Vascular Alterations in Uterine and Ovarian Hemodynamics and Hormonal Analysis throughout Pregnancy Loss in Cows under Heat Stress
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Cow Housing
2.2. Study Design
2.3. B- and Color Modes for Luteal Changes and Uteroovarian Vascular Perfusion
2.4. Data and Image Analysis
2.5. Sampling and Hormonal Assaying
2.6. Statistical Analysis
3. Results
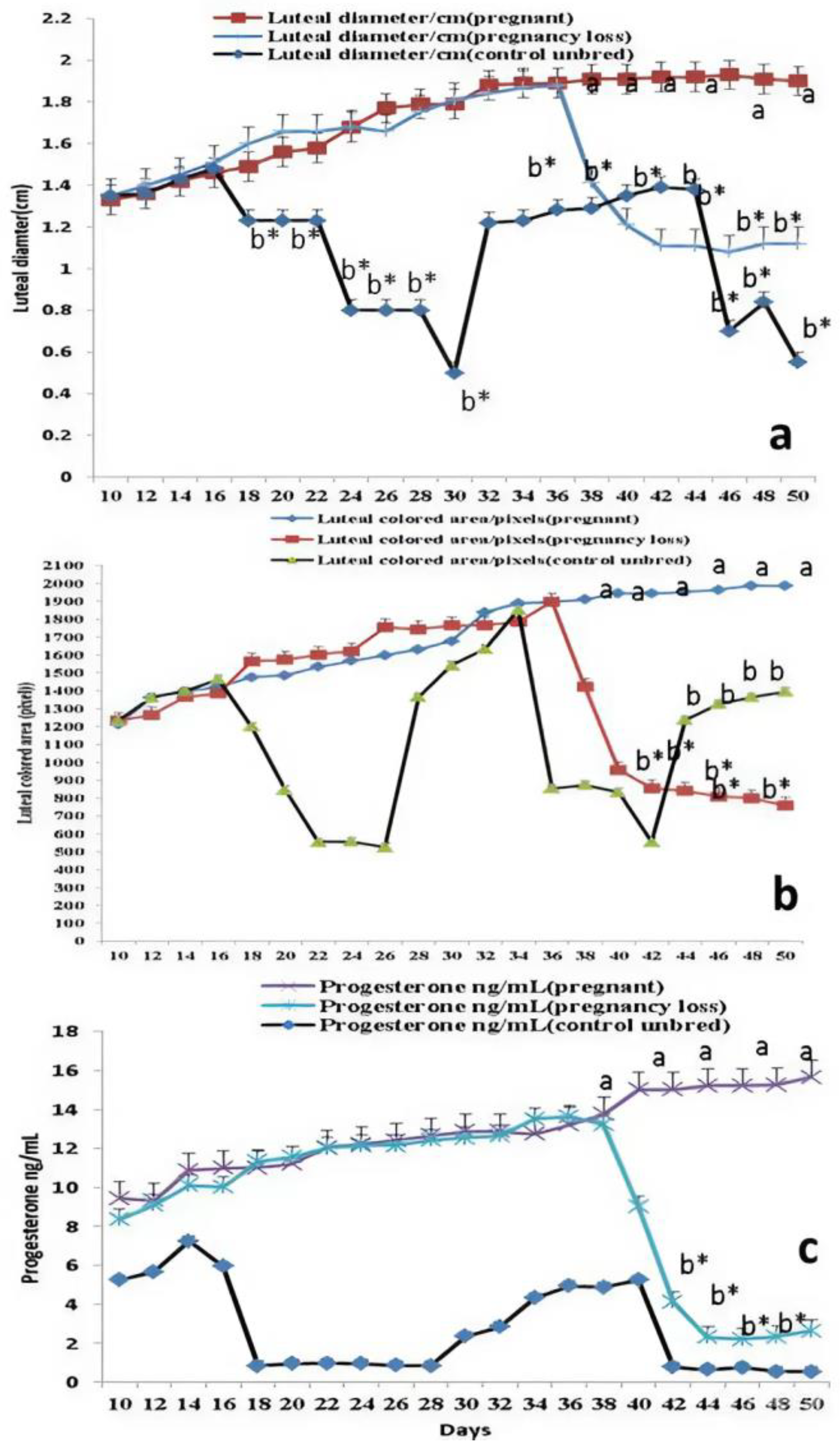
3.1. Luteal Size, Colored Area/Pixels, and Progesterone Levels
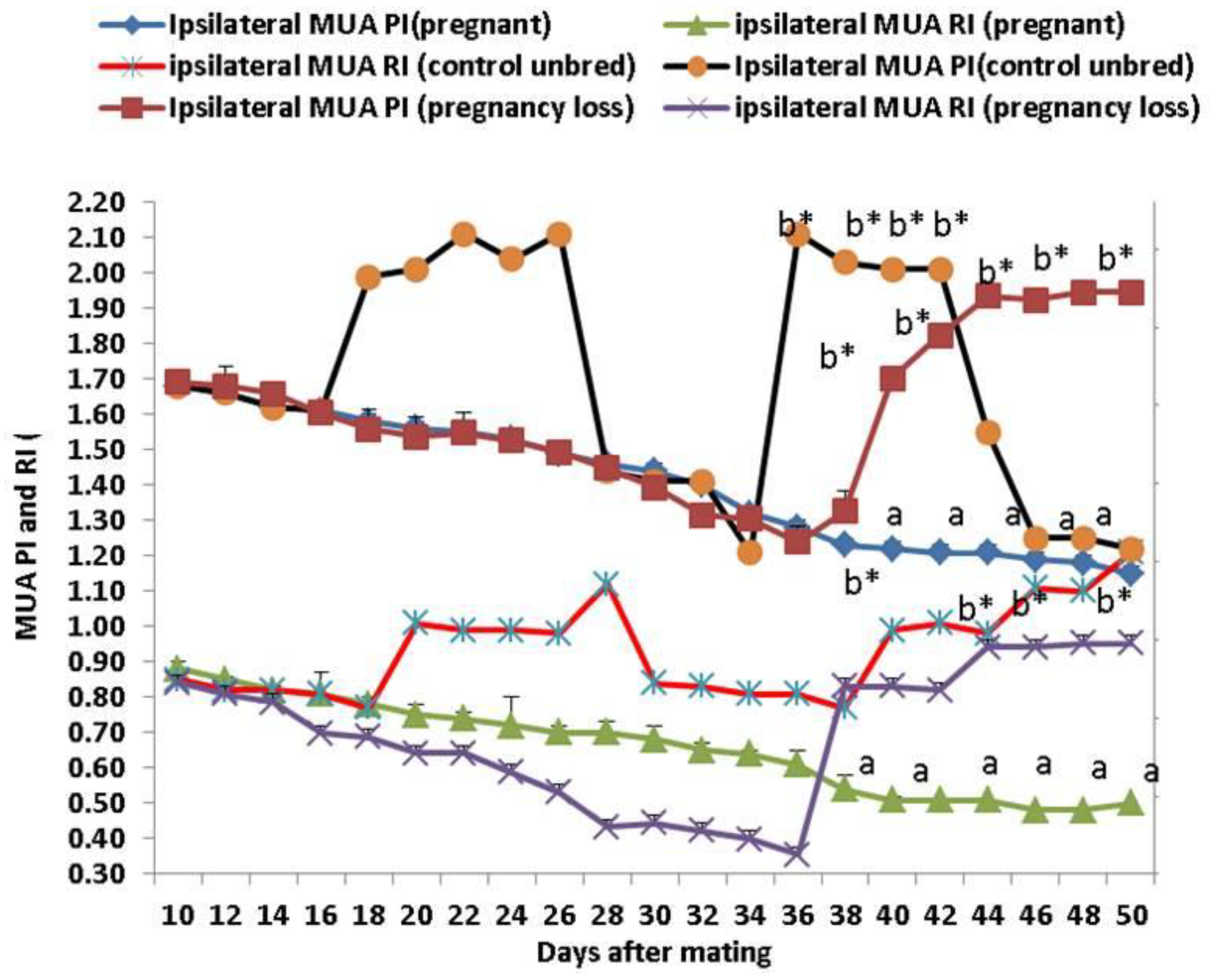
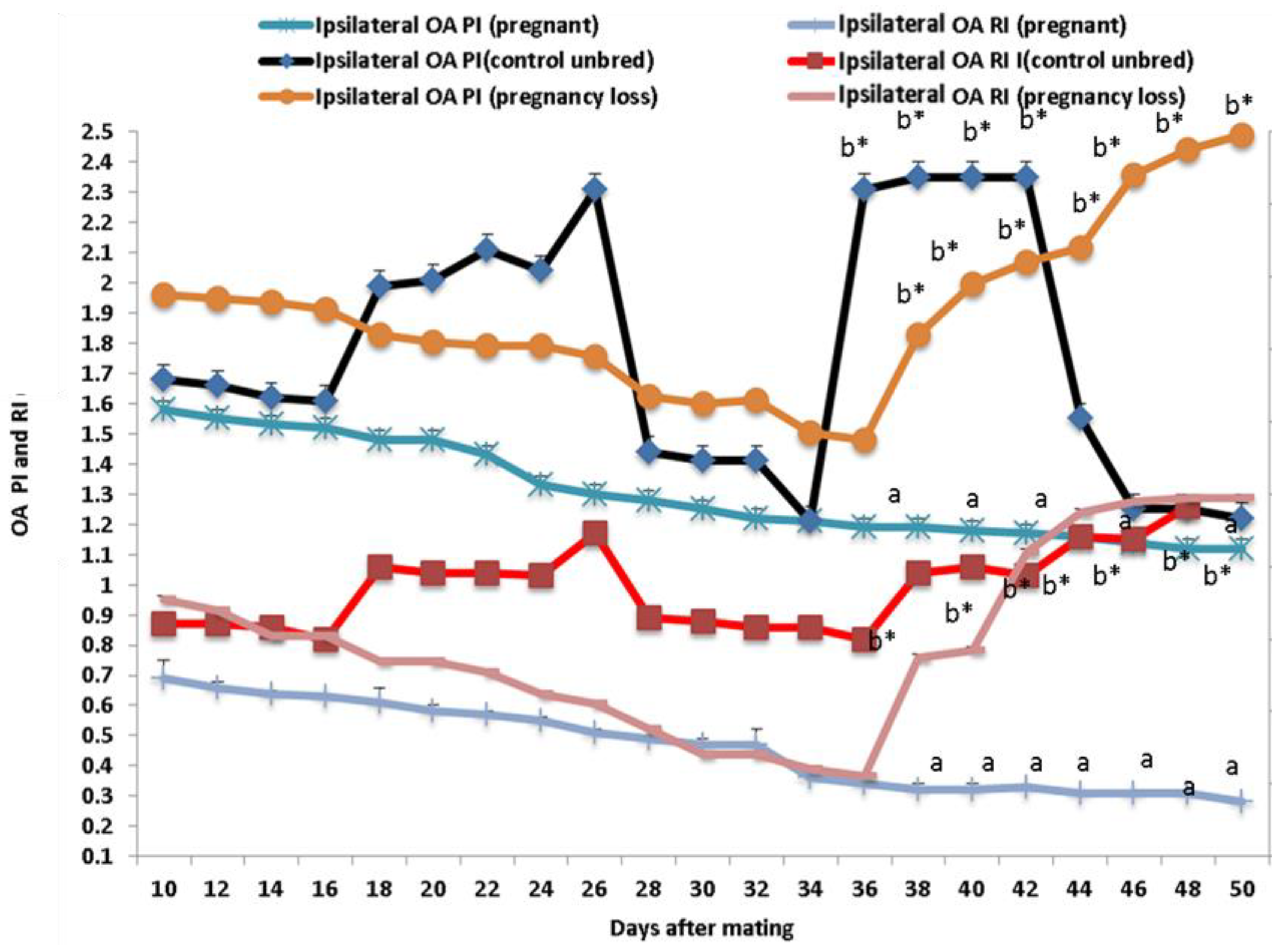
3.2. Ipsilateral Middle Uterine (MUA) and Ovarian Arteries (OA) Doppler Indices
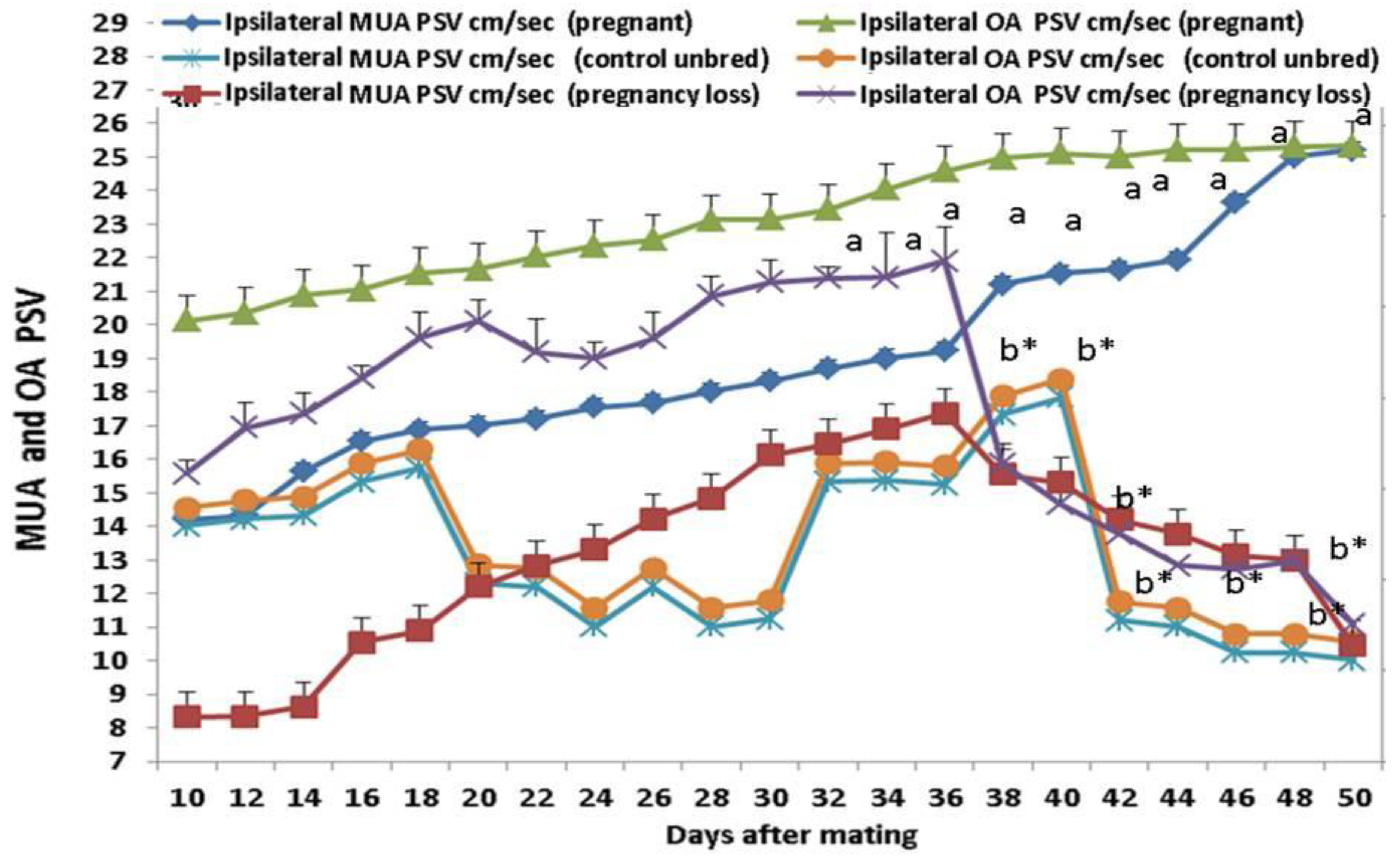
3.3. Doppler Peak Velocity in Both MUA and OA
3.4. Estradiol (E2) and Nitric Oxide Metabolites (NOMs)
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Meadows, C.; Rajala-Schultz, P.J.; Frazer, G.S. A spreadsheet-based model demonstrating the nonuniform economic effects of varying reproductive performance in Ohio dairy herds. J. Dairy Sci. 2005, 88, 1244–1254. [Google Scholar] [CrossRef] [PubMed]
- Santos, J.E.; Bisinotto, R.S.; Ribeiro, E.S.; Lima, F.S.; Greco, L.F.; Staples, C.R.; Thatcher, W.W. Applying nutrition and physiology to improve reproduction in dairy cattle. Soc. Reprod. Fertil. 2010, 67, 387–403. [Google Scholar] [CrossRef]
- Wiltbank, M.C.; Pursley, J.R. The cow as an induced ovulatory: Timed AI after synchronization of ovulation. Theriogenology 2014, 81, 170–185. [Google Scholar] [CrossRef]
- Speckhart, S.L.; Reese, S.T.; Franco, G.A.; Ault, T.B.; Oliveira, R.V.; Oliveira, A.P.; Green, J.A.; Vasconcelos, J.L.M.; Pohler, K.G. Invited Review: Detection and management of pregnancy loss in the cow herd. Appl. Anim. Sci. 2018, 34, 544–557. [Google Scholar] [CrossRef]
- Zeron, Y.; Ocheretny, A.; Kedar, O.; Borochov, A.; Sklan, D.; Arav, A. Seasonal changes in bovine fertility: Relation to developmental competence of oocytes, membrane properties and fatty acid composition of follicles. Reproduction 2001, 121, 447–454. [Google Scholar] [CrossRef]
- Hansen, P.J. Embryonic mortality in cattle from the embryo’s perspective. J. Anim. Sci. 2002, 80 (E. Suppl. 2), E33–E44. [Google Scholar] [CrossRef]
- Sartori, R.; Sartor-Bergfelt, R.; Mertens, S.A.; Guenther, J.N.; Parrish, J.J.; Wiltbank, M.C. Fertilization and early embryonic development in heifers and lactating cows in summer and lactating and dry cows in winter. J. Dairy Sci. 2002, 85, 2803–2812. [Google Scholar] [CrossRef]
- Al-Katanani, Y.M.; Paula-Lopes, F.F.; Hansen, P.J. Effect of season and exposure to heat stress on oocyte competence in Holstein cows. J. Dairy Sci. 2002, 85, 390–396. [Google Scholar] [CrossRef]
