Impact of Rumex nepalensis on Performance, Blood Markers, Immunity, Intestinal Microbiology and Histomorphology in Broiler Chicken
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Material
2.2. Proximate Composition
2.3. Ethical Approval
2.4. Broiler Chicks, Experimental Design and Management
2.5. Measurement of Performance
2.6. Sample Collection
2.7. Serum Biochemical Profile
2.8. Immune and Antioxidant Parameters
2.9. Microbial Examination
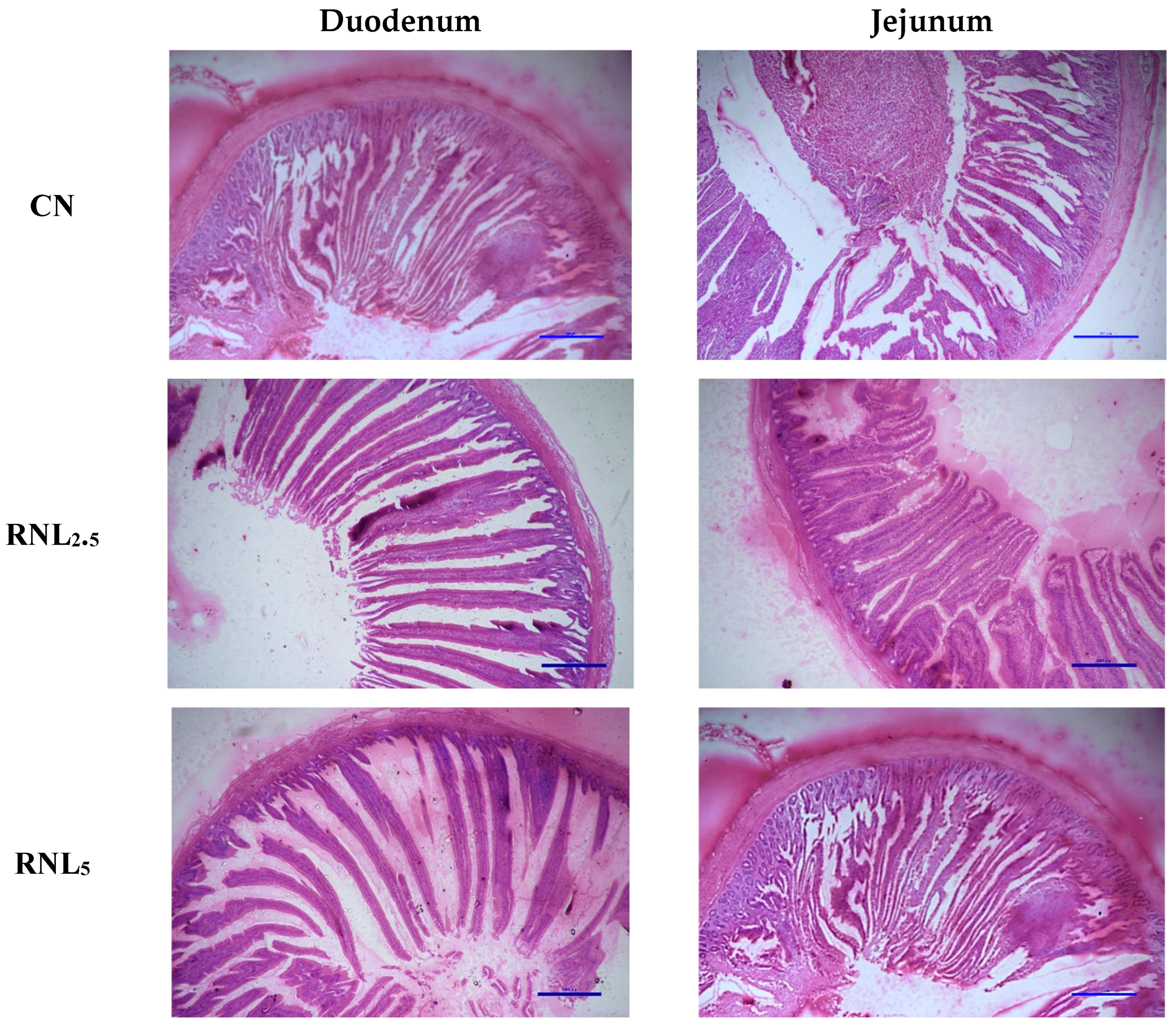
2.10. Histomorphometric Study
2.11. Statistical Analysis
3. Results
3.1. Proximate Composition of RNL
3.2. Growth Performance
3.3. Serum Biochemistry
3.4. Immune and Antioxidant Study
3.5. Microbial Enumeration
3.6. Duodenal and Jejunal Histomorphometry
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kataria, J.M.; Mohan, C.M.; Dey, S.; Dash, B.B.; Dhama, K. Diagnosis and immunoprophylaxis of economically important poultry diseases: A review. Ind. J. Anim. Sci. 2005, 1, 75. [Google Scholar]
- Angelakis, E.; Merhej, V.; Raoult, D. Related actions of probiotics and antibiotics on gut microbiota and weight modification. Lancet Infect. Dis. 2013, 13, 889–899. [Google Scholar] [CrossRef] [PubMed]
- United States Department of Agriculture. Restrictions on Antibiotic Use for Production Purposes in U.S. Livestock Industries Likely to Have Small Effects on Prices and Quantities; United States Department of Agriculture, Economic Research Service: Washington, DC, USA, 2019.
