Beyond Borders: Dirofilaria immitis Infection in Dogs Spreads to Previously Non-Enzootic Areas in Greece—A Serological Survey
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design—Ethical Statement
2.2. Blood Sample Collection and Serological Examination
2.3. Statistical Analyses
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cancrini, G.; Kramer, L. Insect vectors of Dirofilaria spp. In Heartworm Infection in Humans and Animals; Simón, F., Genchi, C., Eds.; Universidad de Salamanca: Salamanca, Spain, 2001; pp. 63–82. [Google Scholar]
- Otranto, D.; Capelli, G.; Genchi, C. Changing distribution patterns of canine vector borne diseases in Italy: Leishmaniosis vs. Dirofilariosis. Parasites Vectors 2009, 2, S2. [Google Scholar] [CrossRef]
- ESDA (European Society of Dirofilariosis and Angiostrongylosis) Guidelines for Clinical Management of Canine Heartworm Disease. Available online: https://www.esda.vet/media/attachments/2021/08/19/canine-heartworm-disease.pdf (accessed on 15 April 2024).
- Otranto, D.; Deplazes, P. Zoonotic nematodes of wild carnivores. Int. J. Parasitol. Parasites Wildl. 2019, 9, 370–383. [Google Scholar] [CrossRef]
- Bowman, D.D.; Atkins, C.E. Heartworm Biology, Treatment, and Control. Vet. Clin. N. Am.—Small Anim. Pract. 2009, 39, 1127–1158. [Google Scholar] [CrossRef]
- McCall, J.W.; Genchi, C.; Kramer, L.H.; Guerrero, J.; Venco, L. Heartworm disease in animals and humans. Adv. Parasitol. 2008, 66, 193–285. [Google Scholar]
- Muro, A.; Genchi, C.; Cordero, M.; Simón, F. Human dirofilariasis in the European union. Parasitol. Today 1999, 15, 386–389. [Google Scholar] [CrossRef]
- Simón, F.; López-Belmonte, J.; Marcos-Atxutegi, C.; Morchón, R.; Martín-Pacho, J.R. What is happening outside North America regarding human dirofilariasis? Vet. Parasitol. 2005, 133, 181–189. [Google Scholar] [CrossRef]
- Simón, F.; Diosdado, A.; Siles-Lucas, M.; Kartashev, V.; González-Miguel, J. Human dirofilariosis in the 21st century: A scoping review of clinical cases reported in the literature. Transbound. Emerg. Dis. 2022, 69, 2424–2439. [Google Scholar] [CrossRef]
- Genchi, C.; Rinaldi, L.; Mortarino, M.; Genchi, M.; Cringoli, G. Climate and Dirofilaria infection in Europe. Vet. Parasitol. 2009, 163, 286–292. [Google Scholar] [CrossRef]