- Cartmill, J.A.; El-Zarkouny, S.Z.; Hensley, B.A.; Lamb, G.C.; Stevenson, J.S. Stage of cycle, incidence, and timing of ovulation, and pregnancy rates in dairy cattle after three timed breeding protocols. J. Dairy Sci. 2001, 84, 1051–1059. [Google Scholar] [CrossRef]
- Wolfenson, D.; Roth, Z.; Meidan, R. Impaired reproduction in heat-stressed cattle: Basic and applied aspects. Anim. Reprod. Sci. 2000, 60–61, 535–547. [Google Scholar] [CrossRef]
- Hansen, P.J. Exploitation of genetic and physiological determinants of embryonic resistance to elevated temperature to improve embryonic survival in dairy cattle during heat stress. Theriogenology 2007, 68, S242–S249. [Google Scholar] [CrossRef] [PubMed]
- Khodaei-Motlagh, M.; Shahneh, A.Z.; Masoumi, R.; Derensis, F. Alterations in reproductive hormones during heat stress in dairy cattle. Afr. J. Biotechnol. 2011, 10, 5552–5558. [Google Scholar]
- Roy, K.S.; Prakash, B.S. Seasonal variation and circadian rhythmicity of the prolactin profile during the summer months in repeat-breeding Murrah buffalo heifers. Reprod. Fertil. Dev. 2007, 19, 596–605. [Google Scholar] [CrossRef] [PubMed]
- Singh, M.; Chaudhari, B.K.; Singh, J.K.; Singh, A.K.; Maurya, P.K. Effects of thermal load on buffalo reproductive performance during summer season. J. Biol. Sci. 2013, 1, 1–8. [Google Scholar]
- Gábor, G.; Kastelic, J.P.; Abonyi-Tóth, Z.; Gábor, P.; Endródi, T.; Balogh, O.G. Pregnancy loss in dairy cattle: Relationships of ultrasound, blood pregnancy-specific protein B, progesterone and production variables. Reprod. Domest. Anim. 2016, 51, 467–473. [Google Scholar] [CrossRef]
- López-Gatius, F.; Santolaria, P.; Yàniz, J.; Rutlant, J.; López-Bèjar, M. Factors affecting pregnancy loss from gestation Day 38 to 90 in lactating dairy cows from a single herd. Theriogenology 2002, 57, 1251–1261. [Google Scholar] [CrossRef] [PubMed]
- Starbuck, M.J.; Dailey, R.A.; Inskeep, E.K. Factors affecting retention of early pregnancy in Dairy cattle. Anim. Reprod. Sci. 2004, 84, 27–39. [Google Scholar] [CrossRef]
- López-Gatius, F.; Hunter, R.H.; Garbayo, J.M.; Santolaria, P.; Yániz, J.; Serrano, B.; de Sousa, N.M.; Beckers, J.F. Plasma concentrations of pregnancy-associated glycoprotein-1 (PAG-1) in high producing dairy cows suffering early fetal loss during the warm season. Theriogenology 2007, 67, 1324–1330. [Google Scholar] [CrossRef] [PubMed]
- Utt, M.D.; Johnson, G.L.; Beal, W.E. The evaluation of corpus luteum blood flow using color-flow Doppler ultrasound for early pregnancy diagnosis in bovine embryo recipients. Theriogenology 2009, 71, 707–715. [Google Scholar] [CrossRef]
- Herzog, K.; Brockhan-Lüdeman, M.; Kaske, M.; Beindorff, N.; Paul, V.; Nieman, H.; Bollwein, H. Luteal blood flow is a more appropriate indicator for luteal function during the bovine estrous cycle than luteal size. Theriogenology 2010, 73, 691–697. [Google Scholar] [CrossRef]
