Dietary Features Are Associated with Differences in the Urinary Microbiome in Clinically Healthy Adult Dogs
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Diet History Collection
2.3. Sample Collection
2.4. DNA Isolation and Amplicon Sequencing
2.5. Processing of Raw DNA Sequence Reads
2.6. Statistical Analysis
3. Results
3.1. Study Participants
3.2. Dietary Histories
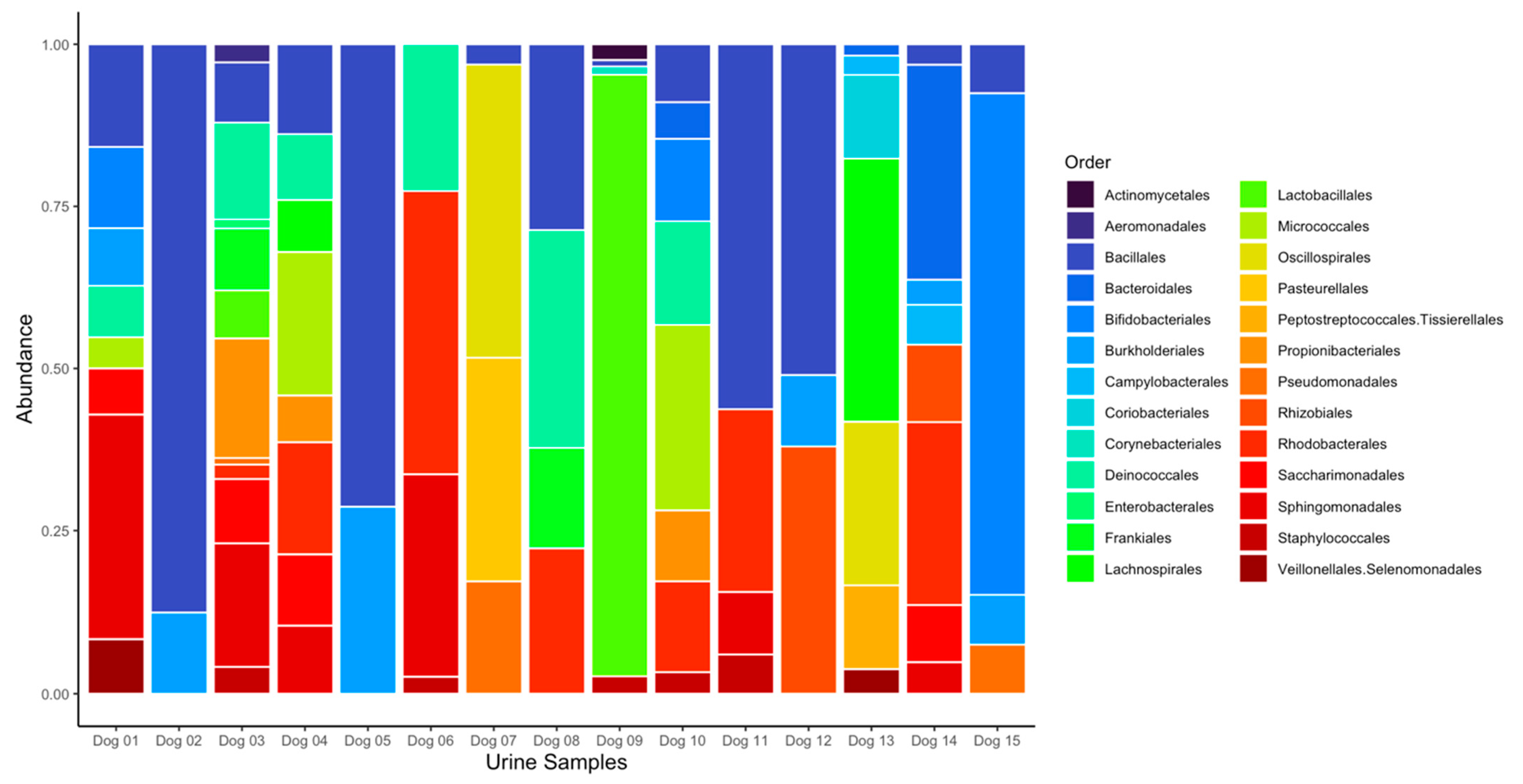
3.3. Data Processing and Bacterial Composition of Urine Samples
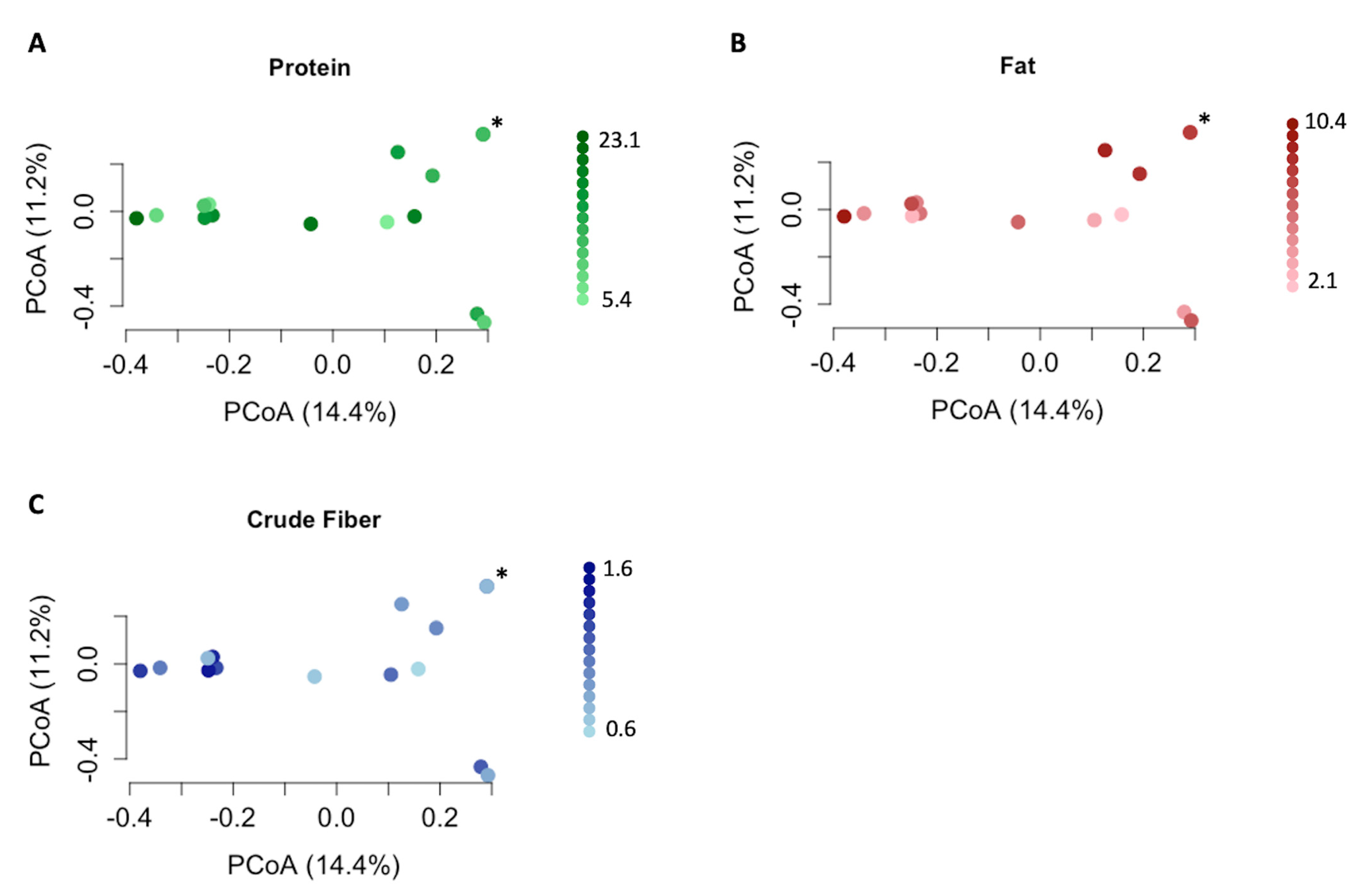
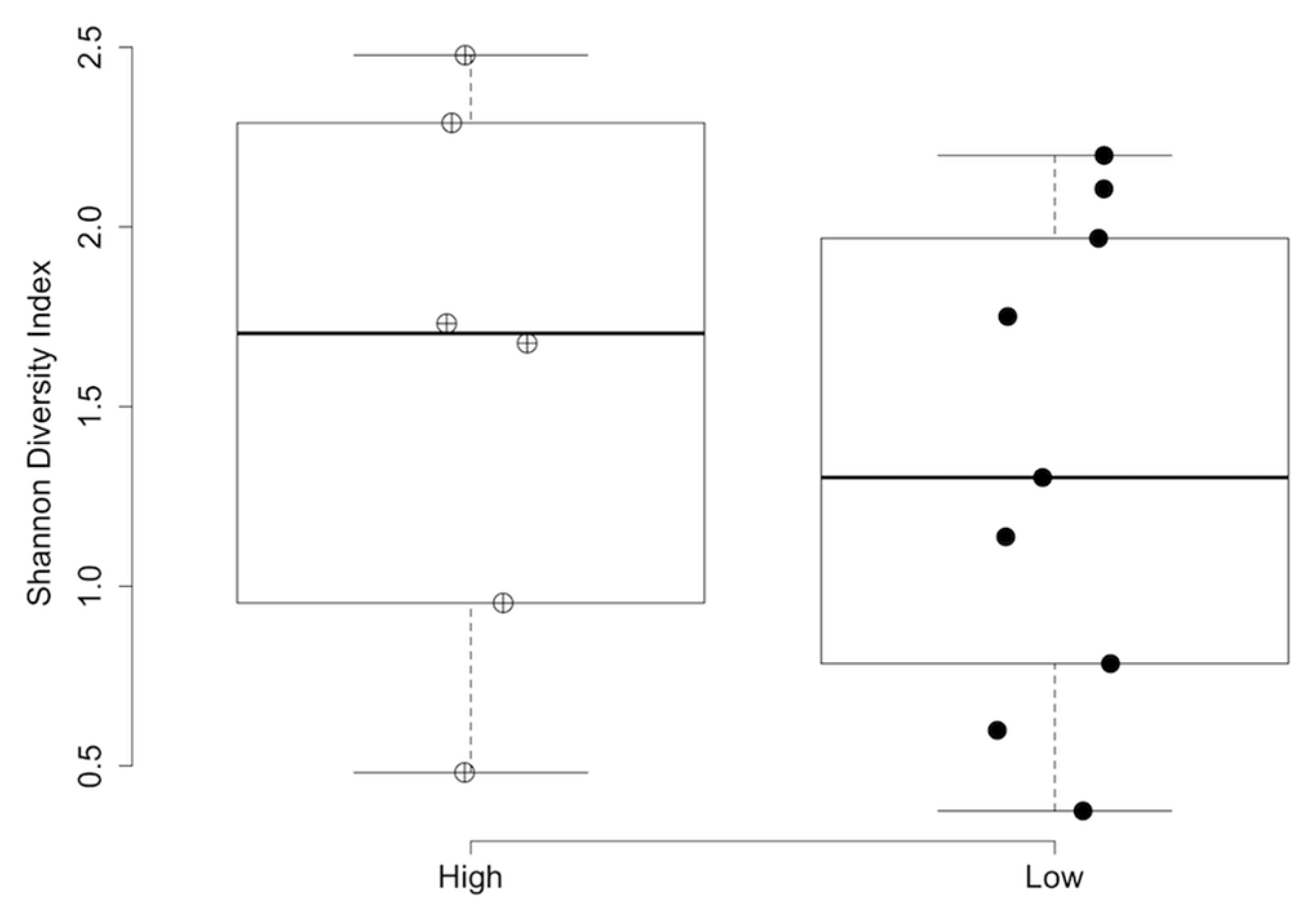
3.4. Impact of Nutritional Features on Urobiome Composition
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wolfe, A.J.; Toh, E.; Shibata, N.; Rong, R.; Kenton, K.; FitzGerald, M.; Mueller, E.R.; Schreckenberger, P.; Dong, Q.; Nelson, D.E.; et al. Evidence of Uncultivated Bacteria in the Adult Female Bladder. J. Clin. Microbiol. 2012, 50, 1376–1383. [Google Scholar] [CrossRef] [PubMed]
- Price, T.; Hilt, E.; Thomas-White, K.; Mueller, E.; Wolfe, A.; Brubaker, L. The Urobiome of Continent Adult Women: A Cross-sectional Study. BJOG Int. J. Obstet. Gynaecol. 2020, 127, 193–201. [Google Scholar] [CrossRef] [PubMed]
- Price, T.K.; Dune, T.; Hilt, E.E.; Thomas-White, K.J.; Kliethermes, S.; Brincat, C.; Brubaker, L.; Wolfe, A.J.; Mueller, E.R.; Schreckenberger, P.C. The Clinical Urine Culture: Enhanced Techniques Improve Detection of Clinically Relevant Microorganisms. J. Clin. Microbiol. 2016, 54, 1216–1222. [Google Scholar] [CrossRef] [PubMed]
- Fouts, D.E.; Pieper, R.; Szpakowski, S.; Pohl, H.; Knoblach, S.; Suh, M.-J.; Huang, S.-T.; Ljungberg, I.; Sprague, B.M.; Lucas, S.K.; et al. Integrated Next-Generation Sequencing of 16S rDNA and Metaproteomics Differentiate the Healthy Urine Microbiome from Asymptomatic Bacteriuria in Neuropathic Bladder Associated with Spinal Cord Injury. J. Transl. Med. 2012, 10, 174. [Google Scholar] [CrossRef]
