Study of the Arrhythmogenic Profile of Dogs with Myxomatous Mitral Valve Disease in Stages B1 and B2
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Oyama, M.A.; Elliott, C.; Loughran, K.A.; Kossar, A.P.; Castillero, E.; Levy, R.J.; Ferrari, G. Comparative pathology of human and canine myxomatous mitral valve degeneration: 5HT and TGF-β mechanisms. Cardiovasc. Pathol. 2020, 46, 107196. [Google Scholar] [CrossRef]
- Borgarelli, M.; Savarino, P.; Crosara, S.; Santilli, R.A.; Chiavegato, D.; Poggi, M.; Bellino, C.; La Rosa, G.; Zanatta, R.; Haggstrom, J.; et al. Survival characteristics and prognostic variables of dogs with mitral regurgitation attributable to myxomatous valve disease. J. Vet. Intern. Med. 2008, 22, 120–128. [Google Scholar] [CrossRef] [PubMed]
- Nelson, R.W.; Couto, C.G. Small Animal Internal Medicine, 5th ed.; Elsevier: Amsterdam, The Netherlands, 2014. [Google Scholar]
- Crosara, S.; Borgarelli, M.; Perego, M.; Häggström, J.; La Rosa, G.; Tarducci, A.; Santilli, R.A. Heart rate variability and arrhythmias evaluated with Holter in dogs with degenerative mitral valve disease. Aust. Vet. J. 2010, 88, 386–392. [Google Scholar] [CrossRef]
- Noszczyk-Nowak, A.; Szałas, A.; Pasławska, U.; Nicpoń, J. Comparison of P-wave dispersion in healthy dogs, dogs with chronic valvular disease and dogs with disturbances of supraventricular conduction. Acta Vet. Scand. 2011, 53, 18. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Riera, A.R.; de Abreu, L.C.; Barbosa-Barros, R.; Grindler, J.; Fernandes-Cardoso, A.; Baranchuk, A. P-wave dispersion: An update. Indian Pacing Electrophysiol. J. 2016, 16, 126–133. [Google Scholar] [CrossRef] [PubMed]
- Dennis, S.G.; Summerfield, N.J.; Boswood, A. Investigation QT-interval dispersion in electrocardiogram of 81 dogs. Vet. Rec. 2002, 151, 77–82. [Google Scholar] [CrossRef]
- Van Der Linde, H.; Van de Water, A.; Loots, W.; Van Deuren, B.; Lu, H.; Van Ammel, K.; Peeters, M.; Gallacher, D. A new method to calculate the beat-to-beat instability of QT duration in drug-induced long QT in anesthetized dogs. J. Pharmacol. Toxicol. Methods 2005, 52, 168–177. [Google Scholar] [CrossRef] [PubMed]
