Detection of Serum IgG Specific for Brachyspira pilosicoli and “Brachyspira canis” in Dogs
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Brachyspira Strains
2.2. Canine Serum Samples
2.3. Antigen Preparation
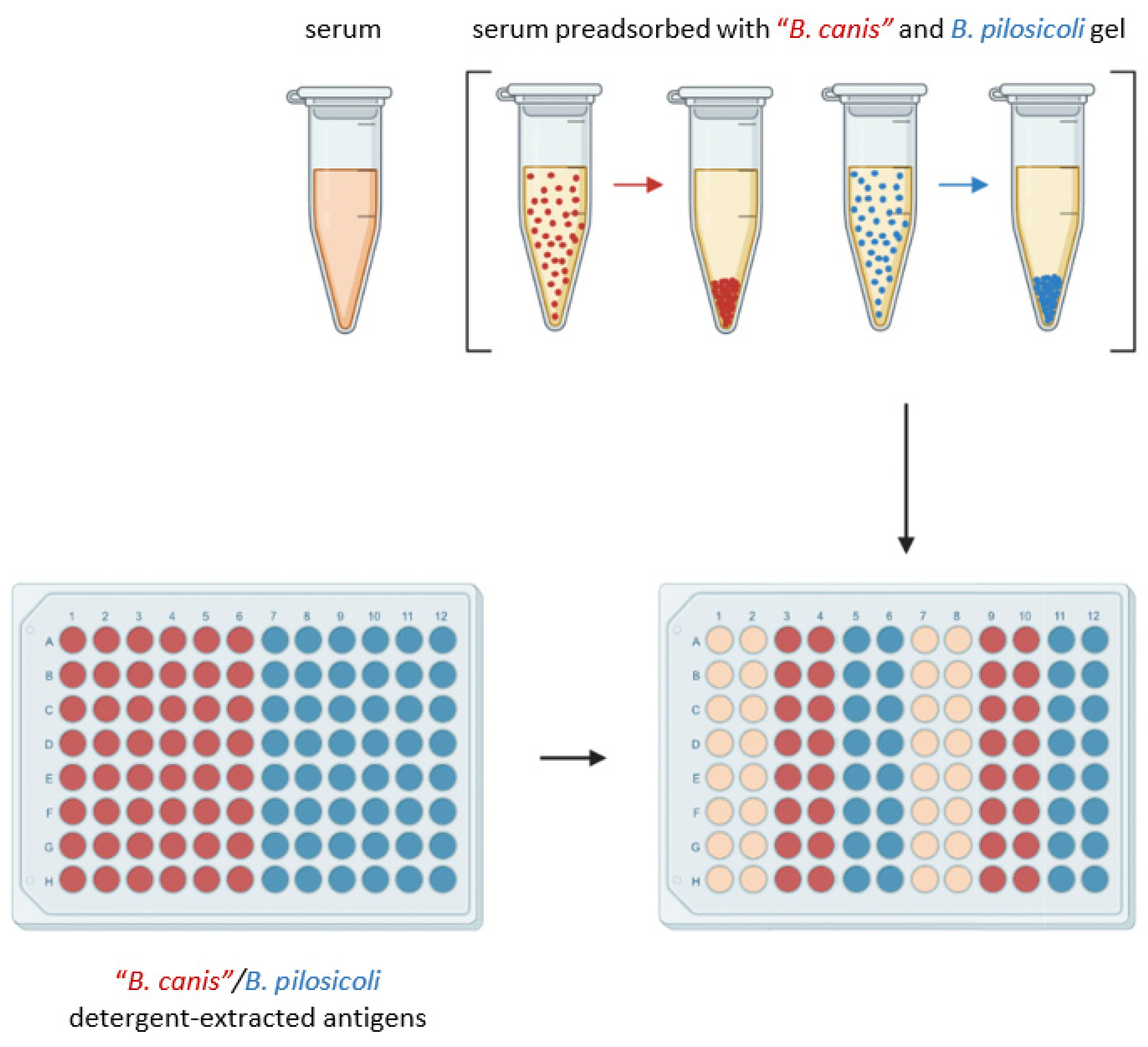
2.4. Gel Preparation
2.5. Enzyme-Linked Immunosorbent Assay with Detergent-Extracted Antigen
2.6. Enzyme-Linked Immunosorbent Assay with Detergent-Extracted, Trichloroacetic Acid-Precipitated Antigen
2.7. SDS-Polyacrylamide Gel Electrophoresis (SDS-PAGE)
2.8. Silver Staining for Lipooligosaccharides
2.9. Statistical Analyses
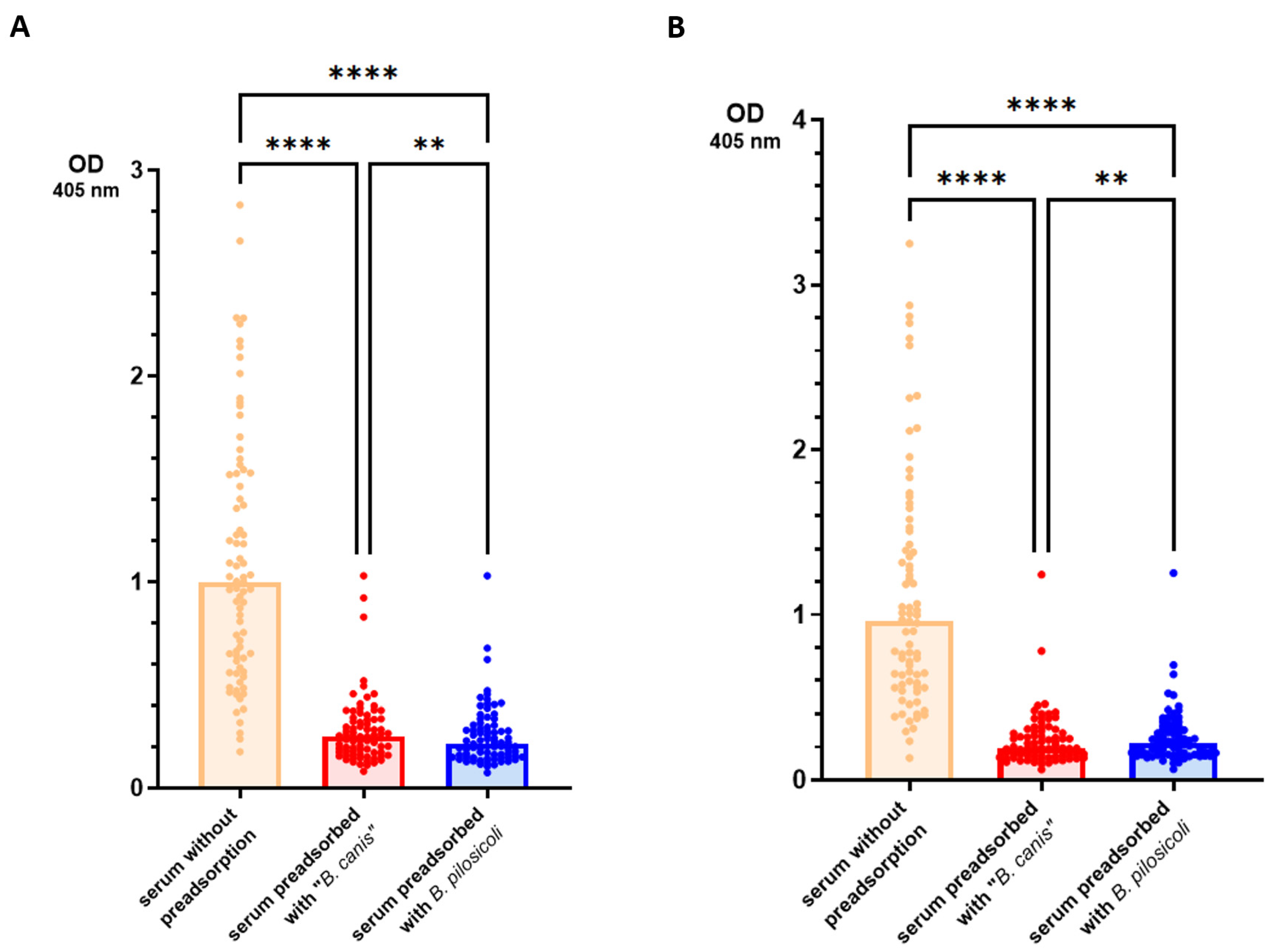
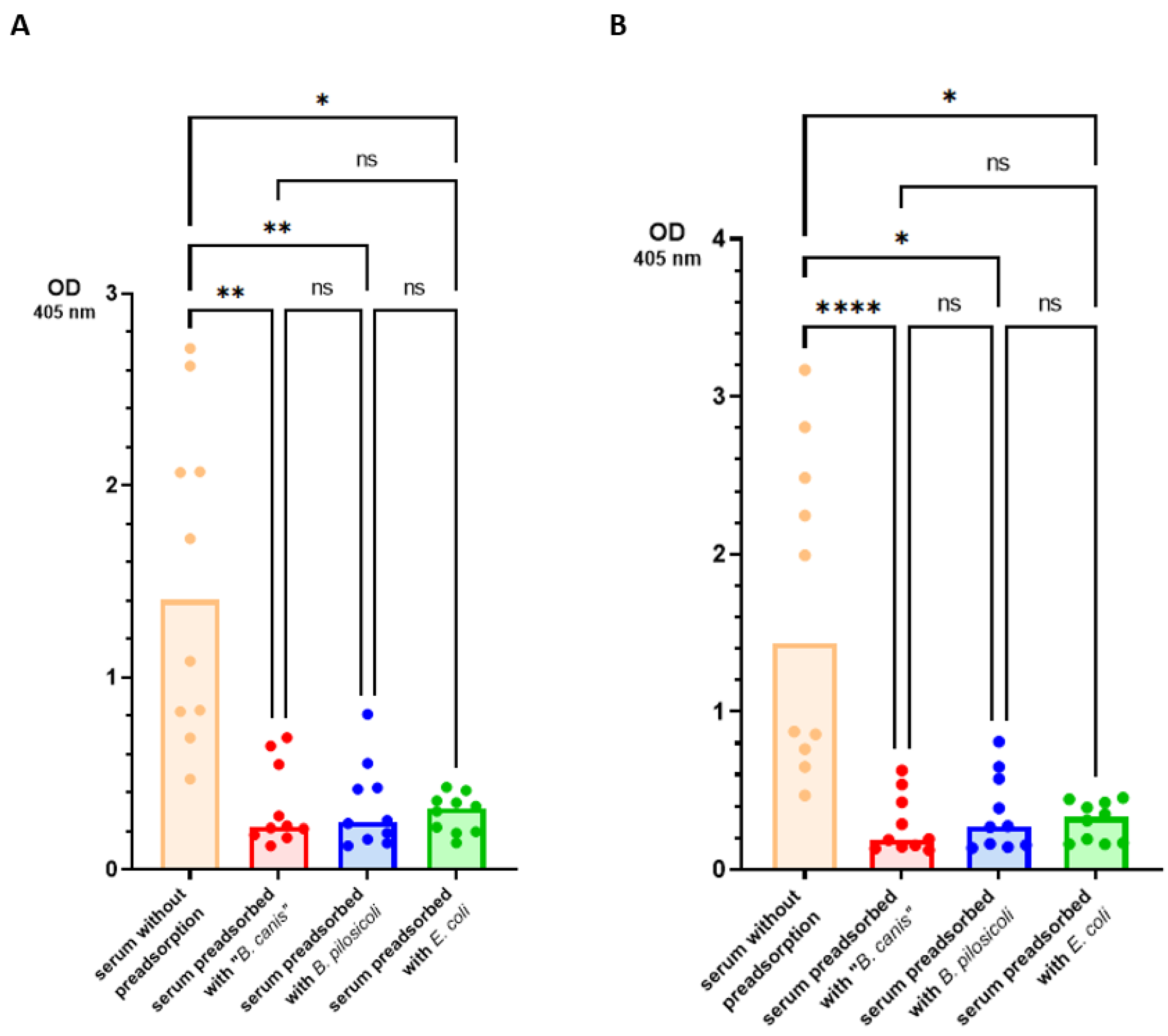
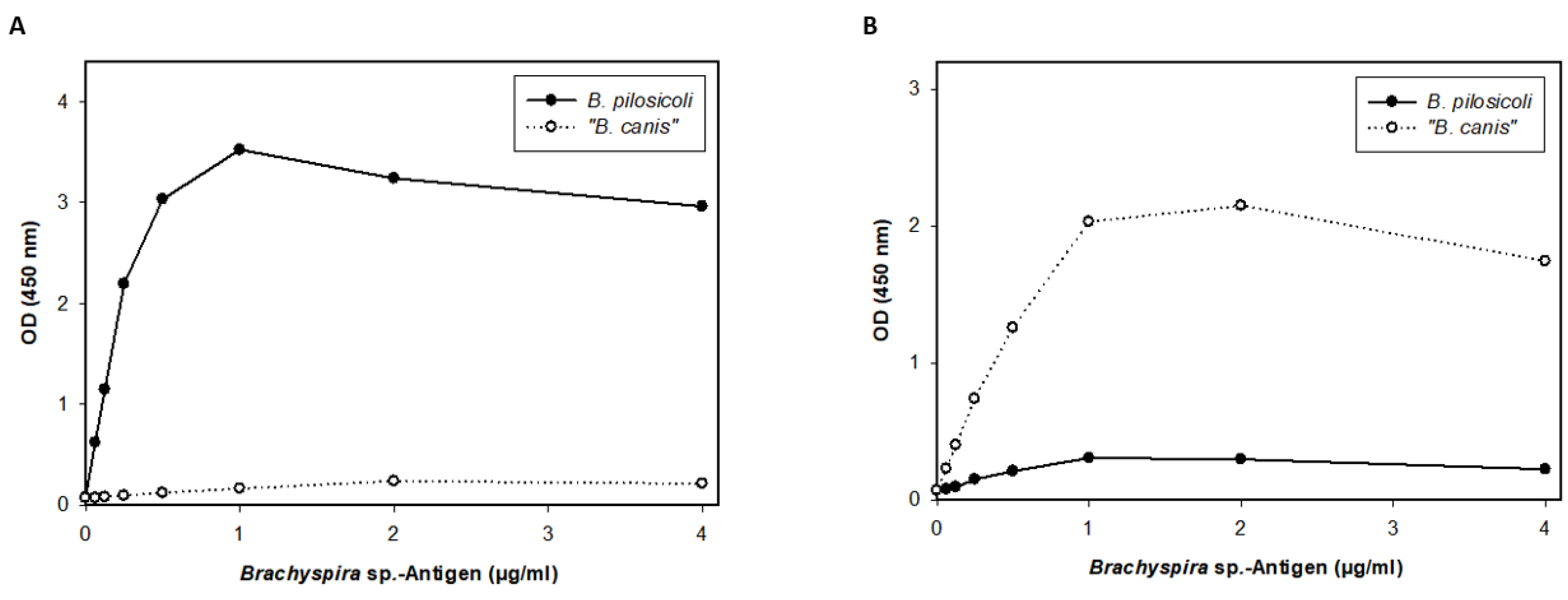
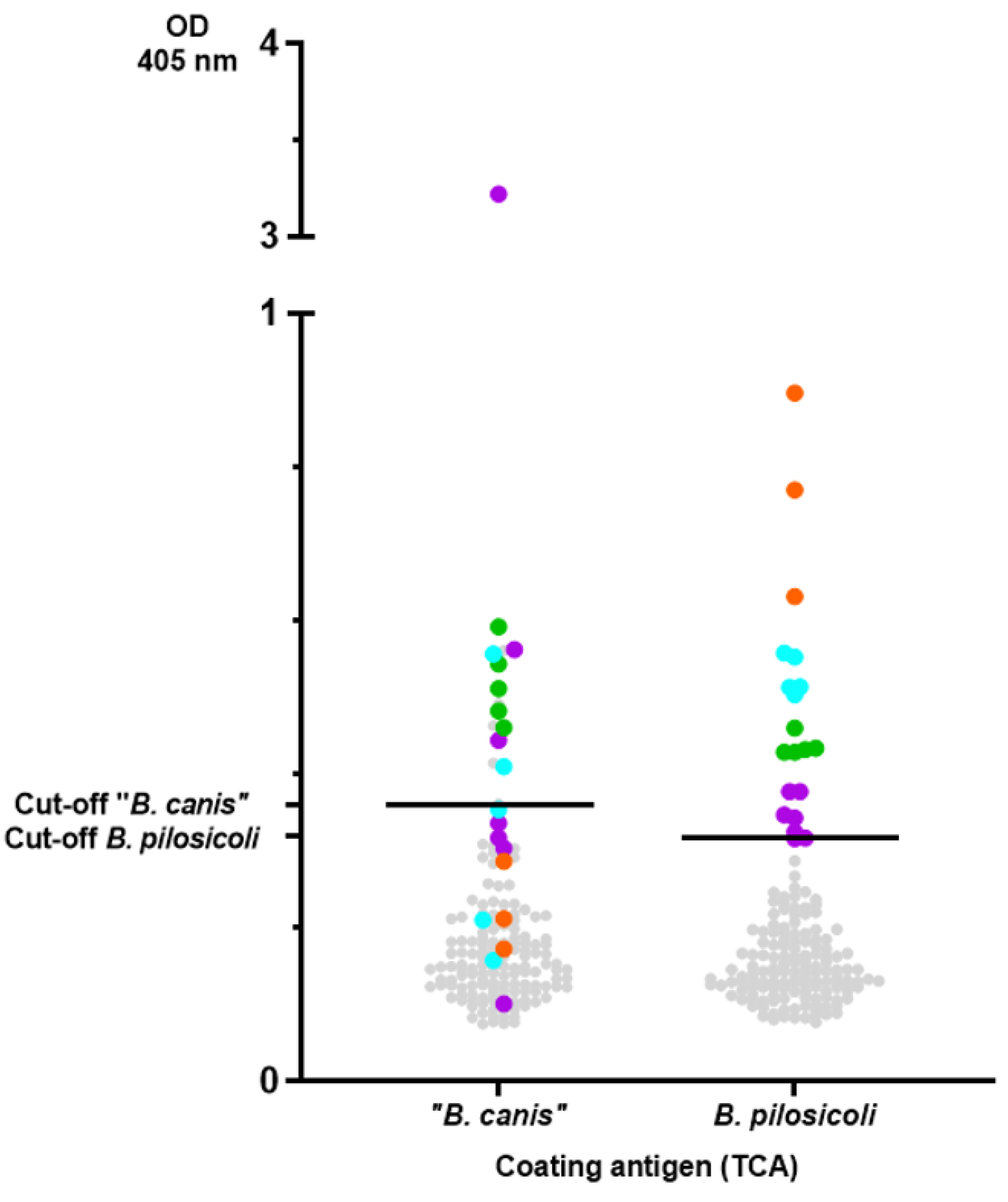
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hampson, D.J. The Spirochete Brachyspira pilosicoli, Enteric Pathogen of Animals and Humans. Clin. Microbiol. Rev. 2018, 31. [Google Scholar] [CrossRef] [PubMed]
- Stephens, C.P.; Hampson, D.J. Experimental infection of broiler breeder hens with the intestinal spirochaete Brachyspira (Serpulina) pilosicoli causes reduced egg production. Avian Pathol. 2002, 31, 169–175. [Google Scholar] [CrossRef] [PubMed]
- La, T.; Phillips, N.D.; Hampson, D.J. An Investigation into the Etiological Agents of Swine Dysentery in Australian Pig Herds. PLoS ONE 2016, 11, e0167424. [Google Scholar] [CrossRef] [PubMed]
