 0.001 bc | 0.0103 ± 0.0024 ac |
| Muscle | ||||
| SOD | U·mg prot.−1 | 9.2 ± 0.2 a | 8.6 ± 0.5 | 11.1 ± 0.3 a |
| CAT | U·mg prot.−1 | 13.7 ± 0.6 a | 10.4 ± 0.7 a | 13.9 ± 2.3 |
| GPx | U·mg prot.−1 | 0.009 ± 0.001 a | 0.009 ± 0.001 b | 0.015 ± 0.001 ab |
| GST | U·mg prot.−1 | 0.032 ± 0.001 a | 0.024 ±0.001 a | 0.032 ± 0.004 b |
| GR | U·mg prot.−1 | 0.0061± 0.0003 | 0.0060 ± 0.0004 | 0.0089 ± 0.0008 |
| TBARS | A535/mg prot. | 0.0076 ± 0.0008 a | 0.0050 ± 0.0006 b | 0.0068 ± 0.0004 ab |
| Item | Unit | Control | FS3 | FS6 |
|---|---|---|---|---|
| Liver | U·mg prot.−1 | 0.073 ± 0.017 | 0.047 ± 0.008 | 0.042 ± 0.007 |
| Brain | U·mg prot.−1 | 0.015 ± 0.002 | 0.008 ± 0.002 | 0.014± 0.002 |
| Heart | U·mg prot.−1 | 0.178 ±0.042 ab | 0.032 ± 0.001 a | 0.063 ± 0.005 b |
| Muscle | U·mg prot.−1 | 0.009 ± 0.001 a | 0.009 ±0.001 b | 0.015 ± 0.001 ab |
| Blood | μkat·L−1 | 715 ± 94 a | 386 ± 41 a | 575 ± 77 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sobeková, A.; Piešová, E.; Maková, Z.; Szabóová, R.; Sopková, D.; Andrejčáková, Z.; Vlčková, R.; Faixová, D.; Faixová, Z. Duration of the Flaxseed Supplementation Affects Antioxidant Defence Mechanisms and the Oxidative Stress of Fattening Pigs. Vet. Sci. 2023, 10, 586. https://doi.org/10.3390/vetsci10090586
Sobeková A, Piešová E, Maková Z, Szabóová R, Sopková D, Andrejčáková Z, Vlčková R, Faixová D, Faixová Z. Duration of the Flaxseed Supplementation Affects Antioxidant Defence Mechanisms and the Oxidative Stress of Fattening Pigs. Veterinary Sciences. 2023; 10(9):586. https://doi.org/10.3390/vetsci10090586
Chicago/Turabian StyleSobeková, Anna, Elena Piešová, Zuzana Maková, Renáta Szabóová, Drahomíra Sopková, Zuzana Andrejčáková, Radoslava Vlčková, Dominika Faixová, and Zita Faixová. 2023. "Duration of the Flaxseed Supplementation Affects Antioxidant Defence Mechanisms and the Oxidative Stress of Fattening Pigs" Veterinary Sciences 10, no. 9: 586. https://doi.org/10.3390/vetsci10090586
APA StyleSobeková, A., Piešová, E., Maková, Z., Szabóová, R., Sopková, D., Andrejčáková, Z., Vlčková, R., Faixová, D., & Faixová, Z. (2023). Duration of the Flaxseed Supplementation Affects Antioxidant Defence Mechanisms and the Oxidative Stress of Fattening Pigs. Veterinary Sciences, 10(9), 586. https://doi.org/10.3390/vetsci10090586