- Hoch, H.; Strickland, K. Canine and Feline Dirofilariasis: Life Cycle, Pathophysiology, and Diagnosis. Compend. Contin. Educ. Vet. 2008, 30, 133–140. [Google Scholar]
- Diakou, A.; Kapantaidakis, E.; Tamvakis, A.; Giannakis, V.; Strus, N. Dirofilaria infections in dogs in different areas of Greece. Parasites Vectors 2016, 9, 508. [Google Scholar] [CrossRef]
- Angelou, A.; Gelasakis, A.I.; Verde, N.; Pantchev, N.; Schaper, R.; Chandrashekar, R.; Papadopoulos, E. Prevalence and risk factors for selected canine vector-borne diseases in Greece. Parasites Vectors 2019, 12, 283. [Google Scholar] [CrossRef]
- Papazahariadou, M.G.; Koutinas, A.F.; Rallis, T.S.; Haralabidis, S.T. Prevalence of Microfilaraemia in Episodic Weakness and Clinically Normal Dogs Belonging to Hunting Breeds. J. Helminthol. 1994, 68, 243–245. [Google Scholar] [CrossRef]
- Founta, A.; Theodoridis, Y.; Frydas, S.; Chliounakis, S. The presence of filarial parasites of dogs in Serrae Province. J. Hell. Vet. Med. Soc. 1999, 50, 315–320. [Google Scholar] [CrossRef][Green Version]
- Lefkaditis, A.M.; Koukeri, E.S. The clinical signs and protocol of treatment of 25 infected dogs with Dirofilaria immitis. Bul. Univ. Stiinte Agric. Med. Vet. Cluj-Napoca Ser. Med. Vet. 2005, 62, 466–468. [Google Scholar]
- Lefkaditis, M.; Koukeri, S.; Cozma, V. An endemic area of Dirofilaria immitis seropositive dogs at the eastern foothills of Mt Olympus, Northern Greece. Helminthologia 2010, 47, 3–7. [Google Scholar] [CrossRef]
- Mederle, N.; Opruti, D.A.; Diakou, A. Epidemiological research in canine dirofilariosis in Thessaloniki area of Greece. Lucr. Ştiinţifice Med. Vet. 2019, 4, 72–75. [Google Scholar]
- Athanasiou, L.V.; Kontos, V.I.; Kritsepi Konstantinou, M.; Polizopoulou, Z.S.; Rousou, X.A.; Christodoulopoulos, G. Cross-Sectional Serosurvey and Factors Associated with Exposure of Dogs to Vector-Borne Pathogens in Greece. Vector-Borne Zoonotic Dis. 2019, 19, 923–928. [Google Scholar] [CrossRef]
- Morelli, S.; Diakou, A.; Frangipane di Regalbono, A.; Colombo, M.; Simonato, G.; Di Cesare, A.; Passarelli, A.; Pezzuto, C.; Tzitzoudi, Z.; Barlaam, A.; et al. Use of In-Clinic Diagnostic Kits for the Detection of Seropositivity to Leishmania infantum and Other Major Vector-Borne Pathogens in Healthy Dogs. Pathogens 2023, 12, 696. [Google Scholar] [CrossRef]