- Hilt, E.E.; McKinley, K.; Pearce, M.M.; Rosenfeld, A.B.; Zilliox, M.J.; Mueller, E.R.; Brubaker, L.; Gai, X.; Wolfe, A.J.; Schreckenberger, P.C. Urine Is Not Sterile: Use of Enhanced Urine Culture Techniques to Detect Resident Bacterial Flora in the Adult Female Bladder. J. Clin. Microbiol. 2014, 52, 871–876. [Google Scholar] [CrossRef] [PubMed]
- Pearce, M.M.; Hilt, E.E.; Rosenfeld, A.B.; Zilliox, M.J.; Thomas-White, K.; Fok, C.; Kliethermes, S.; Schreckenberger, P.C.; Brubaker, L.; Gai, X.; et al. The Female Urinary Microbiome: A Comparison of Women with and without Urgency Urinary Incontinence. mBio 2014, 5, e01283-14. [Google Scholar] [CrossRef]
- Abernethy, M.G.; Rosenfeld, A.; White, J.R.; Mueller, M.G.; Lewicky-Gaupp, C.; Kenton, K. Urinary Microbiome and Cytokine Levels in Women with Interstitial Cystitis. Obstet. Gynecol. 2017, 129, 500–506. [Google Scholar] [CrossRef]
- Pearce, M.M.; Zilliox, M.J.; Rosenfeld, A.B.; Thomas-White, K.J.; Richter, H.E.; Nager, C.W.; Visco, A.G.; Nygaard, I.E.; Barber, M.D.; Schaffer, J.; et al. The Female Urinary Microbiome in Urgency Urinary Incontinence. Am. J. Obstet. Gynecol. 2015, 213, 347.e1–347.e11. [Google Scholar] [CrossRef]
- Zampini, A.; Nguyen, A.H.; Rose, E.; Monga, M.; Miller, A.W. Defining Dysbiosis in Patients with Urolithiasis. Sci. Rep. 2019, 9, 5425. [Google Scholar] [CrossRef]
- Wu, P.; Zhang, G.; Zhao, J.; Chen, J.; Chen, Y.; Huang, W.; Zhong, J.; Zeng, J. Profiling the Urinary Microbiota in Male Patients with Bladder Cancer in China. Front. Cell. Infect. Microbiol. 2018, 8, 167. [Google Scholar] [CrossRef]
- Hourigan, S.K.; Zhu, W.; Wong, W.S.W.; Clemency, N.C.; Provenzano, M.; Vilboux, T.; Niederhuber, J.E.; Deeken, J.; Chung, S.; McDaniel-Wiley, K.; et al. Studying the Urine Microbiome in Superficial Bladder Cancer: Samples Obtained by Midstream Voiding versus Cystoscopy. BMC Urol. 2020, 20, 5. [Google Scholar] [CrossRef] [PubMed]