- Trott, D.J.; Huxtable, C.R.; Hampson, D.J. Experimental infection of newly weaned pigs with human and porcine strains of Serpulina pilosicoli. Infect. Immun. 1996, 64, 4648–4654. [Google Scholar] [CrossRef] [PubMed]
- Hidalgo, A.; Rubio, P.; Osorio, J.; Carvajal, A. Prevalence of Brachyspira pilosicoli and “Brachyspira canis” in dogs and their association with diarrhoea. Vet. Microbiol. 2010, 146, 356–360. [Google Scholar] [CrossRef] [PubMed]
- Prapasarakul, N.; Lugsomya, K.; Disatian, S.; Lekdumrongsak, T.; Banlunara, W.; Chetanachan, P.; Hampson, D.J. Faecal excretion of intestinal spirochaetes by urban dogs, and their pathogenicity in a chick model of intestinal spirochaetosis. Res. Vet. Sci. 2011, 91, e38–e43. [Google Scholar] [CrossRef] [PubMed]
- Fellström, C.; Pettersson, B.; Zimmerman, U.; Gunnarsson, A.; Feinstein, R. Classification of Brachyspira spp. isolated from Swedish Dogs. Anim. Health Res. Rev. 2001, 2, 75–82. [Google Scholar] [CrossRef] [PubMed]
- Pandey, A.; Humbert, M.V.; Jackson, A.; Passey, J.L.; Hampson, D.J.; Cleary, D.W.; La Ragione, R.M.; Christodoulides, M. Evidence of homologous recombination as a driver of diversity in Brachyspira pilosicoli. Microb. Genom. 2020, 6, e000470. [Google Scholar] [CrossRef]
- Halter, M.R.; Joens, L.A. Lipooligosaccharides from Treponema hyodysenteriae and Treponema innocens. Infect. Immun. 1988, 56, 3152–3156. [Google Scholar] [CrossRef]
