Correlation between 146S Antigen Content in Foot-and-Mouth Disease Inactivated Vaccines and Immunogenicity Level and Vaccine Potency Alternative Test Methods
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Viruses and Vaccines
2.2. Quantification of 146S
2.3. Animal Immunization and Challenge
2.4. Serological Assays
2.4.1. Total Anti-FMDV Antibody Assay
2.4.2. Interferon (IFN)-γ Assay
2.5. Statistical Analyses
3. Results
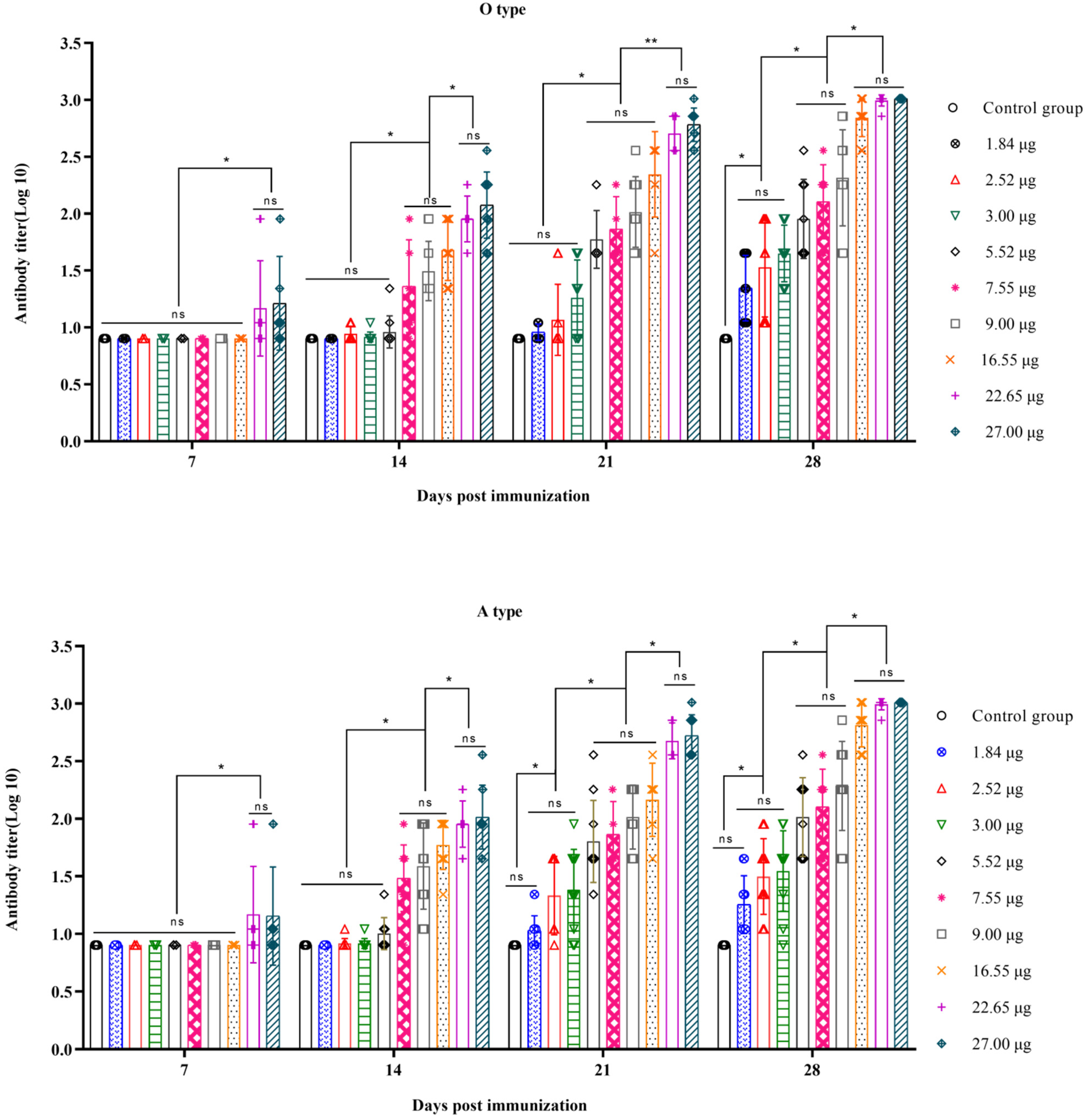
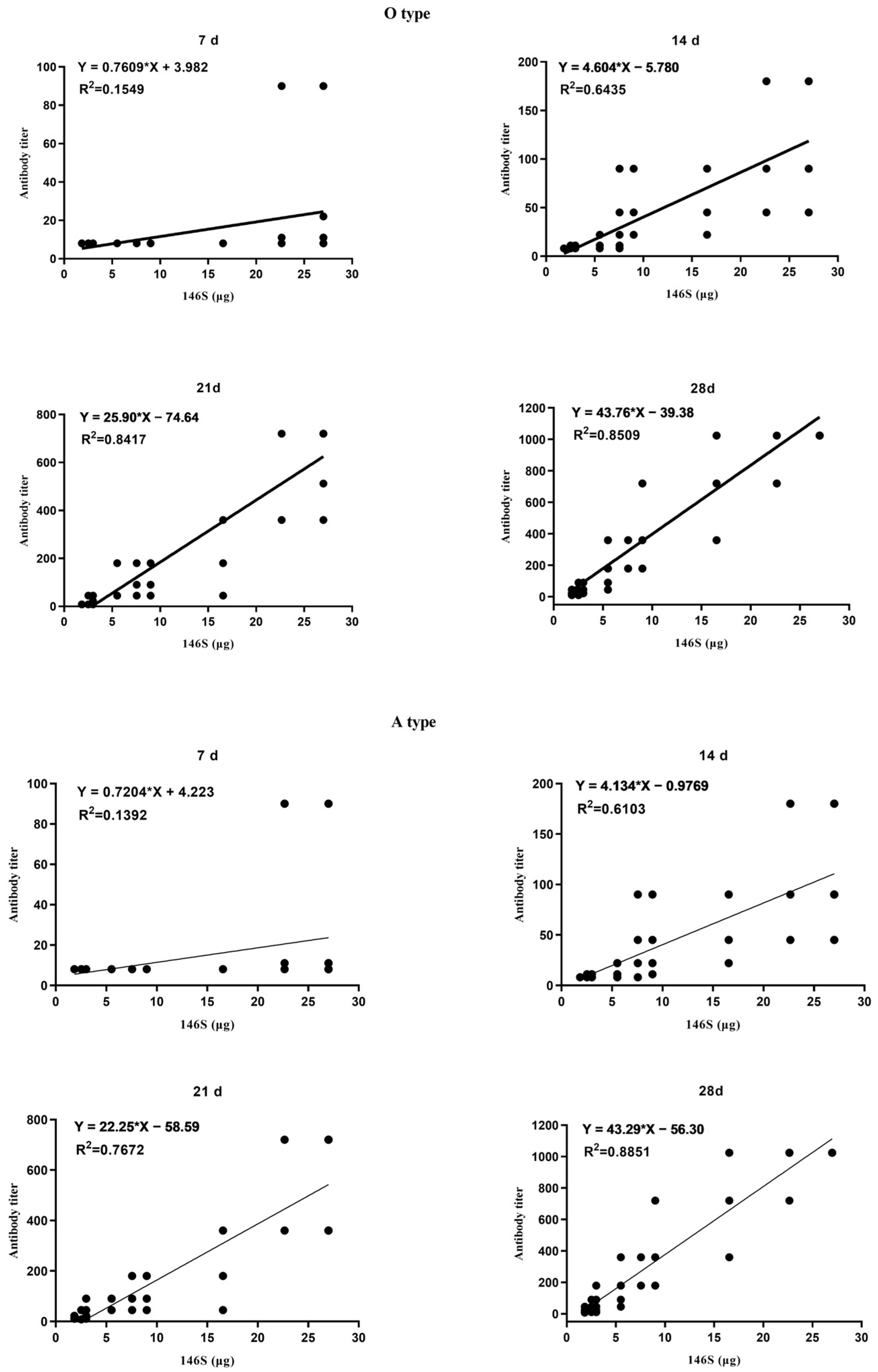
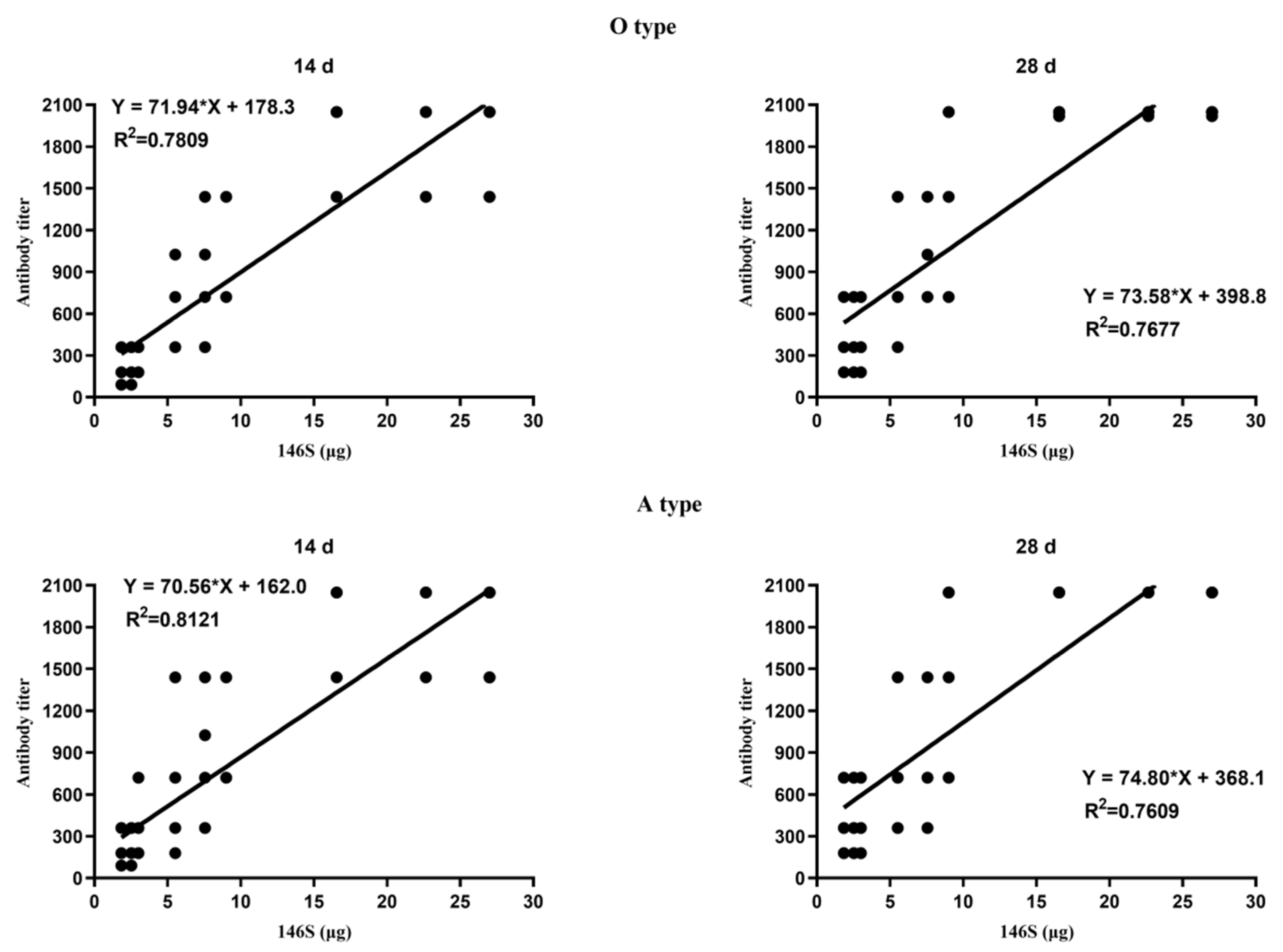
3.1. Correlation between the Quantity of 146S and the Antibody Titer after the First Immunization
3.2. Correlation between the Quantity of 146S and the Qualification Rate after the First Imunization
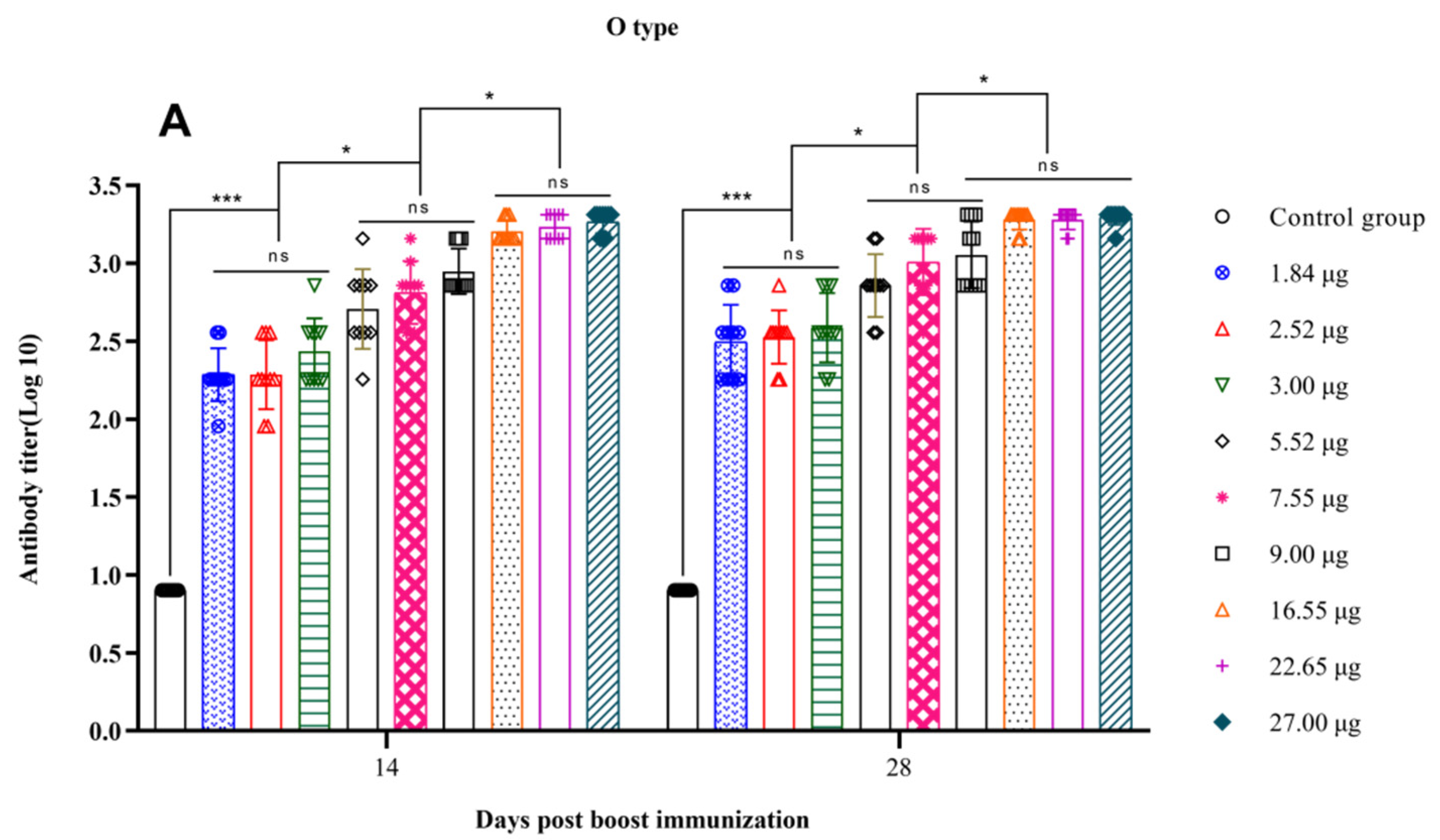