- Rothschild, D.; Weissbrod, O.; Barkan, E.; Kurilshikov, A.; Korem, T.; Zeevi, D.; Costea, P.I.; Godneva, A.; Kalka, I.N.; Bar, N.; et al. Environment Dominates over Host Genetics in Shaping Human Gut Microbiota. Nature 2018, 555, 210–215. [Google Scholar] [CrossRef] [PubMed]
- David, L.A.; Maurice, C.F.; Carmody, R.N.; Gootenberg, D.B.; Button, J.E.; Wolfe, B.E.; Ling, A.V.; Devlin, A.S.; Varma, Y.; Fischbach, M.A.; et al. Diet Rapidly and Reproducibly Alters the Human Gut Microbiome. Nature 2014, 505, 559–563. [Google Scholar] [CrossRef] [PubMed]
- Salazar, A.M.; Neugent, M.L.; De Nisco, N.J.; Mysorekar, I.U. Gut-Bladder Axis Enters the Stage: Implication for Recurrent Urinary Tract Infections. Cell Host Microbe 2022, 30, 1066–1069. [Google Scholar] [CrossRef] [PubMed]
- Schembri, M.A.; Nhu, N.T.K.; Phan, M.-D. Gut–Bladder Axis in Recurrent UTI. Nat. Microbiol. 2022, 7, 601–602. [Google Scholar] [CrossRef] [PubMed]
- Magruder, M.; Sholi, A.N.; Gong, C.; Zhang, L.; Edusei, E.; Huang, J.; Albakry, S.; Satlin, M.J.; Westblade, L.F.; Crawford, C.; et al. Gut Uropathogen Abundance Is a Risk Factor for Development of Bacteriuria and Urinary Tract Infection. Nat. Commun. 2019, 10, 5521. [Google Scholar] [CrossRef] [PubMed]
- Thänert, R.; Reske, K.A.; Hink, T.; Wallace, M.A.; Wang, B.; Schwartz, D.J.; Seiler, S.; Cass, C.; Burnham, C.-A.D.; Dubberke, E.R.; et al. Comparative Genomics of Antibiotic-Resistant Uropathogens Implicates Three Routes for Recurrence of Urinary Tract Infections. mBio 2019, 10, e01977-19. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Ma, L.; Fu, P. Gut Microbiota-Derived Short-Chain Fatty Acids and Kidney Diseases. Drug Des. Dev. Ther. 2017, 11, 3531–3542. [Google Scholar] [CrossRef] [PubMed]
