Therapeutic Effects of Propionibacterium acnes and Lipopolysaccharide from Escherichia coli in Cats with Feline Panleukopenia
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents and Product
2.2. Retrospective Study
Study Period and Data Collection
2.3. Prospective Study
2.3.1. Study Period and Sample Collection
2.3.2. Detection of Antibodies from Serum and Feces
2.4. Statistical Analysis
2.4.1. Statistical Analysis of the Retrospective Study
2.4.2. Statistical Analysis of the Prospective Study
3. Results
3.1. Retrospective Study
3.1.1. Descriptive Analysis
3.1.2. Comparison of the Mean Total White Blood Cell Counts between and within Groups
3.1.3. Investigation of Repeated Measurements
3.1.4. Survival Curve Analysis
3.2. Prospective Study
3.2.1. Descriptive Analysis
3.2.2. Detection of Antibodies in the Serum and Feces
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Liu, J.; Zhang, Z.; Bai, A.; Sha, Y.; Ma, L.; Qin, S.; Chen, F.; Qin, S.; Wu, J. Prophylactic Efficacy of Equine Immunoglobulin F (ab′) 2 Fragments Against Feline Parvovirus. Appl. Biochem. Biotechnol. 2021, 193, 3151–3162. [Google Scholar] [CrossRef] [PubMed]
- Parrish, J.K. Influence of group size and habitat type on reproductive success in Common Murres (Uria aalge). Auk 1995, 112, 390–401. [Google Scholar] [CrossRef]
- Barrs, V.R. Feline panleukopenia: A re-emergent disease. Vet. Clin. N. Am. Small Anim. Pract. 2019, 49, 651–670. [Google Scholar] [CrossRef]
- Steinel, A.; Parrish, C.R.; Bloom, M.E.; Truyen, U. Parvovirus infections in wild carnivores. J. Wildl. Dis. 2001, 37, 594–607. [Google Scholar] [CrossRef]
- Boschetti, N.; Wyss, K.; Mischler, A.; Hostettler, T.; Kempf, C. Stability of minute virus of mice against temperature and sodium hydroxide. Biologicals 2003, 31, 181–185. [Google Scholar] [CrossRef]