- Lee, B.J.; Hampson, D.J. Lipo-oligosaccharide profiles of Serpulina pilosicoli strains and their serological cross-reactivities. J. Med. Microbiol. 1999, 48, 411–415. [Google Scholar] [CrossRef][Green Version]
- Trott, D.J.; Alt, D.P.; Zuerner, R.L.; Wannemuehler, M.J.; Stanton, T.B. The search for Brachyspira outer membrane proteins that interact with the host. Anim. Health Res. Rev. 2001, 2, 19–30. [Google Scholar] [CrossRef] [PubMed]
- Casas, V.; Vadillo, S.; San Juan, C.; Carrascal, M.; Abian, J. The Exposed Proteomes of Brachyspira hyodysenteriae and B. pilosicoli. Front. Microbiol. 2016, 7, 1103. [Google Scholar] [CrossRef] [PubMed]
- Casas, V.; Rodríguez-Asiain, A.; Pinto-Llorente, R.; Vadillo, S.; Carrascal, M.; Abian, J. Brachyspira hyodysenteriae and B. pilosicoli Proteins Recognized by Sera of Challenged Pigs. Front. Microbiol. 2017, 8, 723. [Google Scholar] [CrossRef] [PubMed]
- Duhamel, G.E.; Trott, D.J.; Muniappa, N.; Mathiesen, M.R.; Tarasiuk, K.; Lee, J.I.; Hampson, D.J. Canine intestinal spirochetes consist of Serpulina pilosicoli and a newly identified group provisionally designated “Serpulina canis” sp. nov. J. Clin. Microbiol. 1998, 36, 2264–2270. [Google Scholar] [CrossRef] [PubMed]
- Johansson, K.-E.; Duhamel, G.E.; Bergsjö, B.; Engvall, E.O.; Persson, M.; Pettersson, B.; Fellström, C. Identification of three clusters of canine intestinal spirochaetes by biochemical and 16S rDNA sequence analysis. J. Med. Microbiol. 2004, 53, 345–350. [Google Scholar] [CrossRef]