- Hofmann, M.; Hodžić, A.; Pouliou, N.; Joachim, A. Vector-borne pathogens affecting shelter dogs in eastern Crete, Greece. Parasitol. Res. 2019, 118, 1661–1666. [Google Scholar] [CrossRef]
- Genchi, C.; Mortarino, M.; Rinaldi, L.; Cringoli, G.; Traldi, G.; Genchi, M. Changing climate and changing vector-borne disease distribution: The example of Dirofilaria in Europe. Vet. Parasitol. 2011, 176, 295–299. [Google Scholar] [CrossRef]
- Giangaspero, A.; Marangi, M.; Latrofa, M.S.; Martinelli, D.; Traversa, D.; Otranto, D.; Genchi, C. Evidences of increasing risk of dirofilarioses in southern Italy. Parasitol. Res. 2013, 112, 1357–1361. [Google Scholar] [CrossRef]
- Genchi, C.; Rinaldi, L.; Cascone, C.; Mortarino, M.; Cringoli, G. Is heartworm disease really spreading in Europe? Vet. Parasitol. 2005, 133, 137–148. [Google Scholar] [CrossRef]
- Morchón, R.; Carretón, E.; González-Miguel, J.; Mellado-Hernández, I. Heartworm disease (Dirofilaria immitis) and their vectors in Europe—New distribution trends. Front. Physiol. 2012, 3, 196. [Google Scholar] [CrossRef]
- Tasić-Otašević, S.A.; Trenkić Božinović, M.S.; Gabrielli, S.V.; Genchi, C. Canine and human Dirofilaria infections in the Balkan Peninsula. Vet. Parasitol. 2015, 209, 151–156. [Google Scholar] [CrossRef]
- Morchón, R.; Montoya-Alonso, J.A.; Rodríguez-Escolar, I.; Carretón, E. What Has Happened to Heartworm Disease in Europe in the Last 10 Years? Pathogens 2022, 11, 1042. [Google Scholar] [CrossRef]
- Napoli, E.; De Benedetto, G.; Ciuca, L.; Bosco, A.; Lia, R.P.; Veneziano, V.; Bezerra Santos, M.A.; Otranto, D.; Rinaldi, L.; Brianti, E. New distribution patterns of Dirofilaria immitis in Italy. Front. Vet. Sci. 2023, 10, 1162403. [Google Scholar] [CrossRef]
- Rodríguez-Escolar, I.; Hernández-Lambraño, R.E.; Sánchez-Agudo, J.Á.; Collado-Cuadrado, M.; Sioutas, G.; Papadopoulos, E.; Morchón, R. Ecological niche modeling analysis (Cx. pipiens), potential risk and projection of Dirofilaria spp. infection in Greece. Vet. Parasitol. 2024, 328, 110172. [Google Scholar] [CrossRef]
- Diakou, A.; Di Cesare, A.; Morelli, S.; Colombo, M.; Halos, L.; Simonato, G.; Tamvakis, A.; Beugnet, F.; Paoletti, B.; Traversa, D. Endoparasites and vector-borne pathogens in dogs from Greek islands: Pathogen distribution and zoonotic implications. PLoS Negl. Trop. Dis. 2019, 13, e0007003. [Google Scholar] [CrossRef]
