Long-Term Survival in Canine Hepatosplenic T-Cell Lymphoma Treated with Toceranib Phosphate Following Splenectomy: A Case of Atypical Lymphoma
Abstract
Simple Summary
Abstract
1. Introduction
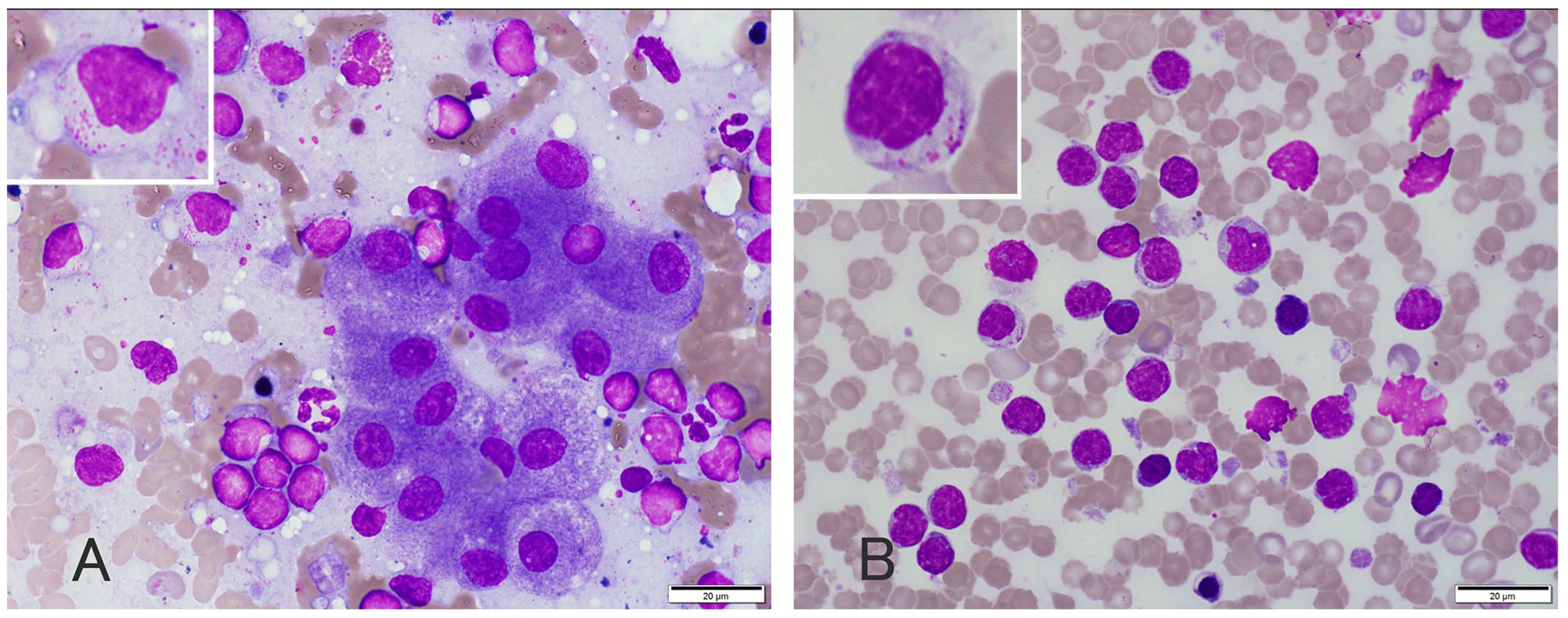
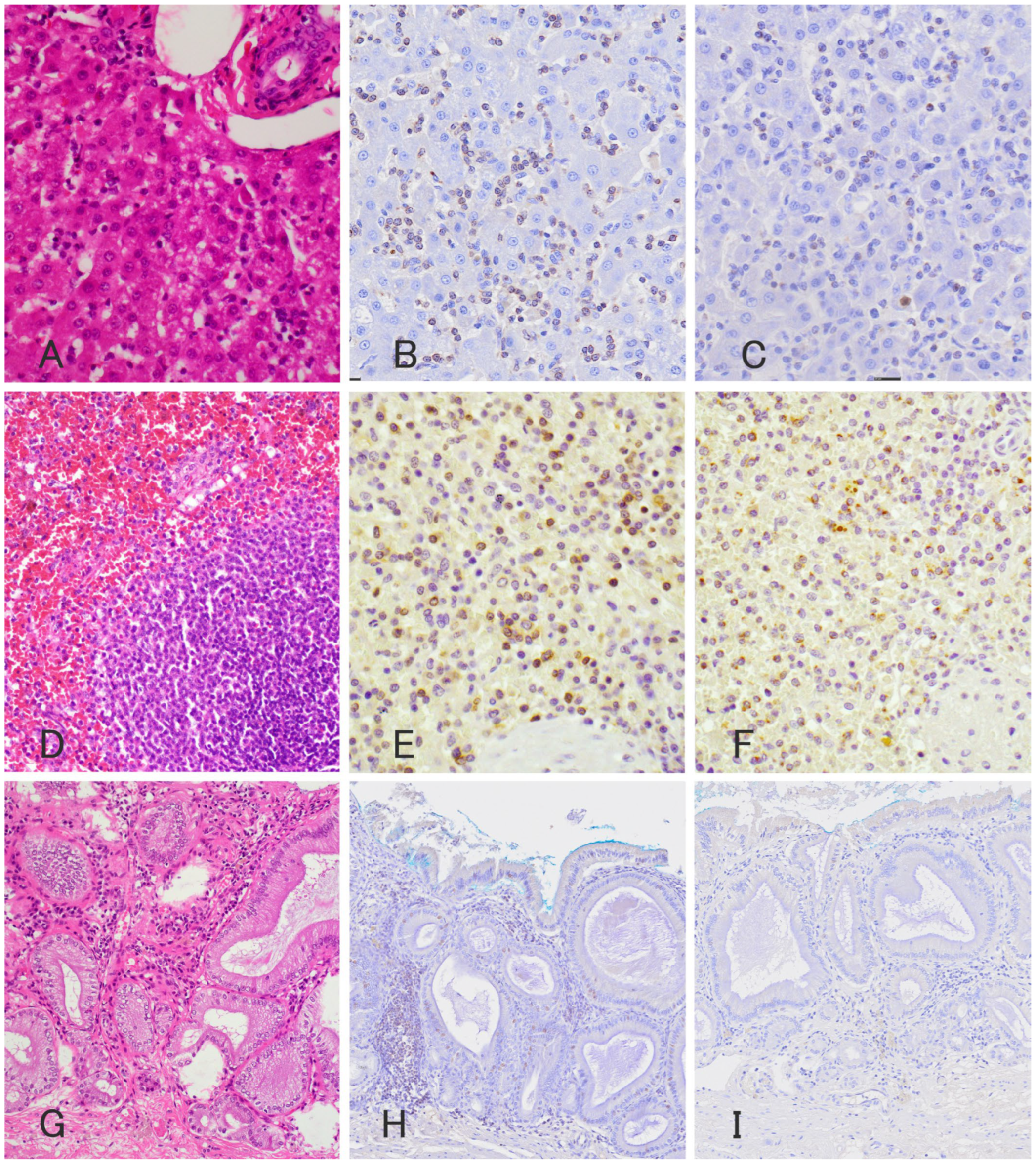
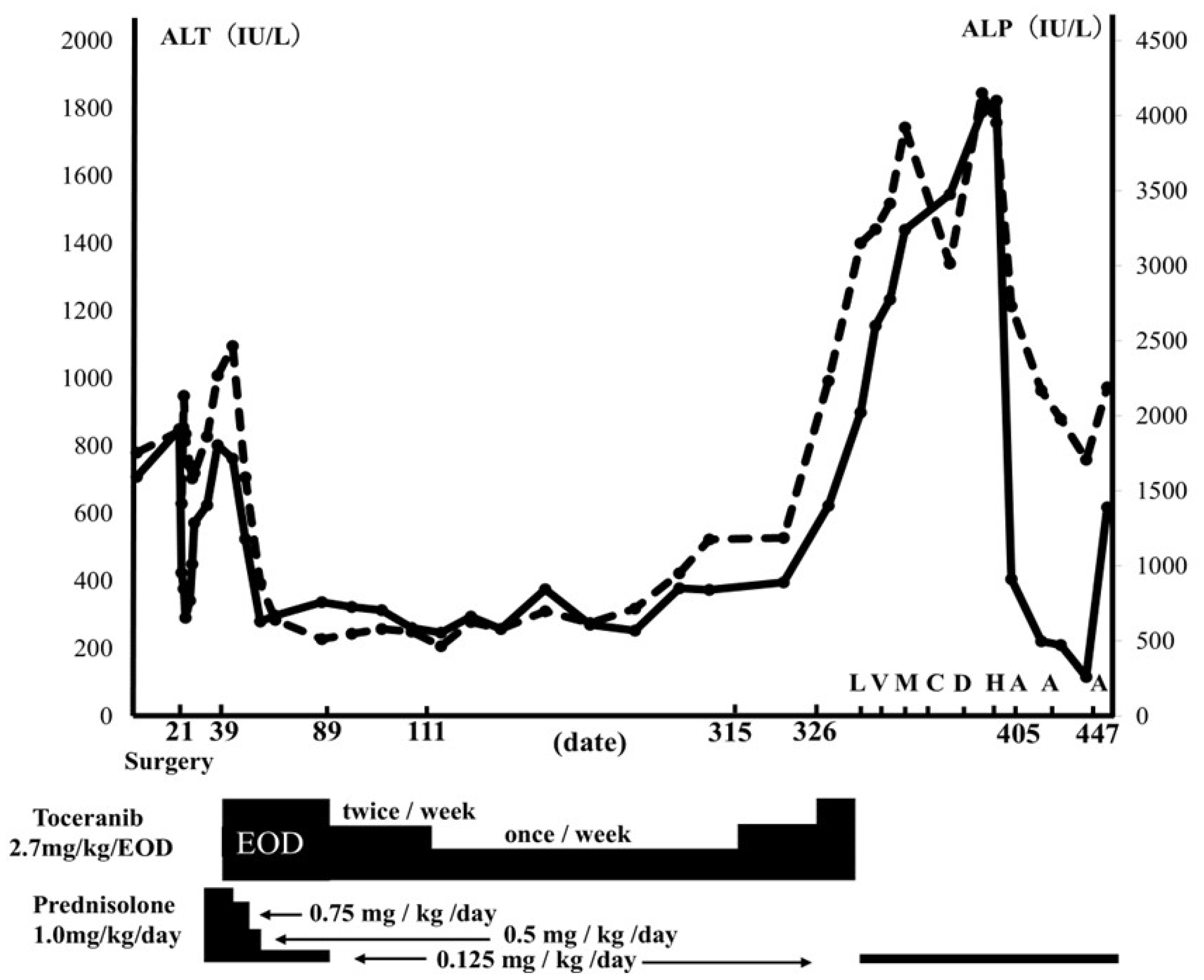
2. Case Presentation
3. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fry, M.M.; Vernau, W.; Pesavento, P.A.; Brömel, C.; Moore, P.F. Hepatosplenic lymphoma in a dog. Vet. Pathol. 2003, 40, 556–562. [Google Scholar] [CrossRef] [PubMed]
- Keller, S.M.; Vernau, W.; Hodges, J.; Kass, P.H.; Vilches-Moure, J.G.; McElliot, V.; Moore, P.F. Hepatosplenic and hepatocytotropic T-cell lymphoma: Two distinct types of T-cell lymphoma in dogs. Vet. Pathol. 2013, 50, 281–290. [Google Scholar] [CrossRef] [PubMed]
- Akiyoshi, M.; Hisasue, M.; Neo, S.; Akiyoshi, M.; Goto-Koshino, Y. A case of hemophagocytic syndrome progressing into large granular lymphoma in a dog. Vet. Clin. Pathol. 2019, 48, 71–77. [Google Scholar] [CrossRef]
- Akiyoshi, M.; Hisasue, M.; Asakawa, M.G.; Neo, S.; Akiyoshi, M. Hepatosplenic lymphoma and visceral mast cell tumor in the liver of a dog with synchronous and multiple primary tumors. Vet. Clin. Pathol. 2022, 51, 414–421. [Google Scholar] [CrossRef] [PubMed]
- London, C.A.; Malpas, P.B.; Wood-Follis, S.L.; Boucher, J.F.; Rusk, A.W.; Rosenberg, M.P.; Henry, C.J.; Mitchener, K.L.; Klein, M.K.; Hintermeister, J.G.; et al. Multi-center, placebo-controlled, double-blind, randomized study of oral toceranib phosphate (SU11654), a receptor tyrosine kinase inhibitor, for the treatment of dogs with recurrent (either local or distant) mast cell tumor following surgical excision. Clin. Cancer Res. 2009, 15, 3856–3865. [Google Scholar] [PubMed]
