Incompetence of Vector Capacity of Rhipicephalus bursa to Transmit Babesia aktasi following Feeding on Clinically Infected Goat with High Level of Parasitemia
Abstract
:Simple Summary
Abstract
1. Introduction
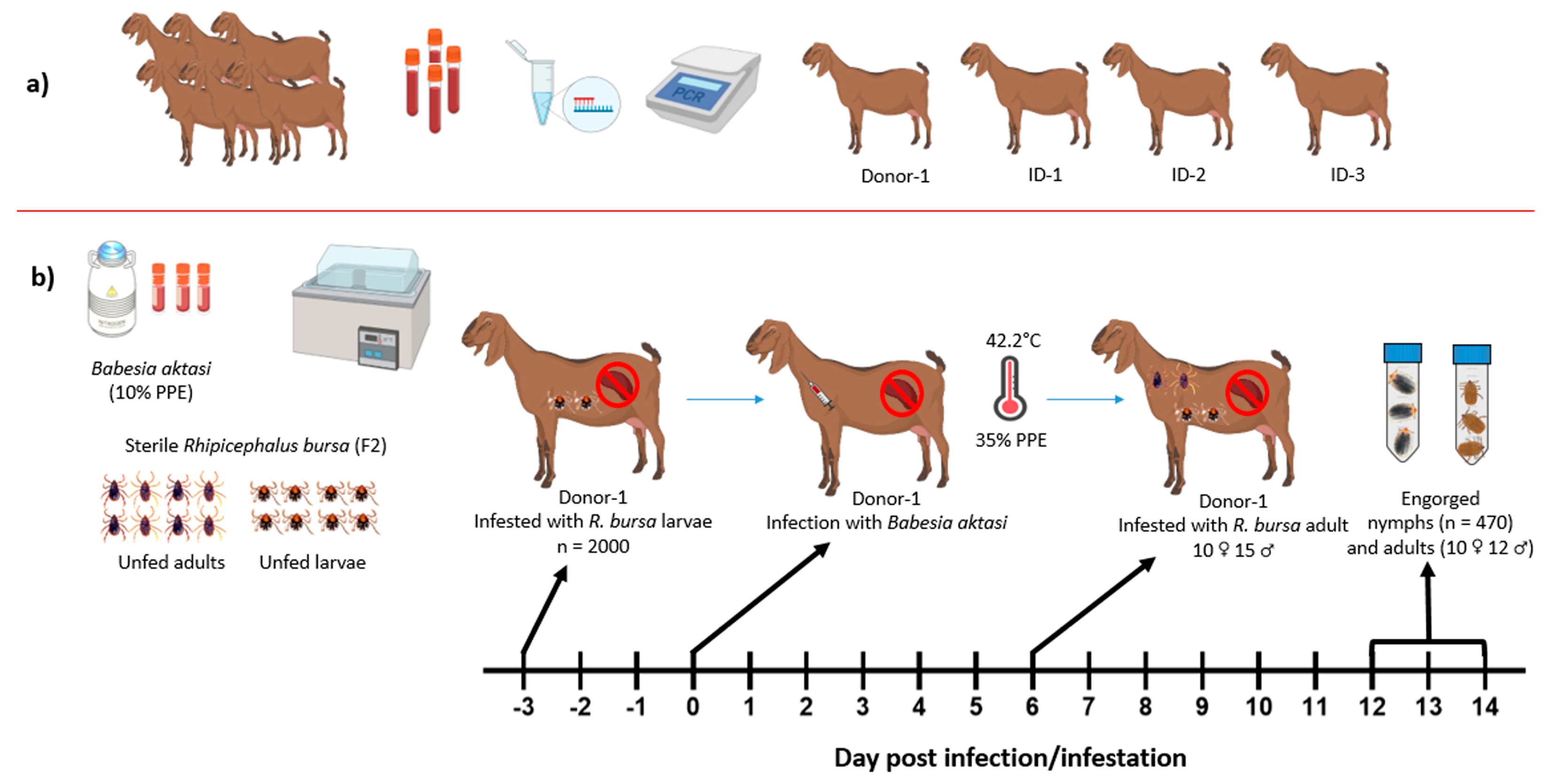
2. Materials and Methods
2.1. Ticks and Parasite
2.2. Selection of Experimental Goats
2.3. Splenectomy and Post-Operative Care
2.4. Transmission Experiments of Babesia aktasi
2.4.1. Experimental Infection of the Donor Goat
2.4.2. Acquisition of B. aktasi by R. bursa in the Clinically Infected Donor Goat
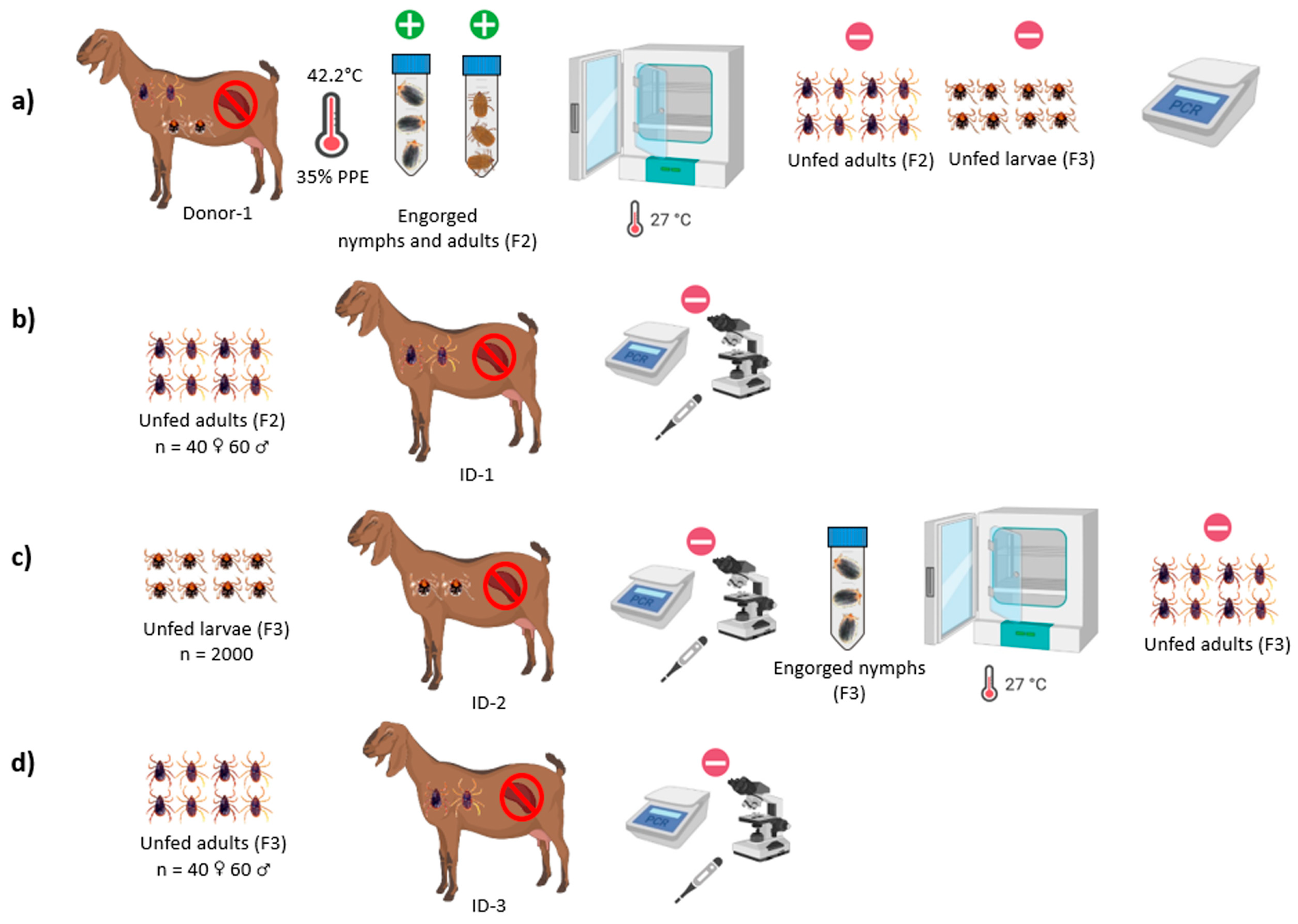
2.4.3. Transstadial and Transovarial Transmission Experiments of B. aktasi to the Recipient Goats by R. bursa
2.5. DNA Isolation from Tick and Blood Samples and nPCR
2.6. Microscopic Detection
3. Results
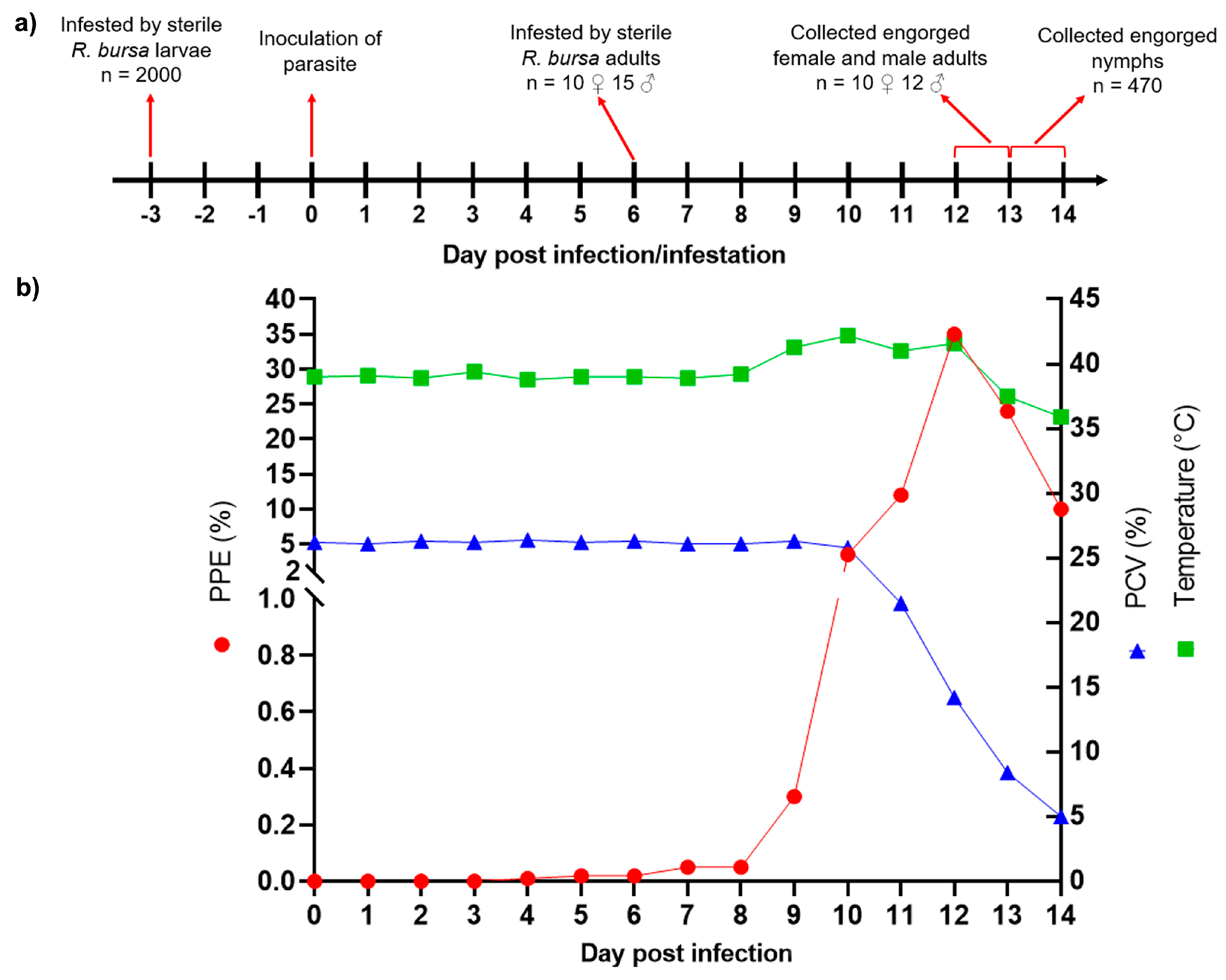
3.1. Experimental Infection of B. aktasi in Donor Goat
3.2. Outcome of Acquisition of B. aktasi by Immature and Adult Stages of R. bursa Fed on the Clinically Infected Donor Goat
3.3. Babesia Aktasi Is Not Transstadially and Transovarially Transmitted to Immune-Suppressed Goats by R. bursa
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gray, J.S.; Estrada-Peña, A.; Zintl, A. Vectors of babesiosis. Annu. Rev. Entomol. 2019, 64, 149–165. [Google Scholar] [CrossRef] [PubMed]
- Schnittger, L.; Ganzinelli, S.; Bhoora, R.; Omondi, D.; Nijhof, A.M.; Florin-Christensen, M. The Piroplasmida Babesia, Cytauxzoon, and Theileria in farm and companion animals: Species compilation, molecular phylogeny, and evolutionary insights. Parasitol. Res. 2022, 121, 1207–1245. [Google Scholar] [CrossRef] [PubMed]