- Oxberry, S.L.; Hampson, D.J. Colonisation of pet shop puppies with Brachyspira pilosicoli. Vet. Microbiol. 2003, 93, 167–174. [Google Scholar] [CrossRef] [PubMed]
- Gothe, J.; Pfetzing, S.; Ulrich, R.; Schrödl, W.; Baums, C.G.; Heilmann, R.M. Brachyspira in dogs: Risk factors of shedding in central Germany and longitudinal study of an infected kennel. BMC Vet. Res. 2024, 20, 136. [Google Scholar] [CrossRef] [PubMed]
- Harms, M.; Schmidt, V.; Heydel, T.; Hauptmann, J.; Ahlers, C.; Bergmann, R.; Baums, C.G. Differentiation of Brachyspira spp. isolated from laying hens using PCR-based methods and matrix-assisted laser desorption/ionization time-of-flight mass spectrometry. J. Vet. Diagn. Investig. 2018, 30, 545–553. [Google Scholar] [CrossRef]
- Fomsgaard, A.; Freudenberg, M.A.; Galanos, C. Modification of the silver staining technique to detect lipopolysaccharide in polyacrylamide gels. J. Clin. Microbiol. 1990, 28, 2627–2631. [Google Scholar] [CrossRef]
- Tsai, C.-M.; Frasch, C.E. A sensitive silver stain for detecting lipopolysaccharides in polyacrylamide gels. Anal. Biochem. 1982, 119, 115–119. [Google Scholar] [CrossRef]
- Lee, J.I.; Hampson, D.J. The prevalence of intestinal spirochetes in dogs. Aust. Vet. J. 1996, 74, 466–467. [Google Scholar] [CrossRef]