- Welch, A.A.; Mulligan, A.; Bingham, S.A.; Khaw, K. Urine pH Is an Indicator of Dietary Acid–Base Load, Fruit and Vegetables and Meat Intakes: Results from the European Prospective Investigation into Cancer and Nutrition (EPIC)-Norfolk Population Study. Br. J. Nutr. 2008, 99, 1335–1343. [Google Scholar] [CrossRef]
- Brachem, C.; Oluwagbemigun, K.; Langenau, J.; Weinhold, L.; Alexy, U.; Schmid, M.; Nöthlings, U. Exploring the Association between Habitual Food Intake and the Urine and Blood Metabolome in Adolescents and Young Adults: A Cohort Study. Mol. Nutr. Food Res. 2022, 66, 2200023. [Google Scholar] [CrossRef]
- Castellano-Escuder, P.; González-Domínguez, R.; Vaillant, M.-F.; Casas-Agustench, P.; Hidalgo-Liberona, N.; Estanyol-Torres, N.; Wilson, T.; Beckmann, M.; Lloyd, A.J.; Oberli, M.; et al. Assessing Adherence to Healthy Dietary Habits Through the Urinary Food Metabolome: Results from a European Two-Center Study. Front. Nutr. 2022, 9, 880770. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Ling, Z.; Tang, C.; Yi, F.; Chen, Y.Q. Moderation Effects of Food Intake on the Relationship between Urinary Microbiota and Urinary Interleukin-8 in Female Type 2 Diabetic Patients. PeerJ 2020, 8, e8481. [Google Scholar] [CrossRef] [PubMed]
- Coffey, E.L.; Gomez, A.M.; Burton, E.N.; Granick, J.L.; Lulich, J.P.; Furrow, E. Characterization of the Urogenital Microbiome in Miniature Schnauzers with and without Calcium Oxalate Urolithiasis. Vet. Intern. Medicne 2022, 36, 1341–1352. [Google Scholar] [CrossRef] [PubMed]
- Burton, E.N.; Cohn, L.A.; Reinero, C.N.; Rindt, H.; Moore, S.G.; Ericsson, A.C. Characterization of the Urinary Microbiome in Healthy Dogs. PLoS ONE 2017, 12, e0177783. [Google Scholar] [CrossRef] [PubMed]
- Melgarejo, T.; Oakley, B.B.; Krumbeck, J.A.; Tang, S.; Krantz, A.; Linde, A. Assessment of Bacterial and Fungal Populations in Urine from Clinically Healthy Dogs Using Next-generation Sequencing. J. Vet. Intern. Med. 2021, 35, 1416–1426. [Google Scholar] [CrossRef] [PubMed]