- Bernabe, L.F.; Portela, R.; Nguyen, S.; Kisseberth, W.C.; Pennell, M.; Yancey, M.F.; London, C.A. Evaluation of the adverse event profile and pharmacodynamics of toceranib phosphate administered to dogs with solid tumors at doses below the maximum tolerated dose. BMC Vet. Res. 2013, 9, 190. [Google Scholar] [CrossRef]
- London, C.; Mathie, T.; Stingle, N.; Clifford, C.; Haney, S.; Klein, M.K.; Beaver, L.; Vickery, K.; Vail, D.M.; Hershey, B.; et al. Preliminary evidence for biologic activity of toceranib phosphate (Palladia®) in solid tumours. Vet. Comp. Oncol. 2012, 10, 194–205. [Google Scholar]
- Pan, X.; Tsimbas, K.; Kurzman, I.D.; Vail, D.M. Safety evaluation of combination CCNU and continuous toceranib phosphate (Palladia®) in tumour-bearing dogs: A phase I dose-finding study. Vet. Comp. Oncol. 2016, 14, 202–209. [Google Scholar] [CrossRef]
- Hernon, T.L.; Friend, E.J.; Chanoit, G.; Black, V.; Meakin, L.B. The effect of flushing of the common bile duct on hepatobiliary markers and short-term outcomes in dogs undergoing cholecystectomy for the management of gall bladder mucocele: A randomized controlled prospective study. Vet. Surg. 2023, 52, 697–703. [Google Scholar] [CrossRef]
- Nakamura, M.; Takahashi, M.; Ohno, K.; Koshino, A.; Nakashima, K.; Setoguchi, A.; Fujino, Y.; Tsujimoto, H. C-reactive protein concentration in dogs with various diseases. J. Vet. Med. Sci. 2008, 70, 127–131. [Google Scholar] [CrossRef]
- Valli, V.E.; Kass, P.H.; San Myint, M.; Scott, F. Canine lymphomas: Association of classification type, disease stage, tumor subtype, mitotic rate, and treatment with survival. Vet. Pathol. 2013, 50, 738–748. [Google Scholar] [CrossRef] [PubMed]
- Elbadawy, M.; Usui, T.; Mori, T.; Tsunedomi, R.; Hazama, S.; Nabeta, R.; Uchide, T.; Fukushima, R.; Yoshida, T.; Shibutani, M.; et al. Establishment of a novel experimental model for muscle-invasive bladder cancer using a dog bladder cancer organoid culture. Cancer Sci. 2019, 110, 2806–2821. [Google Scholar] [CrossRef]