- Sevinc, F.; Sevinc, M.; Ekici, O.D.; Yildiz, R.; Isik, N.; Aydogdu, U. Babesia ovis infections: Detailed clinical and laboratory observations in the pre- and post-treatment periods of 97 field cases. Vet. Parasitol. 2013, 191, 35–43. [Google Scholar] [CrossRef]
- Stuen, S. Haemoparasites—Challenging and wasting infections in small ruminants: A review. Animals 2020, 10, 2179. [Google Scholar] [CrossRef] [PubMed]
- Ozubek, S.; Bastos, R.G.; Alzan, H.F.; Inci, A.; Aktas, M.; Suarez, C.E. Bovine babesiosis in Turkey: Impact, current gaps, and opportunities for intervention. Pathogens 2020, 9, 1041. [Google Scholar] [CrossRef] [PubMed]
- Galon, E.M.; Zafar, I.; Ji, S.; Li, H.; Ma, Z.; Xuan, X. Molecular reports of ruminant Babesia in Southeast Asia. Pathogens 2022, 11, 915. [Google Scholar] [CrossRef] [PubMed]
- Schnittger, L.; Rodriguez, A.E.; Florin-Christensen, M.; Morrison, D.A. Babesia: A world emerging. Infect. Genet. Evol. 2012, 12, 1788–1809. [Google Scholar] [CrossRef] [PubMed]
- Jalovecka, M.; Sojka, D.; Ascencio, M.; Schnittger, L. Babesia life cycle—When phylogeny meets biology. Trends. Parasitol. 2019, 35, 356–368. [Google Scholar] [CrossRef] [PubMed]
- Bonnet, S.I.; Nadal, C. Experimental infection of ticks: An essential tool for the analysis of Babesia species biology and transmission. Pathogens 2021, 10, 1403. [Google Scholar] [CrossRef]
- Friedhoff, K.T. Transmission of Babesia. In Babesiosis of Domestic Animals and Man; CRC Press: Boca Raton, FL, USA, 1988; ISBN 978-1-351-07002-7. [Google Scholar]
- Guan, G.; Ma, M.; Moreau, E.; Liu, J.; Lu, B.; Bai, Q.; Luo, J.; Jorgensen, W.; Chauvin, A.; Yin, H. A new ovine Babesia species transmitted by Hyalomma anatolicum anatolicum. Exp. Parasitol. 2009, 122, 261–267. [Google Scholar] [CrossRef]