- Sałamatin, R.V.; Pavlikovska, T.M.; Sagach, O.S.; Nikolayenko, S.M.; Kornyushin, V.V.; Kharchenko, V.O.; Masny, A.; Cielecka, D.; Konieczna-Sałamatin, J.; Conn, D.B.; et al. Human dirofilariasis due to Dirofilaria repens in Ukraine, an emergent zoonosis: Epidemiological report of 1465 cases. Acta Parasitol. 2013, 58, 592–598. [Google Scholar] [CrossRef]
- Otranto, D.; Dantas-Torres, F.; Brianti, E.; Traversa, D.; Petrić, D.; Genchi, C.; Capelli, G. Vector-borne helminths of dogs and humans in Europe. Parasites Vectors 2013, 6, 16. [Google Scholar] [CrossRef]
- Polizopoulou, Z.S.; Koutinas, A.F.; Saridomichelakis, M.N.; Patsikas, M.N.; Leontidis, L.S.; Roubies, N.A.; Desiris, A.K. Clinical and laboratory observations in 91 dogs infected with Dirofilaria immitis in northern Greece. Vet. Rec. 2000, 146, 466–469. [Google Scholar] [CrossRef]
- Diakou, A.; Soubasis, N.; Chochlios, T.; Oikonomidis, I.L.; Tselekis, D.; Koutinas, C.; Karaiosif, R.; Psaralexi, E.; Tsouloufi, T.K.; Brellou, G.; et al. Canine and feline dirofilariosis in a highly enzootic area: First report of feline dirofilariosis in Greece. Parasitol. Res. 2019, 118, 677–682. [Google Scholar] [CrossRef]
- Mendoza-Roldan, J.; Benelli, G.; Panarese, R.; Iatta, R.; Furlanello, T.; Beugnet, F.; Zatelli, A.; Otranto, D. Leishmania infantum and Dirofilaria immitis infections in Italy, 2009-2019: Changing distribution patterns. Parasites Vectors 2020, 13, 193. [Google Scholar] [CrossRef]
- Mendoza-Roldan, J.A.; Gabrielli, S.; Cascio, A.; Manoj, R.R.S.; Bezerra-Santos, M.A.; Benelli, G.; Brianti, E.; Latrofa, M.S.; Otranto, D. Zoonotic Dirofilaria immitis and Dirofilaria repens infection in humans and an integrative approach to the diagnosis. Acta Trop. 2021, 223, 106083. [Google Scholar] [CrossRef]
- Brianti, E.; Panarese, R.; Napoli, E.; De Benedetto, G.; Gaglio, G.; Bezerra-Santos, M.A.; Mendoza-Roldan, J.A.; Otranto, D. Dirofilaria immitis infection in the Pelagie archipelago: The southernmost hyperendemic focus in Europe. Transbound. Emerg. Dis. 2022, 69, 1274–1280. [Google Scholar] [CrossRef]