- Dianna, V.B.; Kim, M.W. Antibiotic-Free Production and Broiler Chicken Meat Safety. 2018. Available online: https://www.food-safety.com/articles/5971-antibiotic-free-production-and-broiler-chicken-meat-safety (accessed on 12 August 2024).
- Hakimul, H.; Subir, S.; Shariful, I.; Aminul, I.; Rezaul, K.; Mohammad, E.H.K. Sustainable antibiotic-free broiler meat production: Current trends, challenges, and possibilities in a developing country perspective. Biology 2020, 9, 411. [Google Scholar] [CrossRef] [PubMed]
- Letlhogonolo, A.S.; Zahra, M.H.; Tlou, G.M.; Monnye, M. The current status of the alternative use to antibiotics in poultry production: An african perspective. Antibiotics 2020, 9, 594. [Google Scholar] [CrossRef]
- Paintsil, E.K.; Ofori, L.A.; Akenten, C.W.; Fosu, D.; Ofori, S.; Lamshoft, M.; May, J.; Danso, K.O.; Krumkamp, R.; Dekker, D. Antimicrobial usage in commercial and domestic poultry farming in two communities in the Ashanti region of Ghana. Antibiotics 2021, 10, 800. [Google Scholar] [CrossRef]
- Shao, Y.; Wang, Y.; Yuan, Y.; Xie, Y. A systematic review on antibiotics misuse in livestock and aquaculture and regulation implications in China. Sci. Environ. 2021, 798, 149205. [Google Scholar] [CrossRef]
- Swaggerty, C.L.; Bortoluzzi, C.; Lee, A.; Eyng, C.; Pont, D.; Kogut, M.H. Potential replacements for antibiotic growth promoters in poultry: Interactions at the gut level and their impact on host immunity. Recent. Adv. Anim. Nutr. Metab. 2022, 1354, 145–159. [Google Scholar]
- Nhung, N.T.; Chansiripornchai, N.; Carrique-Mas, J.J. Antimicrobial resistance in bacterial poultry pathogens: A review. Front. Vet. Sci. 2017, 4, 126. [Google Scholar] [CrossRef]
- Christy, M.L.; Sampson, M.; Edson, M.; Anthony, O. Antibiotic use in agriculture and its consequential resistance in environmental sources: Potential public health implications. Molecules 2018, 23, 795. [Google Scholar] [CrossRef]
- Oniciuc, E.; Likotrafiti, E.; Alvarez-Molina, A.; Prieto, M.; Santos, J.; AlvarezOrdonez, A. The present and future of whole genome sequencing (WGS) and whole metagenome sequencing (WMS) for surveillance of antimicrobial resistant microorganisms and antimicrobial resistance genes across the food chain. Genes 2018, 9, 268. [Google Scholar] [CrossRef]
- Stanacev, V.; Puvaca, N. Selenium in poultry nutrition and its effect on meat quality. World’s Poult. Sci. J. 2011, 67, 479–484. [Google Scholar]
- Melaku, M.; Zhong, R.; Han, H.; Wan, F.; Yi, B.; Zhang, H. Butyric and citric acids and their salts in poultry nutrition: Effects on gut health and intestinal microbiota. Int. J. Mol. Sci. 2021, 22, 10392. [Google Scholar] [CrossRef] [PubMed]
- Madhupriya, V.; Shamsudeen, P.; Raj Manohar, G.; Senthilkumar, S.; Soundarapandiyan, V.; Moorthy, M. Phyto feed additives in poultry nutrition—A review. Int. J. Sci. Environ. Technol. 2018, 7, 815–822. [Google Scholar]
- Huyghebaert, G.; Ducatelle, R.; Van Immerseel, F. An update on alternatives to antimicrobial growth promoters for broilers. Vet. J. 2011, 187, 182–188. [Google Scholar] [CrossRef] [PubMed]
- Mountzouris, K.C.; Paraskevas, V.; Tsirtsikos, P.; Palamidi, I.; Steiner, T.; Schatzmayr, G.; Fegeros, K. Assessment of a phytogenic feed additive effect on broiler growth performance, nutrient digestibility and caecal microflora composition. Anim. Feed. Sci. Technol. 2011, 168, 223–231. [Google Scholar] [CrossRef]
- Tazi, S.M.; Mukhtar, M.A.; Mohamed, K.A.; Tabidi, M.H. Effect of using black pepper as natural feed additive on performance and carcass quality of broiler chicks. Global advanced research. J. Agri Sci. 2014, 4, 108–113. [Google Scholar]
- Frankic, T.; Volj, C.M.; Salobir, J.; Rezar, V. Use of herbs and spices and their extracts in animal nutrition. Acta Agric. Slov. 2009, 94, 95–102. [Google Scholar] [CrossRef]