- Guan, G.; Moreau, E.; Liu, J.; Hao, X.; Ma, M.; Luo, J.; Chauvin, A.; Yin, H. Babesia sp. BQ1 (Lintan): Molecular evidence of experimental transmission to sheep by Haemaphysalis qinghaiensis and Haemaphysalis longicornis. Parasitol. Int. 2010, 59, 265–267. [Google Scholar] [CrossRef]
- Esmaeilnejad, B.; Tavassoli, M.; Asri-Rezaei, S.; Dalir-Naghadeh, B.; Mardani, K.; Jalilzadeh-Amin, G.; Golabi, M.; Arjmand, J. PCR-based detection of Babesia ovis in Rhipicephalus bursa and small ruminants. J. Parasitol. Res. 2014, 2014, 294704. [Google Scholar] [CrossRef] [PubMed]
- Aydin, M.F.; Aktas, M.; Dumanli, N. Molecular identification of Theileria and Babesia in ticks collected from sheep and goats in the Black Sea Region of Turkey. Parasitol. Res. 2015, 114, 65–69. [Google Scholar] [CrossRef] [PubMed]
- Erster, O.; Roth, A.; Wolkomirsky, R.; Leibovich, B.; Savitzky, I.; Shkap, V. Transmission of Babesia ovis by different Rhipicephalus bursa developmental stages and infected blood injection. Ticks Tick Borne Dis. 2016, 7, 13–19. [Google Scholar] [CrossRef] [PubMed]
- Friedhoff, K.T. Tick-Borne Diseases of sheep and goats caused by Babesia, Theileria or Anaplasma spp. Parassitologia 1997, 39, 99–109. [Google Scholar] [PubMed]
- Yeruham, I.; Hadani, A.; Galker, F. Some epizootiological and clinical aspects of ovine babesiosis caused by Babesia ovis-A review. Vet. Parasitol. 1998, 74, 153–163. [Google Scholar] [CrossRef] [PubMed]
- Yeruham, I.; Hadani, A.; Galker, F. The effect of the ovine host parasitaemia on the development of Babesia ovis (Babes, 1892) in the tick Rhipicephalus bursa (Canestrini and Fanzago, 1877). Vet. Parasitol. 2001, 96, 195–202. [Google Scholar] [CrossRef] [PubMed]