- Acosta, T.J.; Yoshizawa, N.; Ohtani, M.; Miyamoto, A. Local changes in blood flow within the early and midcycle corpus luteum after prostaglandin F(2α) injection in the cow. Biol. Reprod. 2002, 66, 651–658. [Google Scholar] [CrossRef]
- Abdelnaby, E.A.; Abo El-Maaty, A.M.; Ragab, R.S.A.; Seida, A.A. Dynamics of uterine and ovarian arteries flow velocity waveforms and their relation to follicular and luteal growth and blood flow vascularization during the estrous cycle in Friesian cows. Theriogenology 2018, 121, 112–121. [Google Scholar] [CrossRef] [PubMed]
- Alcázar, J.L.; Laparte, C.; López-Garcia, G. Corpus luteum blood flow in abnormal early pregnancy. J. Ultrasound Med. 1996, 15, 645–649. [Google Scholar] [CrossRef] [PubMed]
- Bollwein, H.; Baumgartner, U.; Stolla, R. Transrectal Doppler ultrasonography of uterine blood flow in cows during pregnancy. Theriogenology 2002, 57, 2053–2061. [Google Scholar] [CrossRef]
- Silva, L.A.; Ginther, O.J. Local effects of the conceptus on uterine perfusion during early pregnancy in heifers. Reproduction 2009, 139, 453–463. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, A.M.; Pires, C.R.; Moron, A.F.; Araujo, E.J.; Traina, E.; Mattar, R. Doppler assessment of uterine blood flow in recurrent pregnancy loss. Int. J. Gynaecol. Obstet. 2007, 98, 115–119. [Google Scholar] [CrossRef] [PubMed]
- Hassan, M.; Sattar, A.; Bilal, M.; Avais, M.; Ahmad, N. Evaluation of changes in blood flow of the uterine artery by Doppler ultrasonography during the estrous cycle in lactating Bos indicus cows. Anim. Reprod. Sci. 2017, 184, 78–85. [Google Scholar] [CrossRef]
- Lüttgenau, J.; Bollwein, H. Evaluation of bovine luteal blood flow by using color Doppler ultrasonography. Reprod. Biol. 2014, 14, 103–109. [Google Scholar] [CrossRef]
- Ciernia, L.A.; Perry, G.A.; Smith, M.F.; Rich, J.J.; Northrop, E.J.; Perkins, S.D.; Green, J.A.; Zezeski, A.L.; Geary, T.W. Effect of estradiol preceding and progesterone subsequent to ovulation on proportion of postpartum beef cows pregnant. Anim. Reprod. Sci. 2021, 227, 106723. [Google Scholar] [CrossRef] [PubMed]
- Considine, R.V.; Sinha, M.K. Serum Immunoreactive- Leptin Concentrations in Normal Weight and Obese Humans. N. Engl. J. Med. 1996, 28, 573–581. [Google Scholar] [CrossRef]
- Giustarini, D.; Rossi, R.; Milzani, A.; Dalle-Donne, I. Nitrite and nitrate measurement by Griess reagent in human plasma: Evaluation of interferences and standardization. Methods Enzymol. 2008, 440, 361–380. [Google Scholar] [PubMed]
- Sasser, R.G.; Ruder, C.A.; Ivani, K.A.; Butler, J.E.; Hamilton, W.C. Detection of pregnancy by radioimmunoassay of a novel pregnancy-specific protein in serum of cows and a profile of serum concentrations during gestation. Biol. Reprod. 1986, 35, 936–942. [Google Scholar] [CrossRef] [PubMed]