- Suganya, T.; Senthilkumar, S.; Deepa, K.; Muralidharan, J.; Gomathi, G.; Gobiraju, S. Herbal feed additives in poultry. Int. J. Sci. Environ. Technol. 2016, 5, 1137–1145. [Google Scholar]
- Cuppett, S.L.; Hall, C.A. Antioxidant activity of Labiatae. Adv. Food Nutri. Res. 1998, 42, 245–271. [Google Scholar]
- Jarriyawattanachaikul, W.; Chaveerach, P.; Chokesajjawatee, N. Antimicrobial activity of Thai-Herbal plants against food-borne pathogens E. coli, S. aureus, and C. jejuni. Agric. Agric. Sci. Procedia 2016, 11, 20–24. [Google Scholar] [CrossRef]
- Farooq, U.; Pandith, S.A.; Saggoo, M.I.; Lattoo, S.K. Altitudinal variability in anthraquinone constituents from novel cytotypes of Rumex nepalensis Spreng-a high value medicinal herb of North Western Himalayas. Ind. Crops Prod. 2013, 50, 112–117. [Google Scholar] [CrossRef]
- Anusuya, N.A.; Gomathi, R.A.; Manian, S.E.; Sivaram, V.E.; Menon, A.N. Evaluation of Basellarubra L., Rumex nepalensis Spreng and Commelina benghalensis L. for antioxidant activity. Int. J. Pharm. Sci. 2012, 4, 714–720. [Google Scholar]
- Kensa, M. Floristic study in a Vembanur wetland, Kanyakumari District, Tamilnadu, South India. Plant Sci. Feed. 2011, 1, 194–199. [Google Scholar]
- Al-Kassie, G.A.M. The effect of thyme and cinnamon on the microbial balance in gastro intestinal tract on broiler chicks. Int. J. Poult. Sci. 2010, 9, 495–498. [Google Scholar]
- Daka, D. Antibacterial effect of garlic (Allium sativum) on Staphyloccus aureus: An in vitro study. Afr. J. Biotechnol. 2013, 10, 666–669. [Google Scholar]
- Al-Mariri, A.; Safi, M. In vitro antibacterial activity of several plant extracts and oils against some gram-negative bacteria. Iran. J. Med. Sci. 2014, 39, 36. [Google Scholar]
- Hasan, M.M.; Chowdhury, S.P.; Alam, S.; Hossain, B.; Alam, M.S. Antifungal effects of plant extracts on seed-borne fungi of wheat seed regarding seed germination, Seedling health and vigour index. Pak. J. Biol. Sci. 2005, 8, 1284–1289. [Google Scholar]
- Afzal, R.; Mughal, S.M.; Munir, M.; Sultana, K.; Qureshi, R.; Arshad, M.; Laghari, M.K. Mycoflora associated with seeds of different sunflower cultivars and its management. Pak. J. Bot. 2010, 42, 435–445. [Google Scholar]
- Tagoe, D.N.A.; Nyarko, H.D.; Akpaka, R. A comparison of the antifungal properties of onion (Allium cepa), ginger (Zingiber officinale) and garlic (Allium sativum) against Aspergillus flavus, Aspergillus niger and Cladosporium herbarum. J. Med. Plant Res. 2011, 5, 281–287. [Google Scholar] [CrossRef]
- Willis, W.L.; Wall, D.C.; Isikhuemhen, O.S.; Jackson, J.N.; Ibrahim, S.A.; Hur, S.L.; Anike, F. Effect of level and type of mushroom on performance, blood parameters and natural coccidiosis infection in floor-reared broilers. Open Mycol. J. 2013, 7, 1–6. [Google Scholar] [CrossRef]
- Zyan, K.A.; Elshourbagy, M.A.; Aggour, G.; Abdelfattah, M.A. Molecular identification of E. tenella in broiler chicks in Kalyoubia governorate and evaluation of different strategies for control cecal coccidiosis. Benha Vet. Med. J. 2017, 33, 175–182. [Google Scholar] [CrossRef]
- Hafeez, A.; Ullah, Z.; Khan, R.U.; Qudrat, U.; Naz, S. Effect of diet supplemented with coconut essential oil on performance and villus histomorphology in broiler exposed to avian coccidiosis. Trop. Anim. Health Prod. 2020, 52, 2499–2504. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.H.; Lillehoj, H.S.; Hong, Y.H.; Jang, S.I.; Lillehoj, E.P.; Ionescu, C.; Mazuranok, L.; Bravo, D. In vitro effects of plant and mushroom extracts on immunological function of chicken lymphocytes and macrophages. Br. Poult. Sci. 2010, 51, 213–221. [Google Scholar] [CrossRef] [PubMed]