- Panarese, R.; Iatta, R.; Latrofa, M.S.; Zatelli, A.; Ignjatović Ćupina, A.; Montarsi, F.; Pombi, M.; Mendoza-Roldan, J.A.; Beugnet, F.; Otranto, D. Hyperendemic Dirofilaria immitis infection in a sheltered dog population: An expanding threat in the Mediterranean region. Int. J. Parasitol. 2020, 50, 555–559. [Google Scholar] [CrossRef]
- Morchón, R.; Rodríguez-Escolar, I.; Lambraño, R.E.H.; Agudo, J.Á.S.; Montoya-Alonso, J.A.; Serafín-Pérez, I.; Fernández-Serafín, C.; Carretón, E. Assessment Heartworm Disease in the Canary Islands (Spain): Risk of Transmission in a Hyperendemic Area by Ecological Niche Modeling and Its Future Projection. Animals 2023, 13, 3251. [Google Scholar] [CrossRef]
- Peterson, A.T.; Soberón, J. Species distribution modeling and ecological niche modeling: Getting the Concepts Right. Nat. Conserv. 2012, 10, 102–107. [Google Scholar] [CrossRef]
- Medlock, J.M.; Barrass, I.; Kerrod, E.; Taylor, M.A.; Leach, S. Analysis of climatic predictions for extrinsic incubation of Dirofilaria in the United Kingdom. Vector-Borne Zoonotic Dis. 2007, 7, 4–14. [Google Scholar] [CrossRef]
- Clark, J.D.; Dunn, J.E.; Smith, K.G. A Multivariate Model of Female Black Bear Habitat Use for a Geographic Information System. J. Wildl. Manag. 1993, 57, 90. [Google Scholar] [CrossRef]
- Genchi, C.; Kramer, L.H.; Prieto, G. Epidemiology of canine and feline dirofilariasis: A global view. In Heartworm Infection in Humans and Animals; Simón, F., Genchi, C., Eds.; Universidad de Salamanca: Salamanca, Spain, 2001; pp. 121–133. [Google Scholar]
- Semenza, J.C.; Menne, B. Climate change and infectious diseases in Europe. Lancet Infect. Dis. 2009, 9, 365–375. [Google Scholar] [CrossRef]
- Cancrini, G.; Scaramozzino, P.; Gabrielli, S.; Di Paolo, M.; Toma, L.; Romi, R. Aedes albopictus and Culex pipiens implicated as natural vectors of Dirofilaria repens in central Italy. J. Med. Entomol. 2007, 44, 1064–1066. [Google Scholar] [CrossRef]
- Trotz-Williams, L.A.; Trees, A.J. Systematic review of the distribution of the major vector-borne parasitic infections in dogs and cats in Europe. Vet. Rec. 2003, 152, 97–105. [Google Scholar] [CrossRef]
- Moroni, B.; Rossi, L.; Meneguz, P.G.; Orusa, R.; Zoppi, S.; Robetto, S.; Marucco, F.; Tizzani, P. Dirofilaria immitis in wolves recolonizing northern Italy: Are wolves competent hosts? Parasites Vectors 2020, 13, 482. [Google Scholar] [CrossRef]