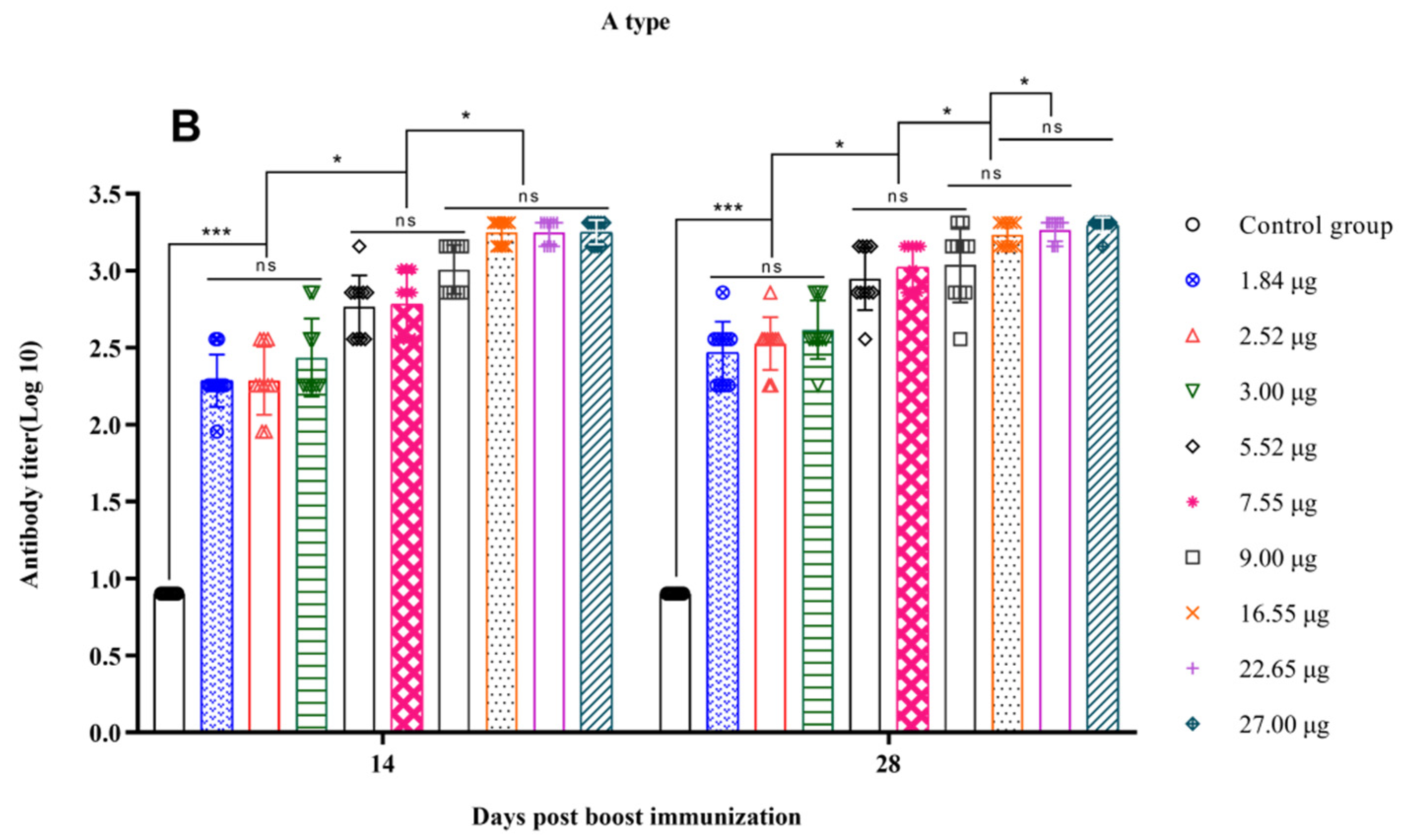
3.3. Correlation between the Quantity of 146S and the Antibody Titer after Boost Immunization
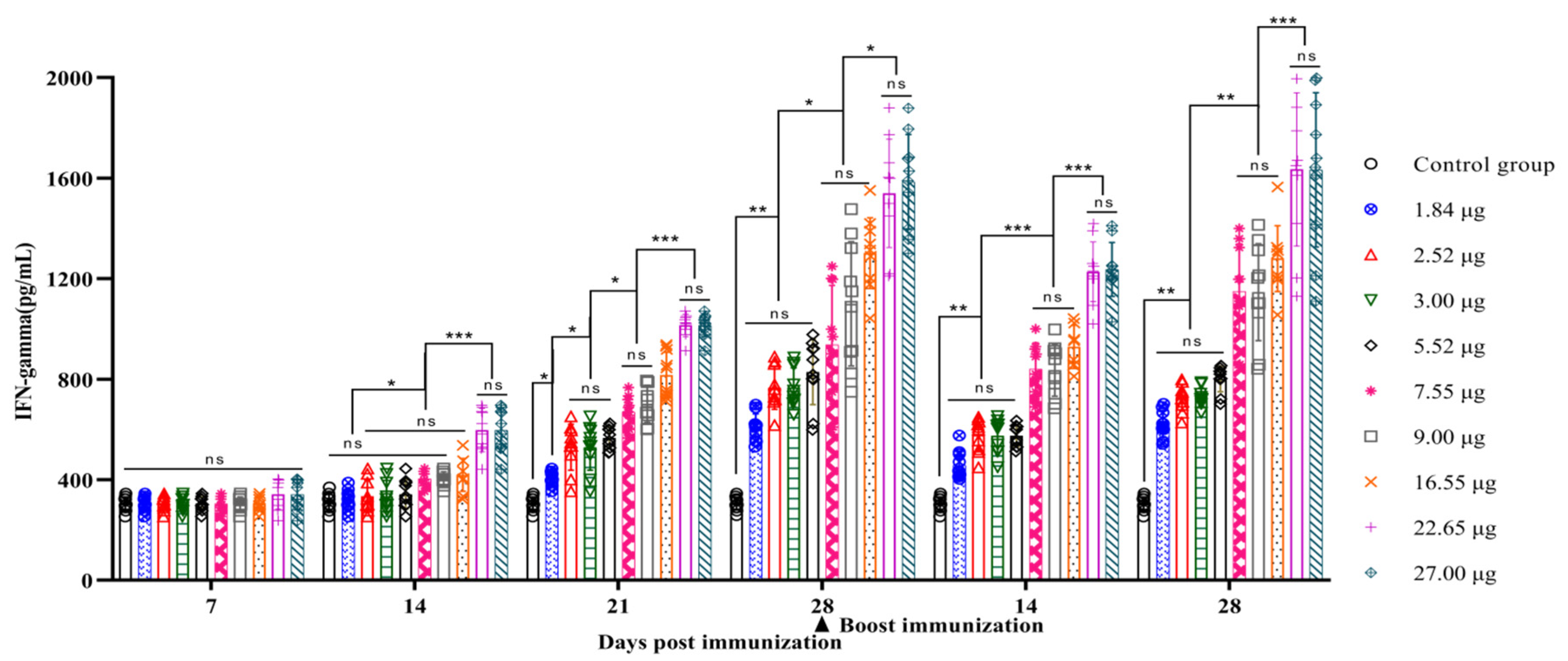
3.4. Correlation between the Quantity of 146S and the IFN-γ Secretion Levels after Immunization
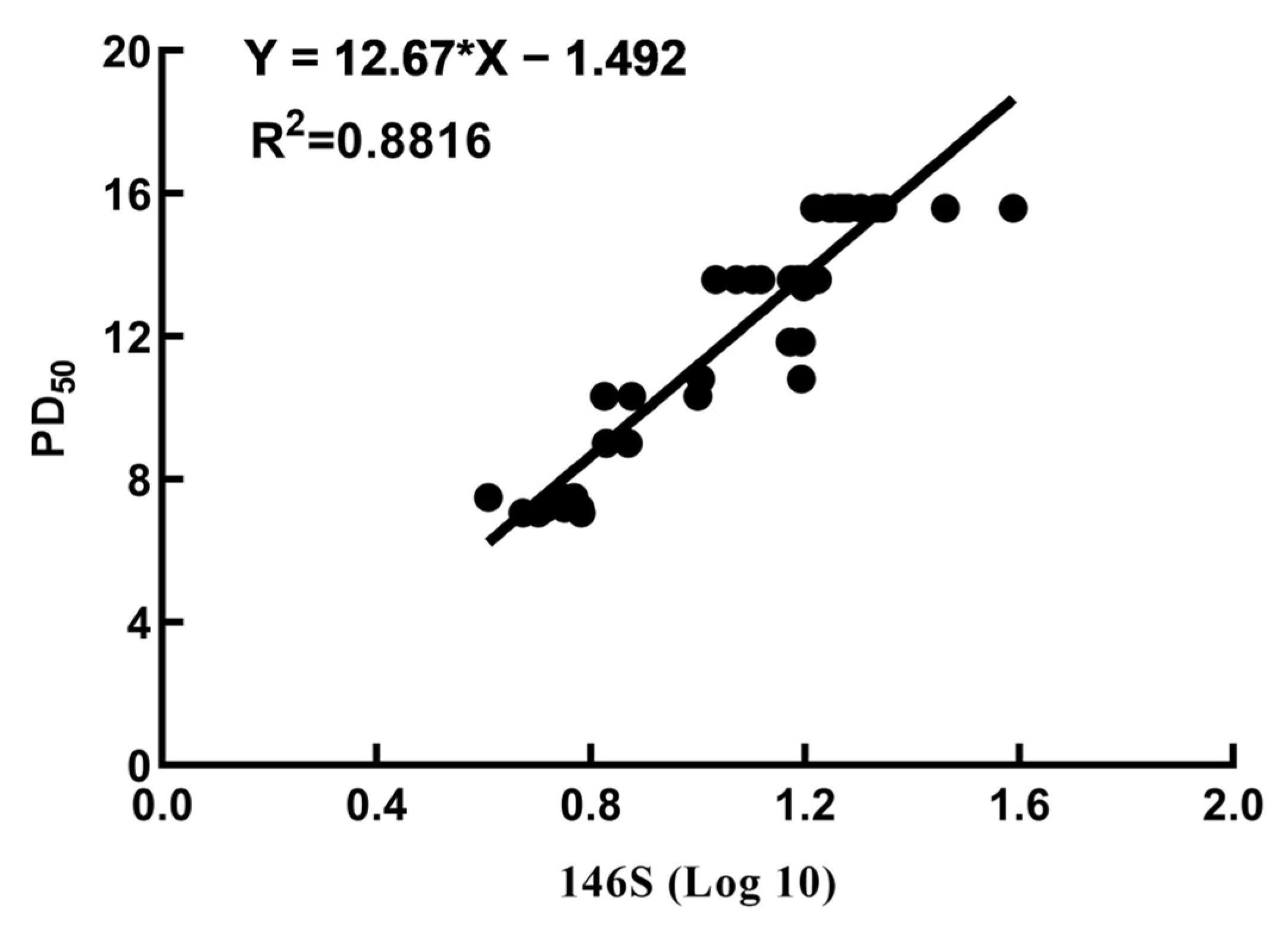
3.5. Correlation between the Quantity of 146S and the PD50
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Grubman, M.J.; Baxt, B. Foot-and-mouth disease. Clin. Microbiol. Rev. 2004, 17, 465–493. [Google Scholar] [CrossRef] [PubMed]
- Sultanov, A.A.; Tyulegenov, S.; Yessembekova, G.N.; Berdikulov, M.A.; Mukhanbetkaliyev, Y.; Akhmetzhanova, A.; Perez, A.M.; Abdrakhmanov, S.K. The progressive control of foot-and-mouth disease (FMD) in the Republic of Kazakhstan: Successes and challenges. Front. Vet. Sci. 2023, 10, 1036121. [Google Scholar] [CrossRef] [PubMed]