- Mrofchak, R.; Madden, C.; Evans, M.V.; Hale, V.L. Evaluating Extraction Methods to Study Canine Urine Microbiota. PLoS ONE 2021, 16, e0253989. [Google Scholar] [CrossRef] [PubMed]
- Mrofchak, R.; Madden, C.; Evans, M.V.; Kisseberth, W.C.; Dhawan, D.; Knapp, D.W.; Hale, V.L. Urine and Fecal Microbiota in a Canine Model of Bladder Cancer and Comparison of Canine and Human Urine Microbiota. All Life 2022, 15, 1245–1263. [Google Scholar] [CrossRef]
- Coelho, L.P.; Kultima, J.R.; Costea, P.I.; Fournier, C.; Pan, Y.; Czarnecki-Maulden, G.; Hayward, M.R.; Forslund, S.K.; Schmidt, T.S.B.; Descombes, P.; et al. Similarity of the Dog and Human Gut Microbiomes in Gene Content and Response to Diet. Microbiome 2018, 6, 72. [Google Scholar] [CrossRef]
- Coffey, E.L.; Gomez, A.M.; Ericsson, A.C.; Burton, E.N.; Granick, J.L.; Lulich, J.P.; Furrow, E. The Impact of Urine Collection Method on Canine Urinary Microbiota Detection: A Cross-Sectional Study. BMC Microbiol. 2023, 23, 101. [Google Scholar] [CrossRef]
- Cline, M.G.; Burns, K.M.; Coe, J.B.; Downing, R.; Durzi, T.; Murphy, M.; Parker, V. 2021 AAHA Nutrition and Weight Management Guidelines for Dogs and Cats. J. Am. Anim. Hosp. Assoc. 2021, 57, 153–178. [Google Scholar] [CrossRef]
- Ruel, M.T. Operationalizing Dietary Diversity: A Review of Measurement Issues and Research Priorities. J. Nutr. 2003, 133, 3911S–3926S. [Google Scholar] [CrossRef] [PubMed]
- Weese, J.S.; Blondeau, J.; Boothe, D.; Guardabassi, L.G.; Gumley, N.; Papich, M.; Jessen, L.R.; Lappin, M.; Rankin, S.; Westropp, J.L.; et al. International Society for Companion Animal Infectious Diseases (ISCAID) Guidelines for the Diagnosis and Management of Bacterial Urinary Tract Infections in Dogs and Cats. Vet. J. 2019, 247, 8–25. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Pieper, R. Urinary Pellet Sample Preparation for Shotgun Proteomic Analysis of Microbial Infection and Host–Pathogen Interactions. Methods Mol. Biol. 2015, 1295, 65–74. [Google Scholar] [CrossRef] [PubMed]