- Markakis, G.; Sioutas, G.; Bitchava, D.; Komnenou, A.; Ganoti, M.; Papadopoulos, E. Is the European badger a new host for Dirofilaria immitis? The first records in Greece. Parasitol. Res. 2024, 123, 118. [Google Scholar] [CrossRef]
- Wixsom, M.J.; Green, S.P.; Corwin, R.M.; Fritzell, E.K. Dirofilaria immitis in coyotes and foxes in Missouri. J. Wildl. Dis. 1991, 27, 166–169. [Google Scholar] [CrossRef]
- Diakou, A.; Migli, D.; Spiridakis, G. Dirofilaria immitis (heartworm) in a golden jackal (Canis aureus) in Greece. In Proceedings of the 13th International Congress on the Zoogeography and Ecology of Greece and Adjacent Regions, Irakleio, Crete, Greece, 7–11 October 2015; p. 27. [Google Scholar]
- Papadopoulos, E.; Komnenou, A.; Poutachides, T.; Heikkinen, P.; Oksanen, A.; Karamanlidis, A.A. Detection of Dirofilaria immitis in a brown bear (Ursus arctos) in Greece. Helminthologia 2017, 54, 257–261. [Google Scholar] [CrossRef]
- Pampiglione, S.; Rivasi, F. Human dirofilariasis due to Dirofilaria (Nochtiella) repens: An update of world literature from 1995 to 2000. Parassitologia 2000, 42, 231–254. [Google Scholar]
- Otranto, D.; Brianti, E.; Gaglio, G.; Dantas-Torres, F.; Azzaro, S.; Giannetto, S. Short report: Human ocular infection with Dirofilaria repens (Railliet and Henry, 1911) in an area endemic for canine dirofilariasis. Am. J. Trop. Med. Hyg. 2011, 84, 1002–1004. [Google Scholar] [CrossRef]
- Tolnai, Z.; Széll, Z.; Sproch, Á.; Szeredi, L.; Sréter, T. Dirofilaria immitis: An emerging parasite in dogs, red foxes and golden jackals in hungary. Vet. Parasitol. 2014, 203, 339–342. [Google Scholar] [CrossRef]
- Courtney, C.H.; Zeng, Q.Y. Comparison of heartworm antigen test kit performance in dogs having low heartworm burdens. Vet. Parasitol. 2001, 96, 317–322. [Google Scholar] [CrossRef]
- Savadelis, M.D.; Roveto, J.L.; Ohmes, C.M.; Hostetler, J.A.; Settje, T.L.; Dzimianski, M.T.; Moorhead, A.R. Evaluation of heat-treating heartworm-positive canine serum samples during treatment with Advantage Multi® for Dogs and doxycycline. Parasites Vectors 2018, 11, 98. [Google Scholar] [CrossRef]
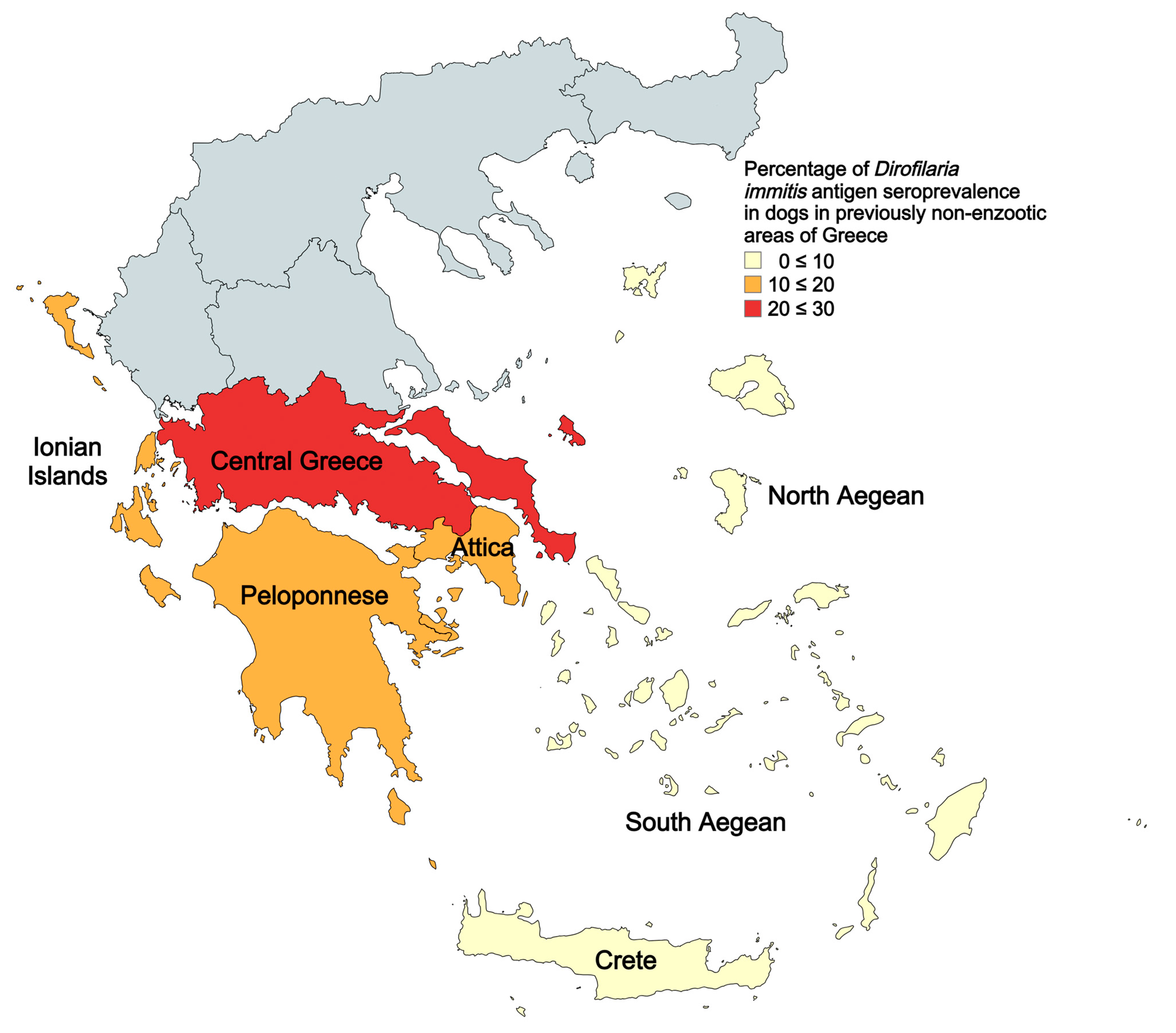
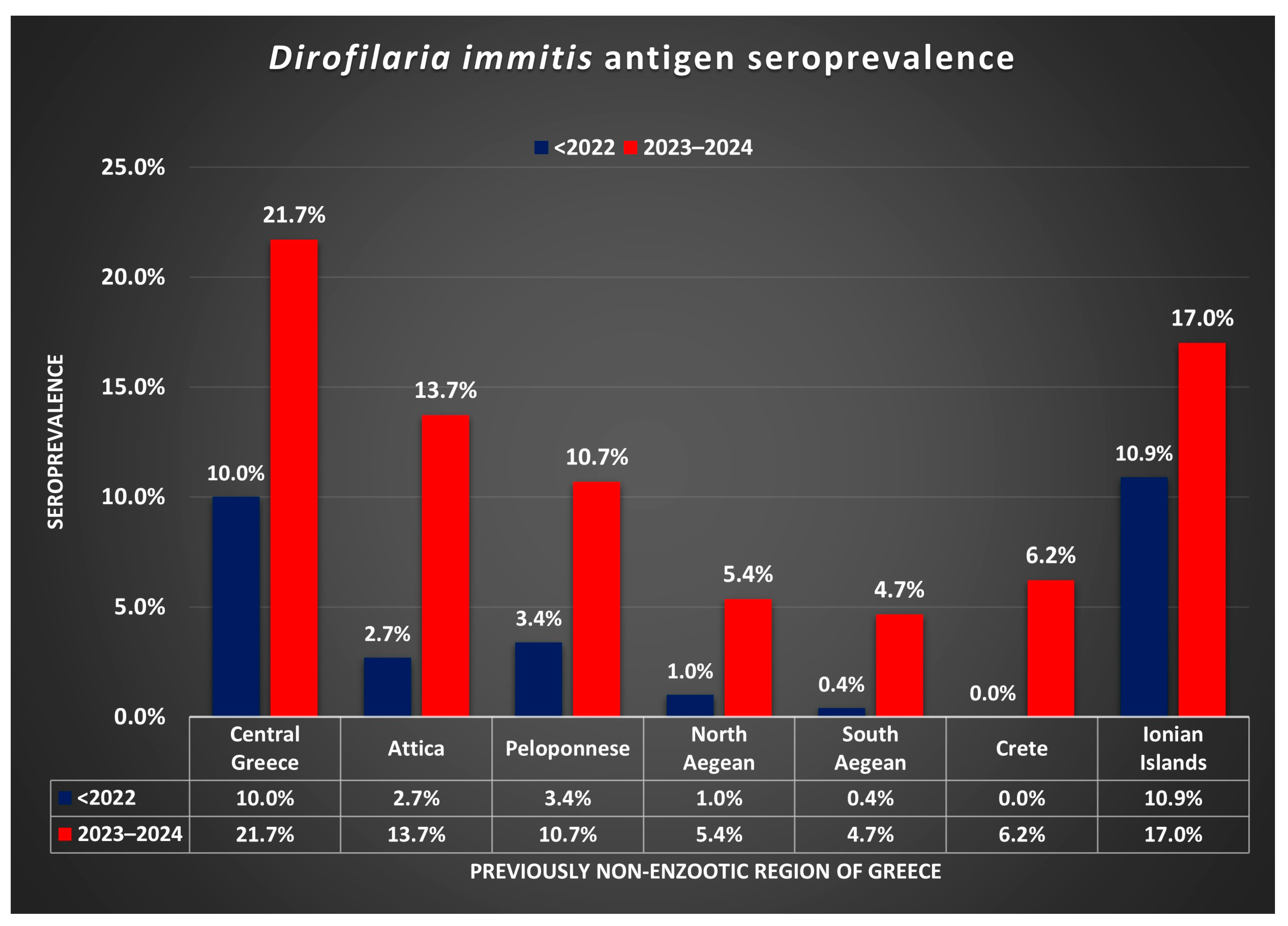