- Elbadawy, M.; Fujisaka, K.; Yamamoto, H.; Tsunedomi, R.; Nagano, H.; Ayame, H.; Ishihara, Y.; Mori, T.; Azakami, D.; Uchide, T.; et al. Establishment of an experimental model of normal dog bladder organoid using a three-dimensional culture method. Biomed. Pharmacother. 2022, 151, 113105. [Google Scholar] [CrossRef]
- Sato, Y.; Elbadawy, M.; Suzuki, K.; Tsunedomi, R.; Nagano, H.; Ishihara, Y.; Yamamoto, H.; Azakami, D.; Uchide, T.; Nabeta, R.; et al. Establishment of an experimental model of canine malignant mesothelioma organoid culture using a three-dimensional culture method. Biomed. Pharmacother. 2023, 162, 114651. [Google Scholar] [CrossRef] [PubMed]
- Veterinary Co-Operative Oncology Group. Veterinary cooperative oncology group—Common terminology criteria for adverse events (VCOG-CTCAE) following chemotherapy or biological antineoplastic therapy in dogs and cats v1.1. Vet. Comp. Oncol. 2016, 14, 417–446. [Google Scholar] [CrossRef] [PubMed]
- Yale, A.D.; Crawford, A.L.; Gramer, I.; Guillén, A.; Desmas, I.; Holmes, E.J. Large granular lymphocyte lymphoma in 65 dogs (2005–2023). Vet. Comp. Oncol. 2024, 22, 115–124. [Google Scholar] [CrossRef]
- Cienava, E.A.; Barnhart, K.F.; Brown, R.; Mansell, J.; Dunstan, R.; Credille, K. Morphologic, immunohistochemical, and molecular characterization of hepatosplenic T-cell lymphoma in a dog. Vet. Clin. Pathol. 2004, 33, 105–110. [Google Scholar] [CrossRef] [PubMed]
- Deravi, N.; Berke, O.; Woods, J.P.; Bienzle, D. Specific immunotypes of canine T cell lymphoma are associated with different outcomes. Vet. Immunol. Immunopathol. 2017, 191, 5–13. [Google Scholar] [CrossRef]
- Suwa, A.; Shimoda, T. Lymphoma-associated hemophagocytic syndrome in six dogs. J. Vet. Med. Sci. 2018, 80, 1271–1276. [Google Scholar] [CrossRef]