- Park, I.J.; Cha, S.Y.; Kang, M.; So, Y.S.; Go, H.G.; Mun, S.P.; Ryu, K.S.; Jang, H.K. Effect of proanthocyanidin-rich extract from Pinusradiata bark on immune response of specific-pathogen-free White Leghorn chickens. Poult. Sci. 2011, 90, 977–982. [Google Scholar] [CrossRef] [PubMed]
- Abou-Elkhair, R.; Ahmed, H.A.; Selim, S. Effects of black pepper (Piper nigrum), turmeric powder (Curcuma longa) and coriander seeds (Coriandrum sativum) and their combinations as feed additives on growth performance, carcass traits, some blood parameters and humoral immune response of broiler chickens. Asian-Australas. J. Anim. Sci. 2014, 27, 847–854. [Google Scholar]
- Zaki, M.M.; Abd El-Ghany, W.A.; Hady, M.M.; Korany, R.M.S. Effect of certain phytobiotics on the immune response of Newcastle disease vaccinated broiler chickens. Asian J. Poult. Sci. 2016, 10, 134–140. [Google Scholar] [CrossRef]
- AOAC. Official Methods of Analysis, 17th ed.; Association of official Analytical Chemists: Gaithersburg, MD, USA, 2000. [Google Scholar]
- Talpatra, S.K.; Roy, S.C.; Sen, K.C. Estimation of Phosphorus, Chlorine, Calcium, Magnesium, Sodium and Potassium in feed stuffs. Indian J. Vet. Anim. Sci. 1940, 10, 243–258. [Google Scholar]
- National Research Council. Nutrient Requirements of Poultry, 9th ed.; The National Academies Press: Washington, DC, USA, 1994. [Google Scholar]
- Kakkar, P.; Das, B.; Viswanathan, P.N. A modified spectrophotometric assay of superoxide dismutase. Indian J. Biochem. Biophys. 1984, 21, 130–132. [Google Scholar]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Speck, M.L. Compendium of Methods for the Micro-Biological Examination of Foods, 2nd ed.; American Public Health Association: New York, NY, USA, 1984. [Google Scholar]
- Thompson, K.; Applegate, T. Feed withdrawal alters small-intestinal morphology and mucus of broilers. Poult. Sci. 2006, 85, 1535–1540. [Google Scholar] [CrossRef]
- Azzam, M.M.; Qaid, M.M.; Al-Mufarrej, S.I.; Al-Garadi, M.A.; Albaadani, H.H.; Alhidary, I.A. Rumex nervosus leaves meal improves body weight gain, duodenal morphology, serum thyroid hormones, and cecal microflora of broiler chickens during the starter period. Poult. Sci. 2020, 99, 5572–5581. [Google Scholar] [CrossRef] [PubMed]
- Suriya, R.; Zulkifli, I.; Alimon, A.R. The effect of dietary inclusion of herbs as growth promoter in broiler chickens. J. Vet. Adv. 2012, 11, 346–350. [Google Scholar] [CrossRef]
- Karangiya, V.K.; Savsani, H.H.; Patil, S.S.; Garg, D.D.; Murthy, K.S.; Ribadiya, N.K.; Vekariya, S.J. Effect of dietary supplementation of garlic, ginger and their combination on feed intake, growth performance and economics in commercial broilers. Vet. World 2016, 9, 245–250. [Google Scholar] [CrossRef] [PubMed]
- Remmal, A.; Achahbar, S.; Bouddine, L.; Chami, N.; Chami, F. In vitro destruction of Eimeria oocysts by essential oils. Vet. Parasitol. 2011, 182, 121–126. [Google Scholar] [CrossRef]
- Abbas, R.Z.; Colwell, D.D.; Gilleard, J. Botanicals: An alternative approach for the control of avian coccidiosis. World’s Poult. Sci. J. 2012, 68, 203–215. [Google Scholar] [CrossRef]
- El-Gendi, G.M. Effect of feeding dietary herbal feed additives on productive and metabolic responses of broiler chicks. Egypt. Poult. Sci. J. 1996, 16, 395–412. [Google Scholar]
- Alloui, N.; Alloui, M.N.; Agabou, A. Application of herbs and phytogenic feed additives in poultry production—A review. Glob. J. Anim. Sci. Res. 2014, 2, 234–243. [Google Scholar]
- Kubkomawa, H.I.; Nafarnda, D.W.; Mukang, S.M.; Tizhe, M.A.; Tuakam, D.K.; Shua, N.J.; Ugwu, C.C.; Opara, M.N.; Neils, J.S.; Okoli, I.C. Ethno-Veterinary health management practices amongst livestock producers in Africa-A review. World J. Agric. Sci. 2013, 1, 252–257. [Google Scholar]