- Caporaso, J.G.; Lauber, C.L.; Walters, W.A.; Berg-Lyons, D.; Lozupone, C.A.; Turnbaugh, P.J.; Fierer, N.; Knight, R. Global Patterns of 16S rRNA Diversity at a Depth of Millions of Sequences per Sample. Proc. Natl. Acad. Sci. USA 2011, 108, 4516–4522. [Google Scholar] [CrossRef] [PubMed]
- Martin, M. Cutadapt Removes Adapter Sequences from High-Throughput Sequencing Reads. EMBnet 2011, 17, 10–12. [Google Scholar] [CrossRef]
- Bolyen, E.; Rideout, J.R.; Dillon, M.R.; Bokulich, N.A.; Abnet, C.C.; Al-Ghalith, G.A.; Alexander, H.; Alm, E.J.; Arumugam, M.; Asnicar, F.; et al. Reproducible, Interactive, Scalable and Extensible Microbiome Data Science Using QIIME 2. Nat. Biotechnol. 2019, 37, 852–857. [Google Scholar] [CrossRef] [PubMed]
- Callahan, B.J.; McMurdie, P.J.; Rosen, M.J.; Han, A.W.; Johnson, A.J.A.; Holmes, S.P. DADA2: High-Resolution Sample Inference from Illumina Amplicon Data. Nat. Methods 2016, 13, 581–583. [Google Scholar] [CrossRef] [PubMed]
- Quast, C.; Pruesse, E.; Yilmaz, P.; Gerken, J.; Schweer, T.; Yarza, P.; Peplies, J.; Glöckner, F.O. The SILVA Ribosomal RNA Gene Database Project: Improved Data Processing and Web-Based Tools. Nucleic Acids Res. 2012, 41, D590–D596. [Google Scholar] [CrossRef] [PubMed]
- Davis, N.M.; Proctor, D.M.; Holmes, S.P.; Relman, D.A.; Callahan, B.J. Simple Statistical Identification and Removal of Contaminant Sequences in Marker-Gene and Metagenomics Data. Microbiome 2018, 6, 226. [Google Scholar] [CrossRef]
- Oksanen, J.; Blanchet, G.; Friendly, M.; Kindt, R.; Legendre, P.; McGlinn, D.; Minchin, P.; O’Hara, R.B.; Simpson, G.; Solymos, P.; et al. Vegan Community Ecology Package. R Package Version 2.5-7. 2020. Available online: https://CRAN.R-project.org/package=vegan (accessed on 1 August 2022).