- Avallone, G.; Rasotto, R.; Chambers, J.K.; Miller, A.D.; Behling-Kelly, E.; Monti, P.; Berlato, D.; Valenti, P.; Roccabianca, P. Review of Histological Grading Systems in Veterinary Medicine. Vet. Pathol. 2021, 58, 809–828. [Google Scholar] [CrossRef]
- Museux, K.; Turinelli, V.; Rosenberg, D.; Rodriguez Piñeiro, I. Chronic lymphopenia and neutropenia in a dog with large granular lymphocytic leukemia. Vet. Clin. Pathol. 2019, 48, 721–724. [Google Scholar] [CrossRef] [PubMed]
- Couto, K.M.; Moore, P.F.; Zwingenberger, A.L.; Willcox, J.L.; Skorupski, K.A. Clinical characteristics and outcome in dogs with small cell T-cell intestinal lymphoma. Vet. Comp. Oncol. 2018, 16, 337–343. [Google Scholar] [CrossRef] [PubMed]
- Lane, J.; Price, J.; Moore, A.; Dandrieux, J.R.S.; Clifford, C.; Curran, K.; Choy, K.; Cannon, C. Low-grade gastrointestinal lymphoma in dogs: 20 cases (2010 to 2016). J. Small Anim. Pract. 2018, 59, 147–153. [Google Scholar] [CrossRef] [PubMed]
- Harrison, J.L.; Turek, B.J.; Brown, D.C.; Bradley, C.; Callahan Clark, J. Cholangitis and Cholangiohepatitis in Dogs: A Descriptive Study of 54 Cases Based on Histopathologic Diagnosis (2004–2014). J. Vet. Intern. Med. 2018, 32, 172–180. [Google Scholar] [CrossRef] [PubMed]
- Jablonski, S.A.; Chen, Y.X.P.; Williams, J.E.; Kendziorski, J.A.; Smedley, R.C. Concurrent hepatopathy in dogs with gallbladder mucocele: Prevalence, predictors, and impact on long-term outcome. J. Vet. Intern. Med. 2024, 38, 176–186. [Google Scholar] [CrossRef] [PubMed]
- Dank, G.; Rassnick, K.M.; Kristal, O.; Rodriguez, C.O.; Clifford, C.A.; Ward, R.; Mallett, C.L.; Gieger, T.; Segev, G. Clinical characteristics, treatment, and outcome of dogs with presumed primary hepatic lymphoma: 18 cases (1992–2008). J. Am. Vet. Med. Assoc. 2011, 239, 966–971. [Google Scholar] [CrossRef]