- Kramer, A.; Schwebke, I.; Kampf, G. How long do nosocomial pathogens persist on inanimate surfaces? A systematic review. BMC Infect. Dis. 2006, 6, 130. [Google Scholar] [CrossRef] [PubMed]
- Mochizuki, M.; Nakatani, H.; Yoshida, M.J.V.M. Inhibitory effects of recombinant feline interferon on the replication of feline enteropathogenic viruses in vitro. Vet. Microbiol. 1994, 39, 145–152. [Google Scholar] [CrossRef] [PubMed]
- Cui, Z.; Li, D.; Yi, S.; Guo, Y.; Dong, G.; Niu, J.; Zhao, H.; Zhang, Y.; Zhang, S.; Cao, L.; et al. Equine immunoglobulin F (ab′) 2 fragments protect cats against feline calicivirus infection. Int. Immunopharmacol. 2019, 75, 105714. [Google Scholar] [CrossRef]
- dos Anjos, D.S.; Assis, A.R.; Fonseca-Alves, C.E.; Babo-Terra, V.J. Clinical, pathological and immunohistochemical evaluation of a primary hemangiosarcoma in a pinscher dog. Acta Sci. Vet. 2016, 44, 6. [Google Scholar] [CrossRef]
- Teixeira, D.; Ishimura, M.E.; Apostolico, J.d.S.; Viel, J.M.; Passarelli, V.C.; Cunha-Neto, E.; Rosa, D.S.; Longo-Maugéri, I.M. Propionibacterium acnes enhances the immunogenicity of HIVBr18 human immunodeficiency virus-1 vaccine. Front. Immunol. 2018, 9, 307834. [Google Scholar] [CrossRef]
- Woodruff, M.; McBride, W.; Dunbar, N. Tumour growth, phagocytic activity and antibody response in Corynebacterium parvum-treated mice. Clin. Exp. Immunol. 1974, 17, 509. [Google Scholar] [PubMed]
- Rollinson, E. Prospects for antiviral chemotherapy in veterinary medicine: 2. Avian, piscine, canine, porcine, bovine and equine virus diseases. Antivir. Chem. Chemother. 1992, 3, 311–326. [Google Scholar] [CrossRef]
- Pappaterra Mendoza, G.; De Antonio, E.M.; Novell Badal, M.; Castillo, M.M.; Fàbrega, J.C.; Puig, J.M. In Vitro and In Vivo Effects of an Immunomodulator Composed of Escherichia Coli Lipopolysaccharide and Propionibacterium granulosum-Inactivated Cells in Pigs. J. Vet. Med. Ser. B 2000, 47, 619–627. [Google Scholar] [CrossRef] [PubMed]