- McMurdie, P.J.; Holmes, S. Phyloseq: An R Package for Reproducible Interactive Analysis and Graphics of Microbiome Census Data. PLoS ONE 2013, 8, e61217. [Google Scholar] [CrossRef]
- Lozupone, C.A.; Knight, R. Species Divergence and the Measurement of Microbial Diversity. FEMS Microbiol. Rev. 2008, 32, 557–578. [Google Scholar] [CrossRef]
- Dufrêne, M.; Legendre, P. Species assemblages and indicator species: The need for a flexible asymmetrical approach. Ecol. Monogr. 1997, 67, 345–366. [Google Scholar] [CrossRef]
- Roberts, D. Labdsv Ordination and Multivariate Analysis for Ecology. R Package Version 1.3. 2007. Available online: http://CRAN.R-project.org/package=labdsv (accessed on 1 August 2022).
- Wickham, H. Elegant Graphics for Data Analysis. 2016. Available online: https://ggplot2.tidyverse.org (accessed on 1 August 2022).
- Pilla, R.; Suchodolski, J.S. The Gut Microbiome of Dogs and Cats, and the Influence of Diet. Vet. Clin. N. Am. Small Anim. Pract. 2021, 51, 605–621. [Google Scholar] [CrossRef]
- Johnson, A.J.; Vangay, P.; Al-Ghalith, G.A.; Hillmann, B.M.; Ward, T.L.; Shields-Cutler, R.R.; Kim, A.D.; Shmagel, A.K.; Syed, A.N.; Walter, J.; et al. Daily Sampling Reveals Personalized Diet-Microbiome Associations in Humans. Cell Host Microbe 2019, 25, 789–802. [Google Scholar] [CrossRef]
- Barratt, M.J.; Lebrilla, C.; Shapiro, H.-Y.; Gordon, J.I. The Gut Microbiota, Food Science, and Human Nutrition: A Timely Marriage. Cell Host Microbe 2017, 22, 134–141. [Google Scholar] [CrossRef] [PubMed]
- Montserrat-Malagarriga, M.; Castillejos, L.; Salas-Mani, A.; Torre, C.; Martín-Orúe, S.M. The Impact of Fiber Source on Digestive Function, Fecal Microbiota, and Immune Response in Adult Dogs. Animals 2024, 14, 196. [Google Scholar] [CrossRef] [PubMed]
- Claesson, M.J.; Jeffery, I.B.; Conde, S.; Power, S.E.; O’Connor, E.M.; Cusack, S.; Harris, H.M.B.; Coakley, M.; Lakshminarayanan, B.; O’Sullivan, O.; et al. Gut Microbiota Composition Correlates with Diet and Health in the Elderly. Nature 2012, 488, 178–184. [Google Scholar] [CrossRef]
- Song, S.J.; Lauber, C.; Costello, E.K.; Lozupone, C.A.; Humphrey, G.; Berg-Lyons, D.; Caporaso, J.G.; Knights, D.; Clemente, J.C.; Nakielny, S.; et al. Cohabiting Family Members Share Microbiota with One Another and with Their Dogs. eLife 2013, 2, e00458. [Google Scholar] [CrossRef]
- Ammitzbøll, N.; Bau, B.P.J.; Bundgaard-Nielsen, C.; Villadsen, A.B.; Jensen, A.-M.; Leutscher, P.D.C.; Glavind, K.; Hagstrøm, S.; Arenholt, L.T.S.; Sørensen, S. Pre- and Postmenopausal Women Have Different Core Urinary Microbiota. Sci. Rep. 2021, 11, 2212. [Google Scholar] [CrossRef]
- Price, T.K.; Wolff, B.; Halverson, T.; Limeira, R.; Brubaker, L.; Dong, Q.; Mueller, E.R.; Wolfe, A.J. Temporal Dynamics of the Adult Female Lower Urinary Tract Microbiota. mBio 2020, 11, e00475-20. [Google Scholar] [CrossRef]