- Altay, K.; Aktas, M.; Dumanli, N. Detection of Babesia ovis by PCR in Rhipicephalus bursa collected from naturally infested sheep and goats. Res. Vet. Sci. 2008, 85, 116–119. [Google Scholar] [CrossRef] [PubMed]
- Liu, A.H.; Yin, H.; Guan, G.Q.; Schnittger, L.; Liu, Z.J.; Ma, M.L.; Dang, Z.S.; Liu, J.L.; Ren, Q.Y.; Bai, Q.; et al. At least two genetically distinct large Babesia species infective to sheep and goats in China. Vet. Parasitol. 2007, 147, 246–251. [Google Scholar] [CrossRef]
- Niu, Q.; Liu, Z.; Yang, J.; Yu, P.; Pan, Y.; Zhai, B.; Luo, J.; Yin, H. Genetic diversity and molecular characterization of Babesia motasi-like in small ruminants and ixodid ticks from China. Infect. Genet. Evol. 2016, 41, 8–15. [Google Scholar] [CrossRef]
- Ozubek, S.; Aktas, M. Molecular evidence of a new Babesia sp. in goats. Vet. Parasitol. 2017, 233, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Ozubek, S.; Ulucesme, M.C.; Aktas, M. Discovery of a novel species infecting goats: Morphological and molecular characterization of Babesia aktasi n. sp. Pathogens 2023, 12, 113. [Google Scholar] [CrossRef] [PubMed]
- Ulucesme, M.C.; Ozubek, S.; Karoglu, A.; Turk, Z.I.; Olmus, I.; Irehan, B.; Aktas, M. Small ruminant piroplasmosis: High prevalence of Babesia aktasi n. sp. in goats in Türkiye. Pathogens 2023, 12, 514. [Google Scholar] [CrossRef]
- Ozubek, S.; Ulucesme, M.C.; Bastos, R.G.; Alzan, H.F.; Laughery, J.M.; Suarez, C.E.; Aktas, M. Experimental infection of non-immunosuppressed and immunosuppressed goats reveals differential pathogenesis of Babesia aktasi n. sp. Front. Cell. Infect. Microbiol. 2023, 13, 1277956. [Google Scholar] [CrossRef] [PubMed]