- Lo, D.-Y.; Hung, C.-N.; Lee, W.-C.; Liao, J.-W.; Blacklaws, B.A.; Chen, T.-H.; Chien, M.-S.; Hsuan, S.-L. Effect of immunostimulation by detoxified E. coli lipopolysaccharide combined with inactivated Propionibacterium granulosum cells on porcine immunity. J. Vet. Med. Sci. 2009, 71, 897–903. [Google Scholar] [CrossRef] [PubMed]
- Isho, B.; Florescu, A.; Wang, A.A.; Gommerman, J.L. Fantastic IgA plasma cells and where to find them. Immunol. Rev. 2021, 303, 119–137. [Google Scholar] [CrossRef] [PubMed]
- Woof, J.; Russell, M. Structure and function relationships in IgA. Mucosal Immunol. 2011, 4, 590–597. [Google Scholar] [CrossRef] [PubMed]
- Pila, P.; Chuammitri, P.; Patchanee, P.; Pringproa, K.; Piyarungsri, K. Evaluation of Bcl-2 as a marker for chronic kidney disease prediction in cats. Front. Vet. Sci. 2023, 9, 1043848. [Google Scholar] [CrossRef] [PubMed]
- Awad, R.A.; Khalil, W.K.; Attallah, A.G.J.V.W. Epidemiology and diagnosis of feline panleukopenia virus in Egypt: Clinical and molecular diagnosis in cats. Vet. World 2018, 11, 578. [Google Scholar] [CrossRef] [PubMed]
- Kruse, B.; Unterer, S.; Horlacher, K.; Sauter-Louis, C.; Hartmann, K.J. Prognostic factors in cats with feline panleukopenia. J. Vet. Intern. Med. 2010, 24, 1271–1276. [Google Scholar] [CrossRef]
- Jacobson, L.S.; Janke, K.J.; Giacinti, J.; Weese, J.S. Diagnostic testing for feline panleukopenia in a shelter setting: A prospective, observational study. J. Feline Med. Surg. 2021, 23, 1192–1199. [Google Scholar] [CrossRef]
- Abd-Eldaim, M.; Beall, M.J.; Kennedy, M.A. Detection of feline panleukopenia virus using a commercial ELISA for canine parvovirus. Vet. Ther. 2009, 10, E1–E6. [Google Scholar] [PubMed]