- De Rensis, F.; Scaramuzzi, R.J. Heat stress and seasonal effects on reproduction in the dairy cow: A review. Theriogenology 2003, 60, 1139–1151. [Google Scholar] [CrossRef] [PubMed]
- Kanazawa, T.; Seki, M.; Ishiyama, K.; Kubo, T.; Kaneda, Y.; Sakaguchi, M.; Izaike, Y.; Takahashi, T. Pregnancy prediction on the day of embryo transfer (Day 7) and Day 14 by measuring luteal blood flow in dairy cows. Theriogenology 2016, 86, 1436–1444. [Google Scholar] [CrossRef]
- Balaro, M.F.A.; Santos, A.S.; Moura, L.F.M.; Fonseca, J.F.; Brandão, F.Z. Luteal dynamic and functionality assessment in dairy goats by luteal blood flow, luteal biometry, and hormonal assay. Theriogenology 2017, 95, 118–126. [Google Scholar] [CrossRef]
- Pinaffi, F.L.V.; Araujo, E.R.; Silva, L.A.; Ginther, O.J. Color-Doppler signals of blood flow in the corpus luteum and vascular perfusion index for ovarian and uterine arteries during expansion of the allantochorion in Bos taurus heifers. Theriogenology 2017, 102, 35–43. [Google Scholar] [CrossRef]
- Jacob, S.; Spencer, N.A.; Bullivant, S.B.; Sellergren, S.A.; Mennella, J.A.; McClintock, M.K. Effects of breastfeeding chemosignals on the human menstrual cycle. Hum. Reprod. 2004, 19, 422–429. [Google Scholar] [CrossRef] [PubMed]
- Scully, S.; Evans, A.C.; Carter, F.; Duffy, P.; Lonergan, P.; Crowe, M.A. Ultrasound monitoring of blood flow and echotexture of the corpus luteum and uterus during early pregnancy of beef heifers. Theriogenology 2015, 83, 449–458. [Google Scholar] [CrossRef]
- Miyamoto, A.; Shirasuna, K.; Wijayagunawardane, M.P.; Watanabe, S.; Hayashi, M.; Yamamoto, D.; Matsui, M.; Acosta, T.J. Blood flow: A key regulatory component of corpus luteum function in the cow. Domest. Anim. Endocrinol. 2005, 29, 329–339. [Google Scholar] [CrossRef]
- Nyman, S.; Gustafsson, H.; Berglund, B. Extent and pattern of pregnancy losses and progesterone levels during gestation in Swedish Red and Swedish Holstein dairy cows. Acta Vet. Scand. 2018, 60, 68. [Google Scholar] [CrossRef]
- Tamanini, C.; De Ambrogi, M. Angiogenesis in Developing Follicle and Corpus Luteum. Reprod. Domest. Anim. 2004, 39, 206–216. [Google Scholar] [CrossRef] [PubMed]
- Abdelnaby, E.A.; Fathi, M.; Salem, N.Y.; Ramadan, E.S.; Yehia, S.G.; Emam, I.A.; Salama, A.; Samir, H.; El-Sherbiny, H.R. Outcomes of dietary alpha-lipoic acid on testicular vascularization, steroid hormones, and seminal quality in aged Baladi bucks. BMC Vet. Res. 2024, 20, 293. [Google Scholar] [CrossRef] [PubMed]
- Abdelnaby, E.A.; Alhaider, A.K.; Ghoneim, I.M.; Salem, N.Y.; Ramadan, E.S.; Farghali, H.A.; Khattab, M.S.; AbdElKader, N.A.; Emam, I.A. Effect of pyometra on vascularity alterations, oxidative stress, histopathology and inflammatory molecules in feline. Reprod. Biol. 2024, 24, 100855. [Google Scholar] [CrossRef] [PubMed]