- Charleston, B.; Rodriguez, L.L. Understanding foot-and-mouth disease virus early pathogenesis and immune responses. Transbound. Emerg. Dis. 2011, 58, 281–282. [Google Scholar] [CrossRef]
- James, A.D.; Rushton, J. The economics of foot and mouth disease. Rev. Sci. Tech. 2002, 21, 637–644. [Google Scholar] [CrossRef]
- Pluimers, F.H.; Akkerman, A.M.; van der Wal, P.; Dekker, A.; Bianchi, A. Lessons from the foot and mouth disease outbreak in The Netherlands in 2001. Rev. Sci. Tech. 2002, 21, 711–721. [Google Scholar] [CrossRef] [PubMed]
- Thompson, D.; Muriel, P.; Russell, D.; Osborne, P.; Bromley, A.; Rowland, M.; Creigh-Tyte, S.; Brown, C. Economic costs of the foot and mouth disease outbreak in the United Kingdom in 2001. Rev. Sci. Tech. 2002, 21, 675–687. [Google Scholar] [CrossRef] [PubMed]
- Knight-Jones, T.J.; Rushton, J. The economic impacts of foot and mouth disease–What are they, how big are they and where do they occur? Prev. Vet. Med. 2013, 112, 161–173. [Google Scholar] [CrossRef]
- Mohamed, F.; Swafford, S.; Petrowski, H.; Bracht, A.; Schmit, B.; Fabian, A.; Pacheco, J.M.; Hartwig, E.; Berninger, M.; Carrillo, C.; et al. Foot-and-mouth disease in feral swine: Susceptibility and transmission. Transbound. Emerg. Dis. 2011, 58, 358–371. [Google Scholar] [CrossRef] [PubMed]
- Jamal, S.M.; Belsham, G.J. Foot-and-mouth disease: Past, present and future. Vet. Res. 2013, 44, 116. [Google Scholar] [CrossRef]