- Kusumawardhani, S.W.; Aji, Y.L.; Widyaastuti, V.M.; Agung, M.; Khoirurroziqin, I.W.W.; Sajuthi, C.K.; Raya, J.S.P.; Tahap, R.N.S.A., III. Retrospective Study of Feline Panleukopenia Virus in Jakarta. In Proceedings of the 20th FAVA CONGRESS & The 15th KIVNAS PDHI, Bali, Indonesia, 1–3 November 2018. [Google Scholar]
- Porporato, F.; Horzinek, M.C.; Hofmann-Lehmann, R.; Ferri, F.; Gerardi, G.; Contiero, B.; Vezzosi, T.; Rocchi, P.; Auriemma, E.; Lutz, H.J.; et al. Survival estimates and outcome predictors for shelter cats with feline panleukopenia virus infection. Am. Vet. Med Assoc. 2018, 253, 188–195. [Google Scholar] [CrossRef] [PubMed]
- Davis, E.G.; Rush, B.R.; Blecha, F.J. Increases in cytokine and antimicrobial peptide gene expression in horses by immunomodulation with Propionibacterium acnes. Vet. Ther. 2003, 4, 5–11. [Google Scholar] [PubMed]
- Koro, A.T.; Sharif, A.Y. The effect of lipopolysaccharide extracted from Escherichia coli on total WBCs, granulocytes and on phagocytic activity in female rats. Iraqi J. Vet. Sci. 2022, 36, 285–289. [Google Scholar] [CrossRef]
- Peters, I.; Calvert, E.; Hall, E.; Day, M.J.C.; Immunology, V. Measurement of immunoglobulin concentrations in the feces of healthy dogs. Clin. Vaccine Immunol. 2004, 11, 841–848. [Google Scholar] [CrossRef]
- Marks, S. Rational approach to diagnosing and managing infectious causes of diarrhea in kittens. In August’s Consultations in Feline Internal Medicine; Elsevier: St. Louis, MO, USA, 2016; Volume 7. [Google Scholar]
- Pietrzak, B.; Tomela, K.; Olejnik-Schmidt, A.; Mackiewicz, A.; Schmidt, M. Secretory IgA in intestinal mucosal secretions as an adaptive barrier against microbial cells. Int. J. Mol. Sci. 2020, 21, 9254. [Google Scholar] [CrossRef]
- Rice, J. Successful Treatment of Feline Panleukopenia: A Guideline for Rescuers and Veterinarians, Part I. J. Vet. Sci. Med. Diagn. 2017, 6, 2. [Google Scholar] [CrossRef]