- Pereira, P.A.; Pinto, J.N.; Passos, F.L.M.; Campello, C.C.; Domingues, S.F.S.; da Silva, L.D.M. Study of the development of uteroplacental and fetal feline circulation by triplex Doppler. Theriogenology 2012, 77, 989–997. [Google Scholar] [CrossRef] [PubMed]
- Scotti, P.L.; Di Salvo, F.; Bocci, C.; Polisca, P.A. Doppler evaluation of maternal and fetal vessels during normal gestation in queen. Theriogenology 2008, 69, 1111–1119. [Google Scholar] [CrossRef]
- Di Salvo, P.; Bocci, F.; Zelli, R.; Polisca, A. Doppler evaluation of maternal and fetal vessels during normal gestation in the bitch. Res. Vet. Sci. 2006, 81, 382–388. [Google Scholar] [CrossRef]
- Nautrup, C.P. Doppler ultrasonography of canine maternal and fetal arteries during normal gestation. J. Reprod. Fertil. 1998, 112, 301–314. [Google Scholar] [CrossRef]
- Tinkanen, H.; Kujansuu, E.C.; Laippola, P. Vascular resistance in uterine and ovarian arteries: Its association with infertility and prognosis of infertility. Eur. J. Obstet. Gynecol. Reprod. Biol. 1994, 57, 111–115. [Google Scholar] [CrossRef]
- Tinkanen, H.; Kujansuu, E.C. The reproducibility of the Doppler ultrasound measurement of uterine artery vascular resistance. Gynecol. Obstet. Invest. 1995, 39, 188–191. [Google Scholar] [CrossRef]
- Tekay, A.; Martikainen, H.; Jouppilas, P. Blood flow changes in uterine and ovarian vasculature and predictive value on transvaginal pulsed color Doppler ultrasonography in an in vitro fertilization program. Hum. Reprod. 1995, 10, 688–693. [Google Scholar] [CrossRef]
- Ardaens, Y.; Gougeon, A.; Lefebvre, C.; Thomas, P.; Leroy, M.; Leroy, J.L.; Dewailly, D. Contribution of ovarian and uterine color Doppler in medically assisted reproduction techniques (ART). Gynecol. Obstet. Fertil. 2002, 30, 663–672. [Google Scholar] [CrossRef] [PubMed]
- Magness, R.R.; Rosenfeld, C.R. Local and systemic estradiol-17 β: Effects on uterine and systemic vasodilation. Am. J. Physiol. 1989, 256, 536–542. [Google Scholar] [CrossRef]
- Magness, R.R.; Phernetton, T.M.; Zheng, J. Systemic and uterine blood flow distribution during prolonged infusion of 17beta-estradiol. American Journal of Physiology 1998, 275, 731–743. [Google Scholar]
- Penotti, M.; Nencioni, T.; Gabrielli, L. Blood flow variations in internal carotid and middle cerebral arteries induced by postmenopausal hormone replacement therapy. Am. J. Obstet. Gynecol. 1993, 169, 1226–1232. [Google Scholar] [CrossRef]
- Hillard, T.; Thomas-Bourne, M.; Whitehead, I.; Tim, J.; Crayford, W.; Collins, P.; Campbell, S. Differential effects of transdermal estradiol and sequential progestogens on impedance to flow within the uterine arteries of postmenopausal women. Fertil. Steril. 1992, 58, 959–963. [Google Scholar] [CrossRef]