- Rasmussen, C.E.; Falk, T.; Zois, N.; Moesgaard, S.; Häggström, J.; Pedersen, H.; Åblad, B.; Nilsen, H.; Olsen, L. Heart Rate, Heart Rate Variability, and Arrhythmias in Dogs with Myxomatous Mitral Valve Disease. J. Vet. Intern. Med. 2012, 26, 76–84. [Google Scholar] [CrossRef] [PubMed]
- Acharya, U.R.; Joseph, K.P.; Kannathal, N.; Lim, C.M.; Suri, J.S. Heart rate variability: A review. Med. Biol. Eng. Comput. 2006, 44, 1031–1051. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, M.S.; Muzzi, R.A.L.; Araújo, R.B.; Muzzi, L.A.L.; Ferreira, D.F.; Nogueira, R.; Silva, E.F. Heart rate variability parameters of myxomatous mitral valve disease in dogs with and without heart failure obtained using 24-hour Holter electrocardiography. Vet. Rec. 2012, 170, 622. [Google Scholar] [CrossRef] [PubMed]
- Cygankiewicz, I.; Zareba, W. Heart rate variability. In Handbok of Clinical Neurology, 1st ed.; Elsevier, B.V.: Amsterdam, The Netherlands, 2013; Volume 117, pp. 379–393. [Google Scholar]
- Taralov, Z.Z.; Terziyski, K.V.; Kostianev, S.S. Heart Rate Variability as a Method for Assessment of the Autonomic Nervous System and the Adaptations to Different Physiological and Pathological Conditions. Folia Medica 2015, 57, 173–180. [Google Scholar] [CrossRef] [PubMed]
- Xhyheri, B.; Manfrini, O.; Mazzolini, M.; Pizzi, C.; Bugiardini, R. Heart Rate Variability Today. Prog. Cardiovasc. Dis. 2012, 55, 321–331. [Google Scholar] [CrossRef] [PubMed]
- Brüler, B.; Jojima, F.; Dittrich, G.; Giannico, A.; Sousa, M. QT instability, an indicator of augmented arrhythmogenesis, increases with the progression of myxomatous mitral valve disease in dogs. J. Vet. Cardiol. 2018, 20, 254–266. [Google Scholar] [CrossRef]
- Keene, B.W.; Atkins, C.E.; Bonagura, J.D.; Fox, P.R.; Häggström, J.; Fuentes, V.L.; Oyama, M.A.; Rush, J.E.; Stepien, R.; Uechi, M. ACVIM consensus guidelines for the diagnosis and treatment of myxomatous mitral valve disease in dogs. J. Vet. Intern. Med. 2019, 33, 1127–1140. [Google Scholar] [CrossRef] [PubMed]