- Yamazaki, H.; Miura, N.; Lai, Y.C.; Takahashi, M.; Goto-Koshino, Y.; Yasuyuki, M.; Nakaichi, M.; Tsujimoto, H.; Setoguchi, A.; Endo, Y. Effects of toceranib phosphate (Palladia) monotherapy on multidrug resistant lymphoma in dogs. J. Vet. Med. Sci. 2017, 79, 1225–1229. [Google Scholar] [CrossRef]
| Day 1 | Unit | Reference Interval | Parameters | Day 1 | Unit | Reference Interval | Parameters | ||
|---|---|---|---|---|---|---|---|---|---|
| RBC | 6.22 | ×106/μL | 5.65–8.87 | - | Total proteins | 7.7 | g/dL | 5.0–7.8 | - |
| PCV | 44.8 | % | 37.3–61.7 | - | Albumin | 3.7 | g/dL | 2.6–4.0 | - |
| Hemoglobin | 14.2 | g/dL | 13.1–20.5 | - | ALT | 708 | IU/L | 17–78 | ↑ |
| MCV | 72 | fL | 61.6–73.5 | - | AST | 170 | IU/L | 17–44 | ↑ |
| MCH | 22.8 | pg | 21.2–25.9 | - | ALP | 1752 | IU/L | 47–254 | ↑ |
| MCHC | 31.7 | g/dL | 32.0–37.9 | ↓ | GGT | 28 | IU/L | 5.0–14 | ↑ |
| Reticulocytes | 26 | ×103/µL | 10–110 | - | Total Bilirubin | 2.2 | mg/dL | 0.1–0.8 | ↑ |
| WBC | 5.3 | ×103/µL | 5.05–16.76 | - | Ammonia | 20 | µg/dL | 16–78 | - |
| Neutrophils | 3.16 | ×103/µL | 2.95–11.64 | - | Glucose | 102 | mg/dL | 78–128 | - |
| Lymphocytes | 1.19 | ×103/µL | 1.05–5.1 | - | Cholesterol | 253 | mg/dL | 115–320 | - |
| Monocytes | 0.66 | ×103/µL | 0.16–1.12 | - | Triglycerides | 131 | mg/dL | 30–133 | - |
| Eossinophils | 0.24 | ×103/µL | 0.06–1.23 | - | Urea | 16.1 | mg/dL | 10.0–29.2 | - |
| Basophils | 0.9 | ×103/µL | 0–0.1 | - | Creatinine | 0.96 | mg/dL | 0.4–1.4 | - |
| Platelets | 166 | ×103/μL | 148–484 | - | Phosphorous | 3.6 | mg/dL | 1.9–5.0 | - |
| PT | 6 | s | 5.0–6.0 | - | Calcium | 10.7 | mg/dL | 9.3–12.1 | - |
| APTT | 12 | s | 11.0–16.0 | - | C-reactive protein | 2.2 | mg/dL | <0.7 | ↑ |
| Fibrinogen | 369 | mg/dL | 128–420 | - | Sodium | 151 | mmol/L | 141.0–152.0 | - |