- Sarrel, P.M. The differential effect of estrogens and progestins on vascular tone. Hum. Reprod. 1999, 5, 205–209. [Google Scholar]
- De Ziegler, D.; Bessis, R.; Frydman, R. Vascular resistance of uterine arteries: Physiological effects of estradiol and progesterone. Fertil. Steril. 1991, 55, 775–779. [Google Scholar] [CrossRef] [PubMed]
- Steer, C.V.; Campbell, S.; Tan, S.L. The use of transvaginal color flow imaging after in vitro fertilization before embryo transfer. Fertil. Steril. 1992, 57, 372–376. [Google Scholar] [CrossRef] [PubMed]
- Cicinelli, E.; Ignarro, L.J.; Lograno, M. Circulating levels of color flow imaging after in vitro fertilization to identify optimum uterine conditions before embryo transfer. Fertil. Steril. 1996, 57, 372–376. [Google Scholar]
- Van-Buren, G.A.; Yang, D.; Clark, E.C. Estrogen-induced uterine vasodilatation is antagonized by L-nitro arginine methyl ester, an inhibitor of nitric oxide synthesis. Am. J. Obstet. Gynecol. 1992, 167, 828–833. [Google Scholar] [CrossRef]
- Jauniaux, E.; Johnson, M.R.; Jurkovic, D. The role of relaxin in the development of the utero-placental circulation in early pregnancy. Obstet. Gynecol. 1994, 84, 338–342. [Google Scholar] [PubMed]
- Alexis, P.M.; Pereira, N.; Murphy, E.M.; Rosenwaks, Z.; Spandorfer, S.D. How low is too low? Cycle day 28 estradiol levels and pregnancy outcomes. Fertil. Steril. 2016, 105, 905–911. [Google Scholar]
- Zarlingo, T.J.; Eis, A.L.; Brockman, D.E.; Kossenjans, W.; Myatt, L. Comparative localization of endothelial and inducible nitric oxide synthase isoforms in haemochorial and epitheliochorial placentae. Placenta 1997, 18, 511–520. [Google Scholar] [CrossRef] [PubMed]
- Magness, R.R.; Sullivan, J.A.; Li, Y.; Phernetton, T.M.; Bird, I.M. Endothelial vasodilator production by uterine and systemic arteries. VI. Ovarian and pregnancy effects on eNOS and NO(x). Am. J. Physiol. Heart Circ. Physiol. 2001, 280, 1692–1698. [Google Scholar] [CrossRef] [PubMed]
- Mateo, R.D.; Wu, M.G.; Bazer, F.W.; Park, J.C.; Shinzato, I.; Kim, S.W. Dietary L-arginine supplementation enhances the reproductive performance of gilts. J. Nutr. 2007, 137, 652–656. [Google Scholar] [CrossRef] [PubMed]
- Chwalisz, K.; Garfield, R.E. Role of nitric oxide in implantation and menstruation. Hum. Reprod. 2000, 15 (Suppl. S3), 96–111. [Google Scholar] [CrossRef] [PubMed]
- Öztürk, E.; Balat, O.; Pehlivan, S.; Uğur, M.G.; Özkan, Y.; Sever, T.E.; Seval Kul, S. Nitric oxide levels and endothelial nitric oxide synthase gene polymorphisms in Turkish women with idiopathic recurrent miscarriage. J. Turk. Ger. Gynecol. Assoc. 2011, 12, 234–238. [Google Scholar]

| Months | T | RH | THI |
|---|---|---|---|
| Nov. | 23.21 | 57.25 | 69.8 |
| Dec. | 22.31 | 54.52 | 67.5 |