- Paton, D.J.; Sumption, K.J.; Charleston, B. Options for control of foot-and-mouth disease: Knowledge, capability and policy. Philos. T R. Soc. B 2009, 364, 2657–2667. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, L.L.; Gay, C.G. Development of vaccines toward the global control and eradication of foot-and-mouth disease. Expert. Rev. Vaccines 2011, 10, 377–387. [Google Scholar] [CrossRef]
- Leon, E.A. Foot-and-mouth disease in pigs: Current epidemiological situation and control methods. Transbound. Emerg. Dis. 2012, 59 (Suppl. 1), 36–49. [Google Scholar] [CrossRef] [PubMed]
- Paton, D.J.; Sinclair, M.; Rodriguez, R. Qualitative assessment of the commodity risk for spread of foot-and-mouth disease associated with international trade in deboned beef. Transbound. Emerg. Dis. 2010, 57, 115–134. [Google Scholar] [CrossRef] [PubMed]
- Brito, B.P.; Rodriguez, L.L.; Hammond, J.M.; Pinto, J.; Perez, A.M. Review of the Global Distribution of Foot-and-Mouth Disease Virus from 2007 to 2014. Transbound Emerg. Dis. 2017, 64, 316–332. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, L.L.; Grubman, M.J. Foot and mouth disease virus vaccines. Vaccine 2009, 27 (Suppl. 4), D90–D94. [Google Scholar] [CrossRef] [PubMed]
- Parida, S. Vaccination against foot-and-mouth disease virus: Strategies and effectiveness. Expert. Rev. Vaccines 2009, 8, 347–365. [Google Scholar] [CrossRef] [PubMed]
- Belsham, G.J. Towards improvements in foot-and-mouth disease vaccine performance. Acta Vet. Scand. 2020, 62, 20. [Google Scholar] [CrossRef] [PubMed]
- OIE. 2018. Available online: http://www.oie.int/fileadmin/Home/eng/Health_standards/tahm/3.01.08_FMD.pdf (accessed on 7 March 2024).