- Aydin, M.F.; Aktas, M.; Dumanli, N. Molecular identification of Theileria and Babesia in sheep and goats in the Black Sea Region in Turkey. Parasitol. Res. 2013, 112, 2817–2824. [Google Scholar] [CrossRef] [PubMed]
- Sevinc, F.; Turgut, K.; Sevinc, M.; Ekici, O.D.; Coskun, A.; Koc, Y.; Erol, M.; Ica, A. Therapeutic and prophylactic efficacy of imidocarb dipropionate on experimental Babesia ovis infection of lambs. Vet. Parasitol. 2007, 149, 65–71. [Google Scholar] [CrossRef]
- Guan, G.; Yin, H.; Luo, J.; Lu, W.; Zhang, Q.; Gao, Y.; Lu, B. Transmission of Babesia sp to sheep with field-collected Haemaphysalis qinghaiensis. Parasitol. Res. 2002, 88, S22–S24. [Google Scholar] [CrossRef]
- Almazán, C.; Bonnet, S.; Cote, M.; Slovák, M.; Park, Y.; Šimo, L. A versatile model of hard tick infestation on laboratory rabbits. JoVE J. Vis. Exp. 2018, 140, e57994. [Google Scholar] [CrossRef]
- Levin, M.L.; Schumacher, L.B.M. Manual for maintenance of multi-host ixodid ticks in the laboratory. Exp. Appl. Acarol. 2016, 70, 343–367. [Google Scholar] [CrossRef]
- Aktas, M.; Vatansever, Z.; Altay, K.; Aydin, M.F.; Dumanli, N. Molecular evidence for Anaplasma phagocytophilum in Ixodes ricinus from Turkey. Trans. R. Soc. Trop. Med. Hyg. 2010, 104, 10–15. [Google Scholar] [CrossRef]
- Georges, K.; Loria, G.R.; Riili, S.; Greco, A.; Caracappa, S.; Jongejan, F.; Sparagano, O. Detection of haemoparasites in cattle by reverse line blot hybridisation with a note on the distribution of ticks in Sicily. Vet. Parasitol. 2001, 99, 273–286. [Google Scholar] [CrossRef]
- Oosthuizen, M.C.; Zweygarth, E.; Collins, N.E.; Troskie, M.; Penzhorn, B.L. Identification of a novel Babesia sp. from a sable antelope (Hippotragus Niger Harris, 1838). J. Clin. Microbiol. 2008, 46, 2247–2251. [Google Scholar] [CrossRef] [PubMed]