- Windisch, W.; Schedle, K.; Plitzner, C.; Kroismayr, A. Use of phytogenic products as feed additives for swine and poultry. J. Anim. Sci. 2008, 86, 140–148. [Google Scholar] [CrossRef]
- Liu, H.N.; Liu, Y.; Hu, L.L.; Suo, Y.L.; Zhang, L.; Jin, F.; Feng, X.A.; Teng, N.; Li, Y. Effects of dietary supplementation of quercetin on performance, egg quality, cecal microflora populations, and antioxidant status in laying hens. Poult. Sci. 2014, 93, 347–353. [Google Scholar] [CrossRef]
- Murali, N.; Kumar-Phillips, G.S.; Rath, N.C.; Marcy, J.; Slavik, M.F. Effect of marinating chicken meat with lemon, green tea and turmeric against food borne bacterial pathogens. Int. J. Poult. Sci. 2012, 11, 326–332. [Google Scholar] [CrossRef]
- Tabatabaei, S.N. Effect of olibanum (Boswellia thurifera) as a feed additive on performance, some blood biochemical and intestinal morphology in broiler chicks. Res. Opin. Anim. Vet. Sci. 2016, 6, 130–134. [Google Scholar]
- Alcicek, A.H.; Bozkurt, M.; Cabuk, M.E. The effect of a mixture of herbal essential oils, an organic acid or a probiotic on broiler performance. S. Afr. J. Anim. Sci. 2004, 34, 217–222. [Google Scholar]
- Mamoun, T.; Mukhtar, A.; Tabidi, M.H. Effect of fenugreek seed powder on the performance, carcass characteristics and some blood serum attributes. Adv. Res. Agric. Vet. Sci. 2014, 1, 6–11. [Google Scholar]
- Singh, J.; Sethi, A.P.S.; Singh, P.; Kaur, J.; Hundal, J.S.; Singh, U. Response garlic supplementation on commercial broiler performance. J. Anim. Nutr. Physiol. 2015, 1, 37–41. [Google Scholar]
- Alqhtani, A.H.; Qaid, M.M.; Al-Garadi, M.A.; Al-abdullatif, A.A.; Alharthi, A.S.; Al-Mufarrej, S.I. Efficacy of Rumex nervosus leaves or Cinnamomum verum bark as natural growth promoters on the growth performance, immune responsiveness, and serum biochemical profile of broiler chickens. Ital. J. Anim. Sci. 2022, 21, 792–801. [Google Scholar] [CrossRef]
- Madubuike, F.N.; Ekenyen, B.U. Heamatology and serum biochemistry characteristics of broiler chicks fed varying dietary levels of Impomea osarifolia leaf meal. Int. J. Poult. Sci. 2006, 5, 9–12. [Google Scholar] [CrossRef][Green Version]
- Prashant, S.; Jonathan, T.; Mauricio, S.; James, S.; Peter, D. Advanced age is a risk factor for post-operative complications and mortality after a pancreaticoduodenectomy: A meta-analysis and systematic review. HPB 2012, 14, 649–657. [Google Scholar] [CrossRef]
- Campbell, T.W. Clinical chemistry of birds. Vet. Hemato. Clin. Chem. 2004, 2, 582–598. [Google Scholar]
- Azis, A.; Abbas, H.; Heryandi, Y.; Kusnadi, E. Thyroid hormone and blood metabolites concentrations of broiler chickens subjected to feeding time restriction. Media Peternak. 2012, 35, 32–37. [Google Scholar] [CrossRef]
- Alqhtani, A.H.; Qaid, M.M.; Al-Mufarrej, S.I.; Al-Garadi, M.A.; Pokoo-Aikins, A. Efficacy and optimal feeding level of Rumex nervosus leaves on blood biochemistry, carcass characteristics, productivity indices, and anticoccidial indicators of broiler chickens infected or not infected with Eimeria tenella. Braz. J. Poult. Sci. 2023, 25, 1–12. [Google Scholar] [CrossRef]
- Thaxton, J.P.; Dozier, W.A.; Branton, S.L.; Morgan, G.W.; Miles, D.W.; Roush, W.B.; Lott, B.D.; Vizzier-Thaxton, Y. Stocking density and physiological adaptive responses of broilers. Poult. Sci. 2006, 85, 819–824. [Google Scholar] [CrossRef] [PubMed]
- Ambrosy, A.P.; Dunn, T.P.; Heidenreich, P.A. Effect of minor liver function test abnormalities and values within the normal range on survival in heart failure. Am. J. Cardiol. 2015, 115, 938–941. [Google Scholar] [CrossRef]
- Murray, R.K.; Granner, D.K.; Mayes, P.A.; Rodwell, V.W. Harper’s Illustrated Biochemistry, 22nd ed.; Appleton and Lange: New York, NY, USA.