- Fayet, M.T.; Fargeaud, D.; Louisot, P.; Stellmann, C.; Roumiantzeff, M. Physical chemical measurement of 140S particles of the foot-and-mouth disease virus. Ann. Inst. Pasteur 1971, 121, 107–118. [Google Scholar]
- Rweyemamu, M.M.; Unehara, O.; Giorgi, W.; Medeiros, R.; Lucca, D.; Baltazar, M. Effect of formaldehyde and binary ethyleneimine (BEI) on the integrity of foot and mouth disease virus capsid. Rev. Sci. Tech. 1989, 8, 747–764. [Google Scholar] [CrossRef] [PubMed]
- Black, L.; Francis, M.J.; Rweyemamu, M.M.; Umebara, O.; Boge, A. The relationship between serum antibody titres and protection from foot and mouth disease in pigs after oil emulsion vaccination. J. Biol. Stand. 1984, 12, 379–389. [Google Scholar] [CrossRef] [PubMed]
- Al Amin, M.; Ali, M.R.; Islam, M.R.; Alam, A.; Shill, D.K.; Rahman, M.S.; Siddique, M.A.; Sultana, M.; Hossain, M.A. Development and serology based efficacy assessment of a trivalent foot-and-mouth disease vaccine. Vaccine 2020, 38, 4970–4978. [Google Scholar] [CrossRef] [PubMed]
- Dar, P.; Kalaivanan, R.; Sied, N.; Mamo, B.; Kishore, S.; Suryanarayana, V.V.; Kondabattula, G. Montanide ISA 201 adjuvanted FMD vaccine induces improved immune responses and protection in cattle. Vaccine 2013, 31, 3327–3332. [Google Scholar] [CrossRef] [PubMed]
- Feng, X.; Ma, J.W.; Sun, S.Q.; Guo, H.C.; Yang, Y.M.; Jin, Y.; Zhou, G.Q.; He, J.J.; Guo, J.H.; Qi, S.Y.; et al. Quantitative Detection of the Foot-And-Mouth Disease Virus Serotype O 146S Antigen for Vaccine Production Using a Double-Antibody Sandwich ELISA and Nonlinear Standard Curves. PLoS ONE 2016, 11, e0149569. [Google Scholar] [CrossRef] [PubMed]
- Kim, A.Y.; Park, S.Y.; Park, S.H.; Kim, J.Y.; Jin, J.S.; Kim, E.S.; Park, J.H.; Ko, Y.J. Comparison of High-Performance Liquid Chromatography with Sucrose Density Gradient Ultracentrifugation for the Quantification of Foot-and-Mouth Disease Vaccine Antigens. Vaccines 2022, 10, 667. [Google Scholar] [CrossRef] [PubMed]
- Reed, L.J.; Muench, H. A simple method of estimating fifty percent endpoint. Am. J. Hyg. 1938, 27, 493–497. [Google Scholar]
- Schober, P.; Boer, C.; Schwarte, L.A. Correlation Coefficients: Appropriate Use and Interpretation. Anesth. Analg. 2018, 126, 1763–1768. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Feng, X.; Jin, Y.; Ma, J.; Cai, H.; Zhang, X. Immunoprotective mechanisms in swine within the “grey zone” in antibody response after immunization with foot-and-mouth disease vaccine. Virus Res. 2016, 220, 39–46. [Google Scholar] [CrossRef] [PubMed]
- Becker, Y. Need for cellular and humoral immune responses in bovines to ensure protection from foot-and-mouth disease virus (FMDV)–A point of view. Virus Genes 1994, 8, 199–214. [Google Scholar] [CrossRef] [PubMed]
- Martins, R.P.; Souza, F.N. Editorial: Cell-mediated immunity in ruminants: Novel approaches and insights. Front. Vet. Sci. 2023, 10, 1177315. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.D.; Hutching, G.; Kitching, P.; Alexandersen, S. The effects of gamma interferon on replication of foot-and-mouth disease virus in persistently infected bovine cells. Arch. Virol. 2002, 147, 2157–2167. [Google Scholar] [CrossRef] [PubMed]
- Kim, W.S.; Zhi, Y.; Guo, H.; Byun, E.B.; Lim, J.H.; Seo, H.S. Promotion of Cellular and Humoral Immunity against Foot-and-Mouth Disease Virus by Immunization with Virus-Like Particles Encapsulated in Monophosphoryl Lipid A and Liposomes. Vaccines 2020, 8, 633. [Google Scholar] [CrossRef] [PubMed]
- Blacksell, S.D.; Siengsanan-Lamont, J.; Kamolsiripichaiporn, S.; Gleeson, L.J.; Windsor, P.A. A history of FMD research and control programmes in Southeast Asia: Lessons from the past informing the future. Epidemiol. Infect. 2019, 147, e171. [Google Scholar] [CrossRef]
- Hammond, J.M.; Maulidi, B.; Henning, N. Targeted FMD Vaccines for Eastern Africa: The AgResults Foot and Mouth Disease Vaccine Challenge Project. Viruses 2021, 13, 1830. [Google Scholar] [CrossRef] [PubMed]
- Brown, F. New approaches to vaccination against foot-and-mouth disease. Vaccine 1992, 10, 1022–1026. [Google Scholar] [CrossRef] [PubMed]