- Šperling, D.; Čada, F.; Čížek, A. Isolation and Characterization of Brachyspira spp. from Dogs in the Czech Republic. Acta Vet. Brno 2010, 79, 437–442. [Google Scholar] [CrossRef]
- Sokolow, S.H.; Rand, C.; Marks, S.L.; Drazenovich, N.L.; Kather, E.J.; Foley, J.E. Epidemiologic evaluation of diarrhea in dogs in an animal shelter. Am. J. Vet. Res. 2005, 66, 1018–1024. [Google Scholar] [CrossRef]
- Trott, D.J.; Combs, B.G.; Mikosza, A.S.; Oxberry, S.L.; Robertson, I.D.; Passey, M.; Taime, J.; Sehuko, R.; Alpers, M.P.; Hampson, D.J. The prevalence of Serpulina pilosicoli in humans and domestic animals in the Eastern Highlands of Papua New Guinea. Epidemiol. Infect. 1997, 119, 369–379. [Google Scholar] [CrossRef]
- Kramomtong, I.; Neramitmansook, W.; Whipp, S.C.; Joens, L.A.; Limawongpranee, S. Comparison of ELISA and selective culture in the diagnosis of swine dysentery in Thailand. Vet. Rec. 1996, 138, 332–333. [Google Scholar] [CrossRef] [PubMed]
- La, T.; Phillips, N.D.; Hampson, D.J. Evaluation of recombinant Bhlp29.7 as an ELISA antigen for detecting pig herds with swine dysentery. Vet. Microbiol. 2009, 133, 98–104. [Google Scholar] [CrossRef]
- Song, Y.; La, T.; Phillips, N.D.; Hampson, D.J. Development of a serological ELISA using a recombinant protein to identify pig herds infected with Brachyspira hyodysenteriae. Vet. J. 2015, 206, 365–370. [Google Scholar] [CrossRef] [PubMed]