| Jan. | 21.01 | 55.62 | 66.6 |
| Feb. | 21.14 | 56.32 | 66.9 |
| Mar. | 24.25 | 44.01 | 69.5 |
| Apr. | 25.32 | 44.5 | 71.2 |
| May. | 26.36 | 46.2 | 71.8 |
| Jun. | 38.11 | 51.25 | 88.9 |
| Jul. | 39.25 | 53.62 | 91.1 |
| Aug. | 42.21 | 61.25 | 97.11 |
| Sep. | 39.52 | 62.01 | 93.25 |
| Oct. | 23.21 | 56.25 | 68.25 |
| Days | PSPB (ng/mL) | |
|---|---|---|
| P | EED | |
| 10 | 20.14 a ± 1.32 | 25.32 a ± 3.25 |
| 12 | 22.22 ab ± 7.52 | 28.24 ab ± 4.62 |
| 14 | 25.21 ab ± 8.25 | 28.52 ab ± 11.65 |
| 16 | 23.41 ab ± 3.45 | 29.32 ab ± 1.85 |
| 18 | 30.18 b ± 1.85 | 34.25 b ± 1.02 |
| 20 | 32.62 b ± 6.95 | 36.52 b ± 1.66 |
| 22 | 33.47 bc ± 2.54 | 38.21 bc ± 2.32 |
| 24 | 33.63 bc ± 2.32 | 38.32 bc ± 1.62 |
| 26 | 38.14 c ± 1.63 | 40.52 c ± 1.22 |
| 28 | 38.12 c ± 1.83 | 41.22 d ± 2.51 |
| 30 | 38.45 c ± 1.15 | 41.54 d ± 2.45 |
| 32 | 40.62 cd ± 2.22 | 46.85 e ± 1.32 |
| 34 | 41.12 d ± 2.11 | 47.22 e ± 1.74 |
| 36 | 41.74 d ± 2.95 | 46.21 e ± 2.25 |
| 38 | 39.93 d ± 2.32 | 40.85 c ± 1.02 |
| 40 | 43.74 de ± 3.55 | 33.82 b* ± 6.75 |
| 42 | 42.55 e ± 2.66 | 22.47 a* ± 1.54 |
| 44 | 43.45 e ± 1.25 | 20.6 a* ± 2.32 |
| 46 | 44.75 e ± 5.26 | 18.25 a* ± 4.02 |
| 48 | 46.12 f ± 1.76 | 19.22 a* ± 1.45 |
| 50 | 47.12 f ± 1.25 | 19.74 a* ± 1.55 |
| p-value | 0.03 | 0.03 |
| Parameter | Ipsi MUA PI(EED) | Ipsi MUA RI(P) | Ipsi MUA RI(EED) | Ipsi OA PI(P) | Ipsi OA PI(EED) | Ipsi OA RI(P) | Ipsi OA RI(EED) |
|---|---|---|---|---|---|---|---|
| Ipsi MUA PI(P) | −0.862 ** | 0.391 ** | −0.672 ** | 0.491 ** | −0.55 ** | 0.66 ** | −0.74 ** |
| Ipsi MUA PI(EED) | −0.581 * | 0.847 * | −0.473 * | 0.39 * | −0.712 * | 0.558 * | |
| Ipsi MUA RI(P) | −0.369 ** | 0.547 ** | −0.431 ** | 0.224 * | −0.411 * | ||
| Ipsi MUA RI(EED) | −0.324 * | 0.732 * | −0.558 * | 0.354 * | |||
| Ipsi OA PI(P) | −0.395 * | 0.466 * | −0.265 * | ||||
| Ipsi OA PI(EED) | −0.651 * | 0.462 * | |||||
| Ipsi OA RI(P) | −0.577 ** |
| Days | Estradiol (pg/mL) | NOMs (µmol/L) | ||||
|---|---|---|---|---|---|---|
| C | P | EED | C | P | EED | |
| 10 | 81.54 ± 1.82 | 127.14 a ± 1.32 | 125.32 a ± 3.25 | 26.36 ± 3.25 | 45.23 a ± 2.31 | 46.32 a ± 2.63 |
| 12 | 83.22 ± 7.72 | 133.22 ab ± 7.52 | 138.24 ab ± 4.62 | 22.36 ± 6.25 | 49.32 ab ± 1.96 | 51.66 ab ± 2.84 |
| 14 | 88.21 ± 8.25 | 135.21 ab ± 8.25 | 134.52 ab ± 11.65 | 27.65 ± 4.25 | 51.32 ab ± 1.88 | 52.32 ab ± 2.93 |
| 16 | 88.41 ± 3.55 | 139.41 ab ± 3.45 | 142.32 ab ± 11.85 | 26.39 ± 1.25 | 52.32 ab ± 2.01 | 54.32 ab ± 1.88 |
| 18 | 78.48 ± 1.85 | 174.18 b ± 1.85 | 168.25 b ± 14.02 | 28.36 ± 2.14 | 52.66 ab ± 3.54 | 54.88 ab ± 1.36 |
| 20 | 97.82 ± 6.95 | 177.62 b ± 6.95 | 174.32 b ± 18.66 | 26.35 ± 2.05 | 52.48 ab ± 3.65 | 57.32 b ± 8.32 |
| 22 | 82.47 ± 1.54 | 182.47 bc ± 21.54 | 185.21 bc ± 21.32 | 24.65 ± 1.65 | 53.66 ab ± 1.65 | 56.77 b ± 7.32 |