| AT | 150 | % | 114–179 | - | Potassium | 4.4 | mmol/L | 3.8–5.0 | - |
| FDPs | 7.1 | µg/mL | 0–5 | ↑ | Chloride | 102 | mmol/L | 102–117 | - |
| D-dimer | 1.6 | µg/mL | 0–2 | - | |||||
| TAT | 0.2 | ng/mL | 0–0.2 | - |
| Chemoterapeutic Agent | Decision | Suppression Rate (%) |
|---|---|---|
| Toceranib | 〇 | 92.70% |
| Carboplatin | 〇 | 79% |
| Masitinib | 〇 | 77.60% |
| Actinomycin D | 〇 | 77.50% |
| Vinblastine | 〇 | 71.50% |
| Doxorubicin | 〇 | 63.80% |
| Cyclophosphamide | 〇 | 60.80% |
| Methotrexate | 〇 | 59.10% |
| Chlorambucil | 〇 | 58.90% |
| Vincristine | 〇 | 57.60% |
| Nimustine | 〇 | 50.20% |
| Lomustine | △ | 37.90% |
| Prednisolone | × | 17.40% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Akiyoshi, M.; Hisasue, M.; Asakawa, M.G.; Neo, S. Long-Term Survival in Canine Hepatosplenic T-Cell Lymphoma Treated with Toceranib Phosphate Following Splenectomy: A Case of Atypical Lymphoma. Vet. Sci. 2024, 11, 458. https://doi.org/10.3390/vetsci11100458
Akiyoshi M, Hisasue M, Asakawa MG, Neo S. Long-Term Survival in Canine Hepatosplenic T-Cell Lymphoma Treated with Toceranib Phosphate Following Splenectomy: A Case of Atypical Lymphoma. Veterinary Sciences. 2024; 11(10):458. https://doi.org/10.3390/vetsci11100458
Chicago/Turabian StyleAkiyoshi, Makoto, Masaharu Hisasue, Midori Goto Asakawa, and Sakurako Neo. 2024. "Long-Term Survival in Canine Hepatosplenic T-Cell Lymphoma Treated with Toceranib Phosphate Following Splenectomy: A Case of Atypical Lymphoma" Veterinary Sciences 11, no. 10: 458. https://doi.org/10.3390/vetsci11100458
APA StyleAkiyoshi, M., Hisasue, M., Asakawa, M. G., & Neo, S. (2024). Long-Term Survival in Canine Hepatosplenic T-Cell Lymphoma Treated with Toceranib Phosphate Following Splenectomy: A Case of Atypical Lymphoma. Veterinary Sciences, 11(10), 458. https://doi.org/10.3390/vetsci11100458