| Regions | Tested Dogs | Positive Dogs | Prevalence of D. immitis (%) | 95% Confidence Interval of Prevalence | ||
|---|---|---|---|---|---|---|
| Lower (%) | Upper (%) | |||||
| 2023–2024 | ||||||
| Central Greece | 106 | 23 | 21.7 | 14.9 | 30.5 | |
| Attica | 401 | 55 | 13.7 | 10.7 | 17.4 | |
| Peloponnese | 487 | 52 | 10.7 | 8.2 | 13.7 | |
| North Aegean islands | 56 | 3 | 5.4 | 1.8 | 14.6 | |
| South Aegean islands | 258 | 12 | 4.7 | 2.7 | 8.0 | |
| Crete | 161 | 10 | 6.2 | 3.4 | 11.1 | |
| Ionian islands | 59 | 10 | 17.0 | 9.5 | 28.5 | |
| Total | 1528 | 165 | 10.8 | 9.3 | 12.5 | |
| <2022 | References | |||||
| Central Greece | 189 | 19 | 10.1 | 6.5 | 15.2 | [13] |
| Attica | 1456 | 39 | 2.7 | 2.0 | 3.6 | [12,13,19] |
| Peloponnese | 266 | 9 | 3.4 | 1.8 | 6.3 | [12,13] |
| North Aegean islands | 95 | 1 | 1.1 | 0.2 | 5.7 | [13,30] |
| South Aegean islands | 286 | 1 | 0.4 | 0.0 | 2.0 | [13,30] |
| Crete | 251 | 0 | 0.0 | 0.0 | 1.5 | [12,13,21] |
| Ionian islands | 55 | 6 | 10.9 | 5.1 | 21.8 | [13] |
| Total | 2598 | 75 | 2.9 | 2.3 | 3.6 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Symeonidou, I.; Sioutas, G.; Gelasakis, A.I.; Bitchava, D.; Kanaki, E.; Papadopoulos, E. Beyond Borders: Dirofilaria immitis Infection in Dogs Spreads to Previously Non-Enzootic Areas in Greece—A Serological Survey. Vet. Sci. 2024, 11, 255. https://doi.org/10.3390/vetsci11060255
Symeonidou I, Sioutas G, Gelasakis AI, Bitchava D, Kanaki E, Papadopoulos E. Beyond Borders: Dirofilaria immitis Infection in Dogs Spreads to Previously Non-Enzootic Areas in Greece—A Serological Survey. Veterinary Sciences. 2024; 11(6):255. https://doi.org/10.3390/vetsci11060255
Chicago/Turabian StyleSymeonidou, Isaia, Georgios Sioutas, Athanasios I. Gelasakis, Dimitra Bitchava, Eleni Kanaki, and Elias Papadopoulos. 2024. "Beyond Borders: Dirofilaria immitis Infection in Dogs Spreads to Previously Non-Enzootic Areas in Greece—A Serological Survey" Veterinary Sciences 11, no. 6: 255. https://doi.org/10.3390/vetsci11060255
APA StyleSymeonidou, I., Sioutas, G., Gelasakis, A. I., Bitchava, D., Kanaki, E., & Papadopoulos, E. (2024). Beyond Borders: Dirofilaria immitis Infection in Dogs Spreads to Previously Non-Enzootic Areas in Greece—A Serological Survey. Veterinary Sciences, 11(6), 255. https://doi.org/10.3390/vetsci11060255