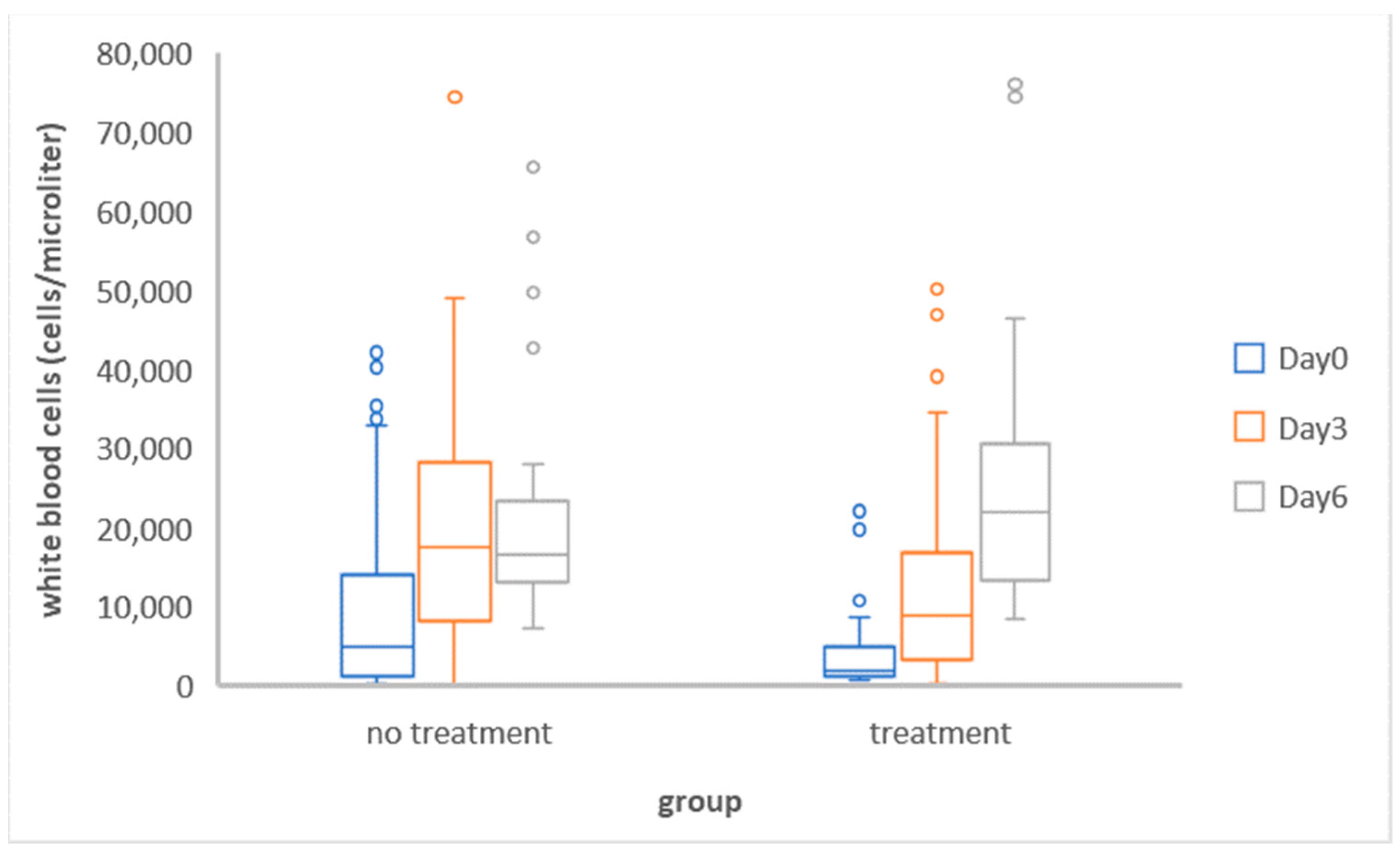
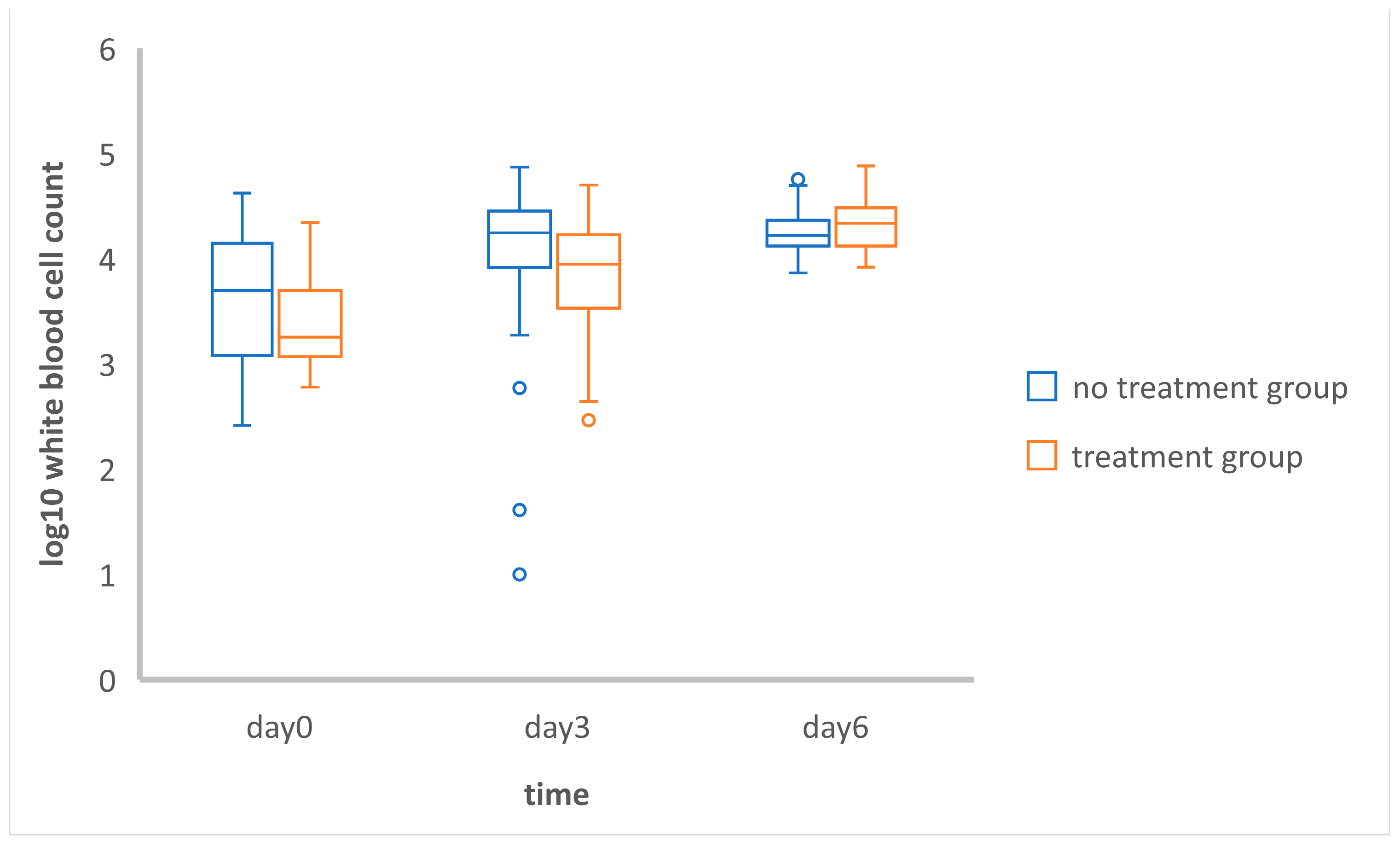
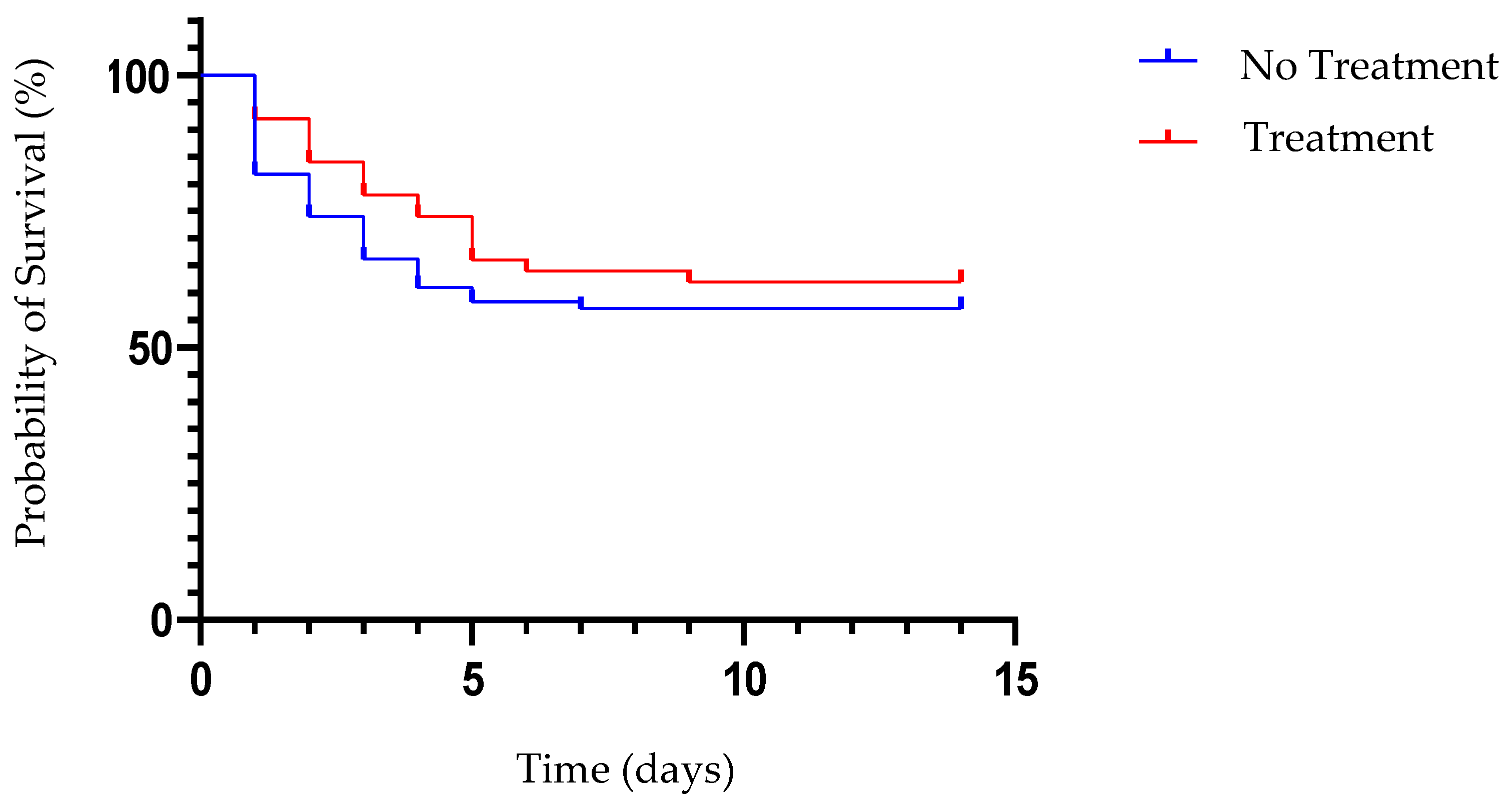
| Group | Day | Mean of Log10 WBC | SD | Mean Difference | SD of Difference | t | p |
|---|---|---|---|---|---|---|---|
| No Treatment | D-0 | 3.64 | 0.64 | −0.40 | 0.99 | −2.70 | <0.05 |
| D-3 | 4.04 | 0.72 | |||||
| D-0 | 3.58 | 0.59 | −0.69 | 0.65 | −5.56 | <0.01 | |
| D-6 | 4.27 | 0.25 | |||||
| D-3 | 4.27 | 0.29 | 0.00 | 0.34 | 0.02 | 0.98 | |
| D-6 | 4.27 | 0.25 | |||||
| Treatment | D-0 | 3.41 | 0.45 | −0.40 | 0.68 | −3.50 | <0.01 |
| D-3 | 3.81 | 0.60 | |||||
| D-0 | 3.31 | 0.39 | −1.04 | 0.46 | −11.14 | <0.01 | |
| D-6 | 4.35 | 0.26 | |||||
| D-3 | 3.71 | 0.66 | −0.64 | 0.71 | −4.39 | <0.01 | |
| D-6 | 4.35 | 0.26 |
| WBC Coefficients | Compared Days | Estimate | SE | Wald | Pr (>|W|) |
|---|---|---|---|---|---|
| No treatment (n = 45) | 0–3 | 0.69 | 0.21 | 10.65 | <0.01 |
| 0–6 | 0.81 | 0.22 | 13.86 | <0.01 | |
| Treatment (n = 35) | 0–3 | 1.28 | 0.34 | 13.96 | <0.01 |
| 0–6 | 1.78 | 0.25 | 49.26 | <0.01 |
| Day | Group | Mean of Log10 Serum IgA | SD | t | p |
|---|---|---|---|---|---|
| 0 | Control (n = 10) | −1.10 | 1.09 | −0.60 | 0.56 |
| Treatment (n = 8) | −0.81 | 0.66 | |||
| 3 | Control (n = 10) | −1.27 | 0.87 | 0.18 | 0.86 |
| Treatment (n = 7) | −1.37 | 0.11 | |||
| 6 | Control (n = 8) | −0.72 | 0.64 | 0.18 | 0.86 |
| Treatment (n = 7) | −0.79 | 0.61 |
| Serum Coefficients | Comparison Days | Estimate | SE | Wald | Pr (>|W|) |
|---|---|---|---|---|---|
| Control | 0–3 | −0.52 | 0.83 | 0.40 | 0.53 |
| 0–6 | 0.51 | 0.50 | 1.06 | 0.30 | |
| Treatment | 0–3 | −2.64 | 0.58 | 20.71 | <0.01 |
| 0–6 | −0.604 | 0.56 | 1.14 | 0.28 |
| Day | Group | Mean of Log10 Feces IgA | sd | t | p |