- Bekker, C.P.J.; de Vos, S.; Taoufik, A.; Sparagano, O.A.E.; Jongejan, F. Simultaneous detection of Anaplasma and Ehrlichia species in ruminants and detection of Ehrlichia ruminantium in Amblyomma variegatum ticks by reverse line blot hybridization. Vet. Microbiol. 2002, 89, 223–238. [Google Scholar] [CrossRef] [PubMed]
- Kawahara, M.; Rikihisa, Y.; Lin, Q.; Isogai, E.; Tahara, K.; Itagaki, A.; Hiramitsu, Y.; Tajima, T. Novel genetic variants of Anaplasma phagocytophilum, Anaplasma bovis, Anaplasma centrale, and a novel Ehrlichia sp. in wild deer and ticks on two major islands in Japan. Appl. Environ. Microbiol. 2006, 72, 1102–1109. [Google Scholar] [CrossRef]
- Black, W.C.; Piesman, J. Phylogeny of hard- and soft-tick taxa (Acari: Ixodida) based on mitochondrial 16S rDNA sequences. Proc. Natl. Acad. Sci. USA 1994, 91, 10034–10038. [Google Scholar] [CrossRef] [PubMed]
- Smith, T.; Kilborne, F.L. Investigations into the Nature, Causation, and Prevention of Texas or Southern Cattle Fever; US Department of Agriculture, Bureau of Animal Industry: Washington, DC, USA, 1893. Available online: https://books.google.co.jp/books?hl=en&lr=&id=YUIWAAAAYAAJ&oi=fnd&pg=PA9&dq=Investigations+into+the+Nature,+Causation,+and+Prevention+of+Texas+or+Southern+Cattle+Fever%3B+US+Department+of+Agriculture,+Bureau+of+Animal+Industry&ots=AG8ZevCeXs&sig=eXC8rHGgsxu5e4Oz3Ixa5D0J35M&redir_esc=y#v=onepage&q=Investigations%20into%20the%20Nature%2C%20Causation%2C%20and%20Prevention%20of%20Texas%20or%20Southern%20Cattle%20Fever%3B%20US%20Department%20of%20Agriculture%2C%20Bureau%20of%20Animal%20Industry&f=false (accessed on 10 May 2024).