- Hampson, D.J.; Robertson, I.D.; La, T.; Oxberry, S.L.; Pethick, D.W. Influences of diet and vaccination on colonisation of pigs by the intestinal spirochaete Brachyspira (Serpulina) pilosicoli. Vet. Microbiol. 2000, 73, 75–84. [Google Scholar] [CrossRef]
- La, T.; Hampson, D.J. Serologic detection of Brachyspira (Serpulina) hyodysenteriae infections. Anim. Health Res. Rev. 2001, 2, 45–52. [Google Scholar] [CrossRef]
- Wannemuehler, M.J.; Hubbard, R.D.; Greer, J.M. Characterization of the major outer membrane antigens of Treponema hyodysenteriae. Infect. Immun. 1988, 56, 3032–3039. [Google Scholar] [CrossRef]
- Egan, I.T.; Harris, D.L.; Joens, L.A. Comparison of the microtitration agglutination test and the enzyme-linked immunosorbent assay for the detection of herds affected with swine dysentery. Am. J. Vet. Res. 1983, 44, 1323–1328. [Google Scholar]
- Trott, D.J.; Alt, D.P.; Zuerner, R.L.; Bulach, D.M.; Wannemuehler, M.J.; Stasko, J.; Townsend, K.M.; Stanton, T.B. Identification and cloning of the gene encoding BmpC: An outer-membrane lipoprotein associated with Brachyspira pilosicoli membrane vesicles. Microbiology (Reading) 2004, 150, 1041–1053. [Google Scholar] [CrossRef]