- Available in Iris. Available online: http://www.iris-kidney.com/guidelines/grading.html (accessed on 27 September 2024).
- Tilley, L.P. Essentials of Canine and Feline Electrocardiography: Interpretation and Treatment, 3rd ed.; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 1992; pp. 21–39. [Google Scholar]
- Santilli, R. Electrocardiography of the Dog & Cat, 2nd ed.; Edra S.p.A.: Milano, Italy, 2018. [Google Scholar]
- Romão, L.M.; Aleixo, A.S.; Romão, F.G.; Lima, M.D.; Tsunemi, M.; Chiacchio, S.B.; de Godoy, M.F.; Lourenço, M.L. Short-term Heart Rate Variability Analysis in Healthy Dogs of Different Ages. Acta Sci. Vet. 2022, 50, 3–9. [Google Scholar] [CrossRef]
- Alfonso, A.; Le Sueur, A.N.V.; Geraldes, S.S.; Guimarães-Okamoto, P.T.C.; Tsunemi, M.H.; Santana, D.F.; Ribeiro, V.R.F.; Melchert, A.; Chiacchio, S.B.; Lourenço, M.L.G. Heart Rate Variability and Electrocardiographic Parameters Predctive of Arrhythmias in Dogs with Stage IV Chronic Kidney Disease Undergoind Intermitent Haemodialysis. Animals 2020, 10, 1829. [Google Scholar] [CrossRef] [PubMed]
- Savarino, P.; Borgarelli, M.; Tarducci, A.; Crosara, S.; Bello, N.M.; Margiocco, M.L. Diagnostic performance of P wave duration in the identification of left atrial enlargement in dogs. J. Small Anim. Pract. 2012, 53, 267–272. [Google Scholar] [CrossRef] [PubMed]
- Brennan, M.; Palaniswami, M.; Kamen, P. Poincare’ plot interpretation using a physiological model of HRV based on a network of oscillators. Am. J. Physiol. Heart Circ. Physiol. 2002, 283, 1873–1886. [Google Scholar] [CrossRef] [PubMed]
- Baisan, R.A.; Vulpe, V.; Ohad, D.G. Short-term heart rate variability in healthy dogs and dogs in various stages of degenerative mitral valve disease evaluated before pharmacotherapy. Vet. J. 2021, 274, 105704. [Google Scholar] [CrossRef] [PubMed]
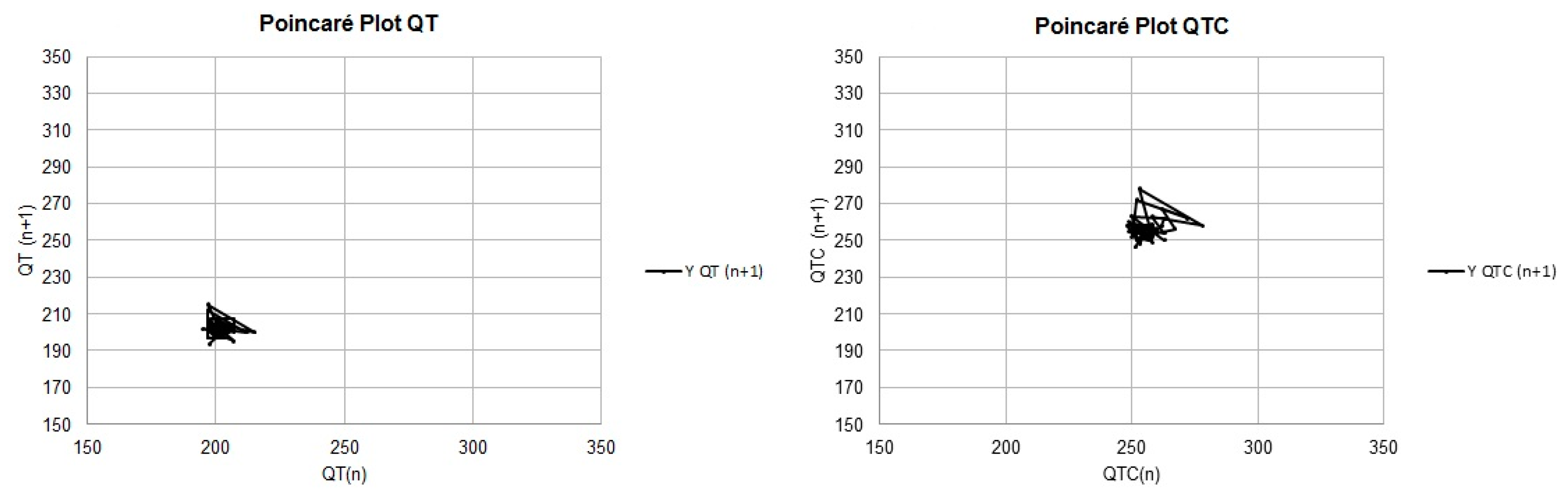
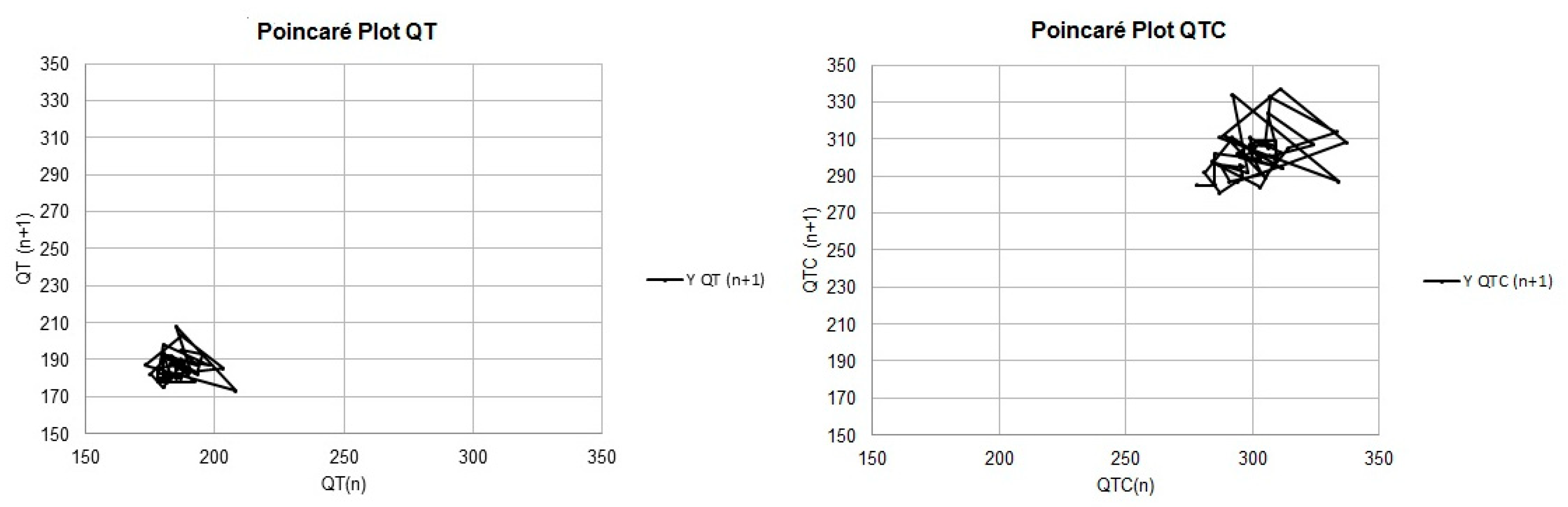
| Variables | B1 | B2 | p-Values |
|---|---|---|---|
| Age | 10.5 | 11.6 | 0.193 |
| Males | 11 (36.7%) | 17 (56.7%) | 0.1957 |
| Females | 19 (63.3%) | 13 (43.4%) | 0.1957 |
| Parameters | Mean | SD | Reference Intervals [20] | Minimum | Median | Maximum | p-Values |