- Beerntsen, B.T.; James, A.A.; Christensen, B.M. Genetics of mosquito vector competence. Microbiol. Mol. Biol. Rev. 2000, 64, 115–137. [Google Scholar] [CrossRef]
- De la Fuente, J.; Antunes, S.; Bonnet, S.; Cabezas-Cruz, A.; Domingos, A.G.; Estrada-Peña, A.; Johnson, N.; Kocan, K.M.; Mansfield, K.L.; Nijhof, A.M. Tick-pathogen interactions and vector competence: Identification of molecular drivers for tick-borne diseases. Front. Cell. Infect. Microbiol. 2017, 7, 114. [Google Scholar] [CrossRef]
- Bajer, A.; Dwużnik-Szarek, D. The specificity of Babesia-tick vector interactions: Recent advances and pitfalls in molecular and field studies. Parasites Vectors 2021, 14, 507. [Google Scholar] [CrossRef] [PubMed]
- Eisen, L. Vector competence studies with hard ticks and Borrelia burgdorferi sensu lato spirochetes: A review. Ticks Tick Borne Dis. 2020, 11, 101359. [Google Scholar] [CrossRef]
- Levin, M.L.; Stanley, H.M.; Hartzer, K.; Snellgrove, A.N. Incompetence of the Asian longhorned tick (Acari: Ixodidae) in transmitting the agent of human granulocytic anaplasmosis in the United States. J. Med. Entomol. 2021, 58, 1419–1423. [Google Scholar] [CrossRef]
- Breuner, N.E.; Ford, S.L.; Hojgaard, A.; Osikowicz, L.M.; Parise, C.M.; Rosales Rizzo, M.F.; Bai, Y.; Levin, M.L.; Eisen, R.J.; Eisen, L. Failure of the Asian longhorned tick, Haemaphysalis longicornis, to serve as an experimental vector of the lyme disease spirochete, Borrelia burgdorferi sensu stricto. Ticks Tick Borne Dis. 2020, 11, 101311. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Xu, R. Ability of Ixodes persulcatus, Haemaphysalis concinna and Dermacentor silvarum ticks to acquire and transstadially transmit Borrelia garinii. Exp. Appl. Acarol. 2003, 31, 151–160. [Google Scholar] [CrossRef]
- Sun, Y.; Xu, R.M.; Guo, T.Y.; Zhang, P.H.; Cao, W.C. Incapability of Haemaphysalis longicornis and Dermacentor nuttalli to acquire and trans-stadial transmit the Lyme spirochetes Borrelia garinii. Acta Parasitol. Med. Entomol. Sin. 2003, 10, 174–180. [Google Scholar]
- Sevinc, F.; Zhou, M.; Cao, S.; Ceylan, O.; Aydin, M.F.; Sevinc, M.; Xuan, X. Haemoparasitic agents associated with ovine babesiosis: A possible negative interaction between Babesia ovis and Theileria ovis. Vet. Parasitol. 2018, 252, 143–147. [Google Scholar] [CrossRef] [PubMed]
- Razmi, G.; Nouroozi, E. Transovarial transmission of Babesia ovis by Rhipicephalus sanguineus and Hyalomma marginatum. Iran. J. Parasitol. 2010, 5, 35–39. [Google Scholar] [PubMed]