- Zhang, P.; Cheng, X.; Duhamel, G.E. Cloning and DNA sequence analysis of an immunogenic glucose-galactose MglB lipoprotein homologue from Brachyspira pilosicoli, the agent of colonic spirochetosis. Infect. Immun. 2000, 68, 4559–4565. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Koontz, L. TCA precipitation. Methods Enzymol. 2014, 541, 3–10. [Google Scholar] [CrossRef]
- Hukari, R.; Helander, I.M.; Vaara, M. Chain length heterogeneity of lipopolysaccharide released from Salmonella typhimurium by ethylenediaminetetraacetic acid or polycations. Eur. J. Biochem. 1986, 154, 673–676. [Google Scholar] [CrossRef]
- Munshi, M.A.; Traub, R.J.; Robertson, I.D.; Mikosza, A.S.J.; Hampson, D.J. Colonization and risk factors for Brachyspira aalborgi and Brachyspira pilosicoli in humans and dogs on tea estates in Assam, India. Epidemiol. Infect. 2004, 132, 137–144. [Google Scholar] [CrossRef]
- Razmyar, J.; Ghavidel, M.; Salari Sedigh, H. Polymerase chain reaction assay targeting nox gene for rapid identification of Brachyspira canis in dogs. Vet. Res. Forum 2019, 10, 67–72. [Google Scholar] [CrossRef] [PubMed]
- Bourgonje, A.R.; Vogl, T.; Segal, E.; Weersma, R.K. Antibody signatures in inflammatory bowel disease: Current developments and future applications. Trends Mol. Med. 2022, 28, 693–705. [Google Scholar] [CrossRef] [PubMed]
- Vujkovic-Cvijin, I.; Welles, H.C.; Ha, C.W.Y.; Huq, L.; Mistry, S.; Brenchley, J.M.; Trinchieri, G.; Devkota, S.; Belkaid, Y. The systemic anti-microbiota IgG repertoire can identify gut bacteria that translocate across gut barrier surfaces. Sci. Transl. Med. 2022, 14, eabl3927. [Google Scholar] [CrossRef]