- Ileke, K.D.; Odeyemi, O.O.; Ashamo, M.O.; Oboh, G. Toxicological and histopathological effects of cheese wood, alstoniaboonei de wild stem bark powder used as cowpea protectant against cowpea bruchid, callosobruchusmaculatu (fab.) coleopteran: Chrysomelidae on albino rats. Ife J. Sci. 2014, 16, 23–33. [Google Scholar]
- Akande, T.O.; Odunsi, A.A.; Rafiu, T.A.; Olaniyi, C.O.; Binuomote, R.T. Growth and serological assessment of broiler chickens fed differently processed castor (Ricinuscommunis Linn.) kernel cake based diets. Afr. J. Agric. Res. 2013, 8, 5161–5165. [Google Scholar]
- Moustafa, N.; Aziza, A.; Orma, O.; Ibrahim, T. Effect of supplementation of broiler diets with essential oils on growth performance, antioxidant status, and general health. Mansoura Vet. Med. J. 2020, 21, 14–20. [Google Scholar] [CrossRef]
- Ahmadi, E.; Shahri, M.M. The antioxidant and anticoagu-lant effects of coumarin and quercetin from cinnamonmethanolic extract, and the assessment of cinnamon pow-der effect on plasma parameters in diabetes, and the dis-infectant activity in diabetic patients. Herb. Med. J. 2020, 4, 103–110. [Google Scholar]
- Gemechu, W.; Woldekidan, S.; Teka, F.; Mohammed, J.; Ashebir, R.; Sisay, B.; Abebe, A.; Meresa, A. Ethnomedicinal uses, phytochemistry and pharmacological activities of Rumex nervosus. JAPLR 2021, 10, 65–69. [Google Scholar] [CrossRef]
- Stanley, D.; Hughes, R.J.; Moore, R.J. Microbiota of the chicken gastrointestinal tract: Influence on health, productivity and disease. Appl. Microbiol. Biotechnol. 2014, 98, 4301–4310. [Google Scholar] [CrossRef] [PubMed]
- Bailey, R.A. Intestinal Microbiota and the Pathogenesis of Dysbacteriosis in Broiler Chickens. Ph.D. Thesis, Institute of Food Research, University of East Anglia, Norwich, UK, 2010. [Google Scholar]
- Lan, Y.; Verstegen, M.W.; Tamminga, S.; Williams, B.A. The role of the commensal gut microbial community in broiler chickens. World’s Poult. Sci. J. 2005, 61, 95–104. [Google Scholar] [CrossRef]
- Allen, H.K.; Stanton, T.B. Altered egos: Antibiotic effects on food animal microbiomes. Annu. Rev. Microbiol. 2014, 68, 297–315. [Google Scholar] [CrossRef]
- Mancabelli, L.; Ferrario, C.; Milani, C.; Mangifesta, M.; Turroni, F.; Duranti, S.; Lugli, G.A.; Viappiani, A.; Ossiprandi, M.C.; van Sinderen, D.; et al. Insights into the biodiversity of the gut microbiota of broiler chickens. Environ. Microbiol. 2016, 18, 4727–4738. [Google Scholar] [CrossRef]
- Manafi, M.; Hedayati, M.; Khalaji, S. Effectiveness of phytogenic feed additive as alternative to bacitracin methylene disalicylate on hematological parameters, intestinal histomorphology and microbial population and production performance of Japanese quails. Asian-Australas. J. Anim. Sci. 2016, 29, 1300. [Google Scholar] [CrossRef]
- Heydarian, M.; Ebrahimnezhad, Y.; Meimandipour, A.; Hosseini, S.A.; Banabazi, M.H. Effects of dietary inclusion of the encapsulated thyme and oregano essential oils mixture and probiotic on growth performance, immune response and intestinal morphology of broiler chickens. Poult. Sci. J. 2020, 8, 17–25. [Google Scholar]