- Jongejan, F.; Su, B.-L.; Yang, H.-J.; Berger, L.; Bevers, J.; Liu, P.-C.; Fang, J.-C.; Cheng, Y.-W.; Kraakman, C.; Plaxton, N. Molecular evidence for the transovarial passage of Babesia gibsoni in Haemaphysalis hystricis (Acari: Ixodidae) ticks from Taiwan: A novel vector for canine babesiosis. Parasites Vectors 2018, 11, 134. [Google Scholar] [CrossRef] [PubMed]
- Kusakisako, K.; Maeda, H.; Galay, R.L.; Matsuo, T.; Tsujio, M.; Umemiya-Shirafuji, R.; Mochizuki, M.; Fujisaki, K.; Tanaka, T. The vector potential of Haemaphysalis longicornis ticks for Babesia microti parasites under experimental condition. J. Protozool. Res. 2015, 25, 8–17. [Google Scholar]
- Ulucesme, M.C.; Ozubek, S.; Aktas, M. Molecular prevalence and genetic diversity based on Msp1a gene of Anaplasma ovis in goats from Türkiye. Life 2023, 13, 1101. [Google Scholar] [CrossRef]
| Tick Stage (Generation) | Source of Ticks | No. of Ticks in Each Pool | No. of Pools | No. of Total Ticks |
|---|---|---|---|---|
| Engorged nymph (F2) | #Donor-1 | 1 * | 20 | 20 |
| Engorged Female carcasses (F2) | #Donor-1 | 1 * | 10 | 10 |
| Unfed adult tick (F2) | #Donor-1 | 3 | 25 | 75 |
| Unfed larvae (F3) | #Donor-1 | 150 | 50 | 7500 |
| Unfed adult ticks (F3) | #ID-2 | 3 | 25 | 75 |
| Tick Stage and Feeding Status | Generation | Transmission Route | No. PCR-Positive Ticks/No. Ticks Tested (% Infection) |
|---|---|---|---|
| Engorged nymphs (individual) | F2 | Transstadial | 20/20 (100%) |
| Unfed adults (pool) | F2 | Transstadial | 0/25 |
| Engorged female carcasses | F2 | - | 10/10 (100%) |
| Unfed larvae (pool) | F3 | Transovarial | 0/50 |
| Unfed adults (pool) | F3 | Transovarial | 0/25 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ulucesme, M.C.; Ozubek, S.; Aktas, M. Incompetence of Vector Capacity of Rhipicephalus bursa to Transmit Babesia aktasi following Feeding on Clinically Infected Goat with High Level of Parasitemia. Vet. Sci. 2024, 11, 309. https://doi.org/10.3390/vetsci11070309
Ulucesme MC, Ozubek S, Aktas M. Incompetence of Vector Capacity of Rhipicephalus bursa to Transmit Babesia aktasi following Feeding on Clinically Infected Goat with High Level of Parasitemia. Veterinary Sciences. 2024; 11(7):309. https://doi.org/10.3390/vetsci11070309
Chicago/Turabian StyleUlucesme, Mehmet Can, Sezayi Ozubek, and Munir Aktas. 2024. "Incompetence of Vector Capacity of Rhipicephalus bursa to Transmit Babesia aktasi following Feeding on Clinically Infected Goat with High Level of Parasitemia" Veterinary Sciences 11, no. 7: 309. https://doi.org/10.3390/vetsci11070309
APA StyleUlucesme, M. C., Ozubek, S., & Aktas, M. (2024). Incompetence of Vector Capacity of Rhipicephalus bursa to Transmit Babesia aktasi following Feeding on Clinically Infected Goat with High Level of Parasitemia. Veterinary Sciences, 11(7), 309. https://doi.org/10.3390/vetsci11070309