- Aidaros, H.A. Regional status and approaches to control and eradication of foot and mouth disease in the Middle East and North Africa. Rev. Sci. Tech. 2002, 21, 451–458. [Google Scholar] [CrossRef] [PubMed]
- Cheng, H.; Chen, J.; Cai, Z.; Du, L.; Hou, J.; Qiao, X.; Zheng, Q. Development of GEM-PA-nanotrap for purification of foot-and-mouth disease virus. Vaccine 2019, 37, 3205–3213. [Google Scholar] [CrossRef] [PubMed]
- Goris, N.; Merkelbach-Peters, P.; Diev, V.I.; Verloo, D.; Zakharov, V.M.; Kraft, H.P.; De Clercq, K. European Pharmacopoeia foot-and-mouth disease vaccine potency testing in cattle: Between test variability and its consequences. Vaccine 2007, 25, 3373–3379. [Google Scholar] [CrossRef] [PubMed]
- Caridi, F.; Vazquez-Calvo, A.; Borrego, B.; McCullough, K.; Summerfield, A.; Sobrino, F.; Martin-Acebes, M.A. Preserved immunogenicity of an inactivated vaccine based on foot-and-mouth disease virus particles with improved stability. Vet. Microbiol. 2017, 203, 275–279. [Google Scholar] [CrossRef] [PubMed]
- Harmsen, M.M.; Fijten, H.P.; Westra, D.F.; Dekker, A. Stabilizing effects of excipients on dissociation of intact (146S) foot-and-mouth disease virions into 12S particles during storage as oil-emulsion vaccine. Vaccine 2015, 33, 2477–2484. [Google Scholar] [CrossRef] [PubMed]
- Meloen, R.H.; Rowlands, D.J.; Brown, F. Comparison of the antibodies elicited by the individual structural polypeptides of foot-and mouth disease and polio viruses. J. Gen. Virol. 1979, 45, 761–763. [Google Scholar] [CrossRef] [PubMed]
- Doel, T.R.; Fletton, B.W.; Staple, R.F. Further developments in the quantification of small RNA viruses by U.V. photometry of sucrose density gradients. Dev. Biol. Stand. 1981, 50, 209–219. [Google Scholar] [PubMed]
- Cancro, M.P.; Tomayko, M.M. Memory B cells and plasma cells: The differentiative continuum of humoral immunity. Immunol. Rev. 2021, 303, 72–82. [Google Scholar] [CrossRef] [PubMed]
- Summerfield, A.; Guzylack-Piriou, L.; Harwood, L.; McCullough, K.C. Innate immune responses against foot-and-mouth disease virus: Current understanding and future directions. Vet. Immunol. Immunopathol. 2009, 128, 205–210. [Google Scholar] [CrossRef]
- Boehm, U.; Klamp, T.; Groot, M.; Howard, J.C. Cellular responses to interferon-gamma. Annu. Rev. Immunol. 1997, 15, 749–795. [Google Scholar] [CrossRef] [PubMed]
- Oh, Y.; Fleming, L.; Statham, B.; Hamblin, P.; Barnett, P.; Paton, D.J.; Park, J.H.; Joo, Y.S.; Parida, S. Interferon-gamma induced by in vitro re-stimulation of CD4+ T-cells correlates with in vivo FMD vaccine induced protection of cattle against disease and persistent infection. PLoS ONE 2012, 7, e44365. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Y.; Yang, R.; Yin, F.; Zhang, H.; Zhai, G.; Sun, S.; Tian, B.; Zeng, Q. Correlation between 146S Antigen Content in Foot-and-Mouth Disease Inactivated Vaccines and Immunogenicity Level and Vaccine Potency Alternative Test Methods. Vet. Sci. 2024, 11, 168. https://doi.org/10.3390/vetsci11040168
Li Y, Yang R, Yin F, Zhang H, Zhai G, Sun S, Tian B, Zeng Q. Correlation between 146S Antigen Content in Foot-and-Mouth Disease Inactivated Vaccines and Immunogenicity Level and Vaccine Potency Alternative Test Methods. Veterinary Sciences. 2024; 11(4):168. https://doi.org/10.3390/vetsci11040168
Chicago/Turabian StyleLi, Yongxia, Ruai Yang, Fu Yin, Haisheng Zhang, Guoyuan Zhai, Shiqi Sun, Bo Tian, and Qiaoying Zeng. 2024. "Correlation between 146S Antigen Content in Foot-and-Mouth Disease Inactivated Vaccines and Immunogenicity Level and Vaccine Potency Alternative Test Methods" Veterinary Sciences 11, no. 4: 168. https://doi.org/10.3390/vetsci11040168
APA StyleLi, Y., Yang, R., Yin, F., Zhang, H., Zhai, G., Sun, S., Tian, B., & Zeng, Q. (2024). Correlation between 146S Antigen Content in Foot-and-Mouth Disease Inactivated Vaccines and Immunogenicity Level and Vaccine Potency Alternative Test Methods. Veterinary Sciences, 11(4), 168. https://doi.org/10.3390/vetsci11040168