- Su, G.; Zhou, X.; Wang, Y.; Chen, D.; Chen, G.; Li, Y.; He, J. Effects of plant essential oil supplementation on growth performance, immune function and antioxidant activities in weaned pigs. Lipids Health Dis. 2018, 17, 139. [Google Scholar] [CrossRef]
- Pluske, J.R.; Thompson, M.J.; Atwood, C.S.; Bird, P.H.; Williams, I.H.; Hartmann, P.E. Maintenance of villus height and crypt depth, and enhancement of disaccharide digestion and monosaccharide absorption, in piglets fed on cows’ whole milk after weaning. Br. J. Nutr. 1996, 76, 409–422. [Google Scholar] [CrossRef]
- Steiner, T. Managing Gut Health: Natural Growth Promoters as a Key to Animal Performance; Nottingham University Press: Nottingham, UK, 2006. [Google Scholar]
- Oladele, O.A.; Emikpe, B.O.; Bakare, H. Effects of dietary garlic (Allium sativum Linn.) supplementation on body weight and gut morphometry of commercial broilers. Int. J. Morphol. 2012, 30, 238–240. [Google Scholar] [CrossRef]

| Ingredients (g/kg) | Starter (7–21 Day) | Finisher (22–42 Day) |
|---|---|---|
| Maize (9.5%) * | 55.98 | 60.45 |
| Soybean meal (48%) * | 34.00 | 24.70 |
| Fish meal (55%) * | 4.00 | 3.00 |
| Mustard oil | 3.00 | 3.50 |
| Limestone | 0.80 | 0.80 |
| Di-calcium phosphate | 1.40 | 1.60 |
| Salt | 0.30 | 0.30 |
| DL-Methionine | 0.11 | 0.10 |
| Lysine | 0.06 | 0.20 |
| Trace mineral Premix 1 | 0.10 | 0.10 |
| Vitamin Premix 2 | 0.15 | 0.15 |
| Choline chloride | 0.05 | 0.05 |
| Toxin binder | 0.05 | 0.05 |
| Total | 100 | 100 |
| Analyzed nutrient | ||
| Crude Protein (%) | 21.93 | 20.01 |
| Calculated nutrient | ||
| Metabolizable Energy (Kcal/kg) | 3052.43 | 3138.25 |
| Calcium | 1.01 | 1.00 |
| Available P | 0.46 | 0.45 |
| Lysine | 1.22 | 1.13 |
| Methionine | 0.49 | 0.49 |
| Parameter | Dietary Treatments 1 | SEM | p-Value | |||
|---|---|---|---|---|---|---|
| CN | RNL2.5 | RNL5 | RNL10 | |||
| BWG, grams | ||||||
| 7–21 d | 456 b | 479 ab | 487 a | 494 a | 5.66 | p < 0.05 |
| 22–42 d | 1303 b | 1315 ab | 1347 a | 1354 a | 8.09 | p < 0.05 |
| 7–42 d | 1759 b | 1794 ab | 1837 a | 1848 a | 13.43 | p < 0.05 |
| FI, grams | ||||||
| 7–21 d | 568 | 575 | 582 | 586 | 5.04 | p > 0.05 |
| 22–42 d | 2506 | 2509 | 2504 | 2509 | 6.71 | p > 0.05 |
| 7–42 d | 3075 | 3084 | 3086 | 3094 | 9.03 | p > 0.05 |
| FCR | ||||||
| 7–21 d | 1.25 a | 1.20 b | 1.20 b | 1.19 b | 0.009 | p < 0.05 |
| 22–42 d | 1.92 a | 1.91 a | 1.86 b | 1.85 b | 0.010 | p < 0.05 |
| 7–42 d | 1.75 a | 1.72 a | 1.68 b | 1.68 b | 0.009 | p < 0.05 |
| Parameter | Dietary Treatments 1 | SEM | p-Value | |||
|---|---|---|---|---|---|---|
| CN | RNL2.5 | RNL5 | RNL10 | |||
| Total Protein (g/dL) | 3.37 | 3.29 | 3.46 | 3.51 | 0.06 | p > 0.05 |
| Glucose (mg/dL) | 225.89 | 215.14 | 205.41 | 196.37 | 6.74 | p > 0.05 |
| Cholesterol (mg/dL) | 143.74 a | 126.82 ab | 115.79 b | 103.25 b | 5.41 | p < 0.05 |