- Farcas, A.K.; Larsen, J.A.; Fascetti, A.J. Evaluation of Fiber Concentration in Dry and Canned Commercial Diets Formulated for Adult Maintenance or All Life Stages of Dogs by Use of Crude Fiber and Total Dietary Fiber Methods. J. Am. Vet. Med. Assoc. 2013, 242, 936–940. [Google Scholar] [CrossRef] [PubMed]


| Variable | Value |
|---|---|
| Age (years) | 3.7 (±1.8) |
| Weight (kg) | 19.3 (±11.2) |
| BCS (scale 1–9) | 5.5 (4.5–6.5) |
| Sex | FS (9), MN (6) |
| USG | 1.042 (1.006–1.045) |
| Urine pH | 7.3 (±0.82) |
| Variable | Value |
|---|---|
| % of RER consumed | 123 ± 49.2 1 |
| Kcal from treats (%) | 7.5 ± 13.2 2 |
| Protein (g/100 kcal) | 7.1 (5.4–23.1) |
| Fat (g/100 kcal) | 3.5 (2.1–10.4) |
| Crude fiber (g/100 kcal) | 1.1 (0.6–2.7) |
| Dogs in Diet Group 1 | 11 |
| Dogs in Diet Group 2 | 4 |
| Unique diet and treat sources | 2 (1–5) 3 |
| Dogs in HDD Group (≥3) | 6 |
| Dogs in LDD Group (<3) | 9 |
| Diversity Metric | Protein | Fat | Crude Fiber | Diet Groups | Dietary Diversity Groups |
|---|---|---|---|---|---|
| Alpha Diversity (p value) | |||||
| Shannon | 1 | 0.23 | 0.39 | 0.018 | 0.61 |
| Inverse Simpson | 0.87 | 0.34 | 0.28 | 0.026 | 0.61 |
| Observed Richness | 0.82 | 0.56 | 0.91 | 0.077 | 0.26 |
| Beta Diversity (p value, R2) | |||||
| Bray–Curtis | 0.73, 0.066 | 0.88, 0.061 | 0.11, 0.084 | 0.017, 0.10 | 0.019, 0.10 |
| WUF | 0.46, 0.067 | 0.46, 0.070 | 0.50, 0.067 | 0.13, 0.090 | 0.51, 0.070 |
| UUF | 0.12, 0.091 | 0.31, 0.080 | 0.63, 0.064 | 0.062, 0.11 | 0.19, 0.090 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Coffey, E.L.; Becker, Z.W.; Gomez, A.M.; Ericsson, A.C.; Churchill, J.A.; Burton, E.N.; Granick, J.L.; Lulich, J.P.; Furrow, E. Dietary Features Are Associated with Differences in the Urinary Microbiome in Clinically Healthy Adult Dogs. Vet. Sci. 2024, 11, 286. https://doi.org/10.3390/vetsci11070286
Coffey EL, Becker ZW, Gomez AM, Ericsson AC, Churchill JA, Burton EN, Granick JL, Lulich JP, Furrow E. Dietary Features Are Associated with Differences in the Urinary Microbiome in Clinically Healthy Adult Dogs. Veterinary Sciences. 2024; 11(7):286. https://doi.org/10.3390/vetsci11070286
Chicago/Turabian StyleCoffey, Emily L., Zoe W. Becker, Andres M. Gomez, Aaron C. Ericsson, Julie A. Churchill, Erin N. Burton, Jennifer L. Granick, Jody P. Lulich, and Eva Furrow. 2024. "Dietary Features Are Associated with Differences in the Urinary Microbiome in Clinically Healthy Adult Dogs" Veterinary Sciences 11, no. 7: 286. https://doi.org/10.3390/vetsci11070286
APA StyleCoffey, E. L., Becker, Z. W., Gomez, A. M., Ericsson, A. C., Churchill, J. A., Burton, E. N., Granick, J. L., Lulich, J. P., & Furrow, E. (2024). Dietary Features Are Associated with Differences in the Urinary Microbiome in Clinically Healthy Adult Dogs. Veterinary Sciences, 11(7), 286. https://doi.org/10.3390/vetsci11070286