|---|---|---|---|---|---|---|---|
| HR (B1) | 124.000 | 25.112 | 60–170 bpm | 78.000 | 123.500 | 169.000 | 0.182 |
| HR (B2) | 123.967 | 25.173 | 60–170 bpm | 79.000 | 124.000 | 174.000 | 0.608 |
| P (ms) (B1) | 52.367 | 4.679 | <40 ms | 43.000 | 52.500 | 62.000 | 0.448 |
| P (ms) (B2) | 53.033 | 6.139 | <40 ms | 43.000 | 52.000 | 70.000 | 0.313 |
| P (mv) (B1) | 0.231 | 0.088 | <0.4 mV | 0.058 | 0.224 | 0.461 | 0.874 |
| P (mv) (B2) | 0.263 | 0.114 | <0.4 mV | 0.070 | 0.248 | 0.547 | 0.032 |
| PR (ms) (B1) | 88.800 | 13.627 | 60–130 ms | 62.000 | 89.500 | 112.000 | 0.567 |
| PR (ms) (B2) | 90.000 | 15.148 | 60–130 ms | 62.000 | 88.500 | 123.000 | 0.035 |
| QRS (ms) (B1) | 56.433 | 7.500 | <70 ms | 45.000 | 57.000 | 73.000 | 0.261 |
| QRS (ms) (B2) | 58.567 | 6.135 | <70 ms | 45.000 | 58.000 | 73.000 | 0.972 |
| QRS (mV) (B1) | 1.060 | 0.402 | <3 mV | 0.125 | 1.068 | 1.820 | 0.995 |
| QRS (mV) (B2) | 1.604 | 0.664 | <3 mV | 0.410 | 1.586 | 3.242 | 0.595 |
| QT (ms) (B1) | 190.700 | 16.449 | 150–240 ms | 167.000 | 187.000 | 237.000 | 0.111 |
| QT (ms) (B2) | 191.767 | 15.281 | 150–240 ms | 170.000 | 190.000 | 230.000 | 0.284 |
| T (mV) (B1) | −0.132 | 0.290 | <0.05–1 mV (+ or −) | −0.715 | −0.146 | −0.781 | 0.016 |
| T (mV) (B2) | −0.106 | 0.339 | <0.05–1 mV (+ or −) | −0.733 | −0.182 | 0.672 | 0.324 |
| Parameters | p-Value |
|---|---|
| P (ms) | 0.6380 |
| P (mV) | 0.2970 |
| PR (ms) | 0.9764 |
| QRS (ms) | 0.2327 |
| QRS (mV) | 0.0004 * |
| QT (ms) | 0.7956 |
| T (mV) | 0.9823 |
| B1 | B2 | ||
|---|---|---|---|
| Parameters 1 | Mean/SD | Mean/SD | p-Values |
| P max | 55.46 ± 4.75 | 59.03 ± 6.07 | 0.0140 * |
| P min | 41.33 ± 5.66 | 44.53 ± 5.14 | 0.0256 * |
| Pd | 14.13 ± 6.76 | 14.50 ± 5.75 | 0.5280 |
| QT max | 199.63 ± 18.48 | 203.60 ± 17.62 | 0.3984 |
| QT min | 183.43 ± 18.83 | 187.43 ± 19.10 | 0.4175 |
| QTd | 16.20 ± 10.14 | 16.16 ± 5.71 | 0.3766 |
| B1 | B2 | ||
|---|---|---|---|
| Parameters 1 | Mean/SD | Mean/SD | p-Values |
| QTa | 189.33 ± 16.27 | 190.01 ± 15.94 | 0.869 |
| QTv | 15.71 ± 14.46 | 32.99 ± 61.71 | 0.358 |
| TI | 6.59 ± 1.30 | 7.34 ± 2.59 | 0.321 |