| 24 | 88.36 ± 6.98 | 188.63 bc ± 28.32 | 192.32 bc ± 17.62 | 28.64 ± 1.22 | 58.31 bc ± 2.14 | 59.32 bc ± 4.62 |
| 26 | 75.62 ± 6.75 | 199.14 c ± 17.63 | 195.52 c ± 14.22 | 27.48 ± 1.25 | 63.12 c ± 1.05 | 62.32 cd ± 2.85 |
| 28 | 77.68 ± 7.11 | 201.12 c ± 11.63 | 207.25 c ± 18.62 | 28.65 ± 1.02 | 62.21 c ± 1.88 | 62.11 cd ± 2.36 |
| 30 | 85.41 ± 3.55 | 211.45 c ± 17.65 | 222.62 cd ± 17.21 | 27.10 ± 1.62 | 67.99 c ± 1.74 | 63.01 cd ± 4.12 |
| 32 | 88.48 ± 1.85 | 221.62 cd ± 18.22 | 228.85 d ± 10.32 | 23.65 ± 1.25 | 68.25 cd ± 1.21 | 64.33 cd ± 1.62 |
| 34 | 87.82 ± 6.95 | 235.12 d ± 20.31 | 250.22 ef ± 14.74 | 26.33 ± 1.66 | 68.32 cd ± 11.31 | 64.32 cd ± 6.85 |
| 36 | 82.67 ± 1.54 | 241.74 d ± 20.95 | 256.21 ef ± 16.25 | 25.32 ± 1.36 | 68.54 cd ± 3.65 | 51.66 d* ± 7.32 |
| 38 | 98.86 ± 6.98 | 243.33 d ± 11.32 | 271.85 g ± 18.02 | 24.65 ± 1.25 | 71.62 d ± 4.22 | 47.57 a* ± 6.66 |
| 40 | 88.55 ± 5.75 | 252.74 de ± 13.55 | 245.62 d ± 17.11 | 29.71 ± 5.22 | 71.88 d ± 6.82 | 47.12 a* ± 4.62 |
| 42 | 77.36 ± 8.77 | 256.55 e ± 2.66 | 229.12 cd ± 18.22 | 28.33 ± 4.25 | 71.21 d ± 1.88 | 45.32 a* ± 0.65 |
| 44 | 84.62 ± 8.78 | 258.45 e ± 11.25 | 210.21 c ± 14.25 | 26.95 ± 5.62 | 72.44 d ± 2.10 | 41.24 a* ± 0.77 |
| 46 | 86.35 ± 9.78 | 259.75 e ± 15.26 | 133.25 ab ± 9.02 | 28.32 ± 2.14 | 72.36 d ± 4.01 | 41.95 a* ± 1.21 |
| 48 | 74.32 ± 2.15 | 277.12 f ± 11.76 | 127.22 a ± 11.45 | 27.36 ± 1.32 | 78.32 e ± 0.54 | 30.14 a* ± 0.88 |
| 50 | 77.98 ± 6.55 | 281.12 f ± 10.25 | 122.74 a ± 1.55 | 25.66 ± 5.26 | 79.55 e ± 1.99 | 30.02 a* ± 0.74 |
| p-value | 0.87 | 0.01 | 0.01 | 0.68 | 0.01 | 0.01 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abdelnaby, E.A.; Alhaider, A.K.; Ghoneim, I.M.; Emam, I.A. Vascular Alterations in Uterine and Ovarian Hemodynamics and Hormonal Analysis throughout Pregnancy Loss in Cows under Heat Stress. Vet. Sci. 2024, 11, 479. https://doi.org/10.3390/vetsci11100479
Abdelnaby EA, Alhaider AK, Ghoneim IM, Emam IA. Vascular Alterations in Uterine and Ovarian Hemodynamics and Hormonal Analysis throughout Pregnancy Loss in Cows under Heat Stress. Veterinary Sciences. 2024; 11(10):479. https://doi.org/10.3390/vetsci11100479
Chicago/Turabian StyleAbdelnaby, Elshymaa A., Abdulrhman K. Alhaider, Ibrahim M. Ghoneim, and Ibrahim A. Emam. 2024. "Vascular Alterations in Uterine and Ovarian Hemodynamics and Hormonal Analysis throughout Pregnancy Loss in Cows under Heat Stress" Veterinary Sciences 11, no. 10: 479. https://doi.org/10.3390/vetsci11100479
APA StyleAbdelnaby, E. A., Alhaider, A. K., Ghoneim, I. M., & Emam, I. A. (2024). Vascular Alterations in Uterine and Ovarian Hemodynamics and Hormonal Analysis throughout Pregnancy Loss in Cows under Heat Stress. Veterinary Sciences, 11(10), 479. https://doi.org/10.3390/vetsci11100479