|---|---|---|---|---|---|
| 0 | Control (n = 10) | −0.55 | 0.57 | 0.06 | 0.95 |
| Treatment (n = 8) | −0.57 | 0.55 | |||
| 3 | Control (n = 10) | −0.62 | 0.50 | −0.09 | 0.93 |
| Treatment (n = 7) | −0.60 | 0.29 | |||
| 6 | Control (n = 8) | −0.51 | 0.69 | −0.36 | 0.74 |
| Treatment (n = 7) | −0.39 | 0.32 |
| Feces Coefficient | Comparison Days | Estimate | SE | Wald | Pr (>|W|) |
|---|---|---|---|---|---|
| Control (n = 10) | 0–3 | −0.01 | 0.55 | 0.00 | 0.98 |
| 0–6 | 0.33 | 0.58 | 0.33 | 0.57 | |
| Treatment (n = 8) | 0–3 | −0.01 | 0.65 | 0.00 | 0.98 |
| 0–6 | 0.70 | 0.63 | 1.24 | 0.26 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chanachaivirada, R.; Chuammitri, P.; Lampa, K.N.; Prachasilchai, W.; Sodarat, C. Therapeutic Effects of Propionibacterium acnes and Lipopolysaccharide from Escherichia coli in Cats with Feline Panleukopenia. Vet. Sci. 2024, 11, 253. https://doi.org/10.3390/vetsci11060253
Chanachaivirada R, Chuammitri P, Lampa KN, Prachasilchai W, Sodarat C. Therapeutic Effects of Propionibacterium acnes and Lipopolysaccharide from Escherichia coli in Cats with Feline Panleukopenia. Veterinary Sciences. 2024; 11(6):253. https://doi.org/10.3390/vetsci11060253
Chicago/Turabian StyleChanachaivirada, Rattanakhon, Phongsakorn Chuammitri, Kannika Na Lampa, Worapat Prachasilchai, and Chollada Sodarat. 2024. "Therapeutic Effects of Propionibacterium acnes and Lipopolysaccharide from Escherichia coli in Cats with Feline Panleukopenia" Veterinary Sciences 11, no. 6: 253. https://doi.org/10.3390/vetsci11060253
APA StyleChanachaivirada, R., Chuammitri, P., Lampa, K. N., Prachasilchai, W., & Sodarat, C. (2024). Therapeutic Effects of Propionibacterium acnes and Lipopolysaccharide from Escherichia coli in Cats with Feline Panleukopenia. Veterinary Sciences, 11(6), 253. https://doi.org/10.3390/vetsci11060253