- Botía-Sánchez, M.; Galicia, G.; Albaladejo-Marico, L.; Toro-Domínguez, D.; Morell, M.; Marcos-Fernández, R.; Margolles, A.; Alarcón-Riquelme, M.E. Gut epithelial barrier dysfunction in lupus triggers a differential humoral response against gut commensals. Front. Immunol. 2023, 14, 1200769. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gothe, J.; Horn, M.; Baums, C.G.; Heilmann, R.M.; Schrödl, W. Detection of Serum IgG Specific for Brachyspira pilosicoli and “Brachyspira canis” in Dogs. Vet. Sci. 2024, 11, 302. https://doi.org/10.3390/vetsci11070302
Gothe J, Horn M, Baums CG, Heilmann RM, Schrödl W. Detection of Serum IgG Specific for Brachyspira pilosicoli and “Brachyspira canis” in Dogs. Veterinary Sciences. 2024; 11(7):302. https://doi.org/10.3390/vetsci11070302
Chicago/Turabian StyleGothe, Julia, Matthias Horn, Christoph G. Baums, Romy M. Heilmann, and Wieland Schrödl. 2024. "Detection of Serum IgG Specific for Brachyspira pilosicoli and “Brachyspira canis” in Dogs" Veterinary Sciences 11, no. 7: 302. https://doi.org/10.3390/vetsci11070302
APA StyleGothe, J., Horn, M., Baums, C. G., Heilmann, R. M., & Schrödl, W. (2024). Detection of Serum IgG Specific for Brachyspira pilosicoli and “Brachyspira canis” in Dogs. Veterinary Sciences, 11(7), 302. https://doi.org/10.3390/vetsci11070302