| LTI | 5.57 ± 0.94 | 5.56 ± 1.22 | 0.615 |
| STI | 2.16 ± 1.03 | 3.10 ± 2.50 | 0.047 * |
| QTca | 271.35 ± 18.28 | 275.58 ± 19.76 | 0.393 |
| QTcv | 169.56 ± 130.02 | 202.23 ± 147.40 | 0.423 |
| TIc | 15.40 ± 5.29 | 16.22 ± 6.01 | 0.580 |
| LTIc | 11.47 ± 3.57 | 12.18 ± 4.26 | 0.686 |
| STIc | 6.16 ± 3.51 | 6.82 ± 3.81 | 0.544 |
| B1 | B2 | ||
|---|---|---|---|
| Parameters 1 | Mean/SD | Mean/SD | p-Values |
| Mean RR | 492.83 ± 88.98 | 487.83 ± 95.06 | 0.6573 |
| Mean HR | 125.90 ± 23.27 | 127.10 ± 22.47 | 0.8397 |
| SDNN | 70.76 ± 42.17 | 70.39 ± 44.30 | 0.9882 |
| rMSSD | 98.60 ± 75.93 | 94.00 ± 75.96 | 0.8130 |
| pNN50 | 43.25 ± 26.56 | 38.84 ± 23.41 | 0.4976 |
| LF | 34.81 ± 17.49 | 36.95 ± 21.35 | 0.7412 |
| HF | 64.68 ± 17.26 | 62.59 ± 21.16 | 0.6761 |
| LF/HF | 0.70 ± 0.72 | 0.85 ± 0.93 | 0.7349 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Santos, B.A.; Corrêa, J.V.; Latini, C.D.; Tsunemi, M.H.; Alfonso, A.; Machado, L.H.d.A.; Lourenço, M.L.G. Study of the Arrhythmogenic Profile of Dogs with Myxomatous Mitral Valve Disease in Stages B1 and B2. Vet. Sci. 2024, 11, 467. https://doi.org/10.3390/vetsci11100467
Santos BA, Corrêa JV, Latini CD, Tsunemi MH, Alfonso A, Machado LHdA, Lourenço MLG. Study of the Arrhythmogenic Profile of Dogs with Myxomatous Mitral Valve Disease in Stages B1 and B2. Veterinary Sciences. 2024; 11(10):467. https://doi.org/10.3390/vetsci11100467
Chicago/Turabian StyleSantos, Beatriz Almeida, Jaqueline Valença Corrêa, Carolina Dragone Latini, Miriam Harumi Tsunemi, Angélica Alfonso, Luiz Henrique de Araújo Machado, and Maria Lucia Gomes Lourenço. 2024. "Study of the Arrhythmogenic Profile of Dogs with Myxomatous Mitral Valve Disease in Stages B1 and B2" Veterinary Sciences 11, no. 10: 467. https://doi.org/10.3390/vetsci11100467
APA StyleSantos, B. A., Corrêa, J. V., Latini, C. D., Tsunemi, M. H., Alfonso, A., Machado, L. H. d. A., & Lourenço, M. L. G. (2024). Study of the Arrhythmogenic Profile of Dogs with Myxomatous Mitral Valve Disease in Stages B1 and B2. Veterinary Sciences, 11(10), 467. https://doi.org/10.3390/vetsci11100467