| ALT (U/L) | 19.30 | 18.27 | 16.15 | 15.02 | 1.22 | p > 0.05 |
| AST (U/L) | 144.78 | 141.19 | 137.74 | 131.30 | 4.99 | p > 0.05 |
| Creatinine (mg/dL) | 0.62 | 0.64 | 0.60 | 0.72 | 0.05 | p > 0.05 |
| Parameter | Dietary Treatments 1 | SEM | p-Value | |||
|---|---|---|---|---|---|---|
| CN | RNL2.5 | RNL5 | RNL10 | |||
| IgG (µg/mL) | 277.40 c | 292.93 bc | 315.62 ab | 332.64 a | 6.64 | p < 0.05 |
| IgM (µg/mL) | 529.74 b | 541.45 b | 553.92 ab | 577.09 a | 5.83 | p < 0.05 |
| SOD (U/mL) | 132.07 b | 135.46 b | 141.63 b | 155.88 a | 3.05 | p < 0.05 |
| MDA (nmol/mL) | 7.11 a | 7.02 ab | 6.90 bc | 6.82 c | 0.04 | p < 0.05 |
| Parameter (cfu/g) | Dietary Treatments 1 | SEM | p-Value | |||
|---|---|---|---|---|---|---|
| CN | RNL2.5 | RNL5 | RNL10 | |||
| Coliform count | 7.16 a | 6.84 ab | 6.78 ab | 6.57 b | 0.080 | p < 0.05 |
| Lactobacilli count | 5.98 | 6.04 | 6.07 | 6.11 | 0.083 | p > 0.05 |
| TPC | 8.93 | 8.75 | 8.84 | 8.71 | 0.064 | p > 0.05 |
| Parameter | Dietary Treatments 1 | SEM | p-Value | |||
|---|---|---|---|---|---|---|
| CN | RNL2.5 | RNL5 | RNL10 | |||
| Duodenum | ||||||
| Villus height (µm) | 1342 c | 1367 bc | 1383 ab | 1396 a | 6.95 | p < 0.05 |
| Crypt depth (µm) | 195 | 186 | 179 | 174 | 3.44 | p > 0.05 |
| VH/CD ratio | 6.89 b | 7.36 ab | 7.76 a | 8.02 a | 0.16 | p < 0.05 |
| Jejunum | ||||||
| Villus height (µm) | 1204 b | 1230 ab | 1247 a | 1256 a | 7.02 | p < 0.05 |
| Crypt depth(µm) | 198 | 191 | 182 | 179 | 3.27 | p > 0.05 |
| VH/CD ratio | 6.09 b | 6.46 ab | 6.85 a | 7.04 a | 0.14 | p < 0.05 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Banday, M.T.; Wani, M.A.; Othman, S.I.; Rudayni, H.A.; Allam, A.A.; Alshahrani, M.Y.; Ibrahim, E.H.; Nabi, S.; Adil, S. Impact of Rumex nepalensis on Performance, Blood Markers, Immunity, Intestinal Microbiology and Histomorphology in Broiler Chicken. Vet. Sci. 2024, 11, 463. https://doi.org/10.3390/vetsci11100463
Banday MT, Wani MA, Othman SI, Rudayni HA, Allam AA, Alshahrani MY, Ibrahim EH, Nabi S, Adil S. Impact of Rumex nepalensis on Performance, Blood Markers, Immunity, Intestinal Microbiology and Histomorphology in Broiler Chicken. Veterinary Sciences. 2024; 11(10):463. https://doi.org/10.3390/vetsci11100463
Chicago/Turabian StyleBanday, Mohammad T., Manzoor A. Wani, Sarah I. Othman, Hassan A. Rudayni, Ahmed A. Allam, Mohammad Y. Alshahrani, Essam H. Ibrahim, Showkat Nabi, and Sheikh Adil. 2024. "Impact of Rumex nepalensis on Performance, Blood Markers, Immunity, Intestinal Microbiology and Histomorphology in Broiler Chicken" Veterinary Sciences 11, no. 10: 463. https://doi.org/10.3390/vetsci11100463
APA StyleBanday, M. T., Wani, M. A., Othman, S. I., Rudayni, H. A., Allam, A. A., Alshahrani, M. Y., Ibrahim, E. H., Nabi, S., & Adil, S. (2024). Impact of Rumex nepalensis on Performance, Blood Markers, Immunity, Intestinal Microbiology and Histomorphology in Broiler Chicken. Veterinary Sciences, 11(10), 463. https://doi.org/10.3390/vetsci11100463





